The Impact of High-Dose Fish Oil Supplementation on Mfsd2a, Aqp4, and Amyloid-β Expression in Retinal Blood Vessels of 5xFAD Alzheimer’s Mouse Model
Abstract
1. Introduction
2. Results
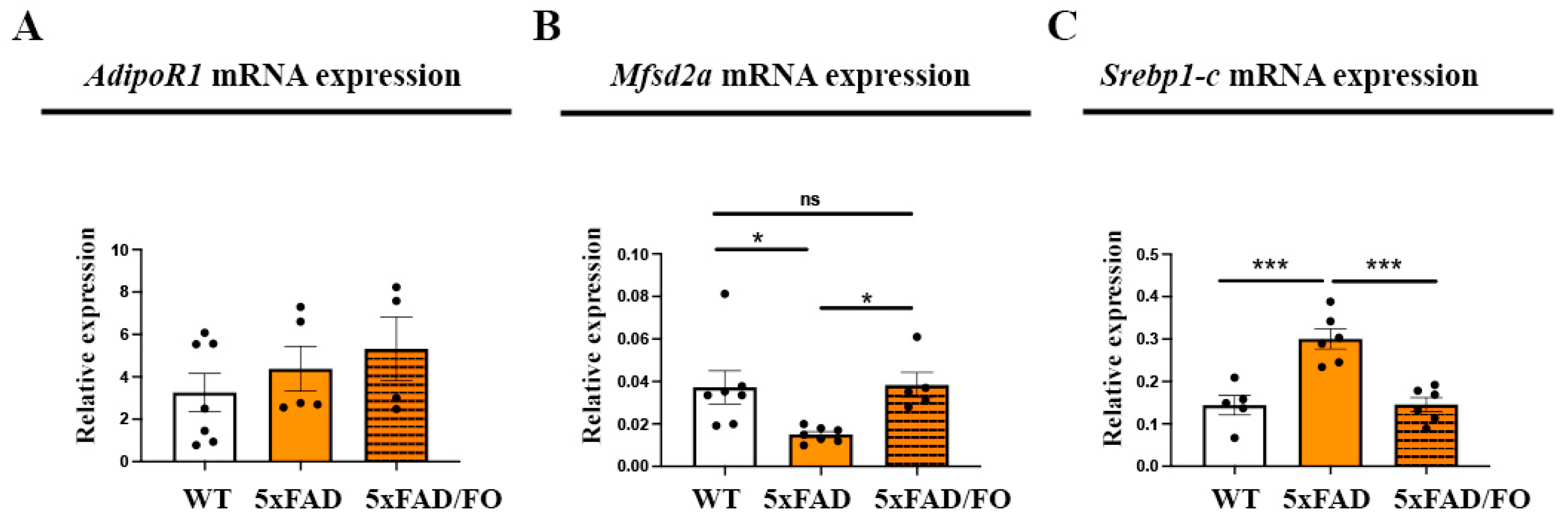
2.1. The Expression Levels of DHA Transporter Mfsd2a in 5xFAD Retinas Were Increased Following the High-Dose FO Treatment
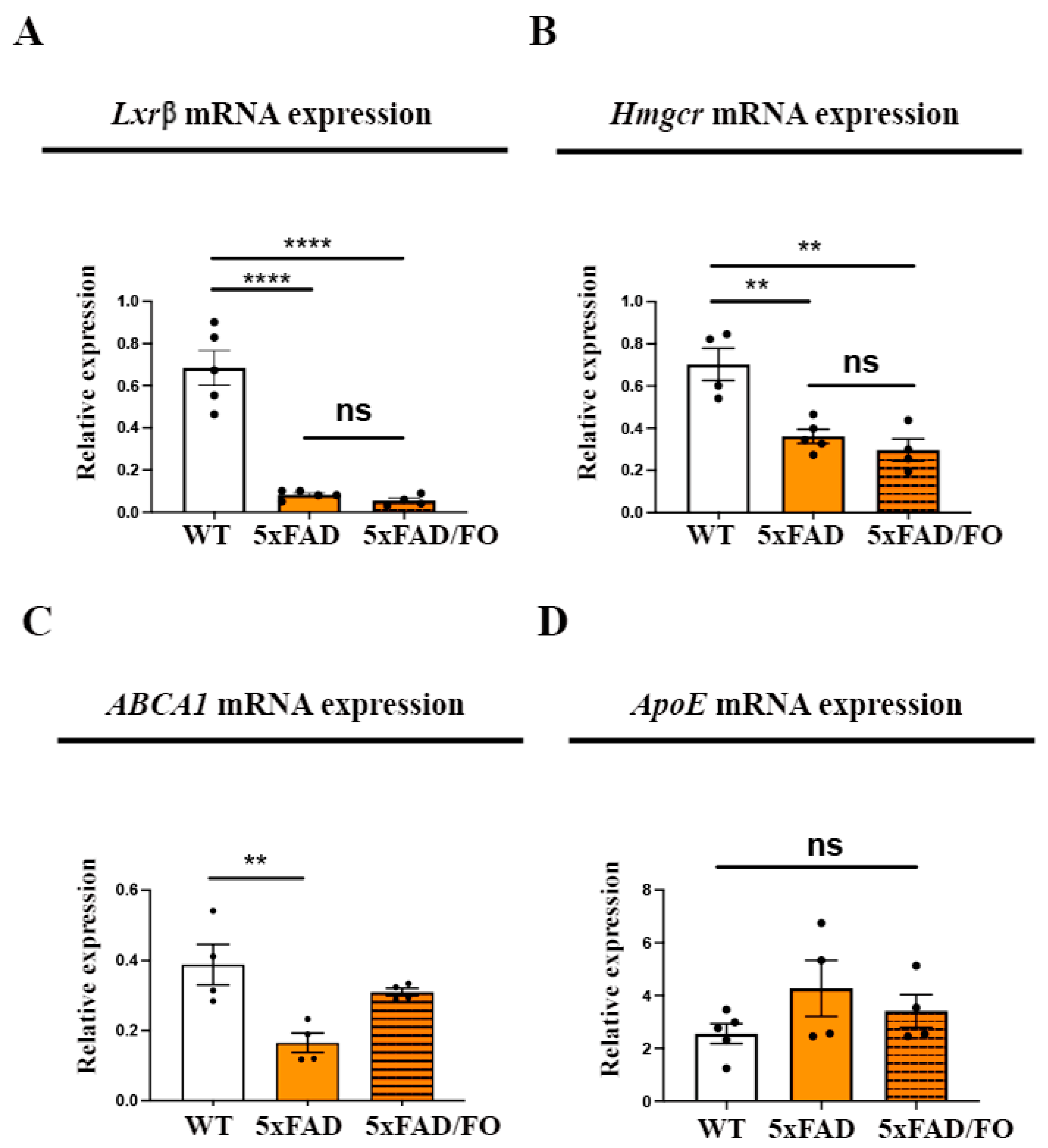
2.2. High-Dose FO Supplementation Did Not Alter the Expression of the Genes Involved in Regulating Cholesterol Synthesis and Transport in 5xFAD Retinas
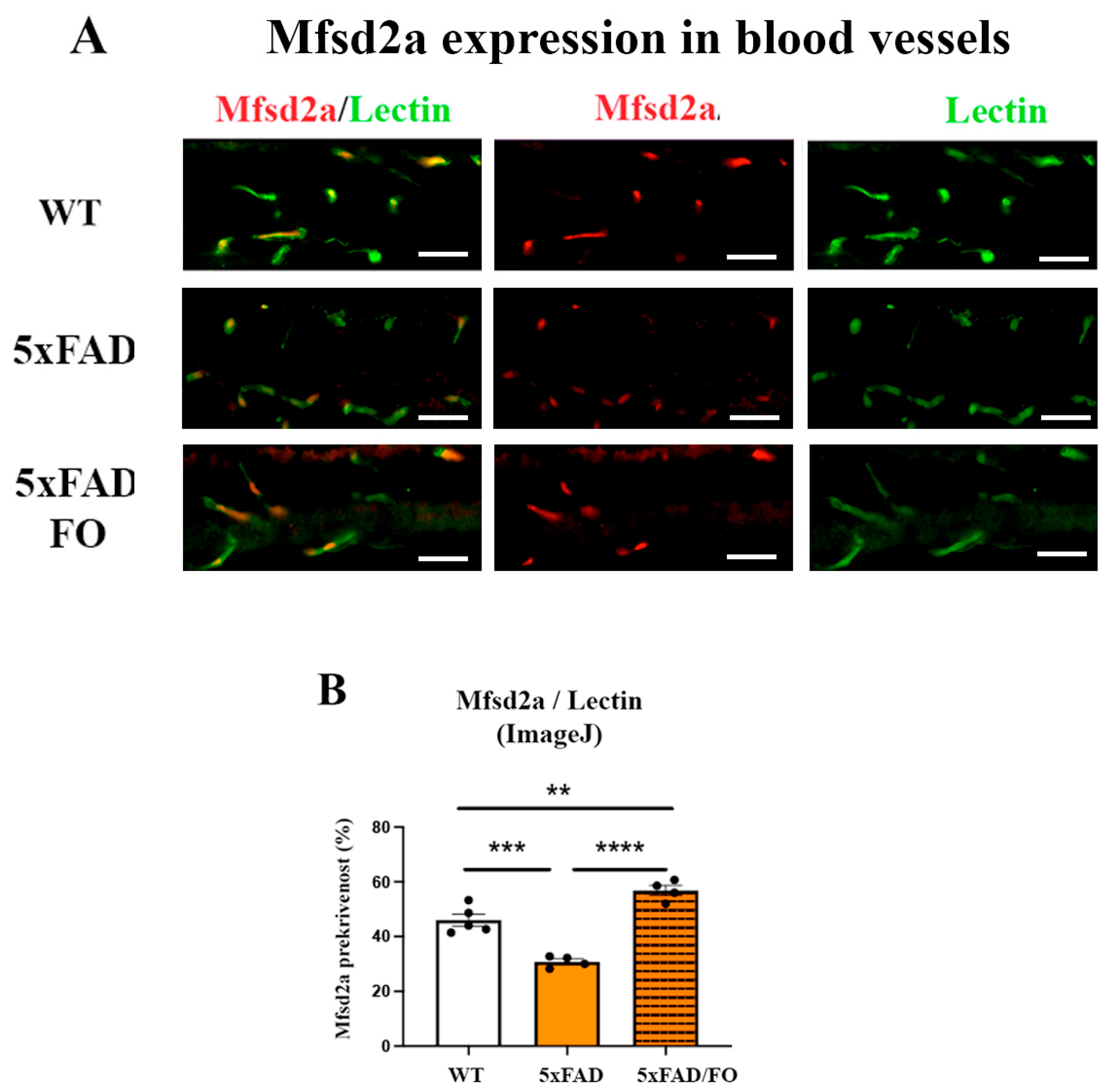
2.3. The High-Dose FO Supplementation Increased Mfsd2a Blood Vessels Coverage in 4M 5xFAD Retinas
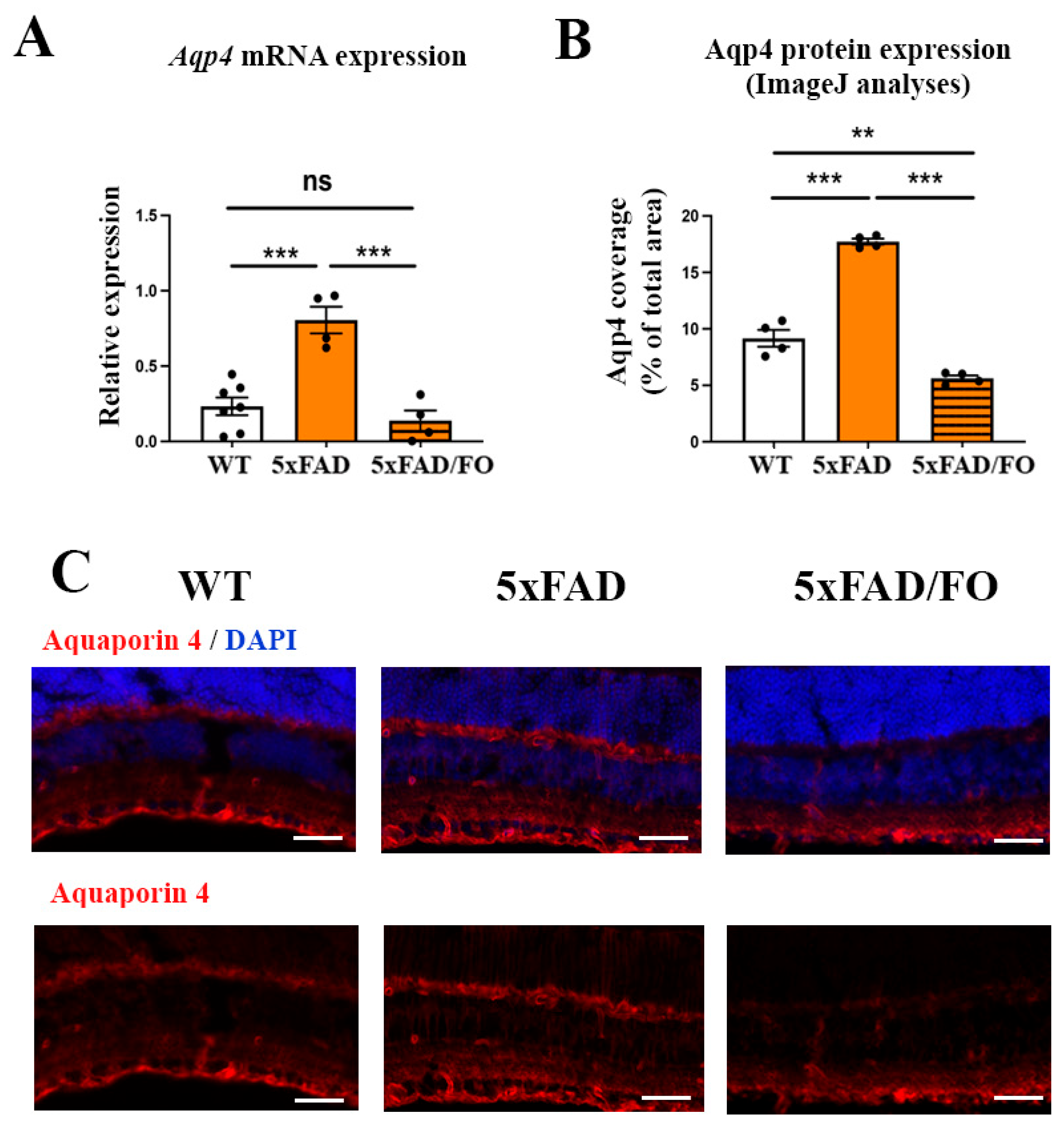
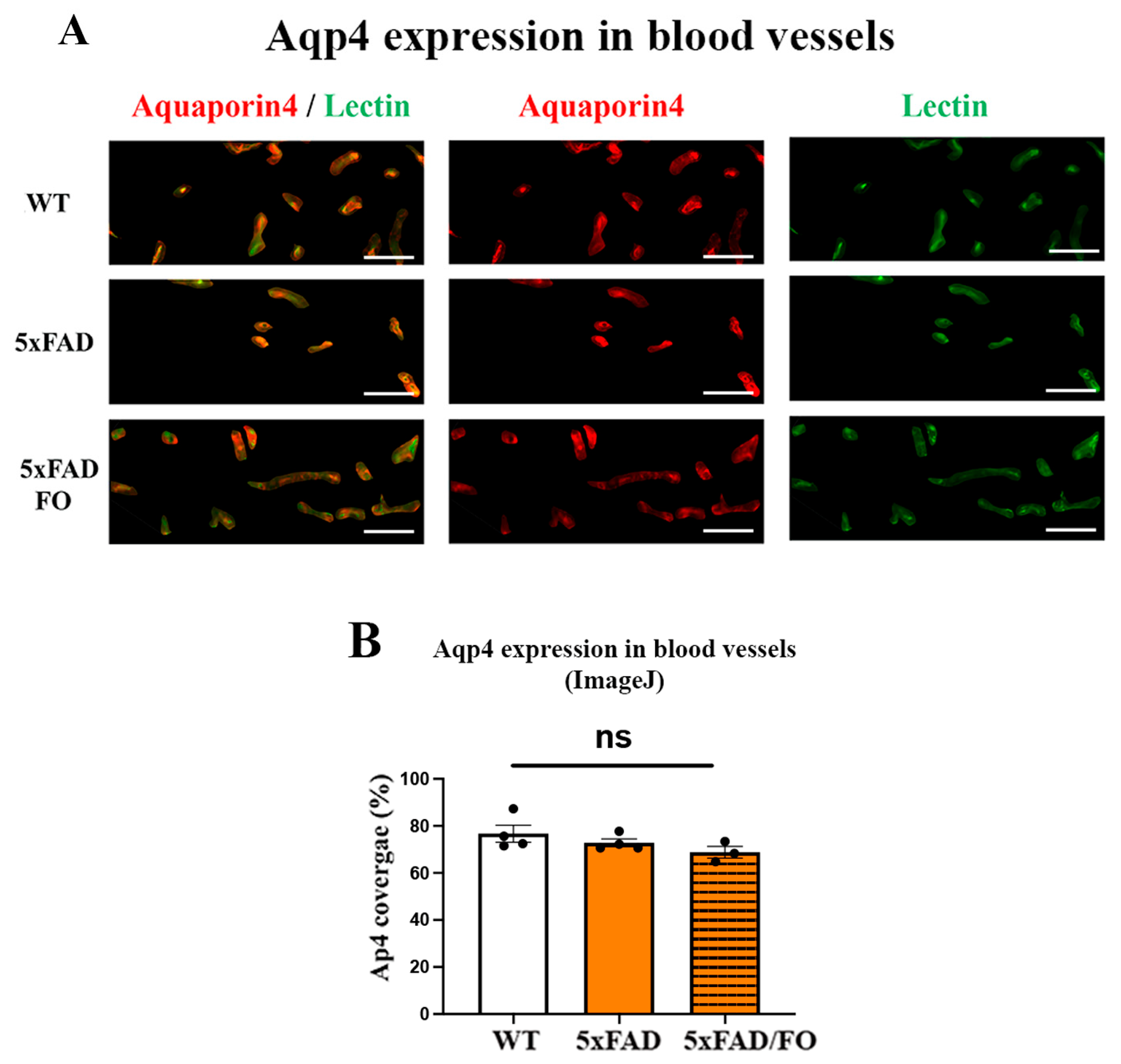
2.4. The High-Dose FO Supplementation Reduced the Levels of Aqp4 Expression in 4M 5xFAD Retinas
2.5. The High-Dose FO Supplementation Did Not Alter the Perivascular Aqp4 Expression in FO Supplemented 4M 5xFAD Retinas
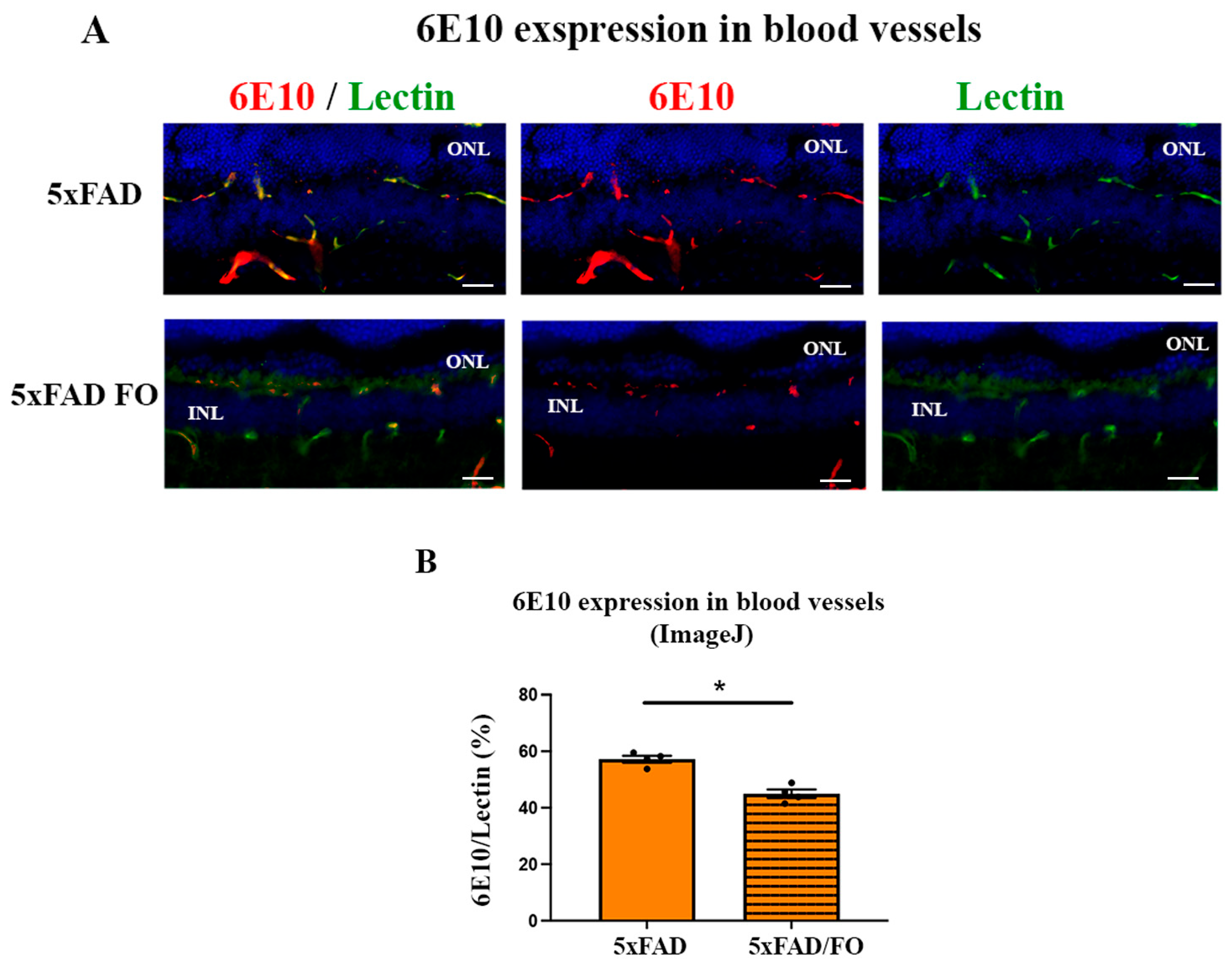
2.6. The High-Dose FO-Supplementation Decreased Amyloid β Accumulation in Retinal Blood Vessels in 4M 5xFAD Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Treatment
4.3. Tissue Collection
4.4. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4.4.1. RNA Isolation and Reverse Transcription
4.4.2. Quantitative RT-PCR (qRT-PCR)
4.5. Immunohistochemistry
4.6. Quantification of Perivascular Aqp4 Expression
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breteler, M.M. Vascular risk factors for Alzheimer’s disease: An epidemiologic perspective. Neurobiol. Aging 2000, 21, 153–160. [Google Scholar] [CrossRef]
- Bell, R.D.; Zlokovic, B.V. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009, 118, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Preston, S.D.; Subash, M.; Carare, R.O. Cerebral amyloid angiopathy in the aetiology and immunotherapy of Alzheimer disease. Alzheimer’s Res. Ther. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Zipser, B.D.; Johanson, C.E.; Gonzalez, L.; Berzin, T.M.; Tavares, R.; Hulette, C.M.; Vitek, M.P.; Hovanesian, V.; Stopa, E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 977–986. [Google Scholar] [CrossRef] [PubMed]
- van de Haar, H.J.; Jansen, J.F.A.; van Osch, M.J.P.; van Buchem, M.A.; Muller, M.; Wong, S.M.; Hofman, P.A.M.; Burgmans, S.; Verhey, F.R.J.; Backes, W.H. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol. Aging 2016, 45, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, V.T. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: Implications for early detection and therapy. FASEB 2011, 25, 5–13. [Google Scholar] [CrossRef]
- Brown, W.R.; Thore, C.R. Review: Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011, 37, 56–74. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Fuchs, D.T.; Sheyn, J.; Wawrowsky, K.; Lahiri, S.; Back, K.L.; Koronyo-Hamaoui, M. Retinal capillary degeneration and blood-retinal barrier disruption in murine models of Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 202. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Rentsendorj, A.; Regis, G.C.; Sheyn, J.; Fuchs, D.T.; Kramerov, A.A.; Ljubimov, A.V.; Dumitrascu, O.M.; Rodriguez, A.R.; et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 2020, 139, 813–836. [Google Scholar] [CrossRef]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.-T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2, e93621. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Fuchs, D.-T.; Sheyn, J.; Jallow, O.; Mandalia, K.; Graham, S.L.; Gupta, V.K.; Mirzaei, M.; Kramerov, A.A.; et al. Retinal arterial Aβ40 deposition is linked with tight junction loss and cerebral amyloid angiopathy in MCI and AD patients. Alzheimer’s Dement. 2023, 19, 5185–5197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zeng, R.; Wu, G.; Hu, Y.; Yu, H. Beyond Vision: A View from Eye to Alzheimer’s Disease and Dementia. J. Prev. Alzheimers Dis. 2024, 11, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.W.; Gu, C. Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation. Neuron 2017, 93, 1325–1333. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Yang, Y.-R.; Xiong, X.-Y.; Liu, J.; Wu, L.-R.; Zhong, Q.; Zhou, K.; Meng, Z.-Y.; Liu, L.; Wang, F.-X.; Gong, Q.-W.; et al. Mfsd2a (Major Facilitator Superfamily Domain Containing 2a) Attenuates Intracerebral Hemorrhage-Induced Blood-Brain Barrier Disruption by Inhibiting Vesicular Transcytosis. J. Am. Heart Ass. 2017, 6, e005811. [Google Scholar] [CrossRef]
- Macura, J.I.; Zivanovic, A.; Perovic, M.; Ciric, J.; Major, T.; Kanazir, S.; Ivkovic, S. The Expression of Major Facilitator Superfamily Domain-Containing Protein2a (Mfsd2a) and Aquaporin 4 Is Altered in the Retinas of a 5xFAD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14092. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The glymphatic system: A beginner’s guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. Paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Wang, X.; Lou, N.; Eberhardt, A.; Yang, Y.; Kusk, P.; Xu, Q.; Forstera, B.; Peng, S.; Shi, M.; Ladron-de-Guevara, A.; et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci. Transl. Med. 2020, 12, eaaw3210. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutri, S.; Logan, A.; Kreida, S.; et al. Targeting aquaporin-4 subcellular localization to treat central nervous system edema. Cell 2020, 181, 784–799.e719. [Google Scholar] [CrossRef]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of Perivascular Localization of Aquaporin-4 with Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017, 74, 91. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Yamamoto, T.; Shimizu, K.; Yoshikazu, U.; Nishizawa, M.; Takahashi, H.; Kakita, A. Characteristics of aquaporin expression surrounding senile plaques and cerebral amyloid angiopathy in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012, 71, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Moftakhar, P.; Lynch, M.D.; Pomakian, J.L.; Vinters, H.V. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 2010, 69, 1201–1209. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef]
- Sinclair, A.J. Docosahexaenoic acid and the brain- what is its role? Asia Pac. J. Clin. Nutr. 2019, 28, 675–688. [Google Scholar] [CrossRef]
- Scott, B.L.; Bazan, N.G. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc. Natl. Acad. Sci. USA 1989, 86, 2903–2907. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zlokovic, B.V. Blood-brain barrier: A dual life of MFSD2A? Neuron 2014, 82, 728–730. [Google Scholar] [CrossRef]
- Ren, H.; Luo, C.; Feng, Y.; Yao, X.; Shi, Z.; Liang, F.; Kang, J.X.; Wan, J.B.; Pei, Z.; Su, H. Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017, 31, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Wan, X.; Yang, L.; Wang, D.; Chen, Z.; Chen, Y.; Liu, M.; Zhang, G.; Wu, J.; Han, H.; et al. Omega-3 Polyunsaturated Fatty Acids Alleviate Traumatic Brain Injury by Regulating the Glymphatic Pathway in Mice. Front. Neurol. 2020, 11, 707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arellanes, I.C.; Choe, N.; Solomon, V.; He, X.; Kavin, B.; Martinez, A.E.; Kono, N.; Buennagel, D.P.; Hazra, N.; Kim, G.; et al. Brain delivery of supplemental docosahexaenoic acid (DHA): A randomized placebo-controlled clinical trial. EBioMedicine 2020, 59, 102883. [Google Scholar] [CrossRef] [PubMed]
- Hooperton, K.E.; Trépanier, M.O.; James, N.C.E.; Chouinard-Watkins, R.; Bazinet, R.P. Fish oil feeding attenuates neuroinflammatory gene expression without concomitant changes in brain eicosanoids and docosanoids in a mouse model of Alzheimer’s disease. Brain Behav. Immunol. 2018, 69, 74–90. [Google Scholar] [CrossRef]
- Macura Jovanovic, I.; Djuricic, I.; Major, T.; Milanovic, D.; Brkic, B.; Sobajic, S.; Kanazir, S.; Ivkovic, S. The high-dose fish oil supplementation increased Mfsd2a expression without altering DHA levels in the healthy retina. J. Funct. Foods 2022, 99, 105302. [Google Scholar] [CrossRef]
- Milanovic, D.; Petrovic, S.; Brkic, M.; Avramovic, V.; Perovic, M.; Ivkovic, S.; Glibetic, M.; Kanazir, S. Short-Term Fish Oil Treatment Changes the Composition of Phospholipids While Not Affecting the Expression of Mfsd2a Omega-3 Transporter in the Brain and Liver of the 5xFAD Mouse Model of Alzheimer’s Disease. Nutrients 2018, 10, 1250. [Google Scholar] [CrossRef]
- Jovic, M.; Lončarević-Vasiljković, N.; Ivković, S.; Dinić, J.; Milanović, D.; Zlokovic, B.; Kanazir, S. Short-term fish oil supplementation applied in presymptomatic stage of Alzheimer’s disease enhances microglial/macrophage barrier and prevents neuritic dystrophy in parietal cortex of 5xFAD mouse model. PLoS ONE 2019, 14, e0216726. [Google Scholar] [CrossRef] [PubMed]
- Casali, B.T.; Corona, A.W.; Mariani, M.M.; Karlo, J.C.; Ghosal, K.; Landreth, G.E. Omega-3 Fatty Acids Augment the Actions of Nuclear Receptor Agonists in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015, 35, 9173–9181. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, P.; Arango-Lievano, M.; Neves, I.D.; Rousset, M.C.; Baranger, K.; Rivera, S.; Jeanneteau, F.; Claeysen, S.; Marchi, N. Cerebrovascular pathology during the progression of experimental Alzheimer’s disease. Neurobiol. Dis. 2016, 88, 107–117. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Chow, K.M.; Derafshi, Z.; Fautsch, M.P.; Hetling, J.R.; Rodgers, D.W.; Hersh, L.B.; Pepperberg, D.R. Reduction of amyloid-beta levels in mouse eye tissues by intra-vitreally delivered neprilysin. Exp. Eye Res. 2015, 138, 134–144. [Google Scholar] [CrossRef]
- Matei, N.; Leahy, S.; Blair, N.P.; Burford, J.; Rahimi, M.; Shahidi, M. Retinal Vascular Physiology Biomarkers in a 5XFAD Mouse Model of Alzheimer’s Disease. Cells 2022, 11, 2413. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Sugasini, D.; Park, J.C.; McAnany, J.J.; Kim, T.H.; Ma, G.; Yao, X.; Antharavally, B.; Oroskar, A.; Oroskar, A.A.; Layden, B.T.; et al. Improvement of retinal function in Alzheimer disease-associated retinopathy by dietary lysophosphatidylcholine-EPA/DHA. Sci. Rep. 2023, 13, 9179. [Google Scholar] [CrossRef]
- Devi, L.; Alldred, M.J.; Ginsberg, S.D.; Ohno, M. Sex- and brain region-specific acceleration of β-amyloidogenesis following behavioral stress in a mouse model of Alzheimer’s disease. Mol. Brain 2010, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.H.; Chan, J.P.; Cazenave-Gassiot, A.; Poh, R.W.; Foo, J.C.; Galam, D.L.; Ghosh, S.; Nguyen, L.N.; Barathi, V.A.; Yeo, S.W.; et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid (DHA) in eye and is important for photoreceptor cell development. J. Biol. Chem. 2016, 291, 10501–10514. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.S.; Calandria, J.M.; Gordon, W.C.; Jun, B.; Zhou, Y.; Gelfman, C.M.; Li, S.; Jin, M.; Knott, E.J.; Chang, B.; et al. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat. Commun. 2015, 6, 6228, Erratum in Nat. Commun. 2015, 6, 7225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Wang, H.-L.; Li, P.-C.; Hong, C.-D.; Chen, A.-Q.; Qiu, Y.-M.; Zeng, A.-P.; Zhou, Y.-F.; Hu, B.; Li, Y.-N. Mfsd2a overexpression alleviates vascular dysfunction in diabetic retinopathy. Pharm. Res. 2021, 171, 105755. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D. Sterol regulatory element-binding proteins: Transcriptional activators of lipid synthesis. Biochem. Soc. Trans. 2002, 30, 1091–1095. [Google Scholar] [CrossRef]
- Tong, J.; Briggs, M.M.; McIntosh, T.J. Water permeability of aquaporin-4 channel depends on bilayer composition, thickness, and elasticity. Biophys. J. 2012, 103, 1899–1908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalaany, N.Y.; Mangelsdorf, D.J. LXRs and FXR: The yin and yang of cholesterol and fat metabolism. Ann. Rev. Physiol. 2006, 68, 159–191. [Google Scholar] [CrossRef]
- Siperstein, M.D.; Fagan, V.M. Feedback control of mevalonate synthesis by dietary cholesterol. JBC 1966, 241, 602–609. [Google Scholar] [CrossRef]
- Mauch, D.H.; Nagler, K.; Schumacher, S.; Goritz, C.; Muller, E.C.; Otto, A.; Pfrieger, F.W. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Lobanova, E.S.; Schuhmann, K.; Finkelstein, S.; Lewis, T.R.; Cady, M.A.; Hao, Y.; Keuthan, C.; Ash, J.D.; Burns, M.E.; Shevchenko, A.; et al. Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health But Not Visual Signal Transduction. J. Neurosci. 2019, 39, 9689–9701. [Google Scholar] [CrossRef] [PubMed]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sagare, A.P.; Ma, Q.; Halliday, M.R.; Kong, P.; Kisler, K.; Winkler, E.A.; Ramanathan, A.; Kanekiyo, T.; Bu, G.; et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 2015, 18, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Vannice, G.; Rasmussen, H. Position of the Academy of Nutrition and Dietetics: Dietary Fatty Acids for Healthy Adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA); Parma, Italy. EFSA J. 2012, 10, 2815. [Google Scholar]
- Patch, C.S.; Hill-Yardin, E.L.; Lewis, M.; Ryan, L.; Daly, E.; Pearce, A.J. The More, the Better: High-Dose Omega-3 Fatty Acids Improve Behavioural and Molecular Outcomes in Preclinical Models in Mild Brain Injury. Curr. Neurol. Neurosci. Rep. 2021, 21, 45. [Google Scholar] [CrossRef]
- Luo, X.-D.; Feng, J.-S.; Yang, Z.; Huang, Q.-T.; Lin, J.-D.; Yang, B.; Su, K.-P.; Pan, J.-Y. High-dose omega-3 polyunsaturated fatty acid supplementation might be more superior than low-dose for major depressive disorder in early therapy period: A network meta-analysis. BMC Psychiatry 2020, 20, 248. [Google Scholar] [CrossRef]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; A Pravda, E.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef]
- Sapieha, P.; Chen, J.; Stahl, A.; Seaward, M.R.; Favazza, T.L.; Juan, A.M.; Hatton, C.J.; Joyal, J.-S.; Krah, N.M.; Dennison, R.J.; et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr. Diabetes 2012, 2, e36. [Google Scholar] [CrossRef]
- Carare, R.O.; Hawkes, C.A.; Jeffrey, M.; Kalaria, R.N.; Weller, R.O. Review: Cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropat. Appl. Neurobiol. 2013, 39, 593–611. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of amyloid-beta peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol. Dis. Drug Targets 2009, 8, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V.; Deane, R.; Sagare, A.P.; Bell, R.D.; Winkler, E.A. Low-density lipoprotein receptor-related protein-1: A serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J. Neurochem. 2010, 115, 1077–1089. [Google Scholar] [CrossRef]
- Liu, B.; Rasool, S.; Yang, Z.; Glabe, C.G.; Schreiber, S.S.; Ge, J.; Tan, Z. Amyloid-peptide vaccinations reduce {beta}-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice. Am. J. Pathol. 2009, 175, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Son, T.; Klatt, D.; Yao, X. Concurrent OCT and OCT angiography of retinal neurovascular degeneration in the 5XFAD Alzheimer’s disease mice. Neurophot 2021, 8, 035002. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.H.; Li, Q.X.; He, Z.; Vingrys, A.J.; Chinnery, H.R.; Mullen, J.; Bui, B.V.; Nguyen, C.T.O. Retinal Functional and Structural Changes in the 5xFAD Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 862. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, O.; He, C.; Soylu-Kucharz, R.; Cai, C.; Lauritzen, A.D.; Aldana, B.I.; Björkqvist, M.; Lauritzen, M.; Kucharz, K. Preserved blood-brain barrier and neurovascular coupling in female 5xFAD model of Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1089005. [Google Scholar] [CrossRef]
- Chan, J.P.; Wong, B.H.; Chin, C.F.; Galam, D.L.A.; Foo, J.C.; Wong, L.C.; Ghosh, S.; Wenk, M.R.; Cazenave-Gassiot, A.; Silver, D.L. The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol. 2018, 16, e2006443. [Google Scholar] [CrossRef]
- Chen, W.; Jump, D.B.; Esselman, W.J.; Busik, J.V. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Investig. Ophthal. Vis. Sci. 2007, 48, 18–26. [Google Scholar] [CrossRef]
- Ou, J.; Tu, H.; Shan, B.; Luk, A.; DeBose-Boyd, R.A.; Bashmakov, Y.; Goldstein, J.L.; Brown, M.S. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc. Natl. Acad. Sci. USA 2001, 98, 6027–6032. [Google Scholar] [CrossRef] [PubMed]
- Boergesen, M.; Pedersen, T.A.; Gross, B.; van Heeringen, S.J.; Hagenbeek, D.; Bindesboll, C.; Caron, S.; Lalloyer, F.; Steffensen, K.R.; Nebb, H.I.; et al. Genome- wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor alpha in mouse liver reveals extensive sharing of binding sites. Mol. Cell. Biol. 2012, 32, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Huang, Y.; Guo, X.R.; Zhang, M.Q.; Yuan, X.S.; Zu, H.B. DHCR24 reverses Alzheimer’s disease-related pathology and cognitive impairment via increasing hippocampal cholesterol levels in 5xFAD mice. Acta Neuropathol. Commun. 2023, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Xiao, N.; Zhang, Y.Z.; Zhao, C.X.; Guo, X.H.; Lu, L.M. Mfsd2a-based pharmacological strategies for drug delivery across the blood-brain barrier. Pharmacol. Res. 2016, 104, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, D.; Shen, Q.; Gao, L.; Zhuang, P.; Zhang, Y.; Guo, H. Storax Inhibits Caveolae-Mediated Transcytosis at Blood-Brain Barrier After Ischemic Stroke in Rats. Front. Pharmacol. 2022, 13, 876235. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Wang, M.X.; Ismail, O.; Braun, M.; Schindler, A.G.; Reemmer, J.; Wang, Z.; Haveliwala, M.A.; O’Boyle, R.P.; Han, W.Y.; et al. Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid β plaque formation in mice. Alzheimer’s Res. Ther. 2022, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Katoozi, S.; Rao, S.B.; Skauli, N.; Froehner, S.C.; Ottersen, O.P.; Adams, M.E.; Amiry-Moghaddam, M. Functional specialization of retinal Müller cell endfeet depends on an interplay between two syntrophin isoforms. Mol. Brain 2020, 13, 40. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, N.; Chen, Y.; Huang, H.; Marshall, C.; Gao, J.; Cai, Z.; Wu, T.; HU, G.; Xiao, M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 2015, 10, 58. [Google Scholar] [CrossRef]
- Rosu, G.C.; Catalin, B.; Balseanu, T.A.; Laurentiu, M.; Claudiu, M.; Kumar-Singh, S.; Daniel, P. Inhibition of Aquaporin 4 Decreases Amyloid Aβ40 Drainage Around Cerebral Vessels. Mol. Neurobiol. 2020, 57, 4720–4734. [Google Scholar] [CrossRef]
- Wang, L.; Mao, X. Role of Retinal Amyloid-β in Neurodegenerative Diseases: Overlapping Mechanisms and Emerging Clinical Applications. Int. J. Mol. Sci. 2021, 22, 2360. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Component | % |
|---|---|
| Protein | 17.2 |
| Carbohydrate | 60.9 |
| Fat | 3.7 |
| PUFA/SFA | 1.3 |
| n-3/n-6 PUFA | 0.05 |
| fiber | 5.6 |
| ash | 7.6 |
| Vitamins and minerals (in adequate amounts) | |
| SFA | 16:0n | palmitic | 22.90 |
| 18:0n | stearic | 2.23 | |
| MUFA | 16:1n-7 | palmitoleic | 11.90 |
| 18:1n-7 | vaccenic | 4.54 | |
| n-6 | 18:2n-6 | linoleic | 1.67 |
| 20:3n-6 | dihomo-gama-linolenic | 0.29 | |
| 20:4n-6 | arachidonic | 1.62 | |
| 22:4n-6 | adrenic | 1.78 | |
| n-3 | 20:5n-3 | EPA | 25.51 |
| 22:5n-3 | DPA | 1.82 | |
| 22:6n-3 | DHA | 15.49 |
| Gene | Orientation | Sequence |
|---|---|---|
| HMGCR | F (5′-3′) | TTG GTC CTT GTT CAC GCT CAT |
| R (3′-5′) | TTC GCC AGA CCC AAG GAA AC | |
| SREBP1-C | F (5′-3′) | ACG GAG CCA TGG ATT GCA |
| R (3′-5′) | AAG TCA CTG TCT TGG TTG TTGATGA | |
| LXRBETA | F (5′-3′) | AGC GTC CAT TCA GAG CAA GTG |
| R (3′-5′) | CAC TCG TGG ACA TCC CAG ATC T | |
| ABCA | F (5′-3′) | AGG CCG CAC CAT TAT TTT GTC |
| R (3′-5′) | GGC AAT TCT GTC CCC AAG GAT | |
| APOE | F (5′-3′) | GGC CCA GGA GGA GAA TCA ATGA G |
| R (3′-5′) | CCT GGC TGG ATA TGG ATG TTG | |
| MFSD2A | F (5′-3′) | AGA AGC AGC AAC TGT CCA TTT |
| R (3′-5′) | CTC GGC CCA CAA AAA GGA TAA T | |
| HPRT | F (5′-3′) | CTC ATG GAC TGA TTA TGG ACA GGA C |
| R (3′-5′) | GCA GGT CAG CAA AGA ACT TAT AGC C | |
| AQP4 | F (5′-3′) R (3′-5′) | AGC AAT TGG ATT TTC CGT TG TGA GCT CCA CAT CAG GAC AG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanovic Macura, I.; Milanovic, D.; Tesic, V.; Major, T.; Perovic, M.; Adzic, M.; Ivkovic, S. The Impact of High-Dose Fish Oil Supplementation on Mfsd2a, Aqp4, and Amyloid-β Expression in Retinal Blood Vessels of 5xFAD Alzheimer’s Mouse Model. Int. J. Mol. Sci. 2024, 25, 9400. https://doi.org/10.3390/ijms25179400
Jovanovic Macura I, Milanovic D, Tesic V, Major T, Perovic M, Adzic M, Ivkovic S. The Impact of High-Dose Fish Oil Supplementation on Mfsd2a, Aqp4, and Amyloid-β Expression in Retinal Blood Vessels of 5xFAD Alzheimer’s Mouse Model. International Journal of Molecular Sciences. 2024; 25(17):9400. https://doi.org/10.3390/ijms25179400
Chicago/Turabian StyleJovanovic Macura, Irena, Desanka Milanovic, Vesna Tesic, Tamara Major, Milka Perovic, Miroslav Adzic, and Sanja Ivkovic. 2024. "The Impact of High-Dose Fish Oil Supplementation on Mfsd2a, Aqp4, and Amyloid-β Expression in Retinal Blood Vessels of 5xFAD Alzheimer’s Mouse Model" International Journal of Molecular Sciences 25, no. 17: 9400. https://doi.org/10.3390/ijms25179400
APA StyleJovanovic Macura, I., Milanovic, D., Tesic, V., Major, T., Perovic, M., Adzic, M., & Ivkovic, S. (2024). The Impact of High-Dose Fish Oil Supplementation on Mfsd2a, Aqp4, and Amyloid-β Expression in Retinal Blood Vessels of 5xFAD Alzheimer’s Mouse Model. International Journal of Molecular Sciences, 25(17), 9400. https://doi.org/10.3390/ijms25179400






