Identification of Grape Laccase Genes and Their Potential Role in Secondary Metabolite Synthesis
Abstract
1. Introduction
2. Results
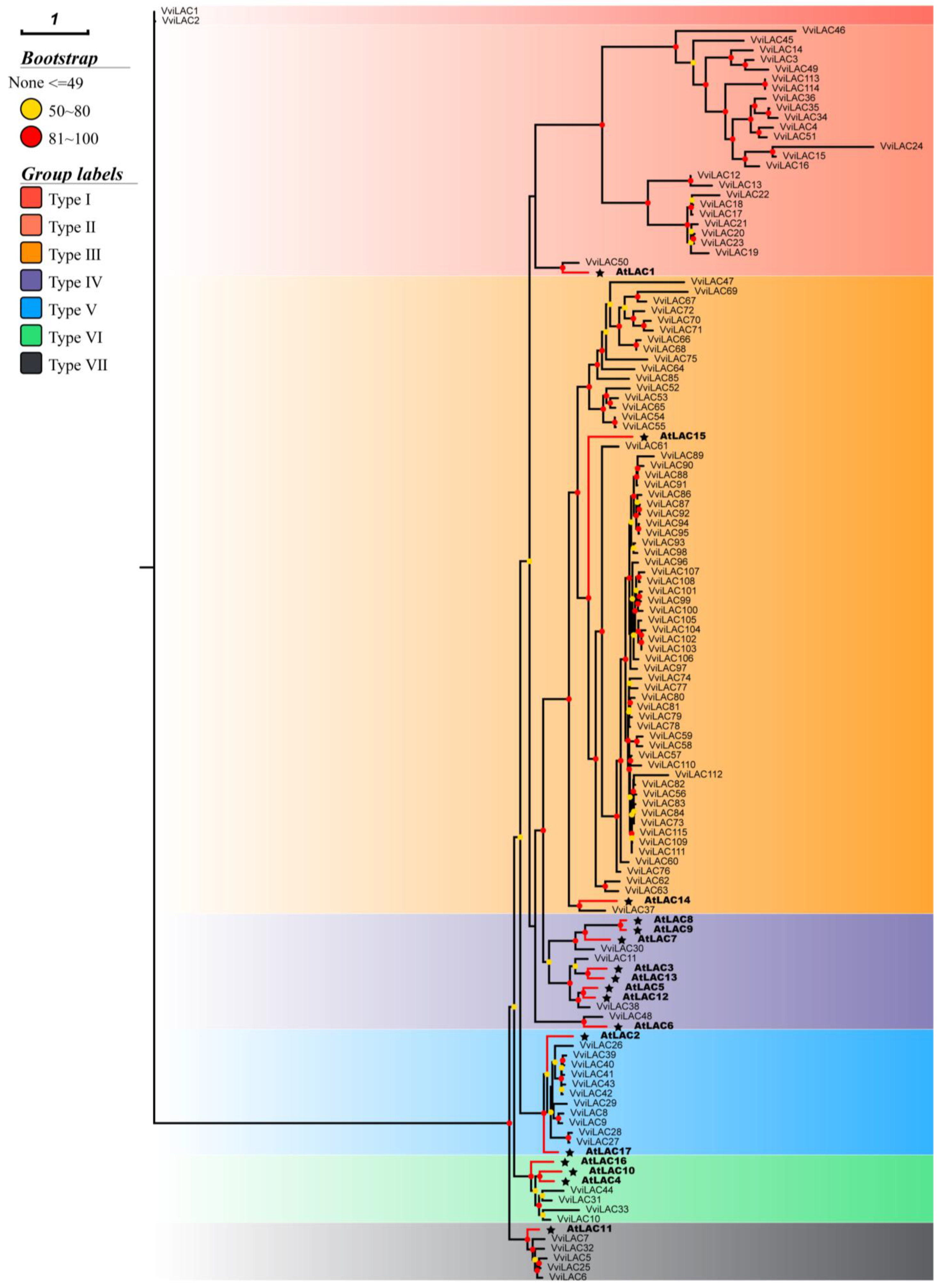
2.1. The Genome of Grape Harbors an Expanded Laccase Gene Family with 115 Members
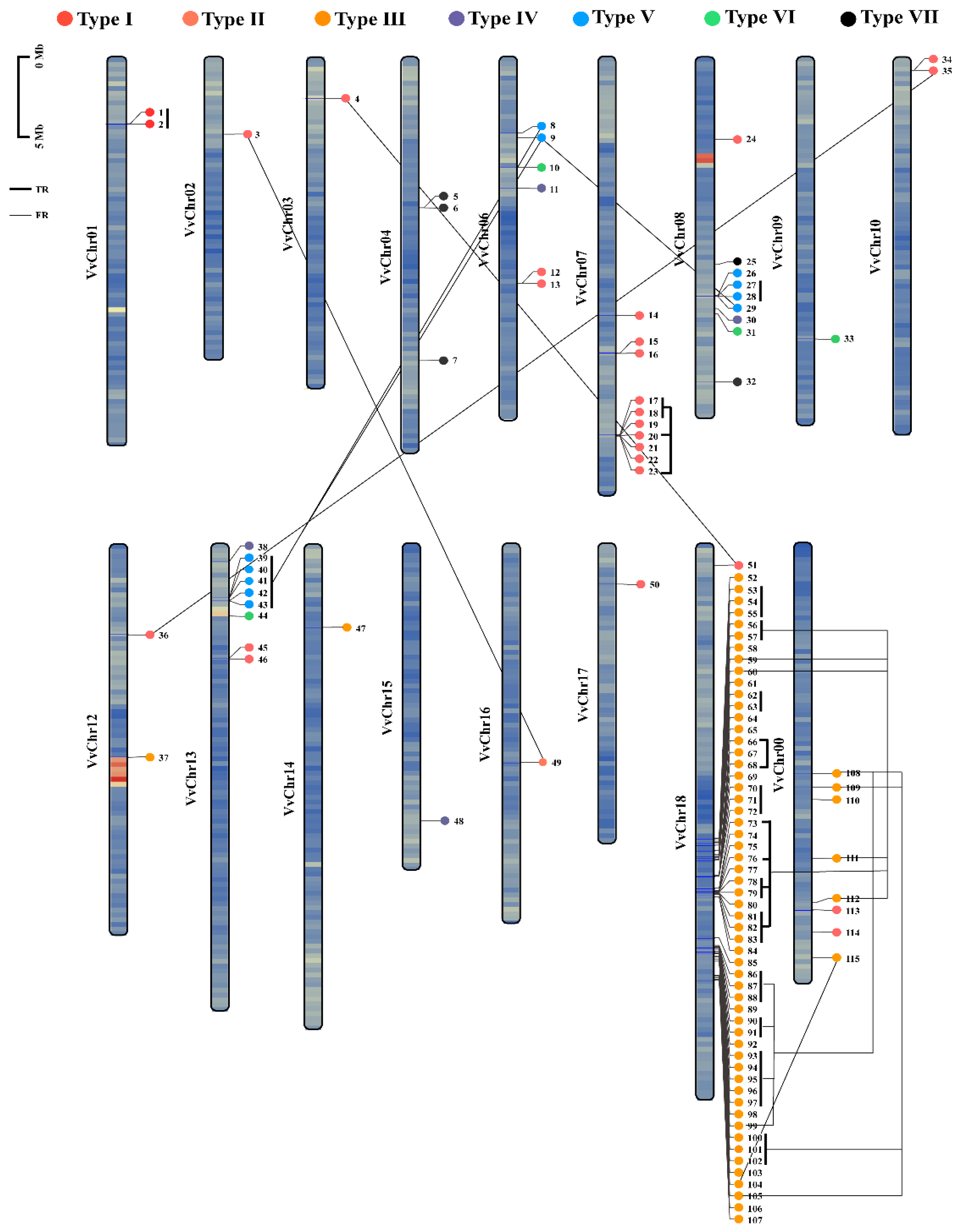
2.2. Expansion of the VviLACs in Grape Was Mainly Caused by Tandem Duplications within Type III Genes
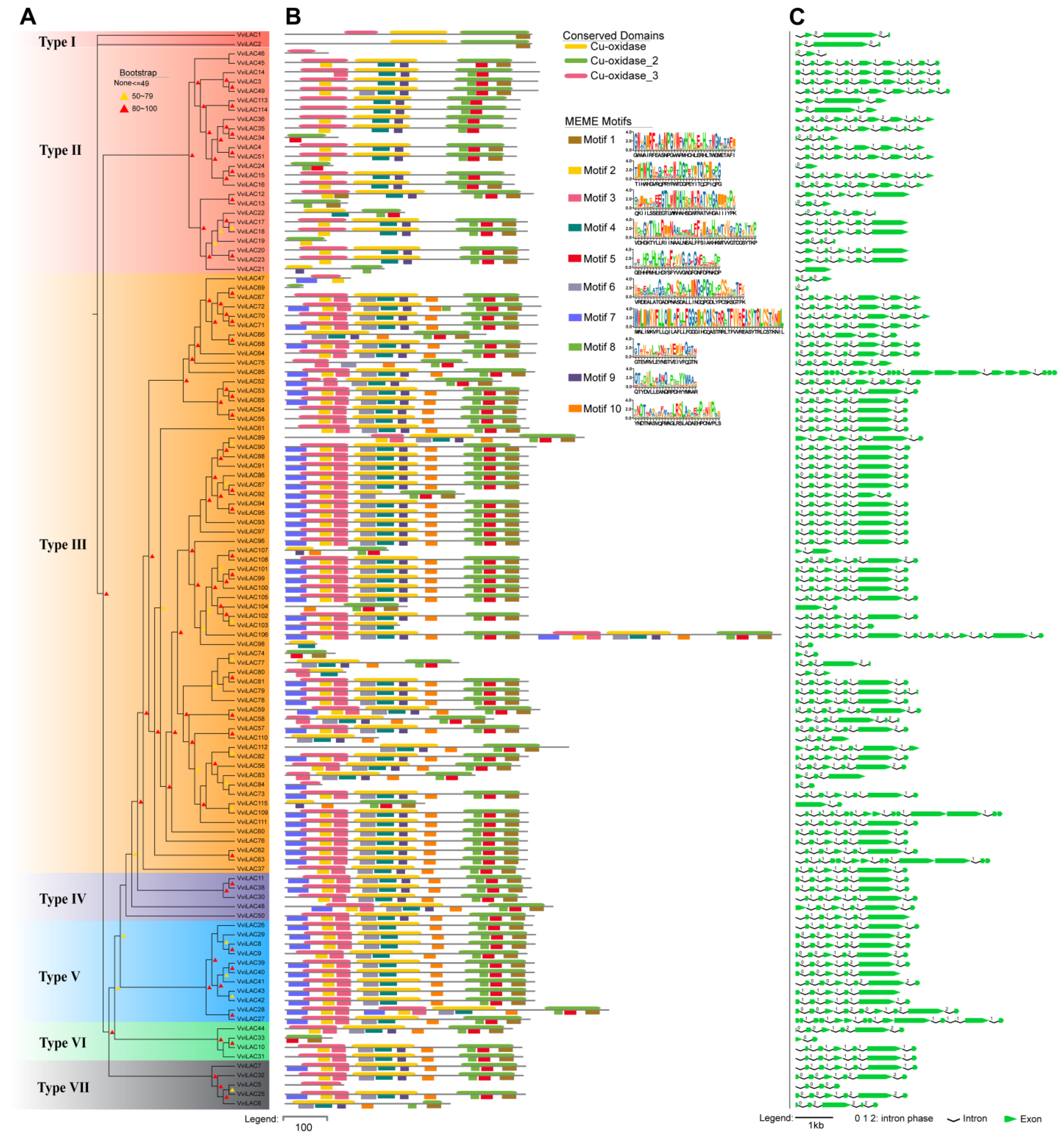
2.3. Gene Structure and Conserved Motifs Analysis with 115 VviLACs
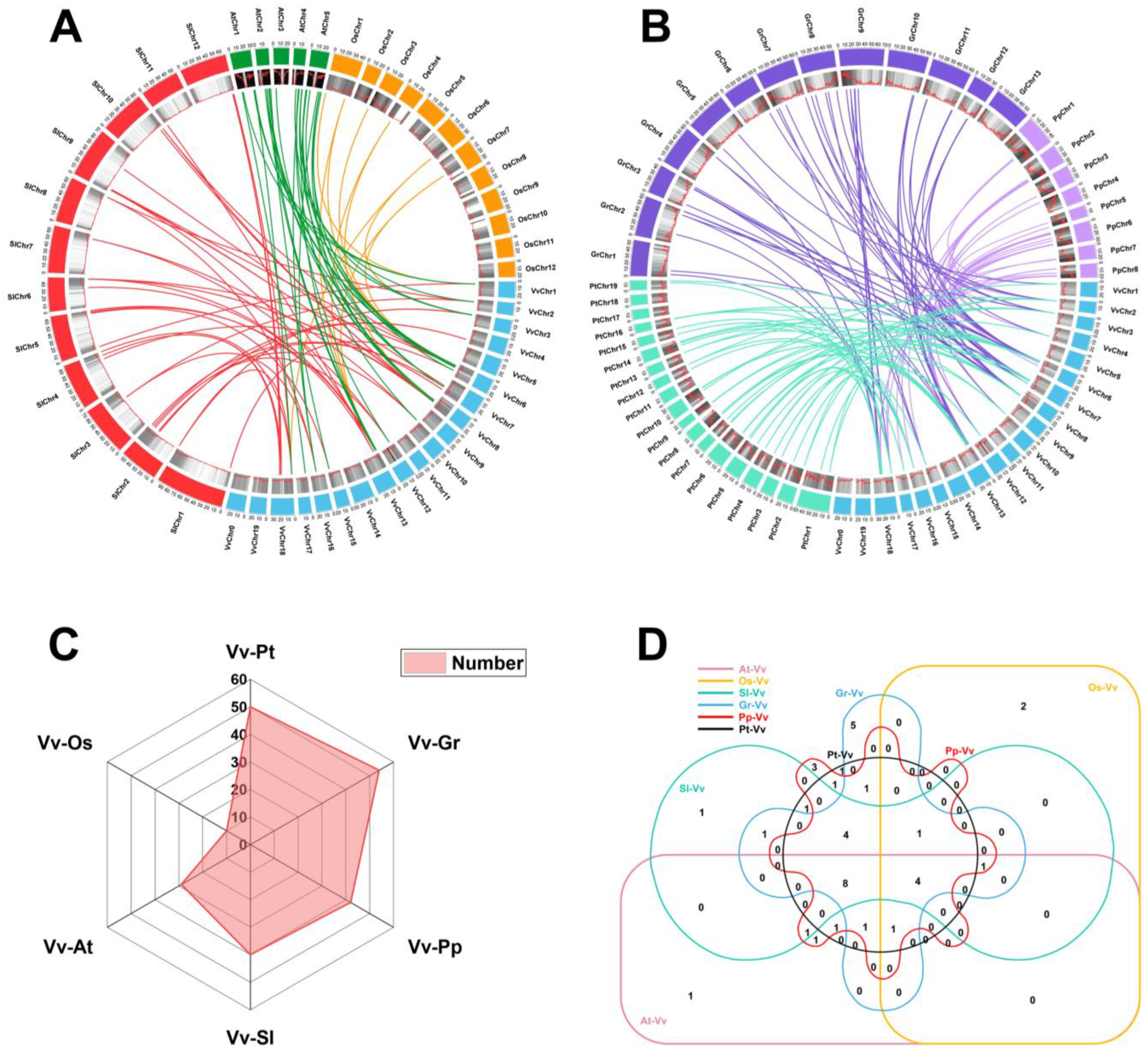
2.4. Multi-Species Collinearity Analysis
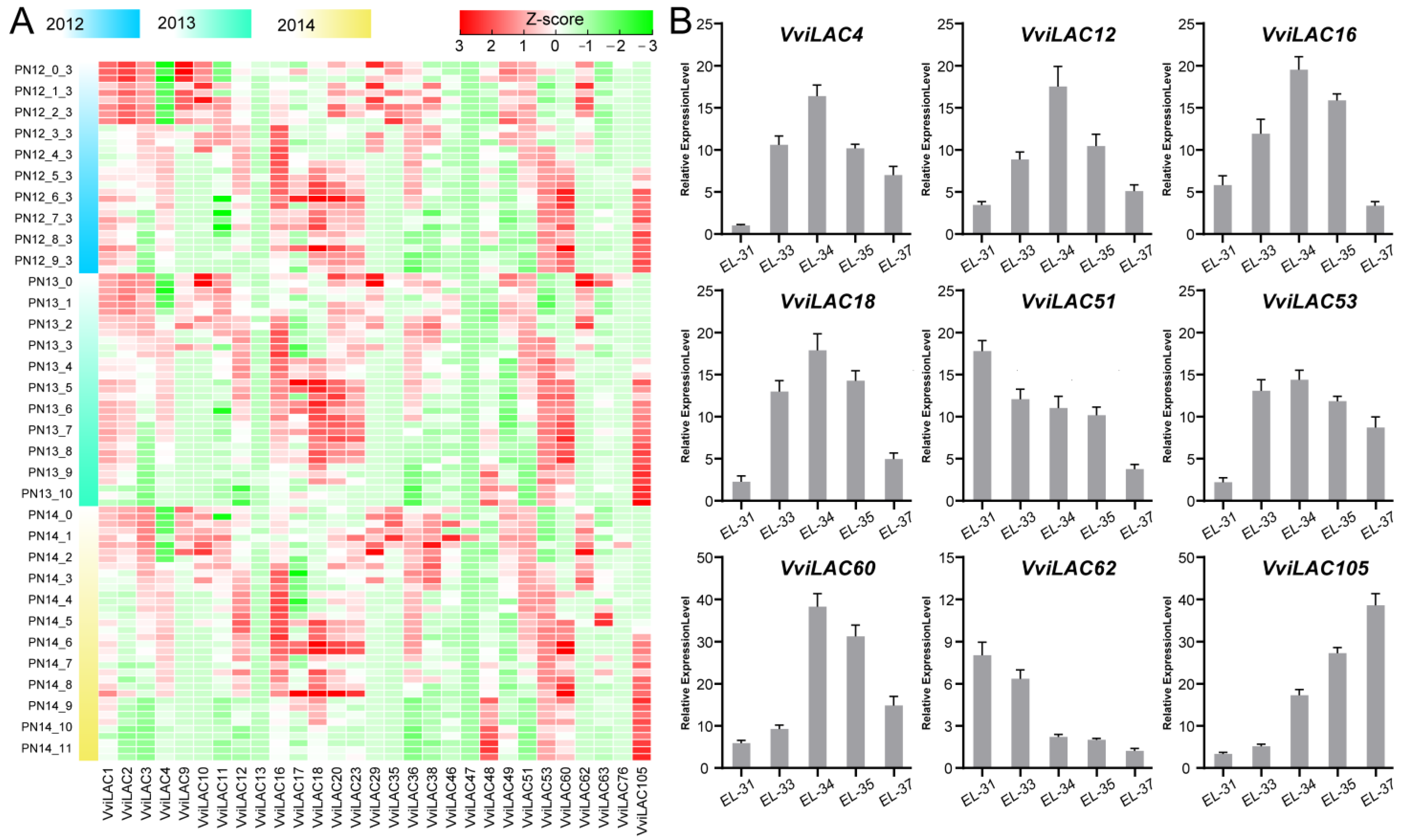
2.5. Expression of VviLACs during Grape Berry Development
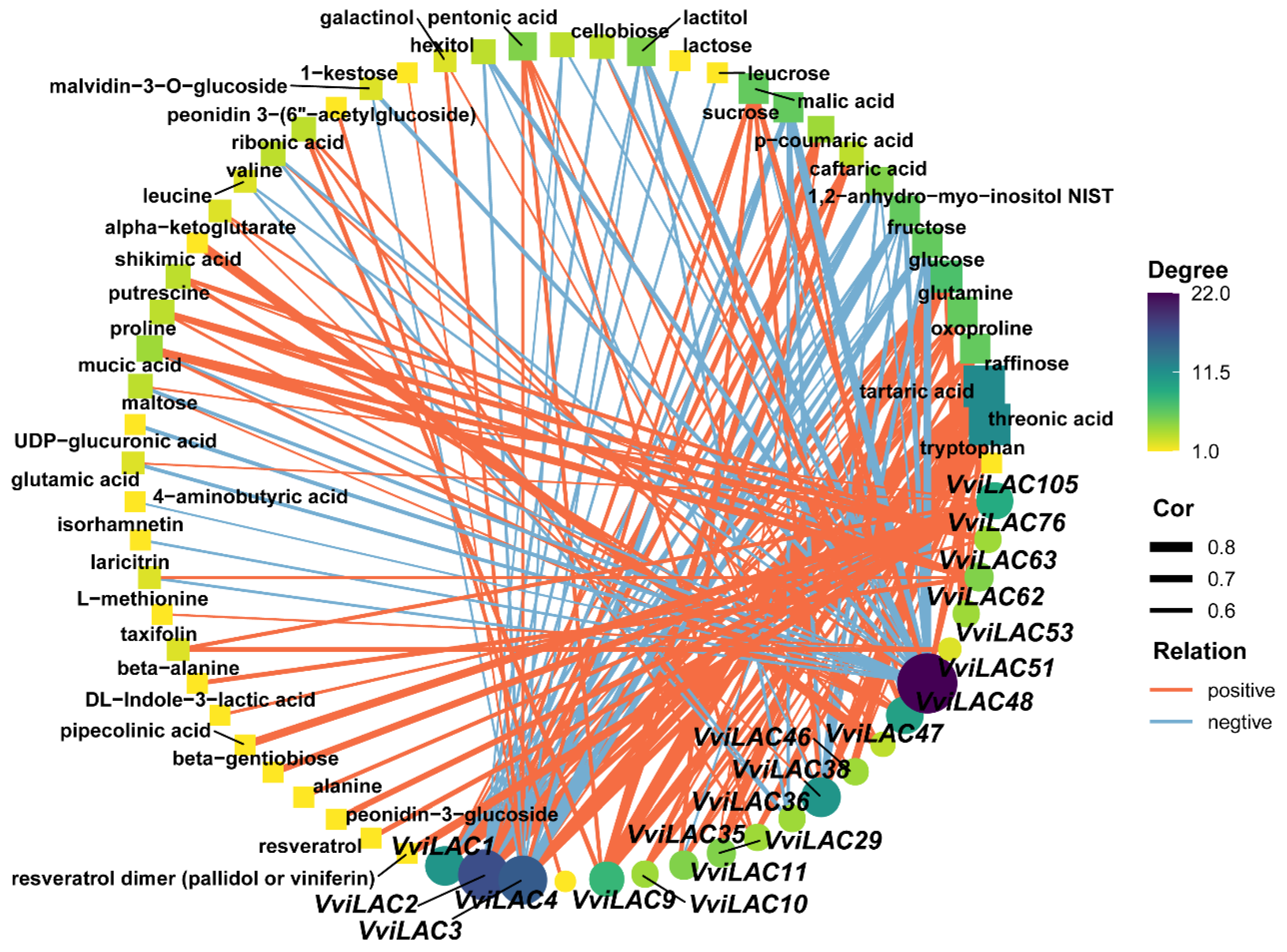
2.6. Relationship between Laccase and Metabolites in Grape Berry Development Stage
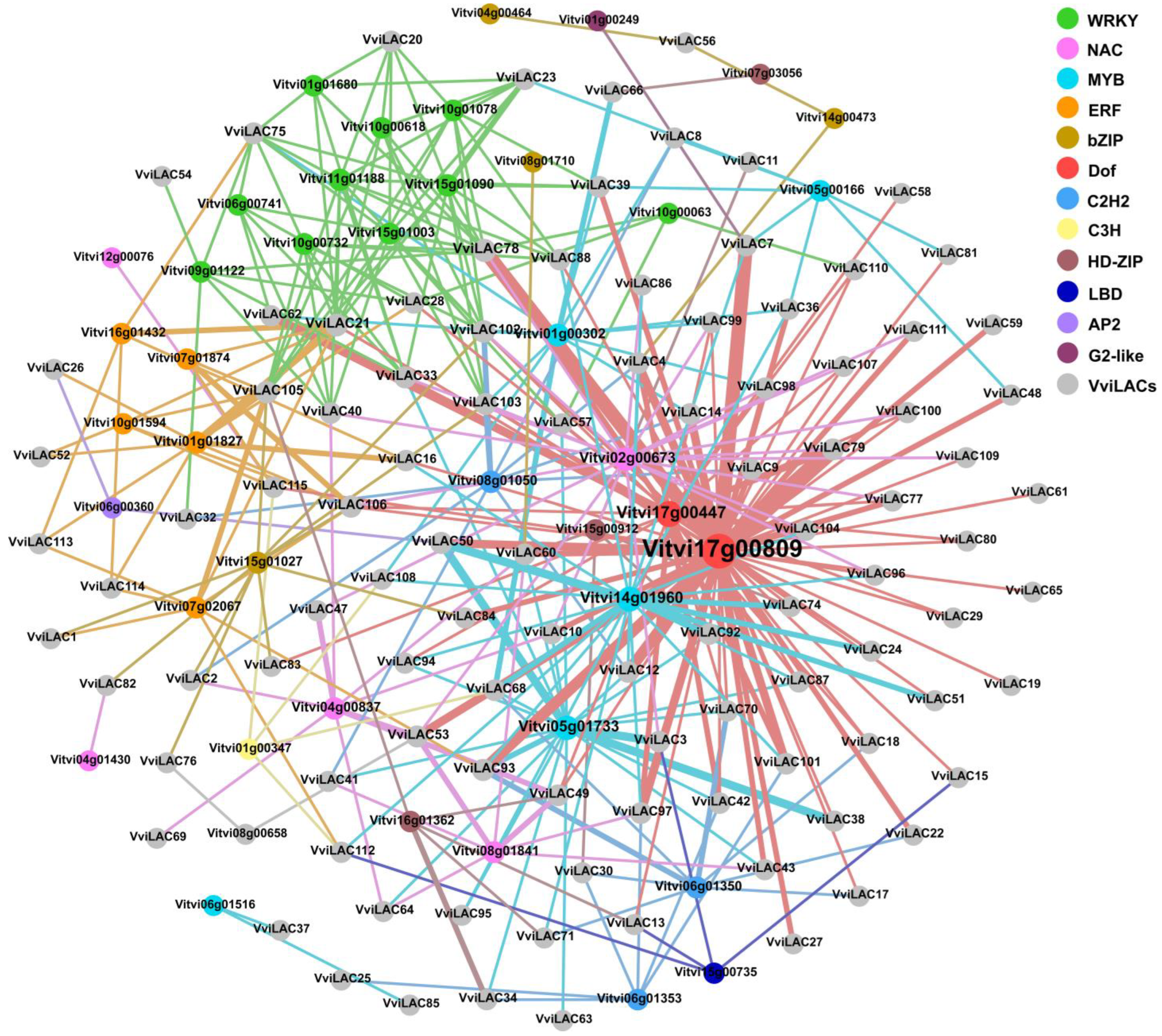
2.7. Predictive Analysis of Candidate Transcription Factors Targeting Laccase Family Genes
3. Discussion
4. Materials and Methods
4.1. Grape Sampling and Developmental Stages
4.2. Identification of VviLACs in the Grapevine Genome
4.3. Bioinformatics Analysis of Laccase Gene Family
4.4. Correlation Analysis of VvLACs Expression and Secondary Metabolites
4.5. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.6. Predictive Analysis of Candidate TFs Targeting Laccase Family Genes
4.7. Significance Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, W.; O’Malley, D.M.; Whetten, R.; Sederoff, R.R. A laccase associated with lignification in loblolly pine xylem. Science 1993, 260, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.-Y.; Dixon, R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Ahsan, R.; Afzal, A.; Jamai, A.; Meksem, K.; El-Shemy, H.A.; Lightfoot, D.A. Multigeneic QTL: The laccase encoded within the soybean Rfs2/rhg1 locus inferred to underlie part of the dual resistance to cyst nematode and sudden death syndrome. Curr. Issues Mol. Biol. 2009, 11, I11. [Google Scholar]
- Ranocha, P.; Chabannes, M.; Chamayou, S.; Danoun, S.; Jauneau, A.; Boudet, A.-M.; Goffner, D. Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol. 2002, 129, 145–155. [Google Scholar] [CrossRef] [PubMed]
- McCaig, B.C.; Meagher, R.B.; Dean, J.F. Gene structure and molecular analysis of the laccase-like multicopper oxidase (LMCO) gene family in Arabidopsis thaliana. Planta 2005, 221, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Turlapati, P.V.; Kim, K.-W.; Davin, L.B.; Lewis, N.G. The laccase multigene family in Arabidopsis thaliana: Towards addressing the mystery of their gene function (s). Planta 2011, 233, 439–470. [Google Scholar] [CrossRef]
- Balasubramanian, V.K.; Rai, K.M.; Thu, S.W.; Hii, M.M.; Mendu, V. Genome-wide identification of multifunctional laccase gene family in cotton (Gossypium spp.); expression and biochemical analysis during fiber development. Sci. Rep. 2016, 6, 34309. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Wang, X.; Shen, Z.; Zheng, L. Comprehensive analysis of rice laccase gene (OsLAC) family and ectopic expression of OsLAC10 enhances tolerance to copper stress in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 209. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Wang, B.; Ding, L.; Wang, Y.; Luo, L.; Zhang, Y.; Kong, W. Genome-wide identification and characterization of laccase gene family in Citrus sinensis. Gene 2019, 689, 114–123. [Google Scholar] [CrossRef]
- Simões, M.S.; Carvalho, G.G.; Ferreira, S.S.; Hernandes-Lopes, J.; de Setta, N.; Cesarino, I. Genome-wide characterization of the laccase gene family in Setaria viridis reveals members potentially involved in lignification. Planta 2020, 251, 46. [Google Scholar] [CrossRef]
- Ping, X.; Wang, T.; Lin, N.; Di, F.; Li, Y.; Jian, H.; Wang, H.; Lu, K.; Li, J.; Xu, X. Genome-wide identification of the LAC gene family and its expression analysis under stress in Brassica napus. Molecules 2019, 24, 1985. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, G.; Ma, C.; Abdullah, M.; Zhang, J.; Zhao, H.; Jin, Q.; Cai, Y.; Lin, Y. Comprehensive genome-wide analysis of the pear (Pyrus bretschneideri) laccase gene (PbLAC) family and functional identification of PbLAC1 involved in lignin biosynthesis. PLoS ONE 2019, 14, e0210892. [Google Scholar] [CrossRef]
- Berthet, S.; Demont-Caulet, N.; Pollet, B.; Bidzinski, P.; Cézard, L.; Le Bris, P.; Borrega, N.; Hervé, J.; Blondet, E.; Balzergue, S.; et al. Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 2011, 23, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef]
- Hu, Q.; Min, L.; Yang, X.; Jin, S.; Zhang, L.; Li, Y.; Ma, Y.; Qi, X.; Li, D.; Liu, H.; et al. Laccase GhLac1 modulates broad-spectrum biotic stress tolerance via manipulating phenylpropanoid pathway and jasmonic acid synthesis. Plant Physiol. 2018, 176, 1808–1823. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, L.; Routaboul, J.-M.; Kerhoas, L.; Caboche, M.; Lepiniec, L.; Debeaujon, I. TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell 2005, 17, 2966–2980. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Davis, E.; Gardner, D.; Cai, X.; Wu, Y. Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis. Planta 2006, 224, 1185–1196. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, X.-L.; Luo, H.-H.; Zhou, J.-J.; Gong, Y.-H.; Li, W.-J.; Shi, Z.-W.; He, Q.; Wu, Q.; Li, L.; et al. An intracellular laccase is responsible for epicatechin-mediated anthocyanin degradation in litchi fruit pericarp. Plant Physiol. 2015, 169, 2391–2408. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- Bagal, U.R.; Leebens-Mack, J.H.; Lorenz, W.W.; Dean, J.F.D. The phenylalanine ammonia lyase (PAL) gene family shows a gymnosperm-specific lineage. BMC Genom. 2012, 13, S1. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Xie, Z.; Jia, H.; Tang, W.; Cui, M.; Fang, J. Function analysis of VvmiR397a and its target genes VvLACs in grape berry development. Acta Hortic. Sin. 2018, 45, 1441. [Google Scholar]
- Huang, Y.; Liang, D.; Xia, H.; Lin, L.-J.; Wang, J.; Lv, X.-L. Lignin and Quercetin Synthesis Underlies Berry Russeting in ‘Sunshine Muscat’ Grape. Biomolecules 2020, 10, 690. [Google Scholar] [CrossRef] [PubMed]
- Aruni, U.V. Role of Laccase as a Virulence Factor in the Infection of Grapes by Botrytis cinerea. Doctoral Thesis, Charles Sturt University, Sydney, Australia, 2017. [Google Scholar]
- Wang, G.-D.; Li, Q.-J.; Luo, B.; Chen, X.-Y. Ex planta phytoremediation of trichlorophenol and phenolic allelochemicals via an engineered secretory laccase. Nat. Biotechnol. 2004, 22, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Lee, C.; Hwang, S.-G.; Park, Y.C.; Lim, H.L.; Jang, C.S. Overexpression of the OsChI1 gene, encoding a putative laccase precursor, increases tolerance to drought and salinity stress in transgenic Arabidopsis. Gene 2014, 552, 98–105. [Google Scholar] [CrossRef]
- Ma, J.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Isolation, purification and characterization of two laccases from carrot (Daucus carota L.) and their response to abiotic and metal ions stresses. Protein J. 2015, 34, 444–452. [Google Scholar] [CrossRef]
- Fasoli, M.; Richter, C.L.; Zenoni, S.; Bertini, E.; Vitulo, N.; Dal Santo, S.; Dokoozlian, N.; Pezzotti, M.; Tornielli, G.B. Timing and Order of the Molecular Events Marking the Onset of Berry Ripening in Grapevine. Plant Physiol. 2018, 178, 1187–1206. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, Y.; Yin, X.; Wang, W.; Li, W.; Jiang, L.; Chen, X.; Wang, B.; Ma, J. Genome-wide identification and expression patterns of the laccase gene family in response to kiwifruit bacterial canker infection. BMC Plant Biol. 2023, 23, 591. [Google Scholar] [CrossRef]
- Shi, J.; Yao, J.; Tong, R.; Wang, S.; Li, M.; Song, C.; Wan, R.; Jiao, J.; Zheng, X. Genome-wide identification of laccase gene family from Punica granatum and functional analysis towards potential involvement in lignin biosynthesis. Horticulturae 2023, 9, 918. [Google Scholar] [CrossRef]
- Arcuri, M.L.; Fialho, L.C.; Vasconcellos Nunes-Laitz, A.; Fuchs-Ferraz, M.C.P.; Wolf, I.R.; Valente, G.T.; Marino, C.L.; Maia, I.G. Genome-wide identification of multifunctional laccase gene family in Eucalyptus grandis: Potential targets for lignin engineering and stress tolerance. Trees 2020, 34, 745–758. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef]
- Cabezas, J.A.; Cervera, M.T.; Ruiz-García, L.; Carreño, J.; Martínez-Zapater, J.M. A genetic analysis of seed and berry weight in grapevine. Genome 2006, 49, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Doligez, A.; Bouquet, A.; Danglot, Y.; Lahogue, F.; Riaz, S.; Meredith, C.; Edwards, K.; This, P. Genetic mapping of grapevine (Vitis vinifera L.) applied to the detection of QTLs for seedlessness and berry weight. Theor. Appl. Genet. 2002, 105, 780–795. [Google Scholar] [CrossRef]
- Bellin, D.; Peressotti, E.; Merdinoglu, D.; Wiedemann-Merdinoglu, S.; Adam-Blondon, A.-F.; Cipriani, G.; Morgante, M.; Testolin, R.; Di Gaspero, G. Resistance to Plasmopara viticola in grapevine ‘Bianca’is controlled by a major dominant gene causing localised necrosis at the infection site. Theor. Appl. Genet. 2009, 120, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R. New horizons for grapevine breeding. Fruit Veg. Cereal Sci. Biotechnol. 2011, 5, 79–100. [Google Scholar]
- Fernandes, J.C.; Goulao, L.F.; Amâncio, S. Regulation of cell wall remodeling in grapevine (Vitis vinifera L.) callus under individual mineral stress deficiency. J. Plant Physiol. 2016, 190, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, G.; Lagreze, J.; Rojas San Martin, B.; Malnoy, M.; Moretto, M.; Moser, C.; Dalla Costa, L. Insights into the cell-wall dynamics in grapevine berries during ripening and in response to biotic and abiotic stresses. Plant Mol. Biol. 2024, 114, 38. [Google Scholar] [CrossRef]
- Schwab, W.; Raab, T. Developmental changes during strawberry fruit ripening and physico-chemical changes during postharvest storage. In Production Practices and Quality Assessment of Food Crops: Quality Handling and Evaluation; Springer: Berlin/Heidelberg, Germany, 2004; pp. 341–369. [Google Scholar]
- Yihui, G.; Song, J.; Du, L.; Vinqvist, M.; Palmer, L.C.; Fillmore, S.; Pang, X.; Zhang, Z. Characterization of laccase from apple fruit during postharvest storage and its response to diphenylamine and 1-methylcyclopropene treatments. Food Chem. 2018, 253, 314–321. [Google Scholar] [CrossRef]
- Liu, B.; Zhong, R.; Wei, J.; Zhang, J.; Luo, H.; Guan, H.; Fang, F.; Pang, X.; Zhang, Z. Genome-wide identification and analysis of the laccase gene family in Litchi chinensis Sonn. provides new insights into pericarp browning. Postharvest Biol. Technol. 2024, 217, 113108. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Q.; Xiao, L.; Wang, Y.; Feng, J.; Bu, Q.; Xiao, Y.; Hao, K.; Guo, M.; Chen, W.; et al. Multiplexed CRISPR/Cas9-Mediated Knockout of Laccase Genes in Salvia miltiorrhiza Revealed Their Roles in Growth, Development, and Metabolism. Front. Plant Sci. 2021, 12, 647768. [Google Scholar] [CrossRef]
- Zhang, L.B.; Yang, W.W.J.; Qiu, T.T. Genome-wide study of Cerrena unicolor 87613 laccase gene family and their mode prediction in association with substrate oxidation. BMC Genomics 2023, 24, 504. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Fu, Q.; Wang, Z.; Liu, X.; Sun, L.; Zhao, Z. Transcriptomic and metabolomic analysis reveals a protein module involved in preharvest apple peel browning. Plant Physiol. 2023, 192, 2102–2122. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Sun, L.; Yang, S.; Fan, X.; Zhang, Y.; Guo, D.; Jiang, J. RNA-sequencing analysis of candidate genes involved in berry development in ‘Summer Black’ grapes and its early bud mutants varieties. Sci. Hortic. 2023, 308, 111568. [Google Scholar] [CrossRef]
- Dry, P.; Coombe, B. Revised Version of “Grapevine Growth Stages—The Modified el System” Viticulture 1; Winetitles Media: Broadview, Australia, 2004. [Google Scholar]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Canaguier, A.; Grimplet, J.; Di Gaspero, G.; Scalabrin, S.; Duchêne, E.; Choisne, N.; Mohellibi, N.; Guichard, C.; Rombauts, S.; Le Clainche, I.; et al. A new version of the grapevine reference genome assembly (12X. v2) and of its annotation (VCost. v3). Genom. Data 2017, 14, 56–62. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.-H. EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res. 2012, 40, W569–W572. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.R.; Yu, Y.H.; Ni, P.Y.; Zhang, G.H.; Guo, D.L. Genome-wide identification of small heat-shock protein (HSP20) gene family in grape and expression profile during berry development. BMC Plant Biol. 2019, 19, 433. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.; Frank, M.; He, Y.; Xia, R. TBtools—An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Ji, X.; Guo, D. Genome-wide identification, characterization, and expression analysis of GDSL-type esterases/lipases gene family in relation to grape berry ripening. Sci. Hortic. 2020, 264, 109162. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, D.; Wang, R.; Kong, N.; Zhang, C.; Yang, C.; Wu, W.; Ma, H.; Chen, Q. Genome-wide analysis of the potato Hsp20 gene family: Identification, genomic organization and expression profiles in response to heat stress. BMC Genom. 2018, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the International AAAI Conference on Web and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhong, H.; Zhang, F.; Zhang, C.; Zhang, S.; Zhou, X.; Wu, X.; Yadav, V. Identification of Grape Laccase Genes and Their Potential Role in Secondary Metabolite Synthesis. Int. J. Mol. Sci. 2024, 25, 10574. https://doi.org/10.3390/ijms251910574
Wang H, Zhong H, Zhang F, Zhang C, Zhang S, Zhou X, Wu X, Yadav V. Identification of Grape Laccase Genes and Their Potential Role in Secondary Metabolite Synthesis. International Journal of Molecular Sciences. 2024; 25(19):10574. https://doi.org/10.3390/ijms251910574
Chicago/Turabian StyleWang, Hao, Haixia Zhong, Fuchun Zhang, Chuan Zhang, Songlin Zhang, Xiaoming Zhou, Xinyu Wu, and Vivek Yadav. 2024. "Identification of Grape Laccase Genes and Their Potential Role in Secondary Metabolite Synthesis" International Journal of Molecular Sciences 25, no. 19: 10574. https://doi.org/10.3390/ijms251910574
APA StyleWang, H., Zhong, H., Zhang, F., Zhang, C., Zhang, S., Zhou, X., Wu, X., & Yadav, V. (2024). Identification of Grape Laccase Genes and Their Potential Role in Secondary Metabolite Synthesis. International Journal of Molecular Sciences, 25(19), 10574. https://doi.org/10.3390/ijms251910574






