Abstract
Doxorubicin (DOX), a commonly used anticancer agent, causes cardiotoxicity that begins with the first dose and may progress to heart failure years after treatment. An inflammatory response associated with neutrophil recruitment has been recognized as a mechanism of DOX-induced cardiotoxicity. This study aimed to validate mRNA expression of the previously identified biomarkers of DOX-induced cardiotoxicity, PGLYRP1, CAMP, MMP9, and CEACAM8, and to assay their protein expression in the peripheral blood of breast cancer patients. Blood samples from 40 breast cancer patients treated with DOX-based chemotherapy were collected before and after the first chemotherapy cycle and > 2 years after treatment. The protein and gene expression of PGLYRP1/Tag7, CAMP/LL37, MMP9/gelatinase B, and CEACAM8/CD66b were determined using ELISA and reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Receiver operating characteristic (ROC) curve analysis was used to determine the diagnostic value of each candidate biomarker. Patients with cardiotoxicity (n = 20) had significantly elevated levels of PGLYRP1, CAMP, MMP9, and CEACAM8 at baseline, after the first dose of DOX-based chemotherapy, and at > 2 years after treatment relative to patients without cardiotoxicity (n = 20). The first dose of DOX induced significantly higher levels of all examined biomarkers in both groups of patients. At > 2 years post treatment, the levels of all but MMP9 dropped below the baseline. There was a good correlation between the expression of mRNA and the target proteins. We demonstrate that circulating levels of PGLYRP1, CAMP, MMP9, and CEACAM8 can predict the cardiotoxicity of DOX. This novel finding may be of value in the early identification of patients at risk for cardiotoxicity.
1. Introduction
Cardiotoxic side effects are serious complications of doxorubicin (DOX) chemotherapy that limit its use in high-dose cancer treatment and strongly impact the quality of life and survival of cancer patients [1,2,3,4]. DOX-induced cardiotoxicity can develop immediately or within months or even years after the completion of therapy [5,6]. Acute DOX cardiotoxicity may develop after a single dose or a single course of treatment, and it often remains asymptomatic, presenting with electrocardiographic abnormalities such as QT interval prolongation and left ventricular dysfunction [7,8,9]. These symptoms usually regress; however, they may also progress into chronic, within a year of treatment, or late cardiotoxicity years or decades after treatment [6]. The long-term cardiovascular morbidity and mortality associated with cancer treatment is attributable to complications including asymptomatic left ventricular (LV) dysfunction, congestive heart failure (CHF), pericarditis, myocardial ischemia, arterial hypertension, atrial and ventricular arrhythmias, and thromboembolic disease [9,10,11,12,13]. The chronic cardiotoxicity is considered irreversible, with poor prognosis and limited treatment options [10,11]. Clinical cardiac toxicity has been reported in 6% of patients treated with anthracyclines and subclinical cardiotoxicity in 18% of the patients after follow-up of 9 years [14]. In a case-control study published in 2023 on 2,196 subjects with various cancers treated with DOX, the cumulative incidence of congestive heart failure (CHF) was 7.4% over 15 years, more than 2 times higher than the matched controls [15].
Patients at increased risk of DOX-induced cardiotoxicity are those who received a high cumulative dose (>250 mg/m2) [11,16]. Additional factors that increase the risk of cardiotoxicity include history of underlying cardiovascular disease, hypertension, diabetes, obesity, genetic susceptibility, individual differences in drug metabolism, and concomitant exposure to trastuzumab and/or radiation [13,17]. Currently, there is no universal definition for anthracycline-induced cardiotoxicity. The diagnosis is made due to a new-onset heart failure (HF) or imaging evidence of a left ventricular ejection fraction (LVEF) decreased by ≥10% or >15% in LV global longitudinal strain (LVGLS) compared to a previous measurement [11,18,19]. The current diagnostic approach lacks the sensitivity to detect early subclinical cardiac dysfunction and cannot reliably predict future outcomes [18,20,21].
Breast cancer is the most common cancer in women and remains the second leading cause of cancer death among women [22]. DOX-induced cardiotoxicity is particularly important in the growing populations of women afflicted with breast cancer, as anthracyclines are still a major component of the treatment of all subtypes of breast cancer [3,23]. A population-based study of breast cancer survivors showed that women who received anthracyclines, including DOX, daunorubicin, or epirubicin, had higher rates of heart failure than did women who received non-anthracycline or no chemotherapy [24]. The risk of CHD in breast cancer patients is higher at 1 year follow-up and persisting over time, up to 20 years after cancer diagnosis [15]. It was reported that DOX-based therapy of breast cancer patients was associated with more than twice the risk of heart failure compared with controls over 15 years [15].
The mechanism of DOX-induced cardiotoxicity is not completely uncovered, but evidence indicates the involvement of multiple mechanisms, such as oxidative stress, mitochondrial dysfunction, DNA damage via inhibition of topoisomerase 2b (Top2b), dysregulation of calcium homeostasis, apoptosis, and inflammation [13,25,26,27,28,29,30,31]. Recent studies have focused on DOX-induced systemic inflammation and endothelial injury, which can possibly trigger the development and progression of cardiomyopathy [16]. DOX-induced cardiotoxicity is dose-dependent, occurring at low doses with asymptomatic, usually reversible, myocardial injury by the first exposure [32,33,34,35]. The subclinical cardiotoxicity may evolve into irreversible symptomatic chronic cardiotoxicity, leading to heart failure with poor prognosis and limited treatment options years after treatment [8,9,11,12,13,25,36]. As the symptomatic heart failure may occur years after treatment, the early recognition of DOX-induced cardiotoxicity or even the prediction of myocardial injury at early doses of chemotherapy can avoid the permanent cardiac damage and reduce cardiovascular mortality [37]. Several studies, including ours [38], have demonstrated a strong link between DOX-induced inflammation and DOX-induced cardiotoxicity [39,40,41,42]. We have found a correlation between DOX cardiotoxicity and elevated circulating levels of chemokines implicated in inflammatory response and immune trafficking (i.e., CCL23, CCL27, and MIF) [38]. It has been shown that DOX-induced myocardial inflammation is associated with the activation of nuclear factor kappa B (NF-κB) and subsequent release of proinflammatory cytokines implicated in cardiac pathogenesis, such as IL-1β, IL-6, TNF-α, and p38 MAPK [43,44,45]. Our previous data showed that the risk of DOX-induced cardiotoxicity was associated with higher levels of circulating markers of hypercoagulability and inflammation [46,47].
Activated neutrophils release the components of the neutrophil granules, including serine proteases, alpha-defensins, and bactericidal proteins, which in addition to their antimicrobial activity are also involved in several inflammation-associated diseases [48,49,50]. Neutrophil granule proteins are predictive of infarct size, LVEF, new cardiovascular events, and death after acute myocardial infarction [51,52]. Neutrophil infiltration in the heart tissue in response to heart injury resulted in upregulation of inflammatory cytokines [53,54,55,56]. There is currently no standardized, minimally invasive, cost-effective, and clinically verified procedure to monitor cardiotoxicity post anthracycline therapy initiation or to detect the early onset of irreversible cardiovascular complications [57]. Previous data indicate that the early subclinical DOX-induced cardiotoxicity in breast cancer patients is associated with increased expression of neutrophil-specific genes (i.e., PGLYRP1, CAMP, MMP9, CEACAM8, MPO), suggesting that neutrophils and their granular proteins are potential biomarkers for DOX-induced cardiotoxicity. We hypothesize that peripheral blood levels of the neutrophil granular proteins peptidoglycan recognition protein 1 (PGLYRP1), cathelicidin antimicrobial peptide (CAMP), matrix metallopeptidase 9 (MMP9), and carcinoembryonic antigen-related cell adhesion molecule 8 (CEACAM8) are associated with a risk of DOX-induced cardiotoxicity in breast cancer patients. The aim of this study was to validate previously determined mRNA biomarkers of DOX-induced presymptomatic cardiotoxicity, PGLYRP1, CAMP, MMP9, and CEACAM8 and to assay their protein expression in breast cancer patients’ circulation. The changes in expression patterns of these markers and their predictive value for DOX-induced acute cardiotoxicity, as well as the correlation between cardiotoxicity risk and biomarker levels, were also analyzed.
2. Results
2.1. Demographic Characteristics of the Study Participants
In this study we analyzed peripheral blood samples from 40 patients with early-stage breast cancer treated with DOX-based chemotherapy for protein and mRNA expression of PGLYRP1, CAMP, MMP9 and CEACAM8. We randomly selected 20 patients with DOX-induced abnormal decrease in LVEF ≥10 percentage points after 4 cycles of chemotherapy from baseline or below 50% (CTX) and 20 patients who maintained normal LVEF or a decrease <10% (NCTX). The incidence of subclinical cardiotoxicity in the all patients enrolled in this study resulting from the administration of DOX-based chemotherapy was 12.5%. We also collected and analyzed the expression of these potential biomarkers from all 40 patients at follow-up time after DOX chemotherapy (median months 24.5–48.2) and evaluated the available ECHO, MUGA and/or 12-lead ECG data.
The characteristics of the patients are presented in Table 1. The median age of all patients was between 50 and 52 years. In the CTX group, 13 patients were treated with neoadjuvant DOX chemotherapy and 7 with adjuvant chemotherapy; in the NCTX group 11 patients were treated with neoadjuvant, and 9 with adjuvant chemotherapy. The median change of LVEF versus baseline among the CTX group after the 4th DOX dose was 11.5% points versus 0.2% in the NCTX group. In CTX group 12 patients had hypertension versus 11 patients in NCTX group. Four patients in each group had diabetes, and smoking was reported in 3 patients in CTX versus 4 in NCTX. There were no significant differences between the two groups of patients with respect to the age, BMI, race, history of hypertension, diabetes, smoking and type of breast cancer.

Table 1.
Demographic characteristics of the study patients.
At 2–4 years follow-up, LVEF >20% decrease to less than 40% with signs of heart failure was recorded in 4 patients from the CTX group. Abnormal ECG at 2–4 years follow-up in the CTX group was found in 3 patients with ventricular enlargement and 3 patients with atrial enlargement (Table 1). At the follow-up there were also the following ECG abnormalities: 7 patients with ST and T abnormalities, 4 patients with QT prolongation, and 5 patients with sinus tachycardia in the CTX group, versus 3 patients with ST and T abnormality, 2 patients with QT prolongation and 1 with sinus tachycardia in the NCTX group (Table 1).
None of the patients had neutropenia before the start of the chemotherapy. At 7 days post 1st cycle, one patient from the CTX group had neutropenia (1.09 K/μL) and one patient from the NCTX group showed neutrophilia (13.8 K/μL). At 14 days after the 1st cycle, 2 patients from the NCTX group had neutropenia (0.02 K and 1.02 K), and 2 patients from the CTX group and 1 patient from the NCTX group had elevated neutrophil count (10.84 K and 11.45 K, and 13.20 K respectively). The neutrophil-to-lymphocyte ratio (NLR) increased after the 1st cycle and kept increasing during the subsequent cycles but was not significantly different between the groups (Table 1).
2.2. Analysis of Gene Expression
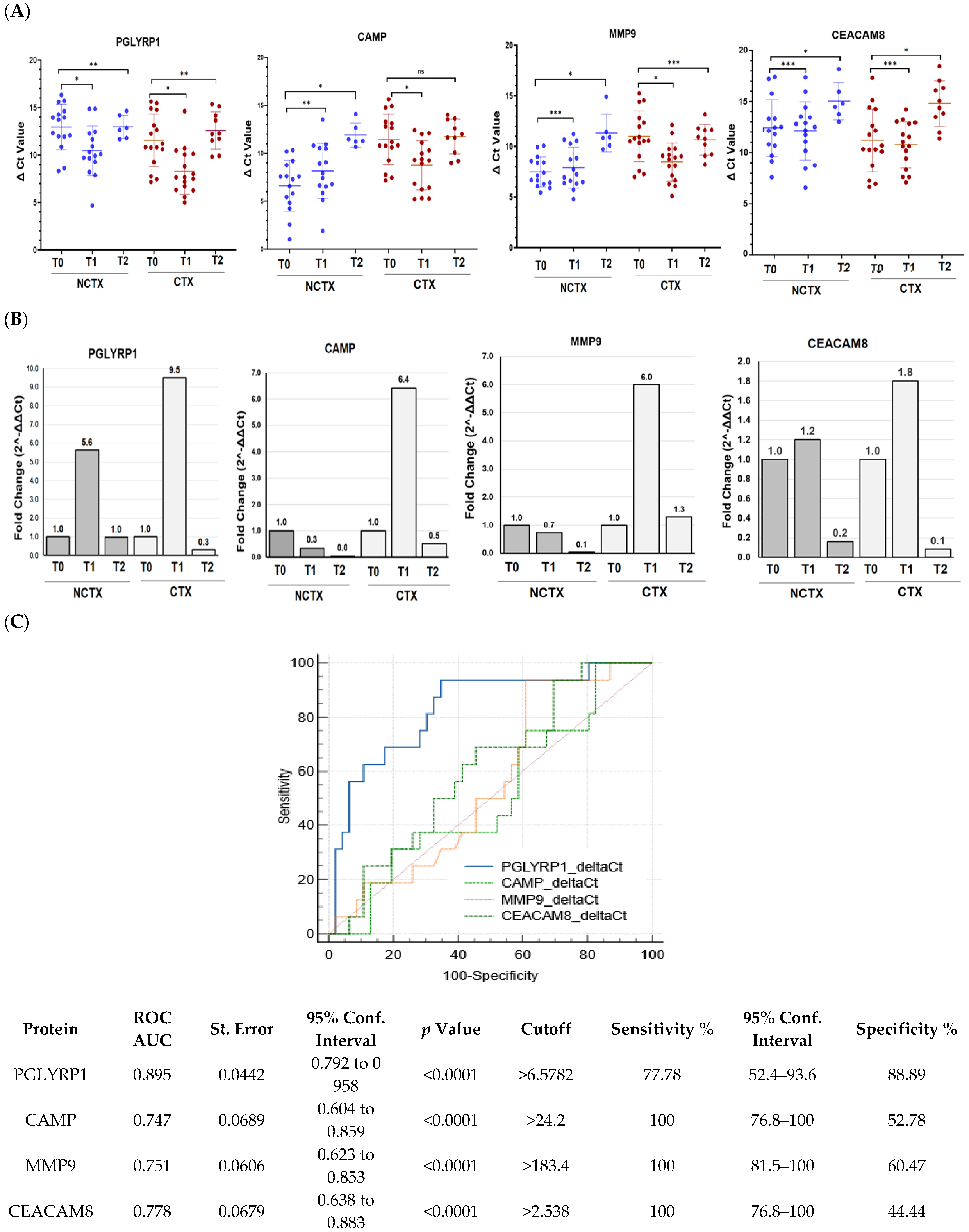
We next determined how gene expression of the neutrophil biomarkers PGLYRP1, CAMP, MMP9, and CEACAM8 correlate with the circulating protein levels. The mRNA expression in PBMCs from 32 breast cancer patients, including 16 with DOX-induced cardiotoxicity and 15 patients without, were examined using real-time PCR. The results showed significant upregulation of the gene expression of PGLYRP1, CAMP, MMP9, and CEACAM8 after the initial DOX chemotherapy versus the baseline (T0) in the CTX group of patients (Figure 1). These data validated our previous findings of increased gene expression of leukocyte markers in breast cancer patients with a risk of DOX-induced cardiotoxicity early after the first DOX dose. At 2–4 years post treatment, mRNA levels of PGLYRP1, CAMP, and CEACAM8 decreased to the baseline levels (Figure 1). The ROC curve analysis constructed to compare the relative mRNAs expressions of the examined biomarkers for DOX-induced cardiotoxicity versus non-cardiotoxicity found the following AUCs (Figure 1C, Supplementary Tables S1–S6): PGLYRP1, 0.779 (p = 0.001); CAMP, 0.562 (p = 0.402); MMP9, 0.610 (p = 0.127); and CEACAM8, 0.516 (p = 0.806). The cutoff values were PGLYRP1 < 10.45, CAMP < 12.43, MMP9 < 10.03, and CEACAM8 < 14.7 (Supplementary Tables S1–S6).
Figure 1.
PGLYRP1, CAMP, MMP9, and CEACAM8 mRNA expression levels are increased in the blood of breast cancer patients treated with DOX-based chemotherapy after the 1st cycle (T1) of DOX-based chemotherapy in comparison with the baseline (T0). (A) Histogram showing the ΔCt values of PGLYRP1, CAMP, MMP9, and CEACAM8 in PBMCs of patients with cardiotoxicity (n = 16) and patients without cardiotoxicity (n = 16). Each dot represents one sample, and the mean ± SD for each group is indicated. (B) Fold change (2^-ΔΔCt) for each of the examined genes comparing T1 and T2, considering the T0 as a calibrator. (C) Receiver operating characteristic (ROC) curves for the ability of blood levels of the four mRNAs to predict DOX-induced cardiotoxicity in breast cancer. NCTX, non-cardiotoxicity; CTX, cardiotoxicity; PGLYRP1, peptidoglycan recognition protein 1; CAMP, cathelicidin antimicrobial peptide; MMP9, matrix metallopeptidase; CEACAM8, carcinoembryonic antigen-related cell adhesion molecule 8. p-value, * < 0.001, ** < 0.01, *** > 0.1.
2.3. Analysis of Protein Expression
In this study, we evaluated the potential of the neutrophil biomarkers PGLYRP1/TAG7, CAMP/LL37, MMP9, and CEACAM8/CD66b to predict DOX-induced cardiotoxicity in patients with early-stage breast cancer. We have determined the differences of the circulating biomarkers among patients with abnormal and normal LVEF at diagnosis (baseline), after the first cycle of DOX-based chemotherapy, and >1year after treatment.
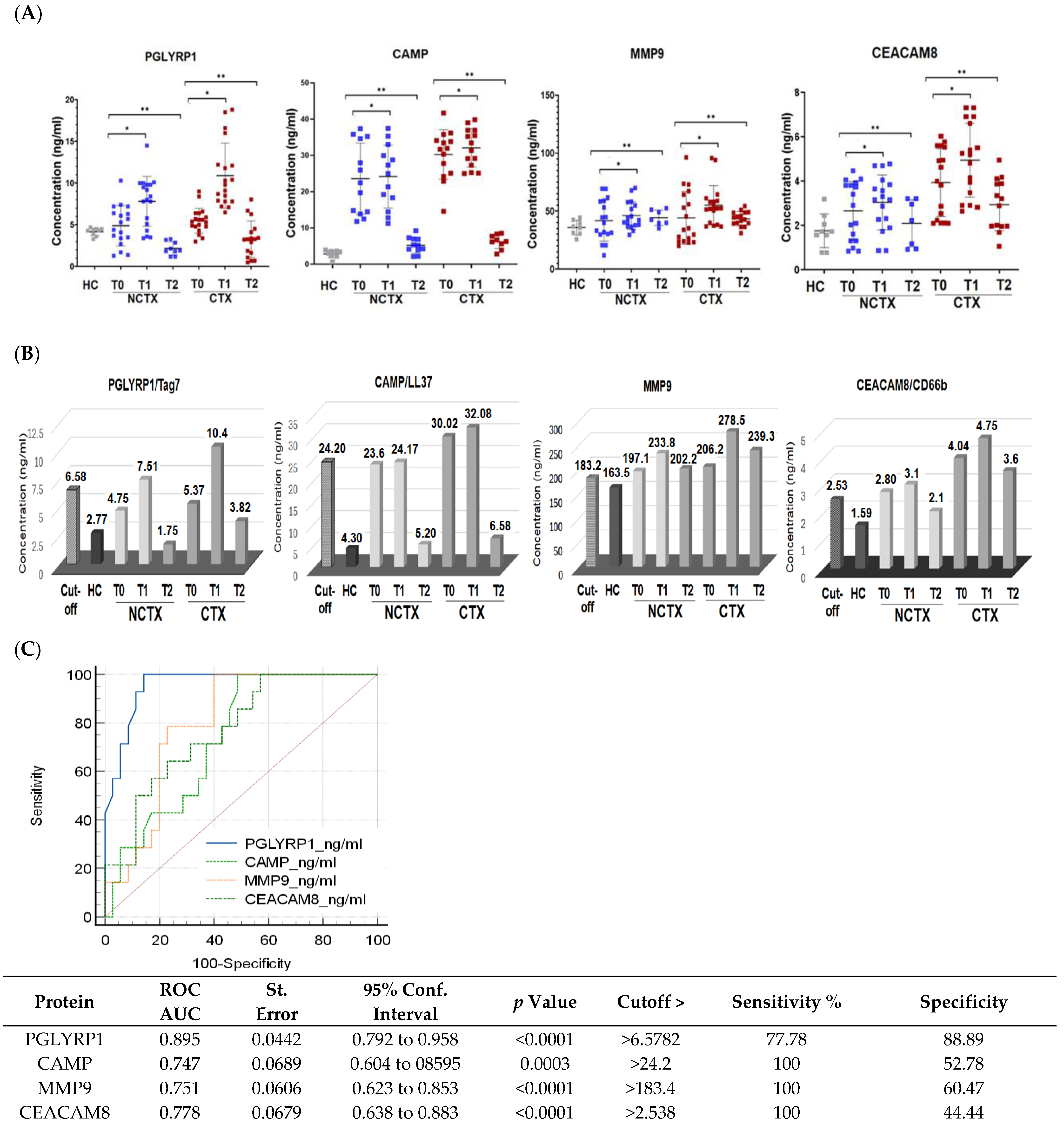
Baseline protein biomarkers: The comparison of the baseline levels (T0) of the examined biomarkers between the two groups of patients using two-sample t-test showed significantly (p < 0.05) higher plasma concentrations of PGLYRP1, CAMP, MMP9, and CEACAM8 in the CTX group of patients versus the NCTX group (Model 1 in Table 2 and Figure 2). After applying ANCOVA modeling with adjustments for age, race, BMI, diabetes, hypertension, and type of breast cancer, the differences were significant only for PGLYRP1 (Model 2 and Model 3, Table 2).

Table 2.
Differences in the circulating protein biomarkers among patients with cardiotoxicity and no cardiotoxicity at baseline and after the first cycle of DOX-based chemotherapy.
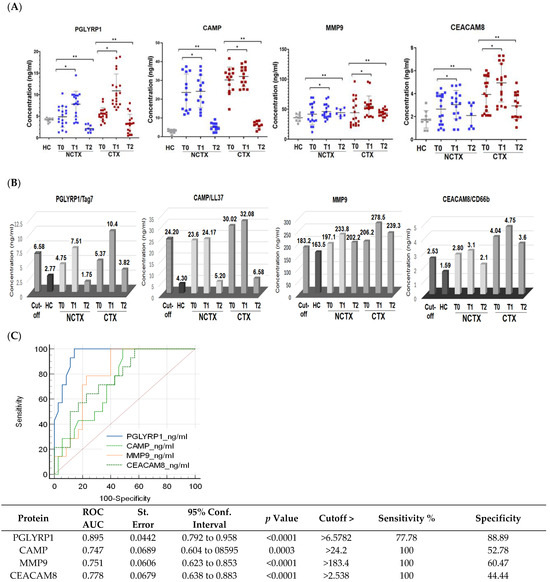
Figure 2.
Protein levels of PGLYRP1, CAMP, MMP9, and CEACAM8 are increased in the plasma of breast cancer patients after the 1st chemotherapy cycle and at >1 year after chemotherapy in comparison with the baseline. (A) Scatter plots demonstrating the comparison of the protein concentrations of the examined proteins in the plasma of breast cancer patients with cardiotoxicity (CTX) and non-cardiotoxicity (NCTX) at baseline (T0), after the first chemotherapy dose (T1), and at >1 year post treatment (T2). Each dot represents one sample, and the mean and SD for each group is indicated. (B) Protein expression levels of the examined markers in comparison with the cutoff values. (C) Receiver operating characteristic (ROC) curves for all examined neutrophil markers at CTX_T1 versus HC + NCTX_T0 + CT_T0. NCTX, non-cardiotoxicity; CTX, cardiotoxicity; PGLYRP1, peptidoglycan recognition protein 1; CAMP, cathelicidin antimicrobial peptide; MMP9, matrix metallopeptidase; CEACAM8 carcinoembryonic antigen-related cell adhesion molecule 8. AUC = area under the curve. p-Value, * < 0.05, ** > 0.01.
Protein biomarkers after the first DOX chemotherapy and at > 1 year: A two-sample t-test was used to compare the levels of the biomarkers at baseline (T0), after the 1st cycle of DOX-based chemotherapy (T1), and at >2 years after treatment (T2) between the two groups of patients, the cardiotoxicity group (CTX) and non-cardiotoxicity group (NCTX). The results showed significantly higher levels of the examined protein markers at baseline (T0), after the 1st cycle of chemotherapy (T1), and at >2 years after treatment (T2) in the group of patients with cardiotoxicity (CTX) versus the group without (NCTX) (Table 2, Model 1). After adjusting for age, race, BMI, diabetes, hypertension, and type of breast cancer in Model 2 and Model 3, only the levels of PGLYRP1 of the CTX group were significantly different from those of the NCTX (p = 0.05) (Model 2 and Model 3, Table 1).
Paired-wise analyses were conducted to determine the changes in the protein markers after the first cycle of chemotherapy (T1) and at >2 years after treatment (T2) in comparison with the baseline (T0) in the two examined groups of patients. In both groups (CTX and NCTX), the plasma levels of PGLYRP1, CAMP, MMP9, and CEACAM8 increased significantly (p < 0.05) after the first chemotherapy cycle in comparison with the baseline (T1-T0) (Table 3) and the cutoff value (Figure 2B). At >2 years post chemotherapy (T2), the protein levels of PGLYRP1 and CAMP dropped below T0 (Table 3) and the cutoff value (Figure 2B) in the CTX group, but they were still higher than the levels of the healthy controls (HCs) (Table 2) and the levels in the NCTX group (Figure 2B, Table 3). At this time point (T2), MMP9 also decreased in comparison with T1; it was higher than the baseline (T2-T0), but not significantly (Table 3, Figure 2B).

Table 3.
Comparison between the means of after–before (T1-T0) and (T2-T0) DOX-based chemotherapy differences in the two groups of breast cancer patients, the group with cardiotoxicity (CTX) and the group with no cardiotoxicity (NCTX).
To assess the diagnostic potential of the four protein biomarker candidates, we used receiver operating characteristic (ROC) curves analysis of the data obtained by ELISA. The ROC curve plots the true positive rate (sensitivity) against the false positive rate (100-specificity). ROC analysis generates an area under the curve (AUC), which is a measure of how well a parameter can distinguish between two diagnostic groups (those with disease/those without disease), where a value of 1 shows perfect performance (sensitivity rises from 0 to 1.0 at 1—specificity of 0, and specificity rises from 0 to 1.0 at sensitivity of 1.0 on an ROC plot). An AUC between 0.7 and 0.9 indicates good diagnostic efficacy. Therefore, the closer the ROC curve is to the upper left corner, the higher the overall accuracy of the test [58]. Youden’s index was used to select the optimal predicted probability cutoff. Using the method suggested by DeLong et al. [59], MedCalc software was used to compare differences in the AUCs. The analysis for each protein biomarker in this study was performed by plotting ELISA data as 0 and 1, where data for “no cardiotoxicity” (HC + NCTX T0 + CTX T0) = 0 and “cardiotoxicity” (CTX T1) =1. The resulting ROC curves are shown in Figure 2C, where the AUCs for PGLYRP1, CAMP, MMP9, and CEACAM8 were 0.895, 0.747, 0.751, and 0.778, respectively, with p < 0.001. The cutoff values calculated by the Youden index were 6.57 ng/mL for PGLYRP1, 24.2 ng/mL for CAMP, 183.4 ng/mL for MMP9, and 2.53 ng/mL for CEACAM8 (Figure 2B, Supplementary Tables S1–S6).
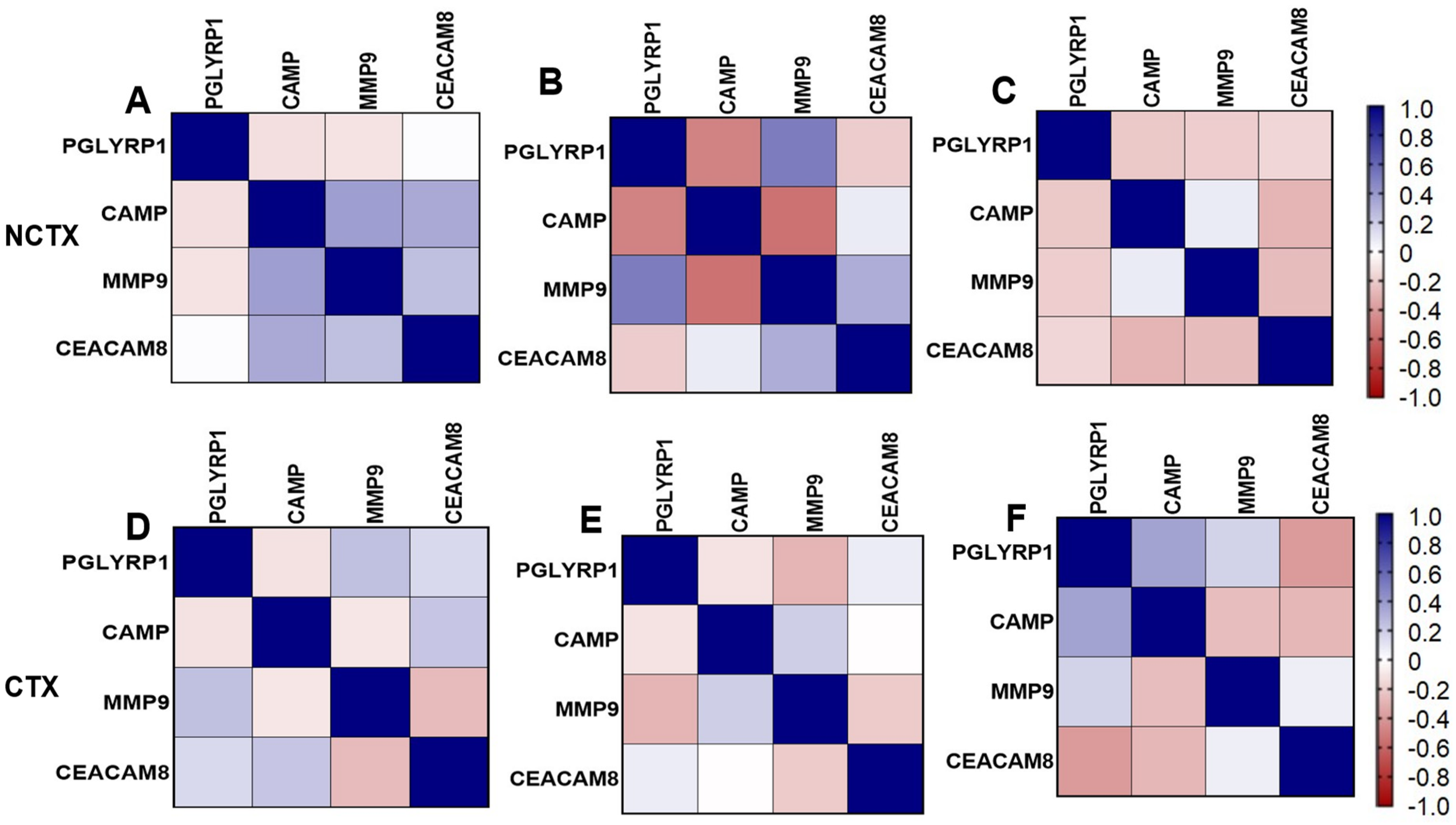
Correlation matrix of markers: Figure 3 depicts the pairwise Pearson correlations matrix between the plasma markers in the two groups of breast cancer patients at different time points (T0 and T1). From the correlation matrix among NCTX patients at both T0 and T1, PGLYRP1, CAMP, MMP9, and CEACAM8 were positively correlated, reflecting similar modulating pathways for those markers.
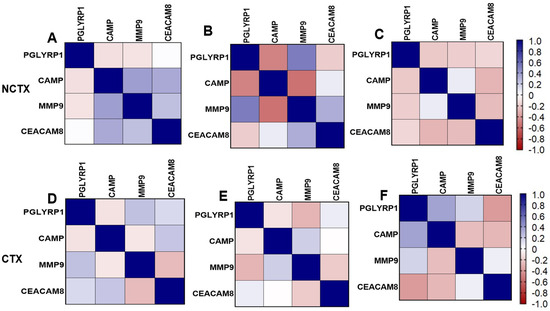
Figure 3.
Pairwise Pearson correlations between plasma markers among the study samples by cardiotoxicity and by time points. CTX, cardiotoxicity; NCTX, non-cardiotoxicity. (A) NCTX at T0; (B) NCTX at T1; (C) NCTX at T2; (D) CTX at T0; (E) CTX at T1; (F) CTX at T2.
3. Discussion
The routine approach in monitoring DOX cardiotoxicity includes serial imaging evaluations of the left ventricle function, which provide limited prognostic efficacy as well as limited sensitivity in monitoring early signs of myocardial injury, cardiac stress and the extent of cardiac remodeling [57,60]. Given these limitations as well as poor cost-effectiveness of this approach [57], the present study aimed to identify novel plasma biomarkers in breast cancer patients treated with DOX-based chemotherapy.
In this study we validated previously determined mRNA biomarkers of DOX-induced cardiotoxicity, PGLYRP1, CAMP, MMP9 and CEACAM8, and demonstrated for the first-time that DOX-based chemotherapy induced elevated circulating levels of their proteins. We found a good correlation between the mRNA expression and the target proteins. The elevated circulating mRNA and protein levels of PGLYRP1/TAG7, CAMP/LL37, MMP9/collagenase IV and CEACAM8/CD66b before and after the first dose of DOX chemotherapy were associated with increased risk for subclinical cardiotoxicity in breast cancer, and the cut-off values were established as predictors for the adverse course of DOX. These findings confirmed our previous data, which suggested that the risk of myocardial injury by the first exposure with DOX in breast cancer may be predicted by the circulating levels of neutrophil-associated proteins PGLYRP1, CAMP, MMP9 and CEACAM8. Taken together, these results indicate that neutrophils through the release of PGLYRP1, CAMP, MMP9 and CEACA8 contribute to DOX-induced cardiotoxicity. These results suggest that targeting these proteins during chemotherapy may decrease the acute DOX-induced cardiotoxicity.
3.1. Previous Studies on the Examined Proteins in Cardiovascular Diseases
The four proteins, we selected and studied are of interest in the context of DOX-induced cardiotoxicity for the following reasons. PGLYRP1, a member of the family of antimicrobial proteins, which bind to peptidoglycan (the main component of bacterial cell wall) and play an important role in antibacterial immunity and inflammation [61,62,63,64]. PGLYRP1 is highly expressed predominantly in neutrophils’ and eosinophils’ granules and its expression was associated with various inflammatory conditions [62,65,66,67], including atherosclerotic disease [68,69,70], acute myocardial infarction [71], rheumatoid arthritis [72], asthma [73], oral inflammation [74,75,76], and cancer [77]. Long-term treatment of mice with recombinant PGLYRP1 was associated with both increased atherogenic lesions and reduced fractional shortening of the left ventricle, suggesting that PGLYRP1 may be a potential biomarker for coronary artery disease and heart failure [78]. Cathelicidins, including CRAMP in mouse/rat, and CAMP/LL-37 in humans are a group of antimicrobial peptides (AMPs), which play essential roles in regulating host antibacterial host defense and immunity [79,80,81]. The immunomodulatory functions of cathelicidins have been documented in a variety of inflammatory diseases, such as atherosclerosis [82,83,84], acute coronary syndrome [85], arthritis [86,87], systemic lupus erythematous [88,89] and diabetes [90]. CAMP was found to aggravate inflammation-related myocardial ischemia/reperfusion (MI/R) injury in experimental mice [91]. Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases secreted by fibroblasts, cardiomyocytes, endothelial cells, and immune cells [92], and their expression is activated by oxidative stress, endothelial dysfunction, and inflammation [93,94,95]. MMPs major function is associated with regulation of extracellular matrix (ECM) turnover and inflammatory signaling [96,97,98], and is involved in in cardiac remodeling and ventricular dilation in heart failure [99]. ECM remodeling plays central role in DOX-induced cardiotoxicity [100,101]. MMP9, collagenase IV is predominantly involved in denaturing basement membrane type IV and V collagens, gelatin, fibronectin and elastin [102], as well as the breakdown of various non-ECM molecules, such as substance P, IL-1β, and myelin basic protein [103]. MMP9 correlated with left ventricular dysfunction after myocardial infarction [104,105], atherosclerosis [106] coronary artery disease [107], and is increased by proinflammatory cytokines, such as interleukin-1 (IL-1) [108], IL-6 [73] and tumor necrosis factor α (TNFα) [74]. CEACAM8 (CD66b) is recognized as a granulocyte activation marker [75], expressed exclusively in humans and two primate species, and absent in mice, and rats [76,77]. CD66-positive neutrophils have been demonstrated in carotid endarterectomy samples from patients with carotid atherosclerosis [109] and patients with psoriasis with an increased risk of cardiovascular diseases [110]. CEACAM8 was one of the plasma proteins related to inflammation identified as key predictive potential biomarkers predictive of heart failure in 3 independent international cohorts [111]. The effect of these biomarkers is mediated and further aggravated by DOX-induced oxidative stress and inflammation specifically in cardiomyocyte, resulting in compromised cardiac structure and left ventricular function decline [93]. Therefore, the panel of circulating biomarkers we have identified in this study presents a strong prognostic utility due to their specific role in DOX- induced cardiotoxicity.
3.2. Neutrophils and Role in Inflammation
Neutrophils are early responders to stimuli that lead to tissue injury, including infection, inflammation, trauma, cancer, and thrombosis [112,113,114,115,116]. Neutrophils have been implicated in the progression of cardiovascular diseases including atherosclerosis, thrombosis, and acute coronary syndrome [117,118,119,120,121]. These cells are the first wave of inflammatory cells that infiltrate the injured heart [121], including DOX-induced heart injury [122,123,124,125]. Degranulation of neutrophils releases reactive oxygen species (ROS), antimicrobial peptides, proteases, and neutrophil extracellular traps (NETs), which are all mechanisms for controlling infection/inflammation [126]. Neutrophil granule proteins have detrimental effects in cardiovascular diseases including acute coronary syndrome, thrombosis, and atherosclerosis [113,127,128,129]. We have shown that circulating biomarkers of inflammation, hypercoagulability, and endothelial injury, including C-reactive protein (CRP), thrombomodulin (TM), and thrombin–antithrombin complex (TAT), correlated with the risk of DOX-induced cardiotoxicity in breast cancer patients [41]. DOX is administered into the systemic circulation, and the first contact the drug makes is with the vascular endothelium, inducing oxidative stress, increased apoptosis, and inflammation [47,130], leading to the recruitment of neutrophils and the subsequent release of neutrophil granule proteins [47,130,131]. Studies have shown that blood neutrophils can infiltrate the myocardium and induce cardiac cell damage by the release of chemokines, cytokines, and adhesion molecules [132]. It has been demonstrated that DOX-induced cardiac neutrophil infiltration and the release of MPO [122,123] and S100a8/a9 from their granules contribute to cardiotoxicity [124]. Our earlier work demonstrated that the early inflammatory response induced by DOX chemotherapy was associated with increased levels of myeloperoxidase (MPO), the most abundant protein in neutrophils [46]. Here, we report that DOX therapy induced increased circulating levels of the neutrophil markers PGLYRP1, CAMP, MMP9, and CEACAM8 early after a single dose of chemotherapy in breast cancer patients. Further, we have found that the late DOX-induced cardiotoxicity is associated with elevated levels of MMP9. MMP9 remained elevated in comparison with the baseline and cut-off at >2 years post chemotherapy in the group of patients with cardiotoxicity, suggesting its role in late cardiotoxicity.
Signs of heart failure and LVEF decrease to <40% was recorded in 4 patients with elevated mRNA and protein expression of PGLYRP1, CAMP, MMP9 and CEACAM8 at baseline and after the initial chemotherapy dose. Elevated expression of the examined proteins at >2 years post treatment correlated with several ECG abnormalities, associated with development of cardiomyopathy, including left ventricular and atrial enlargement, ST and T wave abnormality, QT prolongation and sinus tachycardia [133,134,135,136,137,138] at the follow-up in most of the patients with abnormal LVEF. Systemic inflammation induced by DOX is recognized as a mechanism that triggers the development and progression of cardiomyopathy [47,139,140,141]. Several reports indicate that plasma levels of inflammatory markers increased in chronic heart failure (HF) and could also be subclinical indicators of future HF [44]. Inflammation plays a major role in large arteries stiffening, related to atherosclerosis, arteriosclerosis, endothelial dysfunction, smooth muscle cell migration, vascular calcification, increased activity of metalloproteinases, extracellular matrix degradation, oxidative stress, elastolysis, and degradation of collagen [142,143]. Accordingly, it has been demonstrated that anthracyclines induce accelerated vascular aging-like phenotype, including arterial stiffness, which contributes to premature cardiovascular disease in cancer survivors, exposed to these agents [143]. Children who were treated with anthracycline chemotherapy demonstrate greater arterial stiffness when compared with healthy age-matched controls upon follow-up ranging from 1 to 20 years [144,145,146].
In summary, in this study we have evaluated four predictive biomarkers of DOX-induced cardiotoxicity in breast cancer, identified in our previous microarray study for protein expression. We have demonstrated for the first-time that the risk of DOX-induced cardiotoxicity is associated with increased levels of neutrophil-derived granule proteins PGLYRP1, CAMP, MMP9 and CEACAM8 before the start of DOX chemotherapy and after the initial treatment, suggesting their potential as early predictive biomarkers. These findings provide evidence for the efficacy of the proposed biomarkers in predicting cardiotoxicity of DOX before it is identified by MUGA scan or echocardiogram. Higher circulating levels of PGLYRP1, CAMP, MMP9 and CEACAM8 prior to and after the first dose of DOX chemotherapy may further contribute to progressive cardiovascular disorders in breast cancer survivors and may potentially predict the risk of both acute subclinical and late cardiotoxicity. Early identification of the risk for DOX-cardiotoxicity can help in the design of cardioprotective treatments; avoid the accumulating toxic effects from the subsequent doses during treatment and finally to reduce the morbidity and mortality from cardiotoxicity.
The limitations of this study include the relatively small sample size. Further studies with a larger group of patients, along with the dynamic profile of the suggested markers during the course of DOX chemotherapy in correlation with the risk of cardiotoxicity are needed.
4. Materials and Methods
4.1. Study Population
Women visiting UAMS Winthrop Rockefeller Cancer Institute for diagnosis and treatment of early-stage breast cancer requiring DOX-based chemotherapy were enrolled in this study between 2012 and 2021. Early-stage breast cancer is breast cancer that has not spread beyond the breast or axillary lymph nodes on the same side of the body [147]. This study was approved by the Institutional Review Board (IRB) of UAMS (Protocol #130212) and Central Veterans Healthcare system (CAVHS) IRB (Protocol #1423976-2), where the samples were processed and stored. All patients were treated at the Winthrop Rockefeller Cancer Institute with a predefined protocol which included a combination of DOX (60 mg/m2) with cyclophosphamide (600 mg/m2) in each cycle for 4 cycles every 2 weeks. All participants signed an IRB approved informed consent in which they were informed in detail about the use of their blood samples and medical records for research purposes. The inclusion criteria included early ER+/PR+/Her2-, ER+/PR-/Her2- or triple negative (ER-/PR-/Her2-), stage I to III breast cancers within 18-99 years of age. Participants were ineligible if they were pregnant, or breast feeding and had no prior history of chemotherapy or radiotherapy. At the time of enrollment, demographic data including age, race, body mass index (BMI) were collected. Baseline data on cardiovascular disease risk factors including the presence of cardiovascular disorders, hypertension, diabetes mellitus and smoking were also collected. A cardiovascular disease score was calculated by counting the number of cardiovascular disease risk factors (hypertension, diabetes mellitus, known coronary artery disease, smoking, vitamin D deficiency or elevated cholesterol). Patients with hypertension who were taking antihypertensive medications (β-blockers and ACE inhibitors) prior to chemotherapy continued with the anti-hypertensive regime concomitant with the DOX-based chemotherapy. Patients with diabetes also continued to be treated with insulin or metformin concomitant with the chemotherapy. Healthy volunteers (n = 10) were also consented and enrolled in the study. Eighty-four patients were enrolled initially in this study, and blood samples were collected, plasma and peripheral blood mononuclear cells (PBMCs) were isolated and stored for further analysis. For this study, plasma and PBMCs of 20 subjects with cardiotoxicity at the end of chemotherapy and 20 subjects without were assayed as described further below. Additionally, 10 healthy women visiting UAMS as family members/caregivers were also consented and enrolled in the study.
4.2. Study Endpoints
The aim of this study was to evaluate the predictive value of four neutrophil biomarkers for acute and long-term cardiotoxicity in breast cancer patients treated with DOX-based chemotherapy. Cardiotoxicity was classified as [148,149]: acute cardiotoxicity, immediately after the completion of chemotherapy dose (T1) and late cardiotoxicity which occurs later than one year (2–4 years) after chemotherapy has been completed (T2).
4.3. Assessment of Cardiac Function
Cardiac toxicity was evaluated by clinical assessment of LVEF with mixed multigated acquisition (MUGA) scan and/or transthoracic echocardiography (ECHO). DOX-induced cardiotoxicity was defined as LVEF absolute decrease by >10 percentage points in comparison with the baseline (before the chemotherapy) to below 50%, sustained ventricular arrhythmias, or sudden cardiac death [150,151,152]. A standard 12-lead electrocardiography at rest was performed yearly post chemotherapy for monitoring the risk of cardiotoxicity, and data were compared with previously reported results. The 12-lead ECGs parameters evaluated and reported in a number of studies on chemotherapy-induced cardiotoxicity, include markers of LV hypertrophy and left atrial enlargement, such as QRS complex and T-wave, prolongation of PQ, QRS duration, QT interval, QT apex (QTa) interval, and ST [133,134,135,136,153].
However, after the last dose of DOX-based chemotherapy, most of the patients had undergone treatment with taxane and/or chest wall irradiation, which can affect the results obtained 2–4 years after the completion of DOX chemotherapy.
4.4. Blood Collection
Blood samples (18 mL) were collected in EDTA collection tubes before the start of DOX-based chemotherapy, 2 weeks after the first cycle and at >1 year after DOX. EDTA-anticoagulated blood was centrifuged at 2000 rpm for 15 min and the top layer containing plasma was removed, and aliquoted. The remaining blood was diluted with an equal volume of phosphate-buffered saline (PBS) without Ca2+ and Mg2+ and was layered over Ficoll-Paque Plus (GE Healthcare) and was centrifuged at 2000 rpm for 20 min at room temperature in a swinging-bucket rotor without break. The PBMC interface was removed and washed in PBS. The contaminating red blood cells were lysed by incubation for the PBMC pellet with cold ammonium-chloride-potassium (ACK) lysing buffer (GE Healthcare, Wauwatosa, WI, USA) for 10 min, followed by wash with PBS. The plasma and PBMCs were stored at −80 °C.
4.5. Neutrophil-to-Lymphocyte Ratio (NLR)
The absolute neutrophil and lymphocyte counts were collected, and the neutrophil-to-lymphocyte ratio (NLR) was evaluated at baseline and 2 weeks after the first DOX chemotherapy dose.
4.6. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was isolated from PBMCs using the RNeasy mini kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions. Concentrations (ng/μL) and OD ratios (260/280 nm) of total RNA were determined using the Nanodrop UV/VIS spectrophotometer (Thermo Fisher, Waltham, MA, USA). RNAs with a A260/A280 ratio between 1.85 and 2.10 were used for cDNA conversion. The quantitative conversion of 800 ng of total RNA to single-stranded cDNA in a 20-μL reaction was performed with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Quantitative PCR was performed on 1:10 diluted cDNAs using QuantStudio 12K Flex real-time PCR system software version 1.3 (Applied Biosystems, Watham, MA, USA). Taqman gene expression assays were: Hs00175475_m1 (PGLYRP1), Hs00957562_m1 (MMP9), Hs00189038_m1 (CAMP), Hs00266198_m1 (CEACAM8), Hs99999903_m1 (beta-actin, ACTB) [154]. All quantitative PCRs were performed in a final volume of 10 μL containing 1× of TaqMan Universal PCR Master mix (Applied Biosystems), 1× of each TaqMan Gene Expression Assay (FAM-MGB dyes), and 23 ng cDNA in sterile molecular-grade water. The standard cycling conditions were 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Quantitative PCR was performed in triplicate to ensure quantitative accuracy. The results were analyzed using Expression Suite Software version 1.0 (Applied Biosystems). Relative expression levels were calculated for each sample after normalization against the housekeeping genes beta-actin (ACTB) and GTF2B, using the ΔΔCt method for comparing relative fold expression differences [155]. The data are expressed as fold change (FC). Statistical evaluations were done using one-way ANOVA to compare the gene expression levels of sample groups.
4.7. Enzyme-Linked Immunosorbent Assays (ELISA)
Stored blood plasma samples were thawed on ice and analyzed using commercially available ELISA kits in duplicate. Quantification of four candidate protein biomarkers was conducted using commercially available ELISA assay kits. We used the following ELISA kits and plasma dilutions: Human PGRPs/PGLYRP1 (Invitrogen# EHPGLYRP1, detection range: 6.14–1500 pg/mL, 1:100 diluted); Human CAMP/LL-37 (MyBioSource, # MBS167379, detection range 0.2–60 ng/mL, undiluted samples), Human CEACAM8/CD66B (MyBioSource, Cat# MBS2024215, detection range 15.62–1000 pg/mL, undiluted samples), Human MMP-9 (R and D Systems, #DMP900, detection range: 0.31–20 ng/mL, 1:100 diluted). ELISA results were read using Synergy H1 microplate reader (BioTek, Winooski, VT, USA). The measured optical densities of the original standards in each of the ELISA kits were used to generated 5-parameter standard curves from which the protein concentrations of the target proteins were interpolated. These were then corrected for dilution factors.
4.8. Statistical Analysis
The changes in the blood biomarkers between baseline (T0), after the first DOX dose (T1), and at >1 year post treatment (T2) were determined in association with the risk of DOX-induced cardiotoxicity. The concentration of each protein biomarker in both groups of patients (CTX and NCTX) at each time point (T0, T1, and T2) was presented as means ± SD, and P < 0.05 was considered statistically significant. Two-sample t-tests and chi-square (X2)/Fisher exact tests were performed to evaluate differences in patients’ characteristics between groups. Paired t-tests, as well as analysis of covariance (ANCOVA) with adjustments for race, age, body mass index (BMI), and type of breast cancer were conducted to determined group differences (CTX vs. NCTX) or paired-wise differences for the patients between different time points (T0-T1 and T2-T0). To compare the difference in fold change between CTX and NCTX groups, two-sample t-tests as well as ANCOVA were performed. Analyses were performed using R 4.1.1. Summary descriptive statistics and Pearson correlations were performed using GraphPad Prism version 10.2.3. Normality was assessed using the Kolmogorov–Smirnov test and Shapiro–Wilk test.
Receiver operating characteristic (ROC) curve analysis was used to determine the diagnostic value of each candidate biomarker expression in breast cancer patients with cardiotoxicity (CTX) and non-cardiotoxicity (NCTX) after DOX-based chemotherapy. The performance of each candidate biomarker for discrimination between baseline and one dose of DOX chemotherapy was determined according to ROC “area under the curve” (AUC) values with 95% confidence intervals (95% CI). The cutoff values were determined according to Youden index, and differences in diagnostic performance were analyzed by comparing the ROC curves of MedCalc Software (Version 19.4, Belgium) (www.medcalc.be, accessed on 25 July 2024).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25179735/s1.
Author Contributions
Conceptualization, V.K.T.; formal analysis, V.K.T., A.S., Y.C. and W.Z.; supervision, V.K.T.; writing, V.K.T.; review and editing, V.K.T., G.A., J.Y.W., A.S., S.J.M. and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the following: Lyon Cardiovascular Research Program, Institute on Aging, UAMS and resources within the Pharmacogenomics Analysis Laboratory, Research Service, Central Arkansas Veterans Healthcare System, Little Rock, AR. The Pharmacogenomics Analysis Laboratory is part of the Cooperative Studies Program, Office of Research and Development, Veterans Affairs (The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Veterans Affairs or the United States Government).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of UAMS (Protocol #130212) and the IRB of the Central Veterans Healthcare System (CAVHS) (Protocol #1423976-2).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data generated from this study are available upon reasonable request.
Acknowledgments
We thank all the patients and the personnel of the Cancer Clinical Trials and Regulatory Affairs Office at UAMS. The authors would like to thank Ping-Ching Hsu for her support. The authors would like to thank Weleetka Carter for her assistance in RT-qPCR.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaboré, E.G.; Macdonald, C.; Kaboré, A.; Didier, R.; Arveux, P.; Meda, N.; Boutron-Ruault, M.C.; Guenancia, C. Risk Prediction Models for Cardiotoxicity of Chemotherapy Among Patients with Breast Cancer: A Systematic Review. JAMA Netw. Open 2023, 6, e230569. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; McAndrew, N.P.; Bardia, A.; Press, M.F.; Pegram, M.; Crown, J.P.; Fasching, P.A.; Ejlertsen, B.; Yang, E.H.; Glaspy, J.A.; et al. A careful reassessment of anthracycline use in curable breast cancer. NPJ Breast Cancer 2021, 7, 134. [Google Scholar] [CrossRef]
- Bodai, B.I.; Tuso, P. Breast cancer survivorship: A comprehensive review of long-term medical issues and lifestyle recommendations. Perm. J. 2015, 19, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.N.; Gradishar, W.J. Adjuvant Anthracyclines in Breast Cancer: What Is Their Role? Oncologist 2018, 23, 1153–1161. [Google Scholar] [CrossRef]
- Dulf, P.L.; Mocan, M.; Coadă, C.A.; Dulf, D.V.; Moldovan, R.; Baldea, I.; Farcas, A.D.; Blendea, D.; Filip, A.G. Doxorubicin-induced acute cardiotoxicity is associated with increased oxidative stress, autophagy, and inflammation in a murine model. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 1105–1115. [Google Scholar] [CrossRef]
- Wojtacki, J.; Lewicka-Nowak, E.; Leśniewski-Kmak, K. Anthracycline-induced cardiotoxicity: Clinical course, risk factors, pathogenesis, detection and prevention—Review of the literature. Med. Sci. Monit. 2000, 6, 411–420. [Google Scholar] [PubMed]
- Monsuez, J.J.; Charniot, J.C.; Vignat, N.; Artigou, J.Y. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 2010, 144, 3–15. [Google Scholar] [CrossRef]
- Pai, V.B.; Nahata, M.C. Cardiotoxicity of chemotherapeutic agents: Incidence, treatment and prevention. Drug Saf. 2000, 22, 263–302. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. J. Heart Fail 2017, 19, 9–42. [Google Scholar] [CrossRef]
- Caron, J.; Nohria, A. Cardiac Toxicity from Breast Cancer Treatment: Can We Avoid This? Curr. Oncol. Rep. 2018, 20, 61. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Mata Caballero, R.; Serrano Antolín, J.M.; Jiménez Hernández, R.M.; Talavera Calle, P.; Curcio Ruigómez, A.; Del Castillo Arrojo, S.; Graupner Abad, C.; Cristóbal Varela, C.; Alonso Martín, J.J. Incidence of long-term cardiotoxicity and evolution of the systolic function in patients with breast cancer treated with anthracyclines. Cardiol. J. 2022, 29, 228–234. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Lotrionte, M.; Biondi-Zoccai, G.; Abbate, A.; Lanzetta, G.; D’Ascenzo, F.; Malavasi, V.; Peruzzi, M.; Frati, G.; Palazzoni, G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am. J. Cardiol. 2013, 112, 1980–1984. [Google Scholar] [CrossRef]
- Larsen, C.M.; Garcia Arango, M.; Dasari, H.; Arciniegas Calle, M.; Adjei, E.; Rico Mesa, J.; Scott, C.G.; Thompson, C.A.; Cerhan, J.R.; Haddad, T.C.; et al. Association of Anthracycline with Heart Failure in Patients Treated for Breast Cancer or Lymphoma, 1985–2010. JAMA Netw. Open 2023, 6, e2254669. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Lenihan, D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pract. 2017, 13, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhou, T.; Qiu, B.; Zhang, Y.; Zhou, Y.; Yu, H.; Zhang, J.; Liu, L.; Yuan, L.; Yang, G.; et al. Risk Factors for Anthracycline-Induced Cardiotoxicity. Front. Cardiovasc. Med. 2021, 8, 736854. [Google Scholar] [CrossRef]
- Dean, M.; Kim, M.J.; Dimauro, S.; Tannenbaum, S.; Graham, G.; Liang, B.T.; Kim, A.S. Cardiac and noncardiac biomarkers in patients undergoing anthracycline chemotherapy—A prospective analysis. Cardiooncology 2023, 9, 23. [Google Scholar] [CrossRef]
- Azzam, M.; Wasef, M.; Khalaf, H.; Al-Habbaa, A. 3D-based strain analysis and cardiotoxicity detection in cancer patients received chemotherapy. BMC Cancer 2023, 23, 760. [Google Scholar] [CrossRef]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L., Jr.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef]
- Rossetto, L.; Di Lisi, D.; Madaudo, C.; Sinagra, F.P.; Di Palermo, A.; Triolo, O.F.; Gambino, G.; Ortello, A.; Galassi, A.R.; Novo, G. Right ventricle involvement in patients with breast cancer treated with chemotherapy. Cardiooncology 2024, 10, 24. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Guarneri, V.; de Azambuja, E. Anthracyclines in the treatment of patients with early breast cancer. ESMO Open 2022, 7, 100461. [Google Scholar] [CrossRef]
- Pinder, M.C.; Duan, Z.; Goodwin, J.S.; Hortobagyi, G.N.; Giordano, S.H. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J. Clin. Oncol. 2007, 25, 3808–3815. [Google Scholar] [CrossRef]
- Bhagat, A.; Kleinerman, E.S. Anthracycline-Induced Cardiotoxicity: Causes, Mechanisms, and Prevention. Adv. Exp. Med. Biol. 2020, 1257, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, L.; Du, Y.; Zhang, Y.; Ren, J. Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol. Sci. 2023, 44, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, S.; Dai, Y. Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed. Pharmacother. 2022, 156, 113903. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Mohan, N.; Endo, Y.; Shen, Y.; Wu, W.J. Type IIB DNA topoisomerase is downregulated by trastuzumab and doxorubicin to synergize cardiotoxicity. Oncotarget 2018, 9, 6095–6108. [Google Scholar] [CrossRef]
- Micheletti, P.L.; Carla-da-Silva, J.; Scandolara, T.B.; Kern, R.; Alves, V.D.; Malanowski, J.; Victorino, V.J.; Herrera, A.; Rech, D.; Souza, J.A.O.; et al. Proinflammatory circulating markers: New players for evaluating asymptomatic acute cardiovascular toxicity in breast cancer treatment. J. Chemother. 2021, 33, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Spannbauer, A.; Bergler-Klein, J. Cardio-Oncology: A New Discipline in Medicine and Its Relevance to Hematology. Hamostaseologie 2024, 44, 255–267. [Google Scholar] [CrossRef]
- Sharma, R.; Kashyap, J.; Olanrewaju, O.A.; Jabbar, A.; Someshwar, F.; Saeed, H.; Varrassi, G.; Qadeer, H.A.; Kumar, S.; Cheema, A.Y.; et al. Cardio-Oncology: Managing Cardiovascular Complications of Cancer Therapies. Cureus 2023, 15, e51038. [Google Scholar] [CrossRef] [PubMed]
- Belger, C.; Abrahams, C.; Imamdin, A.; Lecour, S. Doxorubicin-induced cardiotoxicity and risk factors. Int. J. Cardiol. Heart Vasc. 2024, 50, 101332. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, P.; Huang, Y. Anthracycline-induced cardiotoxicity: Mechanisms, monitoring, and prevention. Front. Cardiovasc. Med. 2023, 10, 1242596. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Floyd, J.D.; Nguyen, D.T.; Lobins, R.L.; Bashir, Q.; Doll, D.C.; Perry, M.C. Cardiotoxicity of cancer therapy. J. Clin. Oncol. 2005, 23, 7685–7696. [Google Scholar] [CrossRef]
- Yu, L.R.; Cao, Z.; Makhoul, I.; Daniels, J.R.; Klimberg, S.; Wei, J.Y.; Bai, J.P.; Li, J.; Lathrop, J.T.; Beger, R.D.; et al. Immune response proteins as predictive biomarkers of doxorubicin-induced cardiotoxicity in breast cancer patients. Exp. Biol. Med. 2018, 243, 248–255. [Google Scholar] [CrossRef]
- Feng, P.; Yang, Y.; Liu, N.; Wang, S. Baicalin regulates TLR4/IκBα/NFκB signaling pathway to alleviate inflammation in Doxorubicin related cardiotoxicity. Biochem. Biophys. Res. Commun. 2022, 637, 1–8. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Y.; Huang, K.; Zeng, Q. Heat Shock Protein 22 Attenuates Doxorubicin-Induced Cardiotoxicity via Regulating Inflammation and Apoptosis. Front. Pharmacol. 2020, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Sumneang, N.; Tanajak, P.; Oo, T.T. Toll-like Receptor 4 Inflammatory Perspective on Doxorubicin-Induced Cardiotoxicity. Molecules 2023, 28, 4294. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kotamraju, S.; Konorev, E.; Kalivendi, S.; Joseph, J.; Kalyanaraman, B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: The role of hydrogen peroxide. Biochem. J. 2002, 367, 729–740. [Google Scholar] [CrossRef]
- Reis-Mendes, A.; Padrão, A.I.; Duarte, J.A.; Gonçalves-Monteiro, S.; Duarte-Araújo, M.; Remião, F.; Carvalho, F.; Sousa, E.; Bastos, M.L.; Costa, V.M. Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice. Biomolecules 2021, 11, 1725. [Google Scholar] [CrossRef]
- Todorova, V.K.; Hsu, P.C.; Wei, J.Y.; Lopez-Candales, A.; Chen, J.Z.; Su, L.J.; Makhoul, I. Biomarkers of inflammation, hypercoagulability and endothelial injury predict early asymptomatic doxorubicin-induced cardiotoxicity in breast cancer patients. Am. J. Cancer Res. 2020, 10, 2933–2945. [Google Scholar]
- Todorova, V.K.; Wei, J.Y.; Makhoul, I. Subclinical doxorubicin-induced cardiotoxicity update: Role of neutrophils and endothelium. Am. J. Cancer Res. 2021, 11, 4070–4091. [Google Scholar]
- Stapels, D.A.; Geisbrecht, B.V.; Rooijakkers, S.H. Neutrophil serine proteases in antibacterial defense. Curr. Opin. Microbiol. 2015, 23, 42–48. [Google Scholar] [CrossRef]
- Othman, A.; Sekheri, M.; Filep, J.G. Roles of neutrophil granule proteins in orchestrating inflammation and immunity. FEBS J. 2022, 289, 3932–3953. [Google Scholar] [CrossRef]
- Sheshachalam, A.; Srivastava, N.; Mitchell, T.; Lacy, P.; Eitzen, G. Granule protein processing and regulated secretion in neutrophils. Front. Immunol. 2014, 5, 448. [Google Scholar] [CrossRef]
- Chia, S.; Nagurney, J.T.; Brown, D.F.; Raffel, O.C.; Bamberg, F.; Senatore, F.; Wackers, F.J.; Jang, I.K. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am. J. Cardiol. 2009, 103, 333–337. [Google Scholar] [CrossRef]
- Guasti, L.; Dentali, F.; Castiglioni, L.; Maroni, L.; Marino, F.; Squizzato, A.; Ageno, W.; Gianni, M.; Gaudio, G.; Grandi, A.M.; et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb. Haemost. 2011, 106, 591–599. [Google Scholar] [CrossRef]
- Daseke, M.J., 2nd; Chalise, U.; Becirovic-Agic, M.; Salomon, J.D.; Cook, L.M.; Case, A.J.; Lindsey, M.L. Neutrophil signaling during myocardial infarction wound repair. Cell Signal 2021, 77, 109816. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Hoeffel, G. The Innate Immune Response in Myocardial Infarction, Repair, and Regeneration. Adv. Exp. Med. Biol. 2017, 1003, 251–272. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, S.J.; Hadoke, P.W.; Kozak, A.M.; Small, G.R.; Khaled, H.; Walker, B.R.; Gray, G.A. Improved heart function follows enhanced inflammatory cell recruitment and angiogenesis in 11betaHSD1-deficient mice post-MI. Cardiovasc. Res. 2010, 88, 159–167. [Google Scholar] [CrossRef]
- Marchant, D.J.; Boyd, J.H.; Lin, D.C.; Granville, D.J.; Garmaroudi, F.S.; McManus, B.M. Inflammation in myocardial diseases. Circ. Res. 2012, 110, 126–144. [Google Scholar] [CrossRef]
- Lakhani, H.V.; Pillai, S.S.; Zehra, M.; Dao, B.; Tirona, M.T.; Thompson, E.; Sodhi, K. Detecting early onset of anthracyclines-induced cardiotoxicity using a novel panel of biomarkers in West-Virginian population with breast cancer. Sci. Rep. 2021, 11, 7954. [Google Scholar] [CrossRef] [PubMed]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Geisberg, C.A.; Sawyer, D.B. Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Curr. Hypertens. Rep. 2010, 12, 404–410. [Google Scholar] [CrossRef]
- Kashyap, D.R.; Wang, M.; Liu, L.H.; Boons, G.J.; Gupta, D.; Dziarski, R. Peptidoglycan recognition proteins kill bacteria by activating protein-sensing two-component systems. Nat. Med. 2011, 17, 676–683. [Google Scholar] [CrossRef]
- Liu, C.; Gelius, E.; Liu, G.; Steiner, H.; Dziarski, R. Mammalian peptidoglycan recognition protein binds peptidoglycan with high affinity, is expressed in neutrophils, and inhibits bacterial growth. J. Biol. Chem. 2000, 275, 24490–24499. [Google Scholar] [CrossRef]
- Royet, J.; Dziarski, R. Peptidoglycan recognition proteins: Pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 2007, 5, 264–277. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Philpott, D.J. Peptidoglycan: A critical activator of the mammalian immune system during infection and homeostasis. Immunol. Rev. 2011, 243, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Z.; Gupta, D.; Dziarski, R. Peptidoglycan recognition proteins: A novel family of four human innate immunity pattern recognition molecules. J. Biol. Chem. 2001, 276, 34686–34694. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Platt, K.A.; Gelius, E.; Steiner, H.; Gupta, D. Defect in neutrophil killing and increased susceptibility to infection with nonpathogenic gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood 2003, 102, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Gao, M.; Dai, C.; Meyer, K.S.; Chen, J.; Keeran, K.J.; Nugent, G.Z.; Qu, X.; Yu, Z.X.; Dagur, P.K.; et al. Peptidoglycan recognition protein 1 promotes house dust mite-induced airway inflammation in mice. Am. J. Respir. Cell Mol. Biol. 2013, 49, 902–911. [Google Scholar] [CrossRef]
- Brownell, N.K.; Khera, A.; de Lemos, J.A.; Ayers, C.R.; Rohatgi, A. Association Between Peptidoglycan Recognition Protein-1 and Incident Atherosclerotic Cardiovascular Disease Events: The Dallas Heart Study. J. Am. Coll. Cardiol. 2016, 67, 2310–2312. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Ayers, C.R.; Khera, A.; McGuire, D.K.; Das, S.R.; Matulevicius, S.; Timaran, C.H.; Rosero, E.B.; de Lemos, J.A. The association between peptidoglycan recognition protein-1 and coronary and peripheral atherosclerosis: Observations from the Dallas Heart Study. Atherosclerosis 2009, 203, 569–575. [Google Scholar] [CrossRef]
- Gada, E.; Owens, A.W.; Gore, M.O.; See, R.; Abdullah, S.M.; Ayers, C.R.; Rohatgi, A.; Khera, A.; de Lemos, J.A.; McGuire, D.K. Discordant effects of rosiglitazone on novel inflammatory biomarkers. Am. Heart J. 2013, 165, 609–614. [Google Scholar] [CrossRef]
- Park, H.J.; Noh, J.H.; Eun, J.W.; Koh, Y.S.; Seo, S.M.; Park, W.S.; Lee, J.Y.; Chang, K.; Seung, K.B.; Kim, P.J.; et al. Assessment and diagnostic relevance of novel serum biomarkers for early decision of ST-elevation myocardial infarction. Oncotarget 2015, 6, 12970–12983. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Li, X.; Zhang, L.; Yao, F.; Deng, Z.; Qing, C.; Su, R.; Xu, J.; Guo, Y.; Huang, Z.; et al. Serum PGLYRP-1 is a highly discriminatory biomarker for the diagnosis of rheumatoid arthritis. Mol. Med. Rep. 2019, 19, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Kothari, P.; Pestana, R.; Mesraoua, R.; Elchaki, R.; Khan, K.M.; Dannenberg, A.J.; Falcone, D.J. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J. Immunol. 2014, 192, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Al-Roub, A.; Akhter, N.; Al-Rashed, F.; Wilson, A.; Alzaid, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. TNFα induces matrix metalloproteinase-9 expression in monocytic cells through ACSL1/JNK/ERK/NF-kB signaling pathways. Sci. Rep. 2023, 13, 14351. [Google Scholar] [CrossRef]
- Torsteinsdóttir, I.; Arvidson, N.G.; Hällgren, R.; Håkansson, L. Enhanced expression of integrins and CD66b on peripheral blood neutrophils and eosinophils in patients with rheumatoid arthritis, and the effect of glucocorticoids. Scand. J. Immunol. 1999, 50, 433–439. [Google Scholar] [CrossRef]
- Schmidt, T.; Brodesser, A.; Schnitzler, N.; Grüger, T.; Brandenburg, K.; Zinserling, J.; Zündorf, J. CD66b Overexpression and Loss of C5a Receptors as Surface Markers for Staphylococcus aureus-Induced Neutrophil Dysfunction. PLoS ONE 2015, 10, e0132703. [Google Scholar] [CrossRef]
- Zhou, G.Q.; Zhang, Y.; Hammarström, S. The carcinoembryonic antigen (CEA) gene family in non-human primates. Gene 2001, 264, 105–112. [Google Scholar] [CrossRef]
- Han, Y.; Hua, S.; Chen, Y.; Yang, W.; Zhao, W.; Huang, F.; Qiu, Z.; Yang, C.; Jiang, J.; Su, X.; et al. Circulating PGLYRP1 Levels as a Potential Biomarker for Coronary Artery Disease and Heart Failure. J. Cardiovasc. Pharmacol. 2021, 77, 578–585. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, C.; Hui, L.; Song, Y.; Fu, Y.; Li, M.; Yang, H.; Wu, J.; Sun, J.; Xu, W.; et al. Cathelicidins Target HSP60 To Restrict CVB3 Transmission via Disrupting the Exosome and Reducing Cardiomyocyte Apoptosis. J. Virol. 2023, 97, e0143322. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kulkarni, N.N.; Takahashi, T.; Alimohamadi, H.; Dokoshi, T.; Liu, E.; Shia, M.; Numata, T.; Luo, E.W.; Gombart, A.F.; et al. Increased LL37 in psoriasis and other inflammatory disorders promotes LDL uptake and atherosclerosis. J. Clin. Investig. 2024, 134, e172578. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ohkuma, M.; Someya, A.; Mita, T.; Nagaoka, I. Human Cathelicidin Peptide LL-37 Induces Cell Death in Autophagy-Dysfunctional Endothelial Cells. J. Immunol. 2022, 208, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, P.M.; Lio, W.M.; Yano, J.; Zhao, X.; Zhou, J.; Chyu, K.Y.; Shah, P.K.; Cercek, B.; Dimayuga, P.C. The cathelicidin protein CRAMP is a potential atherosclerosis self-antigen in ApoE(-/-) mice. PLoS ONE 2017, 12, e0187432. [Google Scholar] [CrossRef]
- Chernomordik, F.; Cercek, B.; Lio, W.M.; Mihailovic, P.M.; Yano, J.; Herscovici, R.; Zhao, X.; Zhou, J.; Chyu, K.Y.; Shah, P.K.; et al. The Role of T Cells Reactive to the Cathelicidin Antimicrobial Peptide LL-37 in Acute Coronary Syndrome and Plaque Calcification. Front. Immunol. 2020, 11, 575577. [Google Scholar] [CrossRef] [PubMed]
- Bork, F.; Greve, C.L.; Youn, C.; Chen, S.; NCLeal, V.; Wang, Y.; Fischer, B.; Nasri, M.; Focken, J.; Scheurer, J.; et al. naRNA-LL37 composite DAMPs define sterile NETs as self-propagating drivers of inflammation. EMBO Rep. 2024, 25, 2914–2949. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Maleki, A.R.; Vaithilingam, R.D.; Vadivelu, J.; Sockalingam, S.; Baharuddin, N.A.; Bartold, P.M. Associations between inflammation-related LL-37 with subgingival microbial dysbiosis in rheumatoid arthritis patients. Clin. Oral. Investig. 2022, 26, 4161–4172. [Google Scholar] [CrossRef]
- Zielke, C.; Nielsen, J.E.; Lin, J.S.; Barron, A.E. Between good and evil: Complexation of the human cathelicidin LL-37 with nucleic acids. Biophys. J. 2024, 123, 1316–1328. [Google Scholar] [CrossRef]
- Khosravi-Hafshejani, T.; Ghoreishi, M.; Vera Kellet, C.; Crawford, R.I.; Martinka, M.; Dutz, J.P. Small plaque psoriasis re-visited: A type of psoriasis mediated by a type-I interferon pathway. Exp. Dermatol. 2022, 31, 753–763. [Google Scholar] [CrossRef]
- Meguro, S.; Tomita, M.; Katsuki, T.; Kato, K.; Oh, H.; Ainai, A.; Ito, R.; Kawai, T.; Itoh, H.; Hasegawa, H. Plasma Antimicrobial Peptide LL-37 Level Is Inversely Associated with HDL Cholesterol Level in Patients with Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2014, 2014, 703696. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, J.; Zhai, T.; Hu, J.; Luo, H.; Zhou, H.; Zhang, Q.; Zhou, Z.; Liu, F. Cathelicidin aggravates myocardial ischemia/reperfusion injury via activating TLR4 signaling and P2X(7)R/NLRP3 inflammasome. J. Mol. Cell Cardiol. 2020, 139, 75–86. [Google Scholar] [CrossRef]
- Vanhoutte, D.; Heymans, S. TIMPs and cardiac remodeling: ‘Embracing the MMP-independent-side of the family’. J. Mol. Cell Cardiol. 2010, 48, 445–453. [Google Scholar] [CrossRef]
- Chan, B.Y.H.; Roczkowsky, A.; Cho, W.J.; Poirier, M.; Sergi, C.; Keschrumrus, V.; Churko, J.M.; Granzier, H.; Schulz, R. MMP inhibitors attenuate doxorubicin cardiotoxicity by preventing intracellular and extracellular matrix remodelling. Cardiovasc. Res. 2021, 117, 188–200. [Google Scholar] [CrossRef]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta 2010, 1803, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: Rationale and therapeutic approaches. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- DeLeon-Pennell, K.Y.; Meschiari, C.A.; Jung, M.; Lindsey, M.L. Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. Prog. Mol. Biol. Transl. Sci. 2017, 147, 75–100. [Google Scholar] [CrossRef]
- Leerink, J.M.; van de Ruit, M.; Feijen, E.A.M.; Kremer, L.C.M.; Mavinkurve-Groothuis, A.M.C.; Pinto, Y.M.; Creemers, E.E.; Kok, W.E.M. Extracellular matrix remodeling in animal models of anthracycline-induced cardiomyopathy: A meta-analysis. J. Mol. Med. 2021, 99, 1195–1207. [Google Scholar] [CrossRef]
- Nikitovic, D.; Juranek, I.; Wilks, M.F.; Tzardi, M.; Tsatsakis, A.; Tzanakakis, G.N. Anthracycline-dependent cardiotoxicity and extracellular matrix remodeling. Chest 2014, 146, 1123–1130. [Google Scholar] [CrossRef]
- Vu TH, W.Z. Gelatinase B: Structure, regulation, and function. In Matrix Metalloproteinases; Academic Press: San Diego, CA, USA, 1998; pp. 115–148. [Google Scholar]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef]
- Squire, I.B.; Evans, J.; Ng, L.L.; Loftus, I.M.; Thompson, M.M. Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: Correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J. Card. Fail. 2004, 10, 328–333. [Google Scholar] [CrossRef]
- Ducharme, A.; Frantz, S.; Aikawa, M.; Rabkin, E.; Lindsey, M.; Rohde, L.E.; Schoen, F.J.; Kelly, R.A.; Werb, Z.; Libby, P.; et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Investig. 2000, 106, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, X.; Feng, Y.; Dong, G.; Wang, Y.; Yang, J. The Role of Matrix Metalloproteinase-9 in Atherosclerotic Plaque Instability. Mediators Inflamm. 2020, 2020, 3872367. [Google Scholar] [CrossRef] [PubMed]
- Ben Braiek, A.; Chahed, H.; Dumont, F.; Abdelhak, F.; Hichem, D.; Gamra, H.; Baudin, B. Identification of biomarker panels as predictors of severity in coronary artery disease. J. Cell Mol. Med. 2021, 25, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Kuo, C.T.; Lin, C.C.; Hsieh, H.L.; Yang, C.M. IL-1beta induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br. J. Pharmacol. 2010, 160, 1595–1610. [Google Scholar] [CrossRef]
- Marino, F.; Tozzi, M.; Schembri, L.; Ferraro, S.; Tarallo, A.; Scanzano, A.; Legnaro, M.; Castelli, P.; Cosentino, M. Production of IL-8, VEGF and Elastase by Circulating and Intraplaque Neutrophils in Patients with Carotid Atherosclerosis. PLoS ONE 2015, 10, e0124565. [Google Scholar] [CrossRef]
- Kvist-Hansen, A.; Kaiser, H.; Wang, X.; Krakauer, M.; Gørtz, P.M.; McCauley, B.D.; Zachariae, C.; Becker, C.; Hansen, P.R.; Skov, L. Neutrophil Pathways of Inflammation Characterize the Blood Transcriptomic Signature of Patients with Psoriasis and Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 10818. [Google Scholar] [CrossRef]
- Girerd, N.; Levy, D.; Duarte, K.; Ferreira, J.P.; Ballantyne, C.; Collier, T.; Pizard, A.; Björkman, J.; Butler, J.; Clark, A.; et al. Protein Biomarkers of New-Onset Heart Failure: Insights from the Heart Omics and Ageing Cohort, the Atherosclerosis Risk in Communities Study, and the Framingham Heart Study. Circ. Heart Fail. 2023, 16, e009694. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Neutrophils and Bacterial Immune Evasion. J. Innate Immun. 2018, 10, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Zhu, L. Role of neutrophils in acute viral infection. Immun. Inflamm. Dis. 2021, 9, 1186–1196. [Google Scholar] [CrossRef]
- Mauracher, L.M.; Hell, L.; Moik, F.; Krall, M.; Englisch, C.; Roiß, J.; Grilz, E.; Hofbauer, T.M.; Brostjan, C.; Knapp, S.; et al. Neutrophils in lung cancer patients: Activation potential and neutrophil extracellular trap formation. Res. Pract. Thromb. Haemost. 2023, 7, 100126. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Opneja, A.; Nayak, L. The role of neutrophils in thrombosis. Thromb. Res. 2018, 170, 87–96. [Google Scholar] [CrossRef]
- Ma, Y. Role of Neutrophils in Cardiac Injury and Repair Following Myocardial Infarction. Cells 2021, 10, 1676. [Google Scholar] [CrossRef]
- Sreejit, G.; Johnson, J.; Jaggers, R.M.; Dahdah, A.; Murphy, A.J.; Hanssen, N.M.J.; Nagareddy, P.R. Neutrophils in cardiovascular disease: Warmongers, peacemakers, or both? Cardiovasc. Res. 2022, 118, 2596–2609. [Google Scholar] [CrossRef]
- Luo, J.; Thomassen, J.Q.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Neutrophil counts and cardiovascular disease. Eur. Heart J. 2023, 44, 4953–4964. [Google Scholar] [CrossRef]
- Lee, M.; Lee, E.J.; Kim, R.O.; Pyun, J.M.; Joo, B.E.; Kwon, K.Y.; Roh, H.; Ahn, M.Y.; Lee, K. Systemic immune-inflammation index as a predictor of early stroke progression/recurrence in acute atherosclerotic ischemic stroke. Clin. Neurol. Neurosurg. 2024, 238, 108182. [Google Scholar] [CrossRef]
- Antipenko, S.; Mayfield, N.; Jinno, M.; Gunzer, M.; Ismahil, M.A.; Hamid, T.; Prabhu, S.D.; Rokosh, G. Neutrophils are indispensable for adverse cardiac remodeling in heart failure. J. Mol. Cell Cardiol. 2024, 189, 1–11. [Google Scholar] [CrossRef]
- Sano, S.; Wang, Y.; Ogawa, H.; Horitani, K.; Sano, M.; Polizio, A.H.; Kour, A.; Yura, Y.; Doviak, H.; Walsh, K. TP53-mediated therapy-related clonal hematopoiesis contributes to doxorubicin-induced cardiomyopathy by augmenting a neutrophil-mediated cytotoxic response. JCI Insight 2021, 6, e146076. [Google Scholar] [CrossRef] [PubMed]
- Nettersheim, F.S.; Schlüter, J.D.; Kreuzberg, W.; Mehrkens, D.; Grimm, S.; Nemade, H.; Braumann, S.; Hof, A.; Guthoff, H.; Peters, V.; et al. Myeloperoxidase is a critical mediator of anthracycline-induced cardiomyopathy. Basic Res. Cardiol. 2023, 118, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; He, M.; Wang, Y.L.; Zhang, Y.; Dong, J.; Chen, B.Y.; Li, Y.L.; Zhou, L.J.; Du, L.J.; Liu, Y.; et al. Low-intensity pulsed ultrasound alleviates doxorubicin-induced cardiotoxicity via inhibition of S100a8/a9-mediated cardiac recruitment of neutrophils. Bioeng. Transl. Med. 2023, 8, e10570. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Y.; Xu, X.; Yang, H.; Li, X.; Fu, S.; Guo, Z.; Zhang, J.; Li, H.; Tian, J. Neutrophil extracellular traps mediate cardiomyocyte ferroptosis via the Hippo-Yap pathway to exacerbate doxorubicin-induced cardiotoxicity. Cell Mol. Life Sci. 2024, 81, 122. [Google Scholar] [CrossRef]
- Loh, W.; Vermeren, S. Anti-Inflammatory Neutrophil Functions in the Resolution of Inflammation and Tissue Repair. Cells 2022, 11, 4076. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef]
- Ma, Y.; Yabluchanskiy, A.; Lindsey, M.L. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair. 2013, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F.; Lüscher, T.F.; Camici, G.G.; Liberale, L. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc. Res. 2019, 115, 1266–1285. [Google Scholar] [CrossRef]
- Luu, A.Z.; Chowdhury, B.; Al-Omran, M.; Teoh, H.; Hess, D.A.; Verma, S. Role of Endothelium in Doxorubicin-Induced Cardiomyopathy. JACC Basic. Transl. Sci. 2018, 3, 861–870. [Google Scholar] [CrossRef]
- Dimasi, D.; Sun, W.Y.; Bonder, C.S. Neutrophil interactions with the vascular endothelium. Int. Immunopharmacol. 2013, 17, 1167–1175. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. More friend than foe: The emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Yuzawa, H.; Natori, K.; Wada, R.; Yao, S.; Yano, K.; Akitsu, K.; Koike, H.; Shinohara, M.; Fujino, T.; et al. Early electrocardiographic indices for predicting chronic doxorubicin-induced cardiotoxicity. J. Cardiol. 2021, 77, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.S.; Frey, M.K.; Goliasch, G.; Bartko, P.E.; Prausmüller, S.; Gisslinger, H.; Kornek, G.; Strunk, G.; Raderer, M.; Zielinski, C.; et al. Increased resting heart rate and prognosis in treatment-naïve unselected cancer patients: Results from a prospective observational study. Eur. J. Heart Fail. 2020, 22, 1230–1238. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Soliman, E.Z.; Ehrhardt, M.J.; Lu, L.; Duprez, D.A.; Luepker, R.V.; Armstrong, G.T.; Joshi, V.M.; Green, D.M.; Srivastava, D.; et al. Electrocardiographic abnormalities and mortality in aging survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Am. Heart J. 2017, 189, 19–27. [Google Scholar] [CrossRef]
- Markman, T.M.; Ruble, K.; Loeb, D.; Chen, A.; Zhang, Y.; Beasley, G.S.; Thompson, W.R.; Nazarian, S. Electrophysiological effects of anthracyclines in adult survivors of pediatric malignancy. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Silvetti, E.; Lanza, O.; Romeo, F.; Martino, A.; Fedele, E.; Lanzillo, C.; Crescenzi, C.; Fanisio, F.; Calò, L. The pivotal role of ECG in cardiomyopathies. Front. Cardiovasc. Med. 2023, 10, 1178163. [Google Scholar] [CrossRef]
- Horácek, B.M.; Warren, J.W.; Feild, D.Q.; Feldman, C.L. Statistical and deterministic approaches to designing transformations of electrocardiographic leads. J. Electrocardiol. 2002, 35, 41–52. [Google Scholar] [CrossRef]
- Podyacheva, E.; Danilchuk, M.; Toropova, Y. Molecular mechanisms of endothelial remodeling under doxorubicin treatment. Biomed. Pharmacother. 2023, 162, 114576. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Li, W.; Zhang, Q.; Jiang, Y.; Guo, D.; Sun, X.; Lu, W.; Li, C.; Wang, Y. TFEB-NF-κB inflammatory signaling axis: A novel therapeutic pathway of Dihydrotanshinone I in doxorubicin-induced cardiotoxicity. J. Exp. Clin. Cancer Res. 2020, 39, 93. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef] [PubMed]
- Clayton, Z.S.; Hutton, D.A.; Mahoney, S.A.; Seals, D.R. Anthracycline chemotherapy-mediated vascular dysfunction as a model of accelerated vascular aging. Aging Cancer 2021, 2, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Parr, S.K.; Liang, J.; Schadler, K.L.; Gilchrist, S.C.; Steele, C.C.; Ade, C.J. Anticancer Therapy-Related Increases in Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e015598. [Google Scholar] [CrossRef] [PubMed]
- Budinskaya, K.; Puchnerová, V.; Svačinová, J.; Novák, J.; Hrstková, H.; Nováková, M.; Pekařová, A.; Pekař, M.; Nováková, Z. Non-invasive assessment of vascular system function and damage induced by anthracycline treatment in the pediatric cancer survivors. Physiol. Res. 2017, 66, S553–S560. [Google Scholar] [CrossRef]
- Herceg-Cavrak, V.; Ahel, V.; Batinica, M.; Matec, L.; Kardos, D. Increased arterial stiffness in children treated with anthracyclines for malignant disease. Coll. Antropol. 2011, 35, 389–395. [Google Scholar]
- NIH National Cancer Institute. Early-Stage Breast Cancer. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/early-stage-breast-cancer (accessed on 25 July 2024).
- Lipshultz, S.E.; Lipsitz, S.R.; Sallan, S.E.; Dalton, V.M.; Mone, S.M.; Gelber, R.D.; Colan, S.D. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2005, 23, 2629–2636. [Google Scholar] [CrossRef]
- Russell, R.R.; Alexander, J.; Jain, D.; Poornima, I.G.; Srivastava, A.V.; Storozynsky, E.; Schwartz, R.G. The role and clinical effectiveness of multimodality imaging in the management of cardiac complications of cancer and cancer therapy. J. Nucl. Cardiol. 2016, 23, 856–884. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Chung, R.; Ghosh, A.K.; Banerjee, A. Cardiotoxicity: Precision medicine with imprecise definitions. Open Heart 2018, 5, e000774. [Google Scholar] [CrossRef]
- Mookadam, F.; Sharma, A.; Lee, H.R.; Northfelt, D.W. Intersection of cardiology and oncology clinical practices. Front. Oncol. 2014, 4, 259. [Google Scholar] [CrossRef]
- Pohl, J.; Mincu, R.I.; Mrotzek, S.M.; Wakili, R.; Mahabadi, A.A.; Potthoff, S.K.; Siveke, J.T.; Keller, U.; Landmesser, U.; Rassaf, T.; et al. ECG Scoring for the Evaluation of Therapy-Naïve Cancer Patients to Predict Cardiotoxicity. Cancers 2021, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, L.; Sandford, A.J. Selection of reference genes for gene expression studies in human neutrophils by real-time PCR. BMC Mol. Biol. 2005, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).