Post-Hoc Analysis of Potential Correlates of Protection of a Recombinant SARS-CoV-2 Spike Protein Extracellular Domain Vaccine Formulated with Advax-CpG55.2-Adjuvant
Abstract
:1. Introduction
2. Results
2.1. Evidence of Prior Infection in Trial Participants at Baseline
2.2. SARS-CoV-2 Infections in Trial Participants
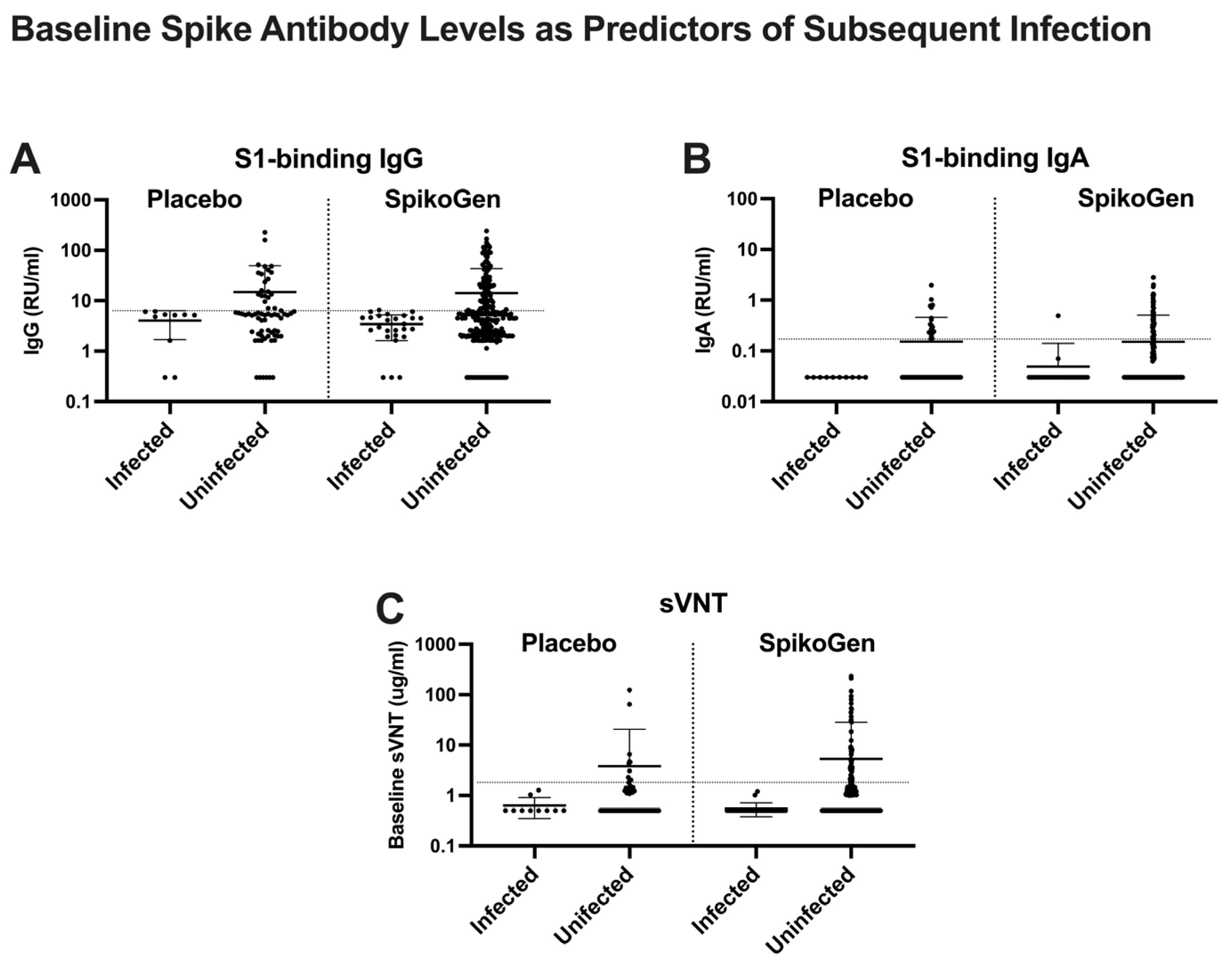
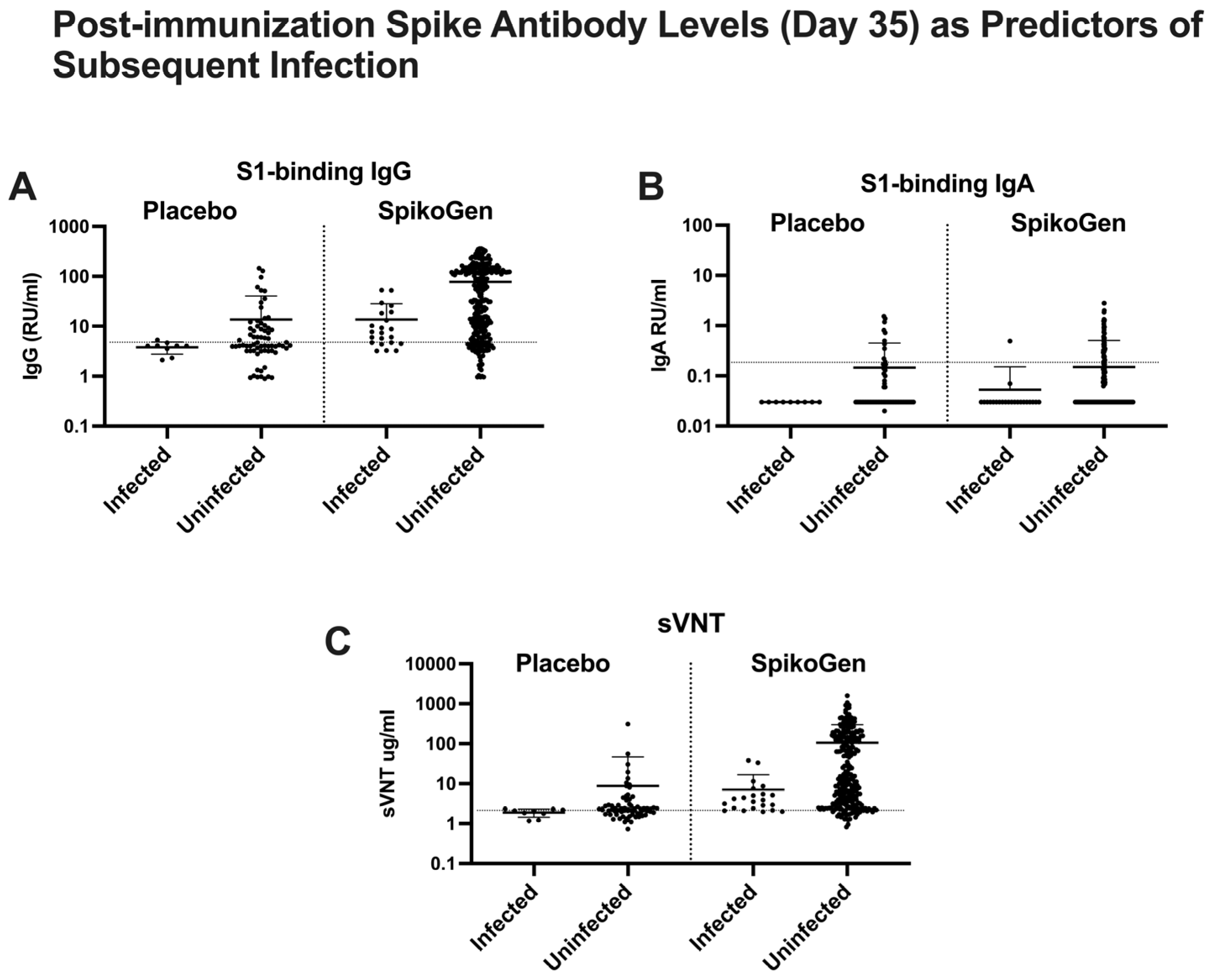
2.3. Putative Immune Correlates of Protection
2.4. Asymptomatic Infections in the Placebo Group between Day 0 and Day 35
3. Discussion
4. Materials and Methods
4.1. Human Trial Samples
4.2. Antibody Measurements
4.3. Statistical Analysis
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, H.C.; Ware, H.; Whelan, M.; Subissi, L.; Li, Z.; Ma, X.; Nardone, A.; Valenciano, M.; Cheng, B.; Noel, K.; et al. SARS-CoV-2 infection in Africa: A systematic review and meta-analysis of standardised seroprevalence studies, from January 2020 to December 2021. BMJ Glob. Health 2022, 7, e008793. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Barnard, D.; Ong, C.H.; Peng, B.H.; Tseng, C.T.; Petrovsky, N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J. Virol. 2015, 89, 2995–3007. [Google Scholar] [CrossRef] [PubMed]
- McPherson, C.; Chubet, R.; Holtz, K.; Honda-Okubo, Y.; Barnard, D.; Cox, M.; Petrovsky, N. Development of a SARS Coronavirus Vaccine from Recombinant Spike Protein Plus Delta Inulin Adjuvant. Methods Mol. Biol. 2016, 1403, 269–284. [Google Scholar]
- Adney, D.R.; Wang, L.; van Doremalen, N.; Shi, W.; Zhang, Y.; Kong, W.-P.; Miller, M.R.; Bushmaker, T.; Scott, D.; de Wit, E.; et al. Efficacy of an Adjuvanted Middle East Respiratory Syndrome Coronavirus Spike Protein Vaccine in Dromedary Camels and Alpacas. Viruses 2019, 11, 212. [Google Scholar] [CrossRef]
- Li, L.; Honda-Okubo, Y.; Huang, Y.; Jang, H.; Carlock, M.A.; Baldwin, J.; Piplani, S.; Bebin-Blackwell, A.G.; Forgacs, D.; Sakamoto, K.; et al. Immunisation of ferrets and mice with recombinant SARS-CoV-2 spike protein formulated with Advax-SM adjuvant protects against COVID-19 infection. Vaccine 2021, 39, 5940–5953. [Google Scholar] [CrossRef]
- Gordon, D.; Kelley, P.; Heinzel, S.; Cooper, P.; Petrovsky, N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled Phase 1 study. Vaccine 2014, 32, 6469–6477. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Saade, F.; Petrovsky, N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 2012, 30, 5373–5381. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Bowen, R.; Barker, M.; Bielefeldt-Ohmann, H.; Petrovsky, N. Advax-CpG55.2-adjuvanted monovalent or trivalent SARS-CoV-2 recombinant spike protein vaccine protects hamsters against heterologous infection with Beta or Delta variants. Vaccine 2023, 41, 7116–7128. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Honda-Okubo, Y.; Baldwin, J.; Bowen, R.; Bielefeldt-Ohmann, H.; Petrovsky, N. Covax-19/Spikogen(R) vaccine based on recombinant spike protein extracellular domain with Advax-CpG55.2 adjuvant provides single dose protection against SARS-CoV-2 infection in hamsters. Vaccine 2022, 40, 3182–3192. [Google Scholar] [CrossRef]
- Tabarsi, P.; Anjidani, N.; Shahpari, R.; Roshanzamir, K.; Fallah, N.; Andre, G.; Petrovsky, N.; Barati, S. Immunogenicity and safety of SpikoGen(R), an adjuvanted recombinant SARS-CoV-2 spike protein vaccine as a homologous and heterologous booster vaccination: A randomized placebo-controlled trial. Immunology 2022, 167, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Siller, A.; Seekircher, L.; Wachter, G.A.; Astl, M.; Tschiderer, L.; Pfeifer, B.; Gaber, M.; Schennach, H.; Schennach, P. Seroprevalence, Waning and Correlates of Anti-SARS-CoV-2 IgG Antibodies in Tyrol, Austria: Large-Scale Study of 35,193 Blood Donors Conducted between June 2020 and September 2021. Viruses 2022, 14, 568. [Google Scholar] [CrossRef] [PubMed]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann. Intern. Med. 2020, 173, 362–367. [Google Scholar] [CrossRef]
- Tabarsi, P.; Anjidani, N.; Shahpari, R.; Mardani, M.; Sabzvari, A.; Yazdani, B.; Kafi, H.; Fallah, N.; Ebrahimi, A.; Taheri, A.; et al. Evaluating the efficacy and safety of SpikoGen(R), an Advax-CpG55.2-adjuvanted severe acute respiratory syndrome coronavirus 2 spike protein vaccine: A phase 3 randomized placebo-controlled trial. Clin. Microbiol. Infect. 2023, 29, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.; Piplani, S.; Sakala, I.G.; Honda-Okubo, Y.; Li, L.; Petrovsky, N. Rapid development of analytical methods for evaluating pandemic vaccines: A COVID-19 perspective. Bioanalysis 2021, 13, 1805–1826. [Google Scholar] [CrossRef]
- Freeman, J.; Olson, K.; Conklin, J.; Shalhoub, V.; Johnson, B.A.; Bopp, N.E.; Fernandez, D.; Menachery, V.D.; Aguilar, P.V. Analytical characterization of the SARS-CoV-2 EURM-017 reference material. Clin. Biochem. 2022, 101, 19–25. [Google Scholar] [CrossRef]
- The National SARS-CoV-2 Serology Assay Evaluation Group. Performance characteristics of five immunoassays for SARS-CoV-2: A head-to-head benchmark comparison. Lancet Infect. Dis. 2020, 20, 1390–1400. [Google Scholar] [CrossRef]
- Goldblatt, D.; Fiore-Gartland, A.; Johnson, M.; Hunt, A.; Bengt, C.; Zavadska, D.; Snipe, H.D.; Brown, J.S.; Workman, L.; Zar, H.J.; et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine 2022, 40, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Cherne, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Tabynov, K.; Turebekov, N.; Babayeva, M.; Fomin, G.; Yerubayev, T.; Yespolov, T.; Li, L.; Renukaradhya, G.J.; Petrovsky, N.; Tabynov, K. An adjuvanted subunit SARS-CoV-2 spike protein vaccine provides protection against Covid-19 infection and transmission. NPJ Vaccines 2022, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.; Stieber, F.; Rao, S.N.; Nikolayevskyy, V.; Manissero, D.; Allen, N.; Boyle, J.; Howard, J. Preliminary Evaluation of QuantiFERON SARS-CoV-2 and QIAreach Anti-SARS-CoV-2 Total Test in Recently Vaccinated Individuals. Infect. Dis. Ther. 2021, 10, 2765–2776. [Google Scholar] [CrossRef]
- Morvan, M.G.; Teque, F.C.; Locher, C.P.; Levy, J.A. The CD8(+) T Cell Noncytotoxic Antiviral Responses. Microbiol. Mol. Biol. Rev. 2021, 85, e00155-20. [Google Scholar] [CrossRef]
- Heitmann, J.S.; Bilich, T.; Tandler, C.; Nelde, A.; Maringer, Y.; Marconato, M.; Reusch, J.; Jäger, S.; Denk, M.; Richter, M.; et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature 2022, 601, 617–622. [Google Scholar] [CrossRef]
- Chu, R.S.; Targoni, O.S.; Krieg, A.M.; Lehmann, P.V.; Harding, C.V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 1997, 186, 1623–1631. [Google Scholar] [CrossRef]
- Murad, Y.M.; Clay, T.M. CpG oligodeoxynucleotides as TLR9 agonists: Therapeutic applications in cancer. BioDrugs 2009, 23, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Monslow, M.A.; Freed, D.C.; Chang, D.; Li, F.; Gindy, M.; Wang, D.; Vora, K.; Espeseth, A.S.; Petrovsky, N.; et al. Novel adjuvants enhance immune responses elicited by a replication-defective human cytomegalovirus vaccine in nonhuman primates. Vaccine 2021, 39, 7446–7456. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.L.; Counoupas, C.; Steain, M.; Ashley, C.; Alca, S.; Hartley-Tassell, L.; Itzstein, M.v.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) is a cellular receptor for delta inulin adjuvant. Immunol Cell Biol. 2024, 102, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Cooper, P.D. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine 2015, 33, 5920–5926. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yang, S.-W.; Joo, J.-S.; Park, M.; Jin, Y.; Kim, J.-W.; Lee, S.-Y.; Lee, S.-V.; Yun, T.-J.; Cho, M.-L.; et al. Sialylated IVIg binding to DC-SIGN(+) Hofbauer cells induces immune tolerance through the caveolin-1/NF-kB pathway and IL-10 secretion. Clin. Immunol. 2023, 246, 109215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Lepenies, B.; Nakamae, S.; Young, B.M.; Santos, R.L.; Raffatellu, M.; Cobb, B.A.; Hiyoshi, H.; Bäumler, A.J. The Vi Capsular Polysaccharide of Salmonella Typhi Promotes Macrophage Phagocytosis by Binding the Human C-Type Lectin DC-SIGN. mBio 2022, 13, e0273322. [Google Scholar] [CrossRef] [PubMed]
- Beirag, N.; Kumar, C.; Madan, T.; Shamji, M.H.; Bulla, R.; Mitchell, D.; Murugaiah, V.; Neto, M.M.; Temperton, N.; Idicula-Thomas, S.; et al. Human surfactant protein D facilitates SARS-CoV-2 pseudotype binding and entry in DC-SIGN expressing cells, and downregulates spike protein induced inflammation. Front. Immunol. 2022, 13, 960733. [Google Scholar] [CrossRef] [PubMed]
- Sprokholt, J.K.; Heineke, M.H.; Kaptein, T.M.; van Hamme, J.L.; Geijtenbeek, T.B.H. DCs facilitate B cell responses against microbial DNA via DC-SIGN. PLoS ONE 2017, 12, e0185580. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Burke, P.C.; Nowacki, A.S.; Gordon, S.M. Risk of Coronavirus Disease 2019 (COVID-19) among those up-to-date and not up-to-date on COVID-19 vaccination by US CDC criteria. PLoS ONE 2023, 18, e0293449. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.K.; Burke, P.C.; Nowacki, A.S.; Simon, J.F.; Hagen, A.; Gordon, S.M. Effectiveness of the Coronavirus Disease 2019 Bivalent Vaccine. Open Forum Infect. Dis. 2023, 10. [Google Scholar] [CrossRef]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Clinical development of SpikoGen®, an Advax-CpG55.2 adjuvanted recombinant spike protein vaccine. Hum. Vaccines Immunother. 2024, 20, 2363016. [Google Scholar] [CrossRef]
- Pal, R.; Ferrari, M.G.; Honda-Okubo, Y.; Wattay, L.; Caple, J.; Navarrete, J.; Andersen, H.; Petrovsky, N. Study of immunogenicity and efficacy against Omicron BA.5 of recombinant protein-based COVID-19 vaccine delivered by intramuscular and mucosal routes in nonhuman primates. Vaccine 2024, 42, 1122–1135. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovsky, N. Post-Hoc Analysis of Potential Correlates of Protection of a Recombinant SARS-CoV-2 Spike Protein Extracellular Domain Vaccine Formulated with Advax-CpG55.2-Adjuvant. Int. J. Mol. Sci. 2024, 25, 9459. https://doi.org/10.3390/ijms25179459
Petrovsky N. Post-Hoc Analysis of Potential Correlates of Protection of a Recombinant SARS-CoV-2 Spike Protein Extracellular Domain Vaccine Formulated with Advax-CpG55.2-Adjuvant. International Journal of Molecular Sciences. 2024; 25(17):9459. https://doi.org/10.3390/ijms25179459
Chicago/Turabian StylePetrovsky, Nikolai. 2024. "Post-Hoc Analysis of Potential Correlates of Protection of a Recombinant SARS-CoV-2 Spike Protein Extracellular Domain Vaccine Formulated with Advax-CpG55.2-Adjuvant" International Journal of Molecular Sciences 25, no. 17: 9459. https://doi.org/10.3390/ijms25179459





