Effects of Melatonin, GM-CSF, IGF-1, and LIF in Culture Media on Embryonic Development: Potential Benefits of Individualization
Abstract
1. Introduction
2. Effect of Supplements in IVF Culture Media on Embryonic Development
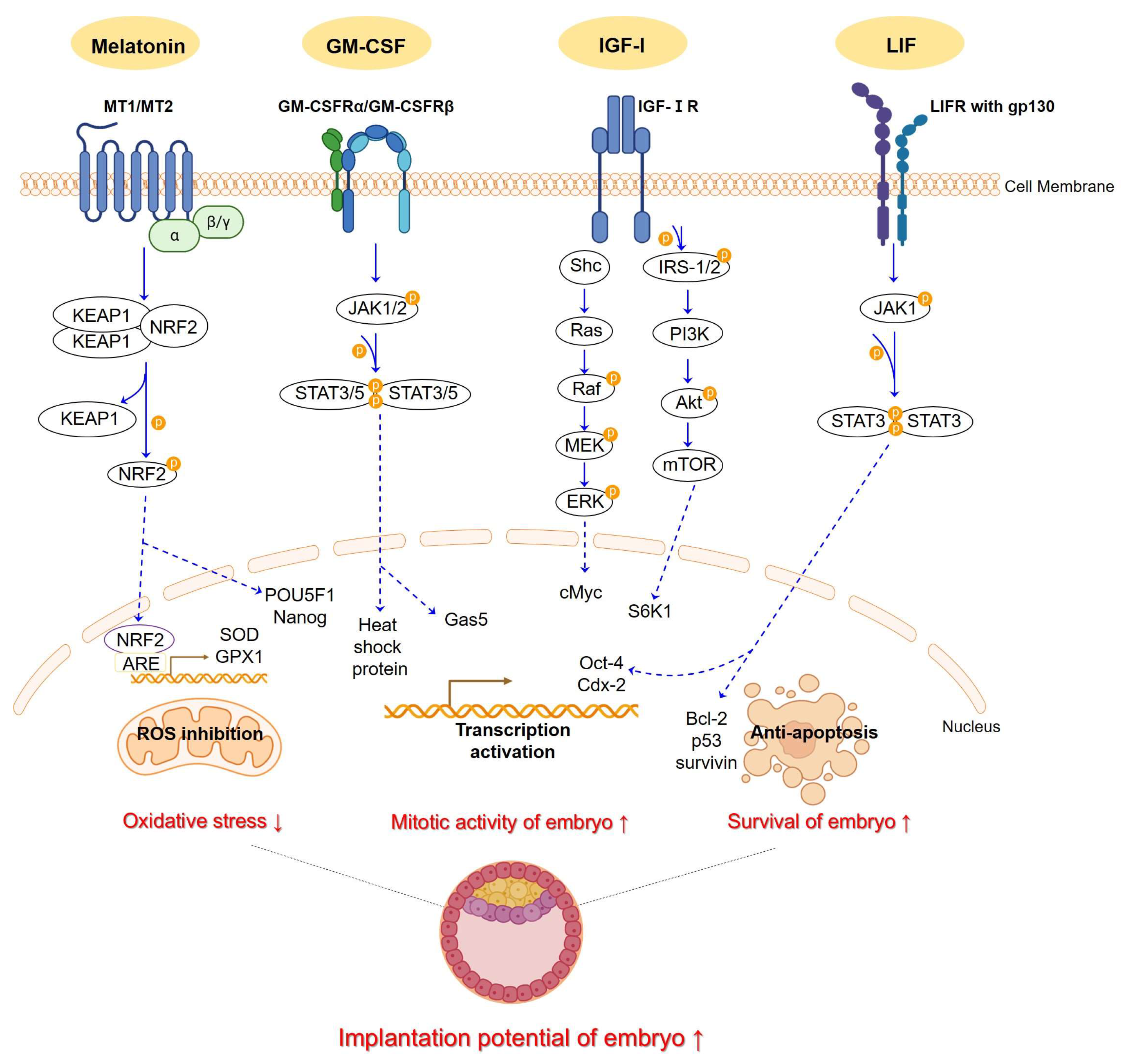
2.1. N-Acetyl-5-Methoxytryptamine (Melatonin)
| Year | Model | Study Type | Dose of Melatonin | Timing of Intervention | Cleavage Development Rate | Blastocyst Development Rate | Embryo Grade | Implantation Rate | Live Birth Rate | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | Human (unexplained infertility) | RCT | 10−7 M | COC | - | - | ↑ day 3 | - | - | [67] |
| Day 3 embryo after vitrified/warmed | - | ↑ | ↑ day 5 | - | - | |||||
| 2022 | Human (RIF) | RCT | 10−9 M | COC or oocyte | ↑ | ↑ | ↑, ↑ day 3, day 5 | ↑ | ↑ | [68] |
| 2013 | Human (PCOS) | RCT | 10−6 M | Immature COC surrounded by compact cumulus cells | → | - | → day 3 | ↑ | - | [69] |
| 2022 | Porcine | animal | 10−9 M | Presumed zygote | ↑ | → | - | - | - | [70] |
| 2014 | Bovine | animal | 10−7 M | Zygote | ↑ | ↑ | - | - | - | [71] |
| 2013 | Murine | animal | 10−7 M | Zygote | ↑ | ↑ | - | - | ↑ | [72] |
2.2. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
| Year | Model | Study Type | Dose of GM-CSF | Timing of Intervention | Cleavage Development Rate | Blastocyst Development Rate | Embryo Grade | Implantation Rate | Live Birth Rate | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | Human (infertility) | Retrospective study | 0.6 ng/mL | After fertilization | →,↑ | →,↑ | → | → | - | [92] |
| 2020 | Human (RIF) | RCT | 2 ng/mL (EmbryoGen, BlastGen) | COC or oocyte | → | ↓ | ↓ | - | → | [93] |
| 2020 | Human (infertility) | Explorative secondary RCT | 2 ng/mL | COC or oocyte | - | - | - | - | → | [95] |
| 2014 | Human (RIF) | RCT | 2 ng/mL (EmbryoGen) | COC or oocyte | - | - | → | ↑ | - | [96] |
| 2013 | Human (unexplained infertility) | Multicenter RCT | 2 ng/mL | COC or oocyte | → | - | → day3 | ↑ | ↑ | [94] |
| → | → | → day3 | ↑ | ↑ | ||||||
| 2008 | Murine | Animal | 2 ng/mL | Isolated blastomere from 2-cell stage | ↑ | → | - | - | - | [97] |
2.2.1. Insulin-like Growth Factor 1 (IGF-Ⅰ)
| Year | Model | Study Type | Dose of IGF-Ⅰ | Timing of Intervention | Cleavage Development Rate | Blastocyst Development Rate | Implantation Rate | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2000 | Human (infertility) | Prospective study | 13 ng/mL | Day 2 embryos of good morphology | → | ↑ | - | [129] |
| 2021 | Cat | Animal | 10 ng/mL, 20 ng/mL (GM-CSF 2 ng/mL) | COC | → | →, ↑ (20 ng/mL) | - | [130] |
| 20 ng/mL (IGF-Ⅱ 20 ng/mL) | COC | → | → | - | ||||
| 20 ng/mL | Oocyte | → | - | - | ||||
| → | - | - | ||||||
| 2019 | Yak-cattle crossbred | Animal | 100 ng/mL (EGF 10 ng/mL, L-cysteine 0.6 mM) | COC | - | ↑ | - | [132] |
| 2013 | Murine | Animal | 10 ng/mL | Zygote | → | ↑ | - | [131] |
| 2013 | Bovine | Animal | 10 ng/mL (EGF 10 ng/mL) | Zygote | → | ↑ | - | [133] |
| 2010 | Bovine | Animal | 500,000 ng/mL (IGF-Ⅱ 10 µg/mL, bFGF 25 µg/mL, TGF- β1 1 µg/mL, GM-CSF 1 µg/mL, LIF µg/mL) | Zygote | - | ↑ | - | [134] |
| 2003 | Bovine | Animal | 50 ng/mL | Zygote | → | ↑ | - | [120] |
2.2.2. Insulin-like Growth Factor 1 (IGF-Ⅰ) Combined with Other Supplements
2.2.3. Leukemia Inhibitory Factor (LIF)
| Year | Model | Study Type | Dose of LIF | Timing of Intervention | Cleavage Development Rate | Blastocyst Development Rate | Embryo Grade | Implantation Rate | Live Birth Rate | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | Human (infertility) | RCT | 5 ng/mL (GM-CSF 2 ng/mL, HB-EGF 5 ng/mL) | After ICSI | → | ↑ | →, ↑ day 3, day 5/6 | ↑, → Fresh, FER | ↑ | [171] |
| 2000 | Human (infertility) | RCT | 1000 IU/mL | Cleavage after vitrified-thawed | → | ↑ | - | - | - | [169] |
| 2021 | Bovine | Animal | 20 ng/mL (FGF2 40 ng/mL, IGF-Ⅰ 20 ng/mL) | Putative zygote | → | ↑ | - | - | - | [172] |
| 2013 | Buffalo | Animal | 100 ng/mL | Presumptive zygote | → | ↑ | - | - | - | [170] |
| 2008 | Murine | Animal | 1500 IU/mL | Isolated blastomere from 2-cell stage | ↑ | → | - | - | - | [97] |
2.2.4. Leukemia Inhibitory Factor (LIF) Combined with Other Supplements
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IVF | in vitro fertilization |
| ART | assisted reproductive technology |
| Nrf2/ARE | nuclear factor erythroid 2-related factor 2/antioxidant-responsive element |
| VEGF | vascular endothelial growth factor |
| ATP | adenosine triphosphate |
| RCT | randomized clinical trial |
| hCG | human chorionic gonadotropin |
| DNA | deoxyribo nucleic acid |
| PCOS | polycystic ovary syndrome |
| IVM | in vitro maturation |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| HSP | heat shock protein |
| GH | growth hormone |
| IGF | insulin-like growth factor |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular signal-regulated kinase |
| IRS | 1insulin receptor substrate |
| EGF | epidermal growth factor |
| bFGF | basic fibroblast growth factor |
| LIF | leukemia inhibitory factor |
| JAK | Janus family tyrosine kinase |
| STAT | signal transducers and activators of transcription |
| PI3K | phosphoinositide 3-kinase |
| Akt | protein kinase B |
| ROS | reactive oxygen species |
| GPCR | G protein-coupled receptor |
| O2− | superoxide anion |
| H2O2 | hydrogen peroxide |
| •OH | hydroxyl radical |
| SOD | superoxide dismutase |
| GSH | glutathione peroxidase |
| Th1 | Type 1 helper T cells |
| Th2 | Type 2 helper T cells |
| HSA | human serum albumin |
| HB-EGF | heparin-binding epidermal growth factor-like growth factor |
| RIF | recurrent implantation failure |
| FGF2 | fibroblast growth factor 2 |
References
- Fauque, P.; Leandri, R.; Merlet, F.; Juillard, J.C.; Epelboin, S.; Guibert, J.; Jouannet, P.; Patrat, C. Pregnancy outcome and live birth after IVF and ICSI according to embryo quality. J. Assist. Reprod. Genet. 2007, 24, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lian, Y.; Li, M.; Chen, L.; Liu, P.; Qiao, J. Does IVF cleavage stage embryo quality affect pregnancy complications and neonatal outcomes in singleton gestations after double embryo transfers? J. Assist. Reprod. Genet. 2014, 31, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Oron, G.; Son, W.Y.; Buckett, W.; Tulandi, T.; Holzer, H. The association between embryo quality and perinatal outcome of singletons born after single embryo transfers: A pilot study. Hum. Reprod. 2014, 29, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Wale, P.L.; Gardner, D.K. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum. Reprod. Update 2016, 22, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Carrell, D.; Cobo, A.; Meseguer, M.; Rubio, C.; Smith, G.D. Optimizing the culture environment and embryo manipulation to help maintain embryo developmental potential. Fertil. Steril. 2016, 105, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Chen, Y.; Shu, Y.; Cheng, Y.; Qiao, J.; Behr, B.; Pera, R.A.; Hsueh, A.J. Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors. PLoS ONE 2012, 7, e49328. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.L.; Barlow, D.H.; Sargent, I.L. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum. Reprod. 1998, 13, 1645–1652. [Google Scholar] [CrossRef]
- Truong, T.T.; Soh, Y.M.; Gardner, D.K. Antioxidants improve mouse preimplantation embryo development and viability. Hum. Reprod. 2016, 31, 1445–1454. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, M.; Hao, F.; Zhao, R.; Teng, X.; He, B.; Zhu, C.; Chen, Z.; Li, K. Effect of hyaluronic acid-enriched transfer medium on frozen-thawed embryo transfer outcomes in RIF patients: A single-centre retrospective study. Front. Endocrinol. 2023, 14, 1170727. [Google Scholar] [CrossRef]
- Rock, J.; Menkin, M.F. In Vitro Fertilization and Cleavage of Human Ovarian Eggs. Science 1944, 100, 105–107. [Google Scholar] [CrossRef]
- Behr, B.; Wang, H. Effects of culture conditions on IVF outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115 (Suppl. S1), S72–S76. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K. The impact of physiological oxygen during culture, and vitrification for cryopreservation, on the outcome of extended culture in human IVF. Reprod. Biomed. Online 2016, 32, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E. Decisions for the IVF laboratory: Comparative analysis of embryo culture incubators. Reprod. Biomed. Online 2014, 28, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.A.; Albert, C.; Marcos, J.; Larreategui, Z.; Bori, L.; Meseguer, M. A propensity score-based, comparative study assessing humid and dry time-lapse incubation, with single-step medium, on embryo development and clinical outcomes. Hum. Reprod. 2022, 37, 1980–1993. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.; Emad, M.; Gad, M.A.; Sabry, M.; Kasem, H.; Mahmoud, M.; Bedaiwy, M.A. Comparing 36.5 degrees C with 37 degrees C for human embryo culture: A prospective randomized controlled trial. Reprod. Biomed. Online 2018, 36, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Hentemann, M.; Mousavi, K.; Bertheussen, K. Differential pH in embryo culture. Fertil. Steril. 2011, 95, 1291–1294. [Google Scholar] [CrossRef]
- Gopichandran, N.; Leese, H.J. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006, 131, 269–277. [Google Scholar] [CrossRef]
- Balaban, B.; Urman, B. Comparison of two sequential media for culturing cleavage-stage embryos and blastocysts: Embryo characteristics and clinical outcome. Reprod. Biomed. Online 2005, 10, 485–491. [Google Scholar] [CrossRef]
- Kleijkers, S.H.; Mantikou, E.; Slappendel, E.; Consten, D.; van Echten-Arends, J.; Wetzels, A.M.; van Wely, M.; Smits, L.J.; van Montfoort, A.P.; Repping, S.; et al. Influence of embryo culture medium (G5 and HTF) on pregnancy and perinatal outcome after IVF: A multicenter RCT. Hum. Reprod. 2016, 31, 2219–2230. [Google Scholar] [CrossRef]
- Castillo, C.M.; Harper, J.; Roberts, S.A.; O’Neill, H.C.; Johnstone, E.D.; Brison, D.R. The impact of selected embryo culture conditions on ART treatment cycle outcomes: A UK national study. Hum. Reprod. Open 2020, 2020, hoz031. [Google Scholar] [CrossRef]
- Stimpfel, M.; Bacer-Kermavner, L.; Jancar, N.; Vrtacnik-Bokal, E. The influence of the type of embryo culture media on the outcome of IVF/ICSI cycles. Taiwan. J. Obstet. Gynecol. 2020, 59, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, B.; Boada, M.; Rodriguez, I.; Coroleu, B.; Barri, P.N.; Veiga, A. Does culture medium influence offspring birth weight? Fertil. Steril. 2013, 100, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Morbeck, D.E.; Baumann, N.A.; Oglesbee, D. Composition of single-step media used for human embryo culture. Fertil. Steril. 2017, 107, 1055–1060.e1051. [Google Scholar] [CrossRef] [PubMed]
- Gruber, I.; Klein, M. Embryo culture media for human IVF: Which possibilities exist? J. Turk. Ger. Gynecol. Assoc. 2011, 12, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Kermack, A.J.; Finn-Sell, S.; Cheong, Y.C.; Brook, N.; Eckert, J.J.; Macklon, N.S.; Houghton, F.D. Amino acid composition of human uterine fluid: Association with age, lifestyle and gynaecological pathology. Hum. Reprod. 2015, 30, 917–924. [Google Scholar] [CrossRef]
- Baltz, J.M. Osmoregulation and cell volume regulation in the preimplantation embryo. Curr. Top. Dev. Biol. 2001, 52, 55–106. [Google Scholar] [CrossRef]
- Devreker, F.; Hardy, K.; Van den Bergh, M.; Vannin, A.S.; Emiliani, S.; Englert, Y. Amino acids promote human blastocyst development in vitro. Hum. Reprod. 2001, 16, 749–756. [Google Scholar] [CrossRef]
- Tarahomi, M.; Vaz, F.M.; van Straalen, J.P.; Schrauwen, F.A.P.; van Wely, M.; Hamer, G.; Repping, S.; Mastenbroek, S. The composition of human preimplantation embryo culture media and their stability during storage and culture. Hum. Reprod. 2019, 34, 1450–1461. [Google Scholar] [CrossRef]
- Rodriguez-Alonso, B.; Maillo, V.; Acuna, O.S.; Lopez-Ubeda, R.; Torrecillas, A.; Simintiras, C.A.; Sturmey, R.; Aviles, M.; Lonergan, P.; Rizos, D. Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Carbohydrate Composition of Bovine Oviduct Fluid. Int. J. Mol. Sci. 2020, 21, 1681. [Google Scholar] [CrossRef]
- Mitchell, M.; Cashman, K.S.; Gardner, D.K.; Thompson, J.G.; Lane, M. Disruption of mitochondrial malate-aspartate shuttle activity in mouse blastocysts impairs viability and fetal growth. Biol. Reprod. 2009, 80, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Morbeck, D.E.; Krisher, R.L.; Herrick, J.R.; Baumann, N.A.; Matern, D.; Moyer, T. Composition of commercial media used for human embryo culture. Fertil. Steril. 2014, 102, 759–766.e759. [Google Scholar] [CrossRef] [PubMed]
- Sydow, A.; Krieg, T.; Ulber, R.; Holtmann, D. Growth medium and electrolyte-How to combine the different requirements on the reaction solution in bioelectrochemical systems using Cupriavidus necator. Eng. Life Sci. 2017, 17, 781–791. [Google Scholar] [CrossRef]
- Salazar, A.; Keusgen, M.; von Hagen, J. Amino acids in the cultivation of mammalian cells. Amino Acids 2016, 48, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.S.; Mann, M.R.; Tremblay, K.D.; Bartolomei, M.S.; Schultz, R.M. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol. Reprod. 2000, 62, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Coutifaris, C.; Sapienza, C.; Mainigi, M. Global DNA methylation levels are altered by modifiable clinical manipulations in assisted reproductive technologies. Clin. Epigenetics 2017, 9, 14. [Google Scholar] [CrossRef]
- Market-Velker, B.A.; Fernandes, A.D.; Mann, M.R. Side-by-side comparison of five commercial media systems in a mouse model: Suboptimal in vitro culture interferes with imprint maintenance. Biol. Reprod. 2010, 83, 938–950. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, Z.; Miao, K.; Yu, Y.; Sui, L.; Tian, J.; An, L. Dynamic integrated analysis of DNA methylation and gene expression profiles in in vivo and in vitro fertilized mouse post-implantation extraembryonic and placental tissues. Mol. Hum. Reprod. 2016, 22, 485–498. [Google Scholar] [CrossRef]
- Reis e Silva, A.R.; Bruno, C.; Fleurot, R.; Daniel, N.; Archilla, C.; Peynot, N.; Lucci, C.M.; Beaujean, N.; Duranthon, V. Alteration of DNA demethylation dynamics by in vitro culture conditions in rabbit pre-implantation embryos. Epigenetics 2012, 7, 440–446. [Google Scholar] [CrossRef][Green Version]
- Perrimon, N.; Pitsouli, C.; Shilo, B.Z. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb. Perspect. Biol. 2012, 4, a005975. [Google Scholar] [CrossRef]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, C.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Yamashita, M.; Tojo, C.; Kondo, M.; Morita, T.; Wakamura, T. Can tryptophan supplement intake at breakfast enhance melatonin secretion at night? J. Physiol. Anthropol. 2017, 36, 20. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef] [PubMed]
- Dies, H.; Cheung, B.; Tang, J.; Rheinstadter, M.C. The organization of melatonin in lipid membranes. Biochim. Biophys. Acta 2015, 1848, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Antoli, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol. Life Sci. 2002, 59, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Nikmard, F.; Hosseini, E.; Bakhtiyari, M.; Ashrafi, M.; Amidi, F.; Aflatoonian, R. The boosting effects of melatonin on the expression of related genes to oocyte maturation and antioxidant pathways: A polycystic ovary syndrome- mouse model. J. Ovarian Res. 2022, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Tamura, E.K.; Cecon, E.; Monteiro, A.W.; Silva, C.L.; Markus, R.P. Melatonin inhibits LPS-induced NO production in rat endothelial cells. J. Pineal Res. 2009, 46, 268–274. [Google Scholar] [CrossRef]
- Glatfelter, G.C.; Jones, A.J.; Rajnarayanan, R.V.; Dubocovich, M.L. Pharmacological Actions of Carbamate Insecticides at Mammalian Melatonin Receptors. J. Pharmacol. Exp. Ther. 2021, 376, 306–321. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, G.A.; Taweechaipaisankul, A.; Lee, S.H.; Qasim, M.; Ahn, C.; Lee, B.C. Melatonin enhances porcine embryo development via the Nrf2/ARE signaling pathway. J. Mol. Endocrinol. 2019, 63, 175–185. [Google Scholar] [CrossRef]
- Mehaisen, G.M.; Saeed, A.M.; Gad, A.; Abass, A.O.; Arafa, M.; El-Sayed, A. Antioxidant Capacity of Melatonin on Preimplantation Development of Fresh and Vitrified Rabbit Embryos: Morphological and Molecular Aspects. PLoS ONE 2015, 10, e0139814. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; Gao, C.; He, C.; Fu, Y.; Ji, P.; Li, Y.; Li, N.; Liu, G. Beneficial effects of melatonin on in vitro bovine embryonic development are mediated by melatonin receptor 1. J. Pineal Res. 2014, 56, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Gergalova, G.; Lykhmus, O.; Kalashnyk, O.; Koval, L.; Chernyshov, V.; Kryukova, E.; Tsetlin, V.; Komisarenko, S.; Skok, M. Mitochondria express alpha7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: Study on isolated mitochondria. PLoS ONE 2012, 7, e31361. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.G.; Partridge, R.J.; Houghton, F.D.; Cox, C.I.; Leese, H.J. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J. Reprod. Fertil. 1996, 106, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Houghton, F.D.; Thompson, J.G.; Kennedy, C.J.; Leese, H.J. Oxygen consumption and energy metabolism of the early mouse embryo. Mol. Reprod. Dev. 1996, 44, 476–485. [Google Scholar] [CrossRef]
- Morado, S.; Cetica, P.; Beconi, M.; Thompson, J.G.; Dalvit, G. Reactive oxygen species production and redox state in parthenogenetic and sperm-mediated bovine oocyte activation. Reproduction 2013, 145, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.S.; Lane, M.; Thompson, J.G. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum. Reprod. 2010, 25, 2762–2773. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.M.; Johnson, M.H. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development 1991, 113, 551–560. [Google Scholar] [CrossRef]
- Thomas, M.; Jain, S.; Kumar, G.P.; Laloraya, M. A programmed oxyradical burst causes hatching of mouse blastocysts. J. Cell Sci. 1997, 110 Pt 14, 1597–1602. [Google Scholar] [CrossRef]
- Ahelik, A.; Mandar, R.; Korrovits, P.; Karits, P.; Talving, E.; Rosenstein, K.; Jaagura, M.; Salumets, A.; Kullisaar, T. Systemic oxidative stress could predict assisted reproductive technique outcome. J. Assist. Reprod. Genet. 2015, 32, 699–704. [Google Scholar] [CrossRef]
- Ali, A.A.; Bilodeau, J.F.; Sirard, M.A. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology 2003, 59, 939–949. [Google Scholar] [CrossRef]
- Swain, J.E.; Cabrera, L.; Xu, X.; Smith, G.D. Microdrop preparation factors influence culture-media osmolality, which can impair mouse embryo preimplantation development. Reprod. Biomed. Online 2012, 24, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Maldonado Rosas, I.; Anagnostopoulou, C.; Cannarella, R.; Boitrelle, F.; Munoz, L.V.; Finelli, R.; Durairajanayagam, D.; Henkel, R.; Saleh, R. Oxidative Stress and Assisted Reproduction: A Comprehensive Review of Its Pathophysiological Role and Strategies for Optimizing Embryo Culture Environment. Antioxidants 2022, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Karagenc, L.; Sertkaya, Z.; Ciray, N.; Ulug, U.; Bahceci, M. Impact of oxygen concentration on embryonic development of mouse zygotes. Reprod. Biomed. Online 2004, 9, 409–417. [Google Scholar] [CrossRef]
- Zhou, G.; Meng, S.; Li, Y.; Ghebre, Y.T.; Cooke, J.P. Optimal ROS Signaling Is Critical for Nuclear Reprogramming. Cell Rep. 2016, 15, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.C.; Tatone, C.; Delle Monache, S.; Marci, R.; Caserta, D.; Colonna, R.; Amicarelli, F. Antioxidant enzymatic defences in human follicular fluid: Characterization and age-dependent changes. Mol. Hum. Reprod. 2003, 9, 639–643. [Google Scholar] [CrossRef]
- Bao, Z.; Li, G.; Wang, R.; Xue, S.; Zeng, Y.; Deng, S. Melatonin Improves Quality of Repeated-Poor and Frozen-Thawed Embryos in Human, a Prospective Clinical Trial. Front. Endocrinol. 2022, 13, 853999. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, K.; Zhang, C.; Chen, B.; Zou, H.; Zou, W.; Xue, R.; Ji, D.; Yu, Z.; Rao, B.; et al. Effect of melatonin on the clinical outcome of patients with repeated cycles after failed cycles of in vitro fertilization and intracytoplasmic sperm injection. Zygote 2022, 30, 471–479. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, E.A.; Kim, H.J.; Choi, W.Y.; Cho, J.H.; Lee, W.S.; Cha, K.Y.; Kim, Y.S.; Lee, D.R.; Yoon, T.K. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod. Biomed. Online 2013, 26, 22–29. [Google Scholar] [CrossRef]
- Martinez, C.A.; Cuello, C.; Parrilla, I.; Maside, C.; Ramis, G.; Cambra, J.M.; Vazquez, J.M.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A. Exogenous Melatonin in the Culture Medium Does Not Affect the Development of In Vivo-Derived Pig Embryos but Substantially Improves the Quality of In Vitro-Produced Embryos. Antioxidants 2022, 11, 1177. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhou, Y.; Tan, D.; Zhu, S.; Dai, Y.; Liu, G. Melatonin improves the quality of in vitro produced (IVP) bovine embryos: Implications for blastocyst development, cryotolerance, and modifications of relevant gene expression. PLoS ONE 2014, 9, e93641. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tian, X.; Zhang, L.; Tan, D.; Reiter, R.J.; Liu, G. Melatonin promotes the in vitro development of pronuclear embryos and increases the efficiency of blastocyst implantation in murine. J. Pineal Res. 2013, 55, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Han, H.B.; Tian, X.Z.; Tan, D.X.; Wang, L.; Zhou, G.B.; Zhu, S.E.; Liu, G.S. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J. Pineal Res. 2012, 52, 305–311. [Google Scholar] [CrossRef] [PubMed]
- McClure, B.J.; Hercus, T.R.; Cambareri, B.A.; Woodcock, J.M.; Bagley, C.J.; Howlett, G.J.; Lopez, A.F. Molecular assembly of the ternary granulocyte-macrophage colony-stimulating factor receptor complex. Blood 2003, 101, 1308–1315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peralta, O.A.; Bucher, D.; Fernandez, A.; Berland, M.; Strobel, P.; Ramirez, A.; Ratto, M.H.; Concha, I. Granulocyte-macrophage colony stimulating factor (GM-CSF) enhances cumulus cell expansion in bovine oocytes. Reprod. Biol. Endocrinol. 2013, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Nicola, N. Guidebook to Cytokines and Their Receptors, 1st ed.; Guidebook series; Oxford University Press: Oxford, NY, USA, 1994; pp. xv, 261p; Illustrations (some color). [Google Scholar]
- Robertson, S.A.; Roberts, C.T.; Farr, K.L.; Dunn, A.R.; Seamark, R.F. Fertility impairment in granulocyte-macrophage colony-stimulating factor-deficient mice. Biol. Reprod. 1999, 60, 251–261. [Google Scholar] [CrossRef]
- Robertson, S.A.; Mayrhofer, G.; Seamark, R.F. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol. Reprod. 1992, 46, 1069–1079. [Google Scholar] [CrossRef]
- Zhao, Y.; Chegini, N. The expression of granulocyte macrophage-colony stimulating factor (GM-CSF) and receptors in human endometrium. Am. J. Reprod. Immunol. 1999, 42, 303–311. [Google Scholar] [CrossRef]
- Zhao, Y.; Chegini, N. Human fallopian tube expresses granulocyte-macrophage colony stimulating factor (GM-CSF) and GM-CSF alpha and beta receptors and contain immunoreactive GM-CSF protein. J. Clin. Endocrinol. Metab. 1994, 79, 662–665. [Google Scholar] [CrossRef]
- Giacomini, G.; Tabibzadeh, S.S.; Satyaswaroop, P.G.; Bonsi, L.; Vitale, L.; Bagnara, G.P.; Strippoli, P.; Jasonni, V.M. Epithelial cells are the major source of biologically active granulocyte macrophage colony-stimulating factor in human endometrium. Hum. Reprod. 1995, 10, 3259–3263. [Google Scholar] [CrossRef]
- Robertson, S.A. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007, 18, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Crainie, M.; Guilbert, L.; Wegmann, T.G. Expression of novel cytokine transcripts in the murine placenta. Biol. Reprod. 1990, 43, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Mayrhofer, G.; Seamark, R.F. Ovarian steroid hormones regulate granulocyte-macrophage colony-stimulating factor synthesis by uterine epithelial cells in the mouse. Biol. Reprod. 1996, 54, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Jokhi, P.P.; King, A.; Loke, Y.W. Production of granulocyte-macrophage colony-stimulating factor by human trophoblast cells and by decidual large granular lymphocytes. Hum. Reprod. 1994, 9, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, C.; Wikland, M.; Robertson, S.A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol. Reprod. 2002, 67, 1817–1823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robertson, S.A.; Sjoblom, C.; Jasper, M.J.; Norman, R.J.; Seamark, R.F. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol. Reprod. 2001, 64, 1206–1215. [Google Scholar] [CrossRef]
- Sjoblom, C.; Wikland, M.; Robertson, S.A. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum. Reprod. 1999, 14, 3069–3076. [Google Scholar] [CrossRef]
- Chin, P.Y.; Macpherson, A.M.; Thompson, J.G.; Lane, M.; Robertson, S.A. Stress response genes are suppressed in mouse preimplantation embryos by granulocyte-macrophage colony-stimulating factor (GM-CSF). Hum. Reprod. 2009, 24, 2997–3009. [Google Scholar] [CrossRef]
- Desai, N.; Kattal, N.; AbdelHafez, F.F.; Szeptycki-Lawson, J.; Goldfarb, J. Granulocyte-macrophage colony stimulating factor (GM-CSF) and co-culture can affect post-thaw development and apoptosis in cryopreserved embryos. J. Assist. Reprod. Genet. 2007, 24, 215–222. [Google Scholar] [CrossRef][Green Version]
- Martinez-Moczygemba, M.; Huston, D.P. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J. Allergy Clin. Immunol. 2003, 112, 653–665, quiz 666. [Google Scholar]
- Chu, D.; Fu, L.; Zhou, W.; Li, Y. Relationship between granulocyte-macrophage colony-stimulating factor, embryo quality, and pregnancy outcomes in women of different ages in fresh transfer cycles: A retrospective study. J. Obstet. Gynaecol. 2020, 40, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.D.; Barry, M.F.; Dunstan, E.V.; Yuen, S.M.; Cameron, L.P.; Knight, E.J.; Norman, R.J.; Hull, M.L. The BlastGen study: A randomized controlled trial of blastocyst media supplemented with granulocyte-macrophage colony-stimulating factor. Reprod. Biomed. Online 2020, 40, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ziebe, S.; Loft, A.; Povlsen, B.B.; Erb, K.; Agerholm, I.; Aasted, M.; Gabrielsen, A.; Hnida, C.; Zobel, D.P.; Munding, B.; et al. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil. Steril. 2013, 99, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Wallberg, K.A.; Munding, B.; Ziebe, S.; Robertson, S.A. GM-CSF does not rescue poor-quality embryos: Secondary analysis of a randomized controlled trial. Arch. Gynecol. Obstet. 2020, 301, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Tevkin, S.; Lokshin, V.; Shishimorova, M.; Polumiskov, V. The frequency of clinical pregnancy and implantation rate after cultivation of embryos in a medium with granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with preceding failed attempts of ART. Gynecol. Endocrinol. 2014, 30 (Suppl. S1), 9–12. [Google Scholar] [CrossRef] [PubMed]
- Sheikholslami, B.; Salehnia, M.; Valojerdi, M.R.; Ramezanzadeh, M. Developmental potential of isolated blastomeres from early mouse embryos in the presence and absence of LIF and GM-CSF. J. Assist. Reprod. Genet. 2008, 25, 7–12. [Google Scholar] [CrossRef][Green Version]
- Bassiouny, Y.A.; Dakhly, D.M.R.; Bayoumi, Y.A.; Hashish, N.M. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil. Steril. 2016, 105, 697–702. [Google Scholar] [CrossRef]
- Dakhly, D.M.R.; Bassiouny, Y.A.; Bayoumi, Y.A.; Hassan, M.A.; Gouda, H.M.; Hassan, A.A. The addition of growth hormone adjuvant therapy to the long down regulation protocol in poor responders undergoing in vitro fertilization: Randomized control trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 161–165. [Google Scholar] [CrossRef]
- Altmae, S.; Mendoza-Tesarik, R.; Mendoza, C.; Mendoza, N.; Cucinelli, F.; Tesarik, J. Effect of Growth Hormone on Uterine Receptivity in Women With Repeated Implantation Failure in an Oocyte Donation Program: A Randomized Controlled Trial. J. Endocr. Soc. 2018, 2, 96–105. [Google Scholar] [CrossRef]
- Hazout, A.; Junca, A.; Menezo, Y.; Demouzon, J.; Cohen-Bacrie, P. Effect of growth hormone on oocyte competence in patients with multiple IVF failures. Reprod. Biomed. Online 2009, 18, 664–670. [Google Scholar] [CrossRef]
- Martin-Estal, I.; de la Garza, R.G.; Castilla-Cortazar, I. Intrauterine Growth Retardation (IUGR) as a Novel Condition of Insulin-Like Growth Factor-1 (IGF-1) Deficiency. Rev. Physiol. Biochem. Pharmacol. 2016, 170, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Kavran, J.M.; McCabe, J.M.; Byrne, P.O.; Connacher, M.K.; Wang, Z.; Ramek, A.; Sarabipour, S.; Shan, Y.; Shaw, D.E.; Hristova, K.; et al. How IGF-1 activates its receptor. Elife 2014, 3, e03772. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, B.; Hillensjo, T.; Nilsson, A.; Tornell, J.; Billig, H. Expression of insulin-like growth factor-I (IGF-I) in the rat fallopian tube: Possible autocrine and paracrine action of fallopian tube-derived IGF-I on the fallopian tube and on the preimplantation embryo. Endocrinology 1993, 133, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Smotrich, D.B.; Stillman, R.J.; Widra, E.A.; Gindoff, P.R.; Kaplan, P.; Graubert, M.; Johnson, K.E. Immunocytochemical localization of growth factors and their receptors in human pre-embryos and Fallopian tubes. Hum. Reprod. 1996, 11, 184–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lighten, A.D.; Hardy, K.; Winston, R.M.; Moore, G.E. Expression of mRNA for the insulin-like growth factors and their receptors in human preimplantation embryos. Mol. Reprod. Dev. 1997, 47, 134–139. [Google Scholar] [CrossRef]
- Lighten, A.D.; Moore, G.E.; Winston, R.M.; Hardy, K. Routine addition of human insulin-like growth factor-I ligand could benefit clinical in-vitro fertilization culture. Hum. Reprod. 1998, 13, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, T.L.; Chegini, N. Immunohistochemical localization of insulin-like growth factor (IGF-I), IGF-I receptor, and IGF binding proteins 1-4 in human fallopian tube at various reproductive stages. Biol. Reprod. 1994, 50, 281–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oosterhuis, G.J.; Vermes, I.; Lambalk, C.B.; Michgelsen, H.W.; Schoemaker, J. Insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations in fluid from human stimulated follicles. Hum. Reprod. 1998, 13, 285–289. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Harvey, M.B.; Kaye, P.L. IGF-2 stimulates growth and metabolism of early mouse embryos. Mech. Dev. 1992, 38, 169–173. [Google Scholar] [CrossRef]
- Adashi, E.Y.; Resnick, C.E.; D’Ercole, A.J.; Svoboda, M.E.; Van Wyk, J.J. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr. Rev. 1985, 6, 400–420. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.J.; Flint, D.J.; Patel, K. Insulin-like growth factor axis during embryonic development. Reproduction 2001, 122, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Godin, P.A.; Nisolle, M.; Donnez, J. Expression of receptors for insulin-like growth factor-I and transforming growth factor-beta in human follicles. Mol. Hum. Reprod. 2000, 6, 137–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Powell-Braxton, L.; Hollingshead, P.; Warburton, C.; Dowd, M.; Pitts-Meek, S.; Dalton, D.; Gillett, N.; Stewart, T.A. IGF-I is required for normal embryonic growth in mice. Genes. Dev. 1993, 7, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Powell-Braxton, L.; Hollingshead, P.; Giltinan, D.; Pitts-Meek, S.; Stewart, T. Inactivation of the IGF-I gene in mice results in perinatal lethality. Ann. N. Y. Acad. Sci. 1993, 692, 300–301. [Google Scholar] [CrossRef]
- White, V.; Jawerbaum, A.; Mazzucco, M.B.; Gauster, M.; Desoye, G.; Hiden, U. IGF2 stimulates fetal growth in a sex- and organ-dependent manner. Pediatr. Res. 2018, 83, 183–189. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C. Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro. Biol. Reprod. 1997, 56, 229–237. [Google Scholar] [CrossRef]
- Pantaleon, M.; Jericho, H.; Rabnott, G.; Kaye, P.L. The role of insulin-like growth factor II and its receptor in mouse preimplantation development. Reprod. Fertil. Dev. 2003, 15, 37–45. [Google Scholar] [CrossRef]
- Sirisathien, S.; Brackett, B.G. TUNEL analyses of bovine blastocysts after culture with EGF and IGF-I. Mol. Reprod. Dev. 2003, 65, 51–56. [Google Scholar] [CrossRef]
- Baker, J.; Liu, J.P.; Robertson, E.J.; Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Novosyadlyy, R.; Wu, Y.; Yakar, S.; LeRoith, D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F.E.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Shelton, J.G.; White, E.R.; Ludwig, D.L.; McCubrey, J.A. Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia 2006, 20, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Ipsa, E.; Cruzat, V.F.; Kagize, J.N.; Yovich, J.L.; Keane, K.N. Growth Hormone and Insulin-Like Growth Factor Action in Reproductive Tissues. Front. Endocrinol. 2019, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chandrakanthan, V.; Day, M.L.; O’Neill, C. Direct evidence for the action of phosphatidylinositol (3,4,5)-trisphosphate-mediated signal transduction in the 2-cell mouse embryo. Biol. Reprod. 2007, 77, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Adrian, C.; Herington, P.E.L. Signal Transduction Mechanisms Underlying Growth Hormone Receptor Action. Open Endocrinol. J. 2012, 6, 13–21. [Google Scholar]
- Shiota, M.; Sugai, N.; Tamura, M.; Yamaguchi, R.; Fukushima, N.; Miyano, T.; Miyazaki, H. Correlation of mitogen-activated protein kinase activities with cell survival and apoptosis in porcine granulosa cells. Zoolog Sci. 2003, 20, 193–201. [Google Scholar] [CrossRef][Green Version]
- Becker, M.A.; Ibrahim, Y.H.; Cui, X.; Lee, A.V.; Yee, D. The IGF pathway regulates ERalpha through a S6K1-dependent mechanism in breast cancer cells. Mol. Endocrinol. 2011, 25, 516–528. [Google Scholar] [CrossRef]
- Spanos, S.; Becker, D.L.; Winston, R.M.; Hardy, K. Anti-apoptotic action of insulin-like growth factor-I during human preimplantation embryo development. Biol. Reprod. 2000, 63, 1413–1420. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, L.; Kozhevnikova, V.; Brusentsev, E.; Jansch, S.; Amstislavsky, S.; Jewgenow, K. IGF-I Medium Supplementation Improves Singly Cultured Cat Oocyte Maturation and Embryo Development In Vitro. Animals 2021, 11, 1909. [Google Scholar] [CrossRef]
- Green, C.J.; Day, M.L. Insulin-like growth factor 1 acts as an autocrine factor to improve early embryogenesis in vitro. Int. J. Dev. Biol. 2013, 57, 837–844. [Google Scholar] [CrossRef]
- Yang, R.-f.; Xiong, X.-r.; Zi, X.-d. Effect of cysteine, insulin-like growth factor-1 and epidermis growth factor during in vitro oocyte maturation and in vitro culture of yak-cattle crossbred embryos. J. Appl. Anim. Res. 2019, 47, 463–466. [Google Scholar] [CrossRef]
- Ahumada, C.J.; Salvador, I.; Cebrian-Serrano, A.; Lopera, R.; Silvestre, M.A. Effect of supplementation of different growth factors in embryo culture medium with a small number of bovine embryos on in vitro embryo development and quality. Animal 2013, 7, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.A.; Tainturier, D.; Pena, M.A.; Martal, J. Effect of the association of IGF-I, IGF-II, bFGF, TGF-beta1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology 2010, 73, 595–604. [Google Scholar] [CrossRef]
- Berishaj, M.; Gao, S.P.; Ahmed, S.; Leslie, K.; Al-Ahmadie, H.; Gerald, W.L.; Bornmann, W.; Bromberg, J.F. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007, 9, R32. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.; Naeth, G.; Heinrich, P.C.; Muller-Newen, G. Novel inhibitors for murine and human leukemia inhibitory factor based on fused soluble receptors. J. Biol. Chem. 2008, 283, 5985–5995. [Google Scholar] [CrossRef] [PubMed]
- Su, R.W.; Jia, B.; Ni, H.; Lei, W.; Yue, S.L.; Feng, X.H.; Deng, W.B.; Liu, J.L.; Zhao, Z.A.; Wang, T.S.; et al. Junctional adhesion molecule 2 mediates the interaction between hatched blastocyst and luminal epithelium: Induction by progesterone and LIF. PLoS ONE 2012, 7, e34325. [Google Scholar] [CrossRef]
- Frezzato, F.; Raggi, F.; Martini, V.; Severin, F.; Trimarco, V.; Visentin, A.; Scomazzon, E.; Accordi, B.; Bresolin, S.; Piazza, F.; et al. HSP70/HSF1 axis, regulated via a PI3K/AKT pathway, is a druggable target in chronic lymphocytic leukemia. Int. J. Cancer 2019, 145, 3089–3100. [Google Scholar] [CrossRef]
- Paling, N.R.; Wheadon, H.; Bone, H.K.; Welham, M.J. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J. Biol. Chem. 2004, 279, 48063–48070. [Google Scholar] [CrossRef]
- Boeuf, H.; Merienne, K.; Jacquot, S.; Duval, D.; Zeniou, M.; Hauss, C.; Reinhardt, B.; Huss-Garcia, Y.; Dierich, A.; Frank, D.A.; et al. The ribosomal S6 kinases, cAMP-responsive element-binding, and STAT3 proteins are regulated by different leukemia inhibitory factor signaling pathways in mouse embryonic stem cells. J. Biol. Chem. 2001, 276, 46204–46211. [Google Scholar] [CrossRef]
- Burdon, T.; Stracey, C.; Chambers, I.; Nichols, J.; Smith, A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 1999, 210, 30–43. [Google Scholar] [CrossRef]
- Cheng, J.G.; Chen, J.R.; Hernandez, L.; Alvord, W.G.; Stewart, C.L. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc. Natl. Acad. Sci. USA 2001, 98, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Takahashi, M.; Carpino, N.; Jou, S.T.; Chao, J.R.; Tanaka, S.; Shigeyoshi, Y.; Parganas, E.; Ihle, J.N. Leukemia inhibitory factor regulates trophoblast giant cell differentiation via Janus kinase 1-signal transducer and activator of transcription 3-suppressor of cytokine signaling 3 pathway. Mol. Endocrinol. 2008, 22, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.; Oxford, J.T.; Jorcyk, C.L. Emerging Perspectives on Leukemia Inhibitory Factor and its Receptor in Cancer. Front. Oncol. 2021, 11, 693724. [Google Scholar] [CrossRef] [PubMed]
- Auernhammer, C.J.; Melmed, S. Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr. Rev. 2000, 21, 313–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lafdil, F.; Kong, X.; Gao, B. Signal transducer and activator of transcription 3 in liver diseases: A novel therapeutic target. Int. J. Biol. Sci. 2011, 7, 536–550. [Google Scholar] [CrossRef]
- Liu, L.; McBride, K.M.; Reich, N.C. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3. Proc. Natl. Acad. Sci. USA 2005, 102, 8150–8155. [Google Scholar] [CrossRef]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef]
- Kujawski, M.; Kortylewski, M.; Lee, H.; Herrmann, A.; Kay, H.; Yu, H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J. Clin. Investig. 2008, 118, 3367–3377. [Google Scholar] [CrossRef]
- Basseres, D.S.; Baldwin, A.S. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 2006, 25, 6817–6830. [Google Scholar] [CrossRef]
- Li, H.; Lee, J.; He, C.; Zou, M.H.; Xie, Z. Suppression of the mTORC1/STAT3/Notch1 pathway by activated AMPK prevents hepatic insulin resistance induced by excess amino acids. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E197–E209. [Google Scholar] [CrossRef]
- Teng, T.S.; Lin, B.; Manser, E.; Ng, D.C.; Cao, X. Stat3 promotes directional cell migration by regulating Rac1 activity via its activator betaPIX. J. Cell Sci. 2009, 122, 4150–4159. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Wright, K.L.; Ma, Y.; Wright, G.M.; Huang, M.; Irby, R.; Briggs, J.; Karras, J.; Cress, W.D.; Pardoll, D.; et al. Role of Stat3 in regulating p53 expression and function. Mol. Cell Biol. 2005, 25, 7432–7440. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhang, Z.G.; Tian, X.Q.; Sun, D.F.; Liang, Q.C.; Zhang, Y.J.; Lu, R.; Chen, Y.X.; Fang, J.Y. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia 2008, 10, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Duval, D.; Malaise, M.; Reinhardt, B.; Kedinger, C.; Boeuf, H. A p38 inhibitor allows to dissociate differentiation and apoptotic processes triggered upon LIF withdrawal in mouse embryonic stem cells. Cell Death Differ. 2004, 11, 331–341. [Google Scholar] [CrossRef]
- van Eijk, M.J.; Mandelbaum, J.; Salat-Baroux, J.; Belaisch-Allart, J.; Plachot, M.; Junca, A.M.; Mummery, C.L. Expression of leukaemia inhibitory factor receptor subunits LIFR beta and gp130 in human oocytes and preimplantation embryos. Mol. Hum. Reprod. 1996, 2, 355–360. [Google Scholar] [CrossRef]
- Margioula-Siarkou, C.; Prapas, Y.; Petousis, S.; Milias, S.; Ravanos, K.; Kalogiannidis, I.; Mavromatidis, G.; Haitoglou, C.; Prapas, N.; Rousso, D. LIF and LIF-R expression in the endometrium of fertile and infertile women: A prospective observational case-control study. Mol. Med. Rep. 2016, 13, 4721–4728. [Google Scholar] [CrossRef]
- Serafini, P.; Rocha, A.M.; Osorio, C.T.; da Silva, I.; Motta, E.L.; Baracat, E.C. Endometrial leukemia inhibitory factor as a predictor of pregnancy after in vitro fertilization. Int. J. Gynaecol. Obstet. 2008, 102, 23–27. [Google Scholar] [CrossRef]
- Hambartsoumian, E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am. J. Reprod. Immunol. 1998, 39, 137–143. [Google Scholar] [CrossRef]
- Steck, T.; Giess, R.; Suetterlin, M.W.; Bolland, M.; Wiest, S.; Poehls, U.G.; Dietl, J. Leukaemia inhibitory factor (LIF) gene mutations in women with unexplained infertility and recurrent failure of implantation after IVF and embryo transfer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 112, 69–73. [Google Scholar] [CrossRef]
- Nichols, J.; Chambers, I.; Taga, T.; Smith, A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development 2001, 128, 2333–2339. [Google Scholar] [CrossRef]
- Kimber, S.J. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction 2005, 130, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Ledee-Bataille, N.; Lapree-Delage, G.; Taupin, J.L.; Dubanchet, S.; Frydman, R.; Chaouat, G. Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum. Reprod. 2002, 17, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Movaghar, B.; Abkenari, S.A.; Nazari, H.; Bakhtiyari, M. Leukemia Inhibitory Factor Enhanced the Developmental and Implantation Compatibility of Mouse Embryos in Co-culture with Human Endometrial Epithelial Cells. Reprod. Dev. Med. 2021, 5, 199–205. [Google Scholar] [CrossRef]
- Mitchell, M.H.; Swanson, R.J.; Oehninger, S. In vivo effect of leukemia inhibitory factor (LIF) and an anti-LIF polyclonal antibody on murine embryo and fetal development following exposure at the time of transcervical blastocyst transfer. Biol. Reprod. 2002, 67, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Q.; Cao, Y.J.; Duan, E.K. Effects of leukaemia inhibitory factor on embryo implantation in the mouse. Cytokine 2000, 12, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, R.; Li, X.; Dai, H.; Han, X.; Wang, X.; Yang, A. LIF in embryo culture medium is a predictive marker for clinical pregnancy following IVF-ET of patients with fallopian tube obstruction. J. Reprod. Immunol. 2020, 141, 103164. [Google Scholar] [CrossRef]
- Liu, Y.L.; Lee, C.I.; Liu, C.H.; Cheng, E.H.; Yang, S.F.; Tsai, H.Y.; Lee, M.S.; Lee, T.H. Association between Leukemia Inhibitory Factor Gene Polymorphism and Clinical Outcomes among Young Women with Poor Ovarian Response to Assisted Reproductive Technology. J. Clin. Med. 2023, 12, 796. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Tsai, H.D.; Chang, C.C.; Hsu, L.W.; Chang, S.C.; Lo, H.Y. Prolonged culture of human cryopreserved embryos with recombinant human leukemia inhibitory factor. J. Assist. Reprod. Genet. 2000, 17, 131–134. [Google Scholar] [CrossRef]
- Eswari, S.; Sai Kumar, G.; Sharma, G.T. Expression of mRNA encoding leukaemia inhibitory factor (LIF) and its receptor (LIFRbeta) in buffalo preimplantation embryos produced in vitro: Markers of successful embryo implantation. Zygote 2013, 21, 203–213. [Google Scholar] [CrossRef]
- Fawzy, M.; Emad, M.; Elsuity, M.A.; Mahran, A.; Abdelrahman, M.Y.; Fetih, A.N.; Abdelghafar, H.; Sabry, M.; Nour, M.; Rasheed, S.M. Cytokines hold promise for human embryo culture in vitro: Results of a randomized clinical trial. Fertil. Steril. 2019, 112, 849–857 e841. [Google Scholar] [CrossRef]
- Stoecklein, K.S.; Ortega, M.S.; Spate, L.D.; Murphy, C.N.; Prather, R.S. Improved cryopreservation of in vitro produced bovine embryos using FGF2, LIF, and IGF1. PLoS ONE 2021, 16, e0243727. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Spate, L.D.; Redel, B.K.; Tian, Y.; Zhou, J.; Prather, R.S.; Roberts, R.M. Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proc. Natl. Acad. Sci. USA 2017, 114, E5796–E5804. [Google Scholar] [CrossRef] [PubMed]
| Component | Role in Embryo Development | Global-Total | CSC | G-TL | 1-Step |
|---|---|---|---|---|---|
| Glucose (mM) | Primary energy source during postcompaction stage | 0.18 | 0.47 | 0.97 | 0.19 |
| Lactate (mM) | Primary energy source during precompaction stage | 4.9 | 5.71 | 10.01 | 4.35 |
| Pyruvate (mM) | 0.24 | 0.28 | 0.55 | 0.22 | |
| Essential amino acids (μM) | |||||
| Arg | Osmolytes | 278 | 292 | 324 | 336 |
| Cys | Buffers of internal pH | 32 | 34 | 26 | 28 |
| His | 76 | 80 | 89 | 90 | |
| Ile | Antioxidants | 182 | 199 | 215 | 204 |
| Leu | Protein synthesis, antioxidants | 177 | 188 | 204 | 206 |
| Lys | Chelators for heavy metals | 154 | 168 | 182 | 182 |
| Met | Osmolytes, chelators for heavy metals | 44 | 50 | 54 | 54 |
| Phe | Antioxidants | 79 | 83 | 91 | 92 |
| Thr | Energy source, osmolytes, antioxidants | 162 | 176 | 184 | 204 |
| Trp | Biosynthetic precursor molecules | 18 | 20 | 21 | 23 |
| Tyr | Protein synthesis | 69 | 75 | 80 | 83 |
| Val | Biosynthetic precursor molecules, antioxidants | 163 | 174 | 200 | 196 |
| Nonessential amino acids (μM) | |||||
| Ala | Buffers of internal pH, antioxidants | 46 | 48 | 63 | 38 |
| Asn | Energy source | 42 | 46 | 40 | 36 |
| Asp | Energy source, osmolytes, chelators for heavy metals | 42 | 43 | 12 | 58 |
| Glu | Energy source, osmolytes | 40 | 41 | 0 | 49 |
| Gln | Energy source, biosynthetic precursor molecules, protein synthesis | 0 | 0 | 10 | 36 |
| Gly | Energy source, osmolytes | 42 | 44 | 185 | 48 |
| Pro | Osmolytes | 55 | 60 | 126 | 66 |
| Ser | Osmolytes, antioxidants | 40 | 42 | 96 | 46 |
| Tau | Antioxidants | 0 | 0 | 48 | 0 |
| Calcium (mM) | Metabolic parameters and macromolecular synthesis through cell-to-cell interaction | 1.6 | 1.9 | 1.0 | 2.1 |
| Magnesium (mM) | 0.24 | 0.78 | 1.62 | 1.78 | |
| Potassium (mM) | High concentrations in oviduct fluid relative to serum | 2.8 | 2.8 | 5.5 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-W.; Kim, S.-W.; Kim, H.-S.; Kang, M.-J.; Kim, S.-A.; Han, J.-Y.; Kim, H.; Ku, S.-Y. Effects of Melatonin, GM-CSF, IGF-1, and LIF in Culture Media on Embryonic Development: Potential Benefits of Individualization. Int. J. Mol. Sci. 2024, 25, 751. https://doi.org/10.3390/ijms25020751
Choi J-W, Kim S-W, Kim H-S, Kang M-J, Kim S-A, Han J-Y, Kim H, Ku S-Y. Effects of Melatonin, GM-CSF, IGF-1, and LIF in Culture Media on Embryonic Development: Potential Benefits of Individualization. International Journal of Molecular Sciences. 2024; 25(2):751. https://doi.org/10.3390/ijms25020751
Chicago/Turabian StyleChoi, Jung-Won, Sung-Woo Kim, Hee-Sun Kim, Moon-Joo Kang, Sung-Ah Kim, Ji-Yeon Han, Hoon Kim, and Seung-Yup Ku. 2024. "Effects of Melatonin, GM-CSF, IGF-1, and LIF in Culture Media on Embryonic Development: Potential Benefits of Individualization" International Journal of Molecular Sciences 25, no. 2: 751. https://doi.org/10.3390/ijms25020751
APA StyleChoi, J.-W., Kim, S.-W., Kim, H.-S., Kang, M.-J., Kim, S.-A., Han, J.-Y., Kim, H., & Ku, S.-Y. (2024). Effects of Melatonin, GM-CSF, IGF-1, and LIF in Culture Media on Embryonic Development: Potential Benefits of Individualization. International Journal of Molecular Sciences, 25(2), 751. https://doi.org/10.3390/ijms25020751






