Promoter of Vegetable Pea PsPIP2-4 Responds to Abiotic Stresses in Transgenic Tobacco
Abstract
:1. Introduction
2. Results
2.1. Structural Analysis of PsPIP2-4
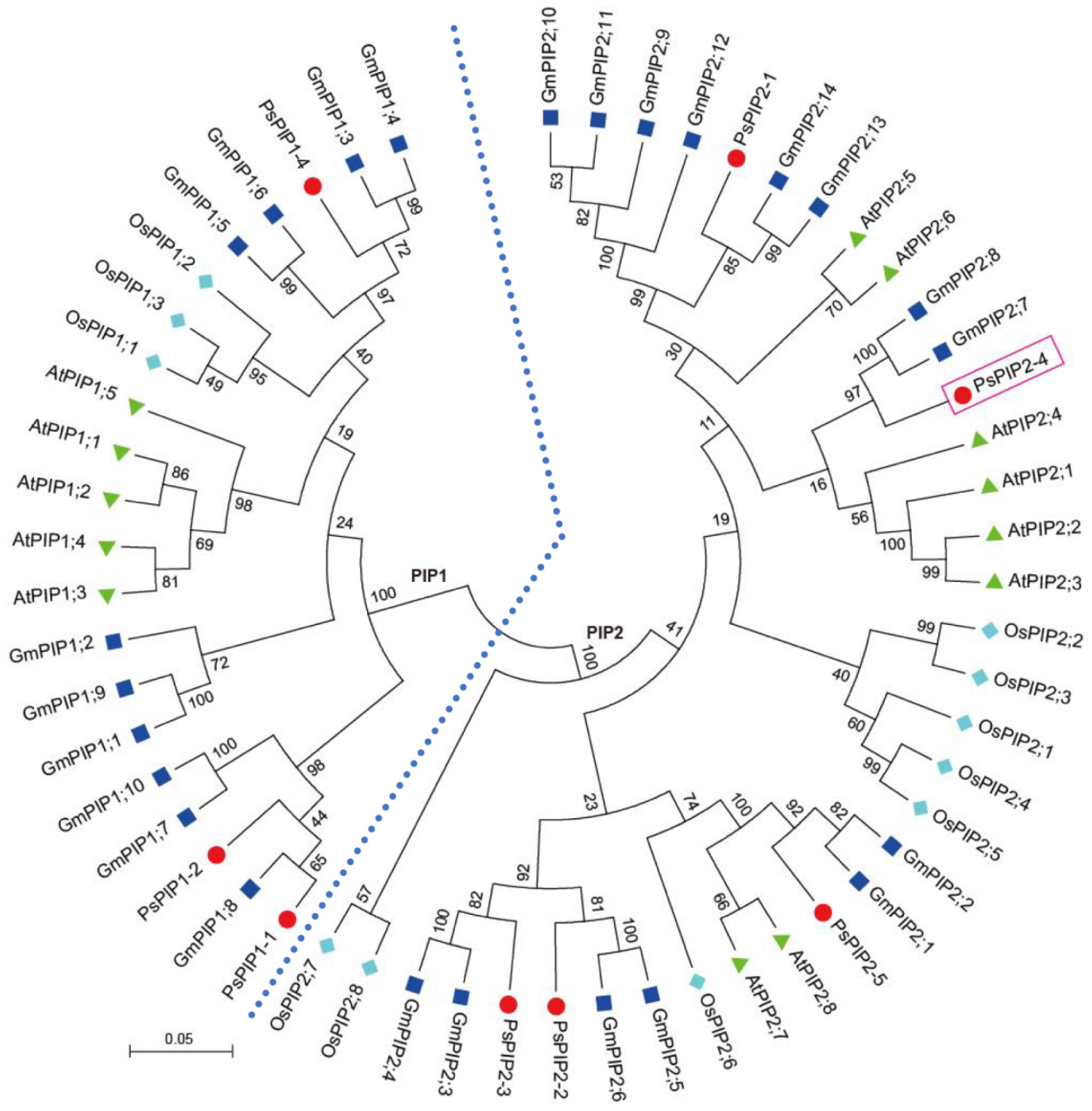
2.2. Phylogenetic Analysis of PsPIP2-4
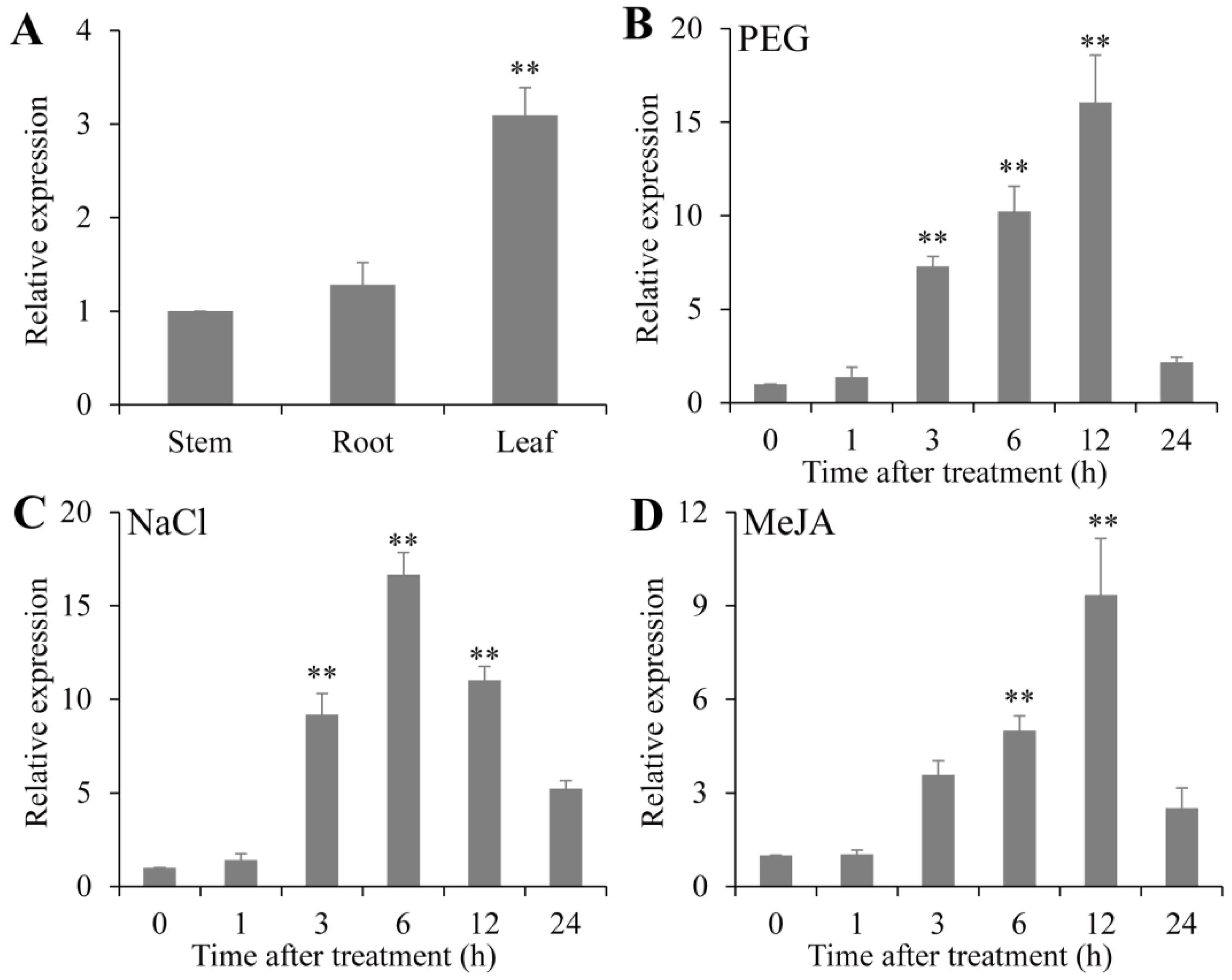
2.3. Expression Pattern Analyses of PsPIP2-4 in Different Tissues and in Response to Different Abiotic Stresses
2.4. Isolation of the PsPIP2-4 Promoter
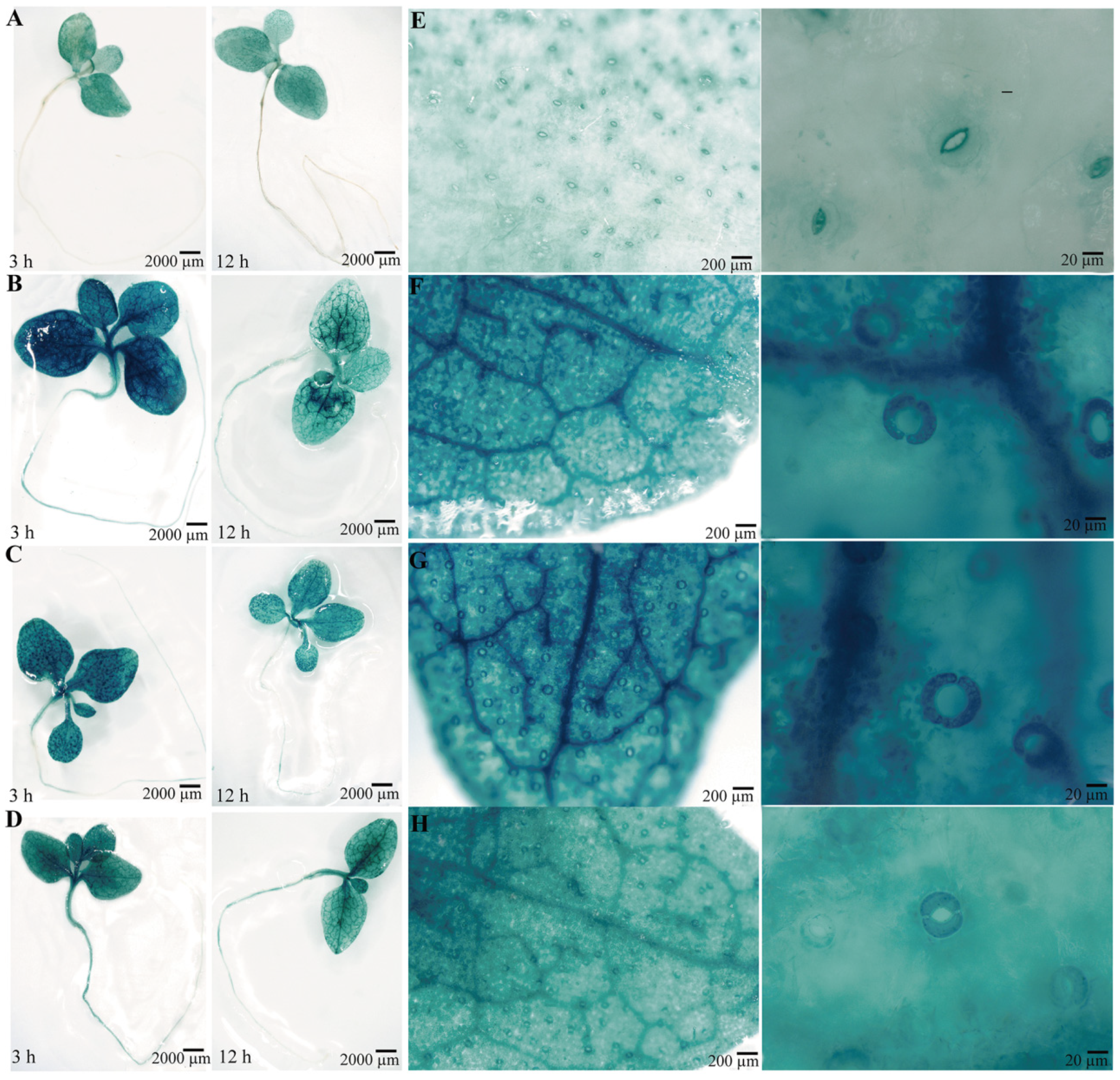
2.5. GUS Activities of the PsPIP2-4 Promoter in Response to Osmotic, Salt, and MeJA Stresses
3. Discussion
4. Materials and Methods
4.1. Vegetable Pea Stress Treatment
4.2. Phylogenetic Analysis
4.3. QRT-PCR
4.4. Promoter Isolation
4.5. Transgenic Tobacco Generation
4.6. Transgenic Tobacco Stress Treatment
4.7. Measurement of GUS Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liu, N.; Zhang, G.; Xu, S.; Mao, W.; Hu, Q.; Gong, Y. Comparative transcriptomic analyses of vegetable and grain pea (Pisum sativum L.) seed development. Front. Plant Sci. 2015, 6, 1039. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, Z.; Wu, C.; Luo, Y.; Li, J.; Wang, P.; Gao, X.; Gao, J.; Feng, B. Identification of differentially expressed genes involved in the molecular mechanism of pericarp elongation and differences in sucrose and starch accumulation between vegetable and grain pea (Pisum sativum L.). Int. J. Mol. Sci. 2019, 20, 6135. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Rasheed, R.; Rizwan, M.; Hussain, I.; Aslam, R.; Qureshi, F.; Hafiza, B.; Bashir, R.; Ali, S. Effect of exogenous taurine on pea (Pisum sativum L.) plants under salinity and iron deficiency stress. Environ. Res. 2023, 223, 115448. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kouser, S.; Asgher, M.; Gandhi, S. Plant aquaporins: A frontward to make crop plants drought resistant. Physiol. Plantarum 2021, 172, 10891105. [Google Scholar] [CrossRef]
- Patel, J.; Mishra, A. Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root architecture, and photosynthesis. Physiol. Plantarum 2021, 172, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Pawłowicz, I.; Masajada, K. Aquaporins as a link between water relations and photosynthetic pathway in abiotic stress tolerance in plants. Gene 2019, 687, 166–172. [Google Scholar] [CrossRef]
- Singh, R.; Deshmukh, R.; Muthamilarasan, M.; Rani, R.; Prasad, M. Versatile roles of aquaporin in physiological processes and stress tolerance in plants. Plant Physiol. Bioch. 2020, 149, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Besserer, A.; Chevalier, A.; Chaumont, F. Insights into plant plasma membrane aquaporin trafficking. Trends Plant Sci. 2013, 18, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Schuurmans, J.; van Dongen, J.; Rutjens, B.; Boonman, A.; Pieterse, C.; Borstlap, A. Members of the aquaporin family in the developing pea seed coat include representatives of the PIP, TIP, and NIP subfamilies. Plant Mol. Biol. 2003, 53, 655–667. [Google Scholar] [CrossRef]
- Song, J.; Ye, G.; Qian, Z.; Ye, Q. Virus-induced plasma membrane aquaporin PsPIP2;1 silencing inhibits plant water transport of Pisum sativum. Bot Stud. 2016, 57, 15. [Google Scholar] [CrossRef]
- Pandey, A.; Sun, T.; Wu, X.; Wang, Z.; Jiang, R.; Zhang, P.; Fang, P.; Xu, P. Aquaporin genes in garden pea and their regulation by the nano-antioxidant fullerol in imbibing embryos under osmotic stress. Veg. Res. 2023, 3, 10. [Google Scholar] [CrossRef]
- Chow, C.; Zheng, H.; Wu, N.; Chien, C.; Huang, H.; Lee, T.; Chiang-Hsieh, Y.; Hou, P.; Yang, T.; Chang, W. PlantPAN 2.0: An update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res. 2016, 44, D1154–D1160. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Ganesan, M. The impact of inducible promoters in transgenic plant production and crop improvement. Plant Gene 2021, 27, 100300. [Google Scholar] [CrossRef]
- Pou, A.; Jeanguenin, L.; Milhiet, T.; Batoko, H.; Chaumont, F.; Hachez, C. Salinity-mediated transcriptional and post-translational regulation of the Arabidopsis aquaporin PIP2;7. Plant Mol. Biol. 2016, 92, 731–744. [Google Scholar] [CrossRef]
- Alexandersson, E.; Danielson, J.; Råde, J.; Moparthi, V.; Fontes, M.; Kjellbom, P.; Johanson, U. Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant J. 2010, 61, 650–660. [Google Scholar] [CrossRef]
- Liu, H.; Yu, X.; Cui, D.; Sun, M.; Sun, W.; Tang, Z.; Kwak, S.; Su, W. The role of water channel proteins and nitric oxide signaling in rice seed germination. Cell Res. 2007, 17, 638–649. [Google Scholar] [CrossRef]
- Yu, Q.; Hu, Y.; Li, J.; Wu, Q.; Lin, Z. Sense and antisense expression of plasma membrane aquaporin BnPIP1 from Brassica napus in tobacco and its effects on plant drought resistance. Plant Sci. 2005, 169, 647–656. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Ma, N.; Gao, J. Regulation of the rose RhPIP2;1 promoter by hormones and abiotic stresses in Arabidopsis. Plant Cell Rep. 2009, 28, 185–196. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Zou, D.; Luo, F.; Wang, X.; Zheng, Y.; Li, X. A cotton gene encoding a plasma membrane aquaporin is involved in seedling development and in response to drought stress. Acta Bioch. Bioph Sin. 2013, 45, 104–114. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, Z.; Xu, B.; Li, J.; Li, Y.; Wang, X.; Wang, A.; Hu, W.; Huang, D.; Wei, Q.; et al. Identification of transcription factors interacting with a 1274 bp promoter of MaPIP1;1 which confers high-level gene expression and drought stress inducibility in transgenic Arabidopsis thaliana. BMC Plant Biol. 2020, 20, 278. [Google Scholar] [CrossRef]
- Stenseth, N.; Andersson, L.; Hoekstra, H. Gregor Johann Mendel and the development of modern evolutionary biology. Proc. Natl. Acad. Sci. USA 2022, 119, e2201327119. [Google Scholar] [CrossRef] [PubMed]
- Kreplak, J.; Madoui, M.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bérard, A.; Vrbová, I.; Fournier, C.; d’Agata, L.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef]
- Yang, T.; Liu, R.; Luo, Y.; Hu, S.; Wang, D.; Wang, C.; Pandey, M.; Ge, S.; Xu, Q.; Li, N.; et al. Improved pea reference genome and pan-genome highlight genomic features and evolutionary characteristics. Nat. Genet. 2022, 54, 1553–1563. [Google Scholar] [CrossRef]
- Liu, N.; Lyu, X.; Zhang, X.; Zhang, G.; Zhang, Z.; Guan, X.; Chen, X.; Yang, X.; Feng, Z.; Gao, Q.; et al. Reference genome sequence and population genomic analysis of peas provide insights into the genetic basis of Mendelian and other agronomic traits. Nat. Genet. 2024, 56, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Nasir, G.; Zaidi, S.; Tabassum, N. A review on nutritional composition, health benefits and potential applications of by-products from pea processing. Biomass Convers. Bior. 2022, 14, 10829–10842. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Kitagawa, Y.; Calamita, G.; Ding, X. Plant aquaporins: Their roles beyond water transport. Crop J. 2024, 12, 641–655. [Google Scholar] [CrossRef]
- Hub, J.; De Groot, B. Mechanism of selectivity in aquaporins and aquaglyceroporins. Proc. Natl. Acad. Sci. USA 2008, 105, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Hove, R.; Bhave, M. Plant aquaporins with non-aqua functions: Deciphering the signature sequences. Plant Mol. Biol. 2011, 75, 413–430. [Google Scholar] [CrossRef]
- Fetter, K.; Van Wilder, V.; Moshelion, M.; Chaumont, F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 2004, 16, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Groszmann, M.; Osborn, H.; Evans, J. Carbon dioxide and water transport through plant aquaporins. Plant Cell Environ. 2017, 40, 938–961. [Google Scholar] [CrossRef]
- Shibasaka, M.; Horie, T.; Katsuhara, M. Mechanisms activating latent functions of PIP aquaporin water channels via the interaction between PIP1 and PIP2 proteins. Plant Cell Physiol. 2021, 62, 92–99. [Google Scholar] [CrossRef]
- Buoso, S.; Musetti, R.; Marroni, F.; Calderan, A.; Schmidt, W.; Santi, S. Infection by phloem-limited phytoplasma affects mineral nutrient homeostasis in tomato leaf tissues. J. Plant Physiol. 2022, 271, 153659. [Google Scholar] [CrossRef] [PubMed]
- Paluch-Lubawa, E.; Polcyn, W. Tissue-specific accumulation of PIP aquaporins of a particular heteromeric composition is part of the maize response to mycorrhiza and drought. Sci. Rep. 2024, 14, 21712. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, C.; Liu, R.; Han, Q.; Vandeleur, R.; Du, J.; Tyerman, S.; Shou, H. Constitutive overexpression of soybean plasma membrane intrinsic protein GmPIP1;6 confers salt tolerance. BMC Plant Biol. 2014, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Dong, C.; Liu, R.; Zhou, B.; Wang, C.; Shou, H. Roles of soybean plasma membrane intrinsic protein GmPIP2;9 in drought tolerance and seed development. Front. Plant Sci. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Hoai, P.; Qiu, J.; Groszmann, M.; De Rosa, A.; Tyerman, S.; Byrt, C. Arabidopsis plasma membrane intrinsic protein (AtPIP2; 1) is implicated in a salinity conditional influence on seed germination. Funct. Plant Biol. 2023, 50, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Cramer, P. Organization and regulation of gene transcription. Nature 2019, 573, 45–54. [Google Scholar] [CrossRef]
- Sumbur, B.; Zhou, M.; Dorjee, T.; Bing, J.; Ha, S.; Xu, X.; Zhou, Y.; Gao, F. Chemical and transcriptomic analyses of leaf cuticular wax metabolism in Ammopiptanthus mongolicus under osmotic stress. Biomolecules 2024, 14, 227. [Google Scholar] [CrossRef]
- Finn, R.; Cerda, J. Evolution and functional diversity of aquaporins. Biol. Bul. 2015, 229, 6–23. [Google Scholar] [CrossRef]
- Schmitz, R.; Grotewold, E.; Stam, M. Cis-regulatory sequences in plants, their importance, discovery, and future challenges. Plant Cell 2022, 34, 718–741. [Google Scholar] [CrossRef]
- An, Y.; Jiao, X.; Yang, S.; Wang, S.; Chen, N.; Huang, L.; Jiang, C.; Lu, M.; Zhang, J. Evaluation of novel promoters for vascular tissue-specific gene expression in Populus. Plant Sci. 2024, 344, 112083. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, S.; Luo, L.; Lu, M.; An, H. Structural feature of RrGGP2 promoter and functional analysis of RrNAC56 regulating RrGGP2 expression and ascorbate synthesis via stress-inducible cis-elements in Rosa roxburghii Tratt. Int. J. Biol. Macromol. 2024, 282, 136584. [Google Scholar] [CrossRef]
- Ruan, X.; Xiong, X.; Li, J. Identification and application of an exocarp-preferential promoter for genetic engineering of tomato fruit. Hortic. Res. 2024, 11, uhae035. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Lu, Y.; Su, X.; Chen, Y.; Shen, Y.; Lin, J.; Li, X. In vivo single-particle tracking of the aquaporin AtPIP2;1 in stomata reveals cell type-specific dynamics. Plant Physiol. 2021, 185, 1666–1681. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Milhiet, T.; Parent, B.; Meziane, A.; Tardieu, F.; Chaumont, F. The plasma membrane aquaporin ZmPIP2;5 enhances the sensitivity of stomatal closure to water deficit. Plant Cell Environ. 2022, 45, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Grondin, A.; Rodrigues, O.; Verdoucq, L.; Merlot, S.; Leonhardt, N.; Maurel, C. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 2015, 27, 1945–1954. [Google Scholar] [CrossRef]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporin facilitate hydrogen peroxide entry into guard cells to mediate ABA-and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Song, Y.; Gong, Z. Integrative regulatory mechanisms of stomatal movements under changing climate. J. Integr. Plant Biol. 2024, 66, 368–393. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Fox, A.; Chaumont, F. Multifaceted role and regulation of aquaporins for efficient stomatal movements. Plant Cell Environ. 2024, 47, 3330–3343. [Google Scholar] [CrossRef]
- Giri, J.; Vij, S.; Dansana, P.; Tyagi, A. Rice A20/AN1 zinc-finger containing stress-associated proteins (SAP1/11) and a receptor-like cytoplasmic kinase (OsRLCK253) interact via A20 zinc-finger and confer abiotic stress tolerance in transgenic Arabidopsis plants. New Phytol. 2011, 191, 721–732. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Teng, F.; Cen, H.; Yan, J.; Lin, S.; Li, D.; Zhang, W. Heterogeneous expression of Osa-MIR156bc increases abiotic stress resistance and forage quality of alfalfa. Crop J. 2021, 9, 1135–1144. [Google Scholar] [CrossRef]
- Jang, J.; Kim, D.; Kim, Y.; Kim, J.; Kang, H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol. Biol. 2004, 54, 713–725. [Google Scholar] [CrossRef]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Liu, N.; Zhang, G.; Niu, F.; Xu, S.; Gong, Y. Investigation of the AQP family in soybean and the promoter activity of TIP2;6 in heat stress and hormone responses. Int. J. Mol. Sci. 2019, 20, 262. [Google Scholar] [CrossRef]
- Die, J.; Román, B.; Nadal, S.; González-Verdejo, C. Evaluation of candidate reference genes for expression studies in Pisum sativum under different experimental conditions. Planta 2010, 232, 145–153. [Google Scholar] [CrossRef]
- Knopkiewicz, M.; Wojtaszek, P. Validation of reference genes for gene expression analysis using quantitative polymerase chain reaction in pea lines (Pisum sativum) with different lodging susceptibility. Ann. Appl. Biol. 2019, 174, 86–91. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gao, H.; Zhang, L.; Tang, W.; Wei, G.; Sun, J.; Xiong, W. Expression of foxtail millet bZIP transcription factor SibZIP67 enhances drought tolerance in Arabidopsis. Biomolecules 2024, 14, 958. [Google Scholar] [CrossRef]
- Dorjee, T.; Cui, Y.; Zhang, Y.; Liu, Q.; Li, X.; Sumbur, B.; Yan, H.; Bing, K.; Geng, Y.; Zhou, Y.; et al. Characterization of NAC gene family in Ammopiptanthus mongolicus and functional analysis of AmNAC24, an osmotic and cold-stress-induced NAC gene. Biomolecules 2024, 14, 182. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Horsch, R.; Fry, J.; Hofman, N.; Eichholtz, D.; Rogers, S.; Fraley, R. A simple method of transferring genes into plants. Science 1985, 277, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Niedbała, G.; Niazian, M.; Sabbatini, P. Modeling agrobacterium-mediated gene transformation of tobacco (Nicotiana tabacum) a model plant for gene transformation studies. Front. Plant Sci. 2021, 12, 695110. [Google Scholar] [CrossRef]
- Jefferson, R.; Kavanagh, T.; Bevan, M. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1985, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, X.; Kou, M.; Yan, H.; Gao, R.; Li, C.; Song, W.; Zhang, Y.; Wang, X.; Liu, Y.; et al. The sweetpotato GIGANTEA gene promoter is co-regulated by phytohormones and abiotic stresses in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 168, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, J.; Yu, Q.; Lan, H. Promoter activity and transcriptome analyses decipher functions of CgbHLH001 gene (Chenopodium glaucum L.) in response to abiotic stress. BMC Plant Biol. 2023, 23, 116. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, C.; Wang, X.; Chen, H. Seed-specific activity of the Arabidopsis β-glucosidase 19 promoter in transgenic Arabidopsis and tobacco. Plant Cell Rep. 2021, 40, 213–221. [Google Scholar] [CrossRef] [PubMed]



| Element Name | Core Sequence | Number | Location (bp) | Function | |
|---|---|---|---|---|---|
| (+) Strand | (−) Strand | ||||
| CGTCA motif | CGTCA | 1 | 191 | MeJA responsiveness | |
| TGACG motif | TGACG | 1 | 191 | MeJA responsiveness | |
| DRE core | GCCGAC | 1 | 233 | Drought responsiveness | |
| TC-rich repeats | ATTCTCTAAC | 1 | 513 | Defense and stress responsiveness | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Liu, N.; Bu, Y.; Zhang, G.; Wang, B.; Gong, Y. Promoter of Vegetable Pea PsPIP2-4 Responds to Abiotic Stresses in Transgenic Tobacco. Int. J. Mol. Sci. 2024, 25, 13574. https://doi.org/10.3390/ijms252413574
Feng Z, Liu N, Bu Y, Zhang G, Wang B, Gong Y. Promoter of Vegetable Pea PsPIP2-4 Responds to Abiotic Stresses in Transgenic Tobacco. International Journal of Molecular Sciences. 2024; 25(24):13574. https://doi.org/10.3390/ijms252413574
Chicago/Turabian StyleFeng, Zhijuan, Na Liu, Yuanpeng Bu, Guwen Zhang, Bin Wang, and Yaming Gong. 2024. "Promoter of Vegetable Pea PsPIP2-4 Responds to Abiotic Stresses in Transgenic Tobacco" International Journal of Molecular Sciences 25, no. 24: 13574. https://doi.org/10.3390/ijms252413574
APA StyleFeng, Z., Liu, N., Bu, Y., Zhang, G., Wang, B., & Gong, Y. (2024). Promoter of Vegetable Pea PsPIP2-4 Responds to Abiotic Stresses in Transgenic Tobacco. International Journal of Molecular Sciences, 25(24), 13574. https://doi.org/10.3390/ijms252413574





