L-Arginine and Taurisolo® Effects on Brain Hypoperfusion–Reperfusion Damage in Hypertensive Rats
Abstract
:1. Introduction
2. Results
2.1. Mean Arterial Blood Pressure (MABP)
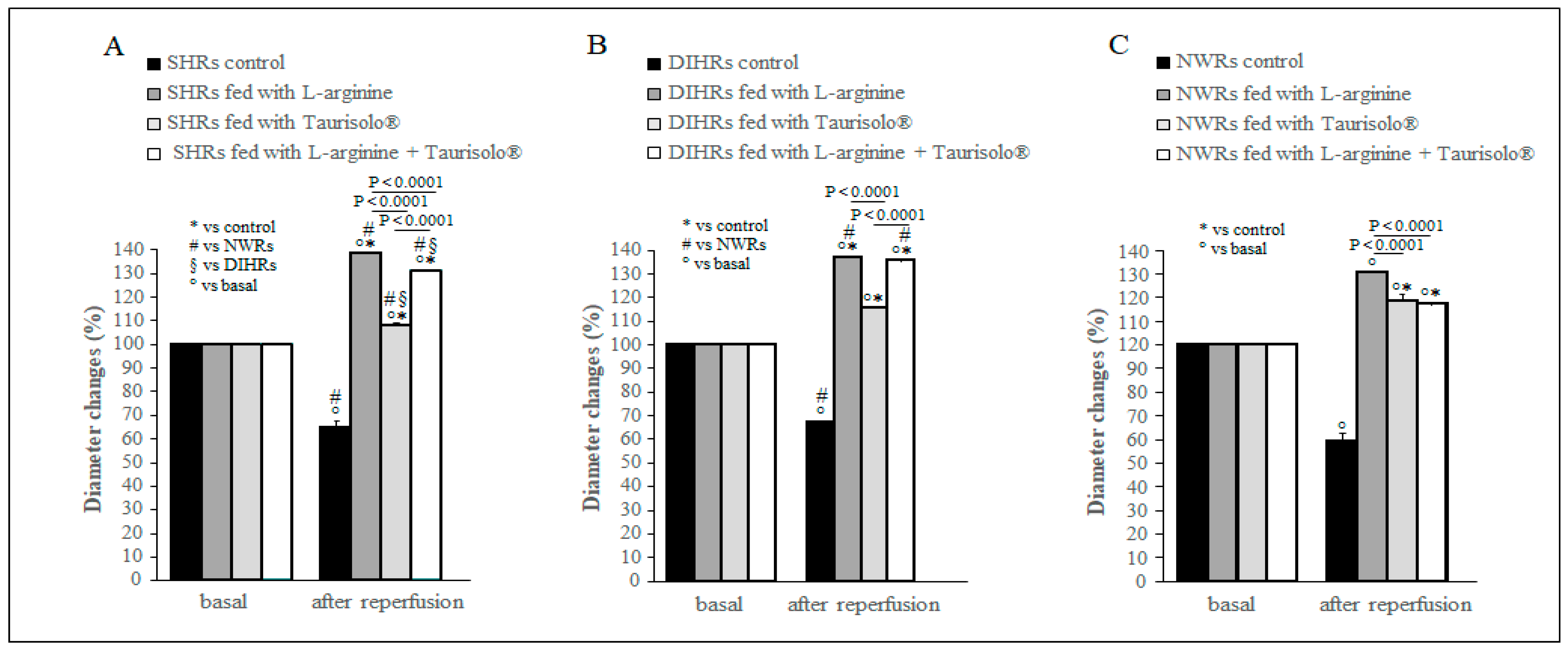
2.2. Arteriolar Diameter Changes
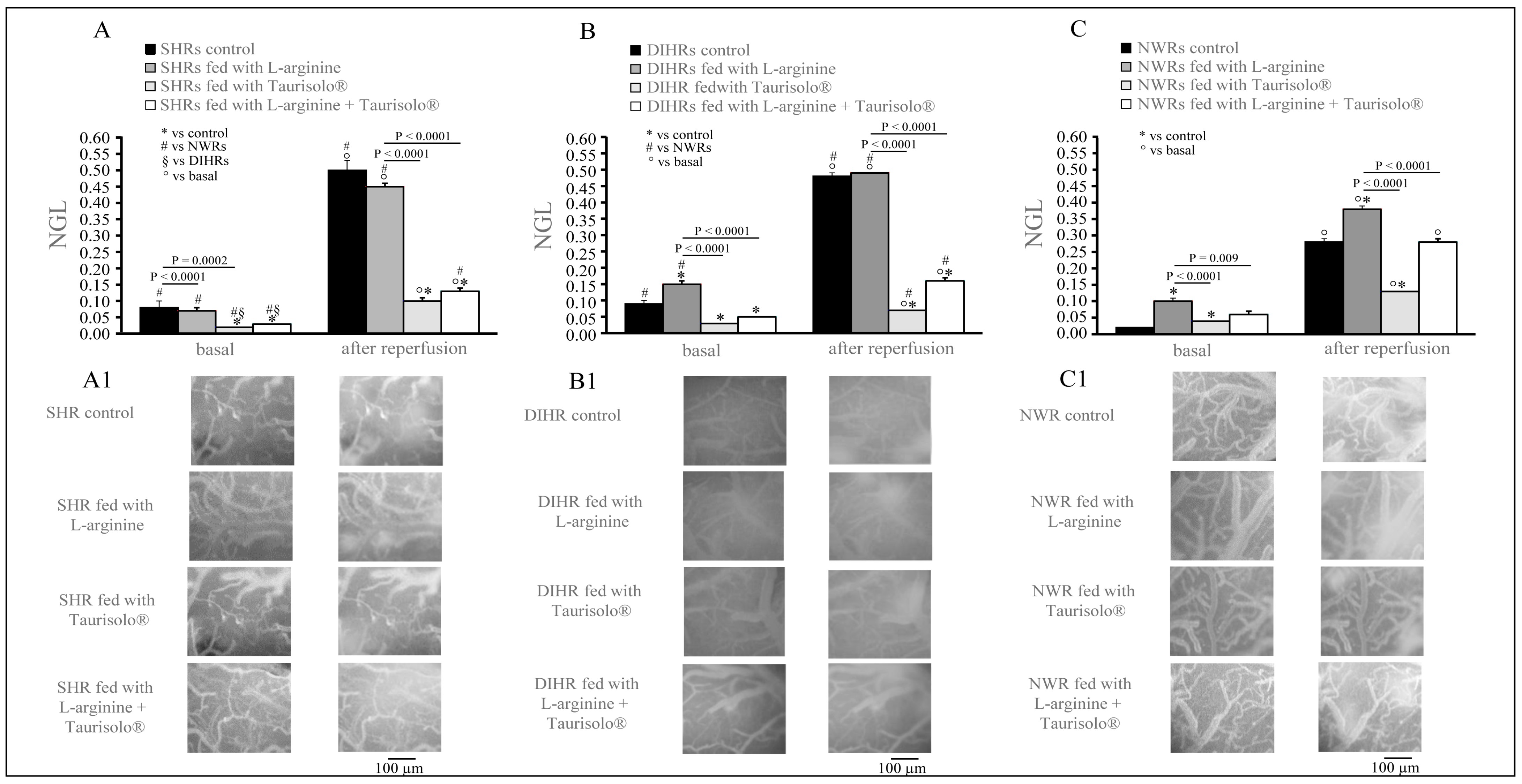
2.3. Microvascular Permeability Changes
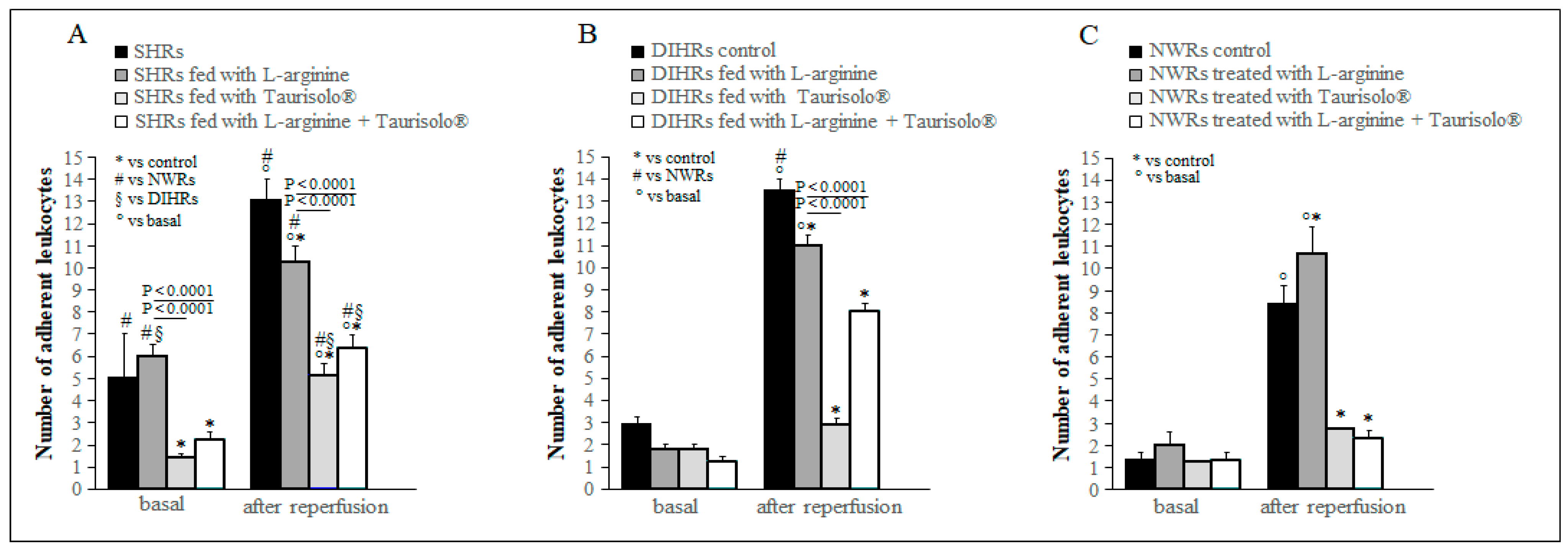
2.4. Leukocyte Adhesion
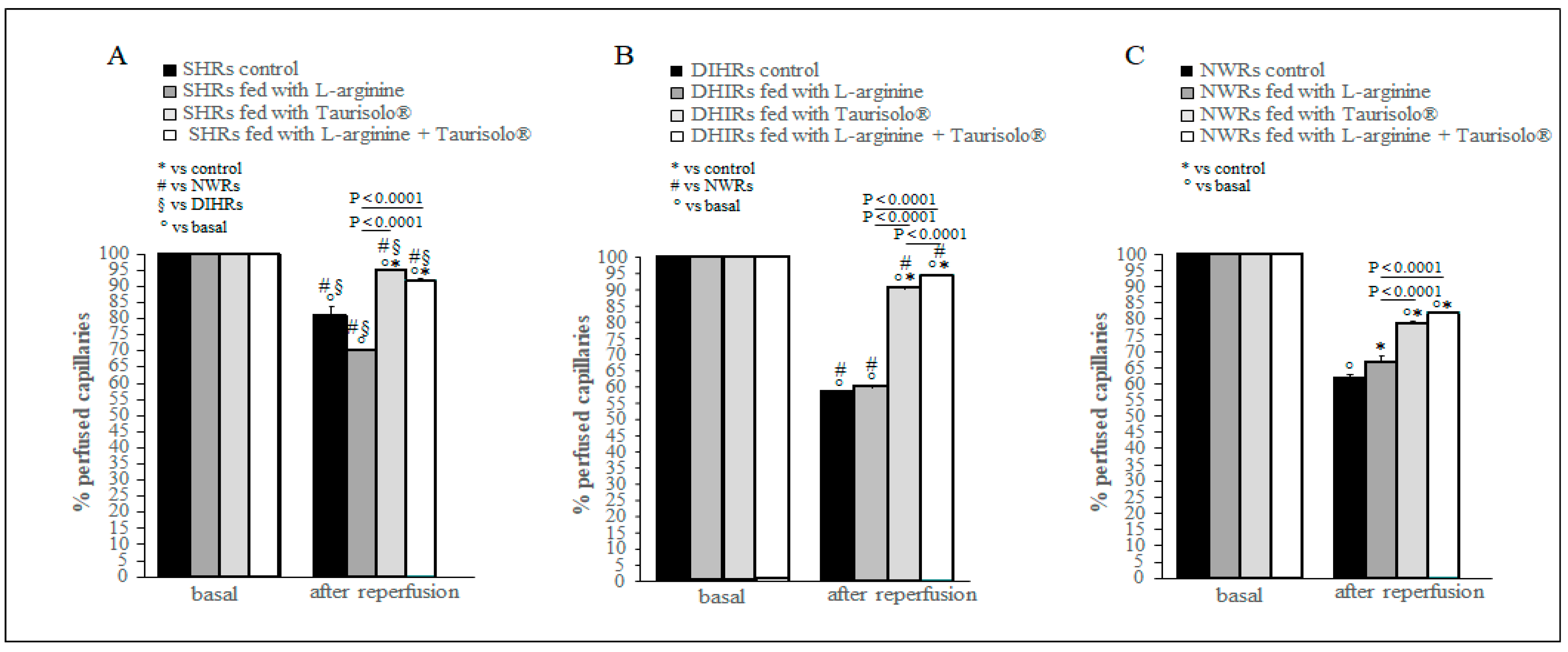
2.5. Capillary Perfusion Changes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Animal Preparation
4.3. Quantification of Microvascular Parameters
4.4. Drugs
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Davisson, R.L. Hypertension and Cerebrovascular Dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef]
- Kim, H.L. Arterial stiffness and hypertension. Clin. Hypertens. 2023, 29, 31. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2019, 75, 285–292. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Carvalho, C.; Moreira, P.L. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef]
- Rueckschloss, U.; Duerrschmidt, N.; Morawietz, H. NADPH Oxidase in Endothelial Cells: Impact on Atherosclerosis. Antioxid. Redox Signal. 2003, 5, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.J.; Liu, C.-A.; Huang, B.; Tseng, A.H.; Wang, D.L. Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Pan, S. Molecular Mechanisms Responsible for the Atheroprotective Effects of Laminar Shear Stress. Antioxid. Redox Signal. 2009, 11, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Touyz, R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006, 71, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflügers Arch.-Eur. J. Physiol. 2010, 459, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Brunssen, C.; Morawietz, H. Contribution of lectin-like oxidized low-density lipoprotein receptor-1 and LOX-1 modulating compounds to vascular diseases. Vasc. Pharmacol. 2018, 107, 1–11. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Mattson, D.L. Role of L-arginine in nitric oxide production in health and hypertension. Clin. Exp. Pharmacol. Physiol. 2009, 36, 249–255. [Google Scholar] [CrossRef]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Annunziata, G.; Jimenez-García, M.; Tejada, S.; Moranta, D.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Sureda, A.; Novellino, E.; Capó, X. Grape polyphenols ameliorate muscle decline reducing oxidative stress and oxidative damage in aged rats. Nutrients 2020, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of grape pomace polyphenolic extract (Taurisolo®) in reducing TMAO serum levels in humans: Preliminary results from a randomized, placebo-controlled, cross-over study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Tacconi, E.; Palma, G.; De Biase, D.; Luciano, A.; Barbieri, M.; de Nigris, F.; Bruzzese, F. Microbiota Effect on Trimethylamine N-Oxide Production: From Cancer to Fitness—A Practical Preventing Recommendation and Therapies. Nutrients 2023, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Hassan, S.T.S.; Tenore, G.C.; Novellino, E. Effect of Grape Pomace Polyphenols With or Without Pectin on TMAO Serum Levels Assessed by LC/MS-Based Assay: A Preliminary Clinical Study on Overweight/Obese Subjects. Front. Pharmacol. 2019, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Lapi, D.; Stornaiuolo, M.; Sabatino, L.; Sommella, E.; Tenore, G.; Daglia, M.; Scuri, R.; Di Maro, M.; Colantuoni, A.; Novellino, E. The Pomace Extract Taurisolo Protects Rat Brain From Ischemia-Reperfusion Injury. Front. Cell. Neurosci. 2020, 28, 14. [Google Scholar] [CrossRef] [PubMed]
- Lapi, D.; Marchiafava, P.L.; Colantuoni, A. Pial microvascular responses to transient bilateral common carotid artery occlusion: Effects of hypertonic glycerol. J. Vasc. Res. 2008, 45, 89–102. [Google Scholar] [CrossRef]
- Strandgaard, S.; Paulson, O.B. Cerebral blood flow and its pathophysiology in hypertension. Am. J. Hypertens. 1989, 2, 486–492. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef]
- Sullivan, J.C.; Pollock, J.S. Coupled and Uncoupled NOS: Separate But Equal? Circ. Res. 2006, 98, 717–719. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef]
- Martelli, A.; Flori, L.; Gorica, E.; Piragine, E.; Saviano, A.; Annunziata, G.; Di Minno, M.N.D.; Ciampaglia, R.; Calcaterra, I.; Maione, F.; et al. Vascular effects of the polyphenolic nutraceutical supplement Taurisolo®: Focus on the protection of the endothelial function. Nutrients 2021, 13, 1540. [Google Scholar] [CrossRef] [PubMed]
- Lapi, D.; Varanini, M.; Galasso, L.; Di Maro, M.; Federighi, G.; Del Seppia, C.; Colantuoni, A.; Scuri, R. Effects of Mandibular Extension on Pial Arteriolar Diameter Changes in Glucocorticoid-Induced Hypertensive Rats. Front. Physiol. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Miczke, A.; Suliburska, J.; Pupek-Musialik, D.; Ostrowska, L.; Jabłecka, A.; Krejpcio, Z.; Skrypnik, D.; Bogdański, P. Effect of L-arginine supplementation on insulin resistance and serum adiponectin concentration in rats with fat diet. Int. J. Clin. Exp. Med. 2015, 8, 10358–10366. [Google Scholar] [PubMed]
- Luckl, J.; Keating, J.; Greenberg, J.H. Alpha-chloralose is a Suitable Anesthetic for Chronic Focal Cerebral Ischemia Studies in the Rat: A comparative study. Brain Res. 2008, 1191, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Ngai, A.C.; Ko, K.R.; Morii, S.; Winn, H.R. Effect of sciatic nerve stimulation on pial arterioles in rats. Am. J. Physiol.-Heart Circ. Physiol. 1988, 254, H133–H139. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, A.G.; Fehér, G.; Weigle, C.G.; Knuese, D.E.; Kampine, J.P. Video microscopy of cerebrocortical capillary flow: Response to hypotension and intracranial hypertension. Am. J. Physiol. 1985, 268, H2202–H2210. [Google Scholar] [CrossRef] [PubMed]
- Morii, S.; Ngai, A.C.; Winn, H.R. Reactivity of rat pial arterioles and venules to adenosine and carbon dioxine: With detailed description of the closed cranial window technique in rats. J. Cereb. Blood Flow Metab. 1986, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Lapi, D.; Marchiafava, P.L.; Colantuoni, A. Geometric characteristics of arterial network of rat pial microcirculation. J. Vasc. Res. 2008, 45, 69–77. [Google Scholar] [CrossRef]
- Watanabe, S. In vivo fluorometric measurement of cerebral oxidative stress using 2 -7-dichlorofluorescein (DCF). Keio J. Med. 1998, 47, 92–98. [Google Scholar] [CrossRef]

| Animal Sub-Groups | Mean Arterial Blood Pressure (MABP) mmHg | |
|---|---|---|
| Before Treatment | After Treatment | |
| SHRs (control) | 175 ± 1.40 | 193 ± 4.00 * |
| SHRs FED WITH L-ARGININE | 178 ± 2.00 | 130 ± 2.00 *⬪ |
| SHRs FED WITH TAURISOLO | 175 ± 3.90 | 160 ± 3.60 *⬪ |
| SHRs FED WITH L-ARGININE + TAURISOLO | 171 ± 2.00 | 132 ± 5.40 *⬪ |
| DIHRs (control) | 123 ± 1.03 | 182 ± 1.58 * |
| DIHRs FED WITH L-ARGININE | 123 ± 1.04 | 125 ± 1.08 ⬪ |
| DIHRs FED WITH TAURISOLO | 123 ± 1.19 | 167 ± 1.03 *⬪ |
| DIHRs FED WITH L-ARGININE + TAURISOLO | 122 ± 0.75 | 121 ± 1.47 ⬪ |
| NWRs (control) | 122 ± 0.71 | 127 ± 4.02 |
| NWRs FED WITH L-ARGININE | 121 ± 0.63 | 110 ± 0.82 *⬪ |
| NWRs FED WITH TAURISOLO | 124 ± 1.03 | 122 ± 0.63 |
| NWRs FED WITH L-ARGININE + TAURISOLO | 124 ± 0.95 | 118 ± 1.03 ⬪ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapi, D.; Tenore, G.C.; Federighi, G.; Chiurazzi, M.; Nunziato, S.; Lonardo, M.S.; Stornaiuolo, M.; Colantuoni, A.; Novellino, E.; Scuri, R. L-Arginine and Taurisolo® Effects on Brain Hypoperfusion–Reperfusion Damage in Hypertensive Rats. Int. J. Mol. Sci. 2024, 25, 10868. https://doi.org/10.3390/ijms251910868
Lapi D, Tenore GC, Federighi G, Chiurazzi M, Nunziato S, Lonardo MS, Stornaiuolo M, Colantuoni A, Novellino E, Scuri R. L-Arginine and Taurisolo® Effects on Brain Hypoperfusion–Reperfusion Damage in Hypertensive Rats. International Journal of Molecular Sciences. 2024; 25(19):10868. https://doi.org/10.3390/ijms251910868
Chicago/Turabian StyleLapi, Dominga, Gian Carlo Tenore, Giuseppe Federighi, Martina Chiurazzi, Santo Nunziato, Maria S. Lonardo, Mariano Stornaiuolo, Antonio Colantuoni, Ettore Novellino, and Rossana Scuri. 2024. "L-Arginine and Taurisolo® Effects on Brain Hypoperfusion–Reperfusion Damage in Hypertensive Rats" International Journal of Molecular Sciences 25, no. 19: 10868. https://doi.org/10.3390/ijms251910868








