Childhood-Onset ANCA-Associated Vasculitis: From Genetic Studies to Advances in Pathogenesis, Classification and Novel Therapeutic Approaches
Abstract
1. Introduction
2. Clinical Presentation of Childhood AAV
2.1. GPA [14]
2.2. MPA
2.3. EGPA
2.4. AAV-Associated Glomerulonephritis in Children
3. Outcomes in Childhood AAV
4. Serological Markers Associated with AAV
4.1. Characteristic ANCA
4.2. Other ANCA Associated with AAV
4.3. Other Antibodies with Clinical Relevance in Paediatric AAV
5. Classification of Childhood AAV
6. Pathogenesis of Childhood-Onset AAV: From Genetic Factors to Dysregulation of the Immune Pathways
7. Genetic Associations with AAV
7.1. GWAS in GPA/MPA
7.2. Candidate-Gene Association Studies in GPA/MPA
7.2.1. HLA Region
7.2.2. Non-HLA Region
7.3. Genetic Associations with EGPA
7.3.1. GWAS in EGPA
7.3.2. Candidate-Gene Association Studies in EGPA
8. Environmental Factors Associated with AAV
Epigenetics
9. Dysregulation of Immune Pathways Involved in AAV Pathogenesis
Pathogenesis of AAV-Related Glomerulonephritis
10. Diagnosis of Childhood AAV
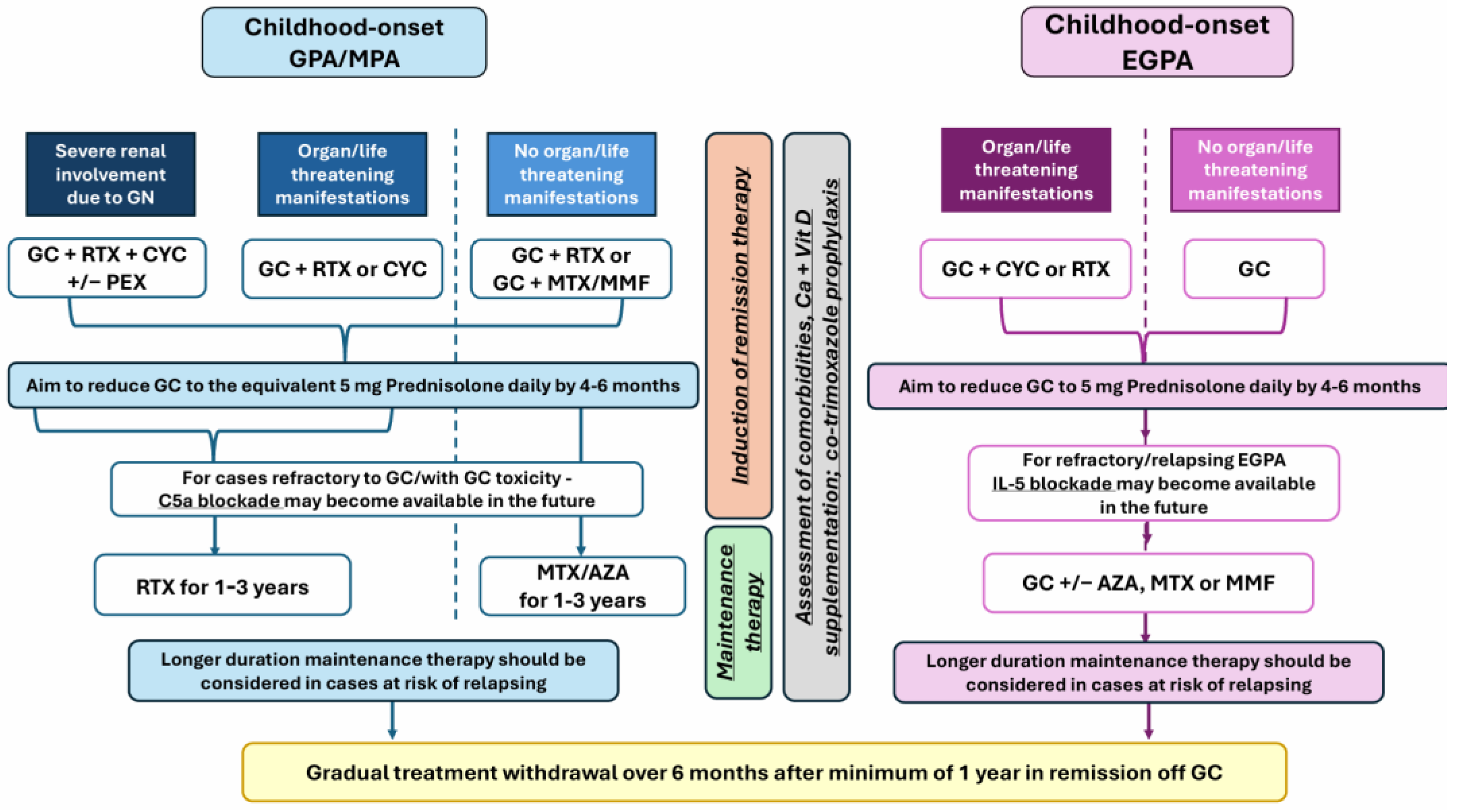
11. Management of Paediatric AAV
12. New Emerging Therapies
13. Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Alba, M.A.; Jennette, J.C.; Falk, R.J. Pathogenesis of ANCA-Associated Pulmonary Vasculitis. Semin. Respir. Crit. Care Med. 2018, 39, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Leacy, E.; Brady, G.; A.Little, M. Pathogenesis of ANCA-associated vasculitis: An emerging role for immunometabolism. Rheumatology 2020, 59, iii33–iii41. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Jawa, P.; Derebail, V.K.; Falk, R.J. Treatment Updates in Antineutrophil Cytoplasmic Autoantibodies (ANCA) Vasculitis. Kidney360 2021, 2, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Mossberg, M.; Segelmark, M.; Kahn, R.; Englund, M.; Mohammad, A.J. Epidemiology of primary systemic vasculitis in children: A population-based study from southern Sweden. Scand. J. Rheumatol. 2018, 47, 295–302. [Google Scholar] [CrossRef]
- Hirano, D.; Ishikawa, T.; Inaba, A.; Sato, M.; Shinozaki, T.; Iijima, K.; Ito, S. Epidemiology and clinical features of childhood-onset anti-neutrophil cytoplasmic antibody-associated vasculitis: A clinicopathological analysis. Pediatr. Nephrol. 2019, 34, 1425–1433. [Google Scholar] [CrossRef]
- Sacri, A.S.; Chambaraud, T.; Ranchin, B.; Florkin, B.; See, H.; Decramer, S.; Flodrops, H.; Ulinski, T.; Allain-Launay, E.; Boyer, O.; et al. Clinical characteristics and outcomes of childhood-onset ANCA-associated vasculitis: A French nationwide study. Nephrol. Dial. Transplant. 2015, 30 (Suppl. 1), i104–i112. [Google Scholar] [CrossRef]
- Grisaru, S.; Yuen, G.W.; Miettunen, P.M.; Hamiwka, L.A. Incidence of Wegener’s granulomatosis in children. J. Rheumatol. 2010, 37, 440–442. [Google Scholar] [CrossRef]
- Nilsen, A.T.; Karlsen, C.; Bakland, G.; Watts, R.; Luqmani, R.; Koldingsnes, W. Increasing incidence and prevalence of ANCA-associated vasculitis in Northern Norway. Rheumatology 2020, 59, 2316–2324. [Google Scholar] [CrossRef]
- Mohammad, A.J. An update on the epidemiology of ANCA-associated vasculitis. Rheumatology 2020, 59, iii42–iii50. [Google Scholar] [CrossRef]
- de Graeff, N.; Groot, N.; Brogan, P.; Ozen, S.; Avcin, T.; Bader-Meunier, B.; Dolezalova, P.; Feldman, B.M.; Kone-Paut, I.; Lahdenne, P.; et al. European consensus-based recommendations for the diagnosis and treatment of rare paediatric vasculitides—The SHARE initiative. Rheumatology 2019, 58, 656–671. [Google Scholar] [CrossRef]
- Cabral, D.A.; Canter, D.L.; Muscal, E.; Nanda, K.; Wahezi, D.M.; Spalding, S.J.; Twilt, M.; Benseler, S.M.; Campillo, S.; Charuvanij, S.; et al. Comparing Presenting Clinical Features in 48 Children With Microscopic Polyangiitis to 183 Children Who Have Granulomatosis With Polyangiitis (Wegener’s): An ARChiVe Cohort Study. Arthritis Rheumatol. 2016, 68, 2514–2526. [Google Scholar] [CrossRef] [PubMed]
- Kaya Akca, U.; Batu, E.D.; Jelusic, M.; Calatroni, M.; Bakry, R.; Frkovic, M.; Vinsova, N.; Campos, R.T.; Horne, A.; Caglayan, S.; et al. Comparison of EULAR/PRINTO/PReS Ankara 2008 and 2022 ACR/EULAR classification criteria for granulomatosis with polyangiitis in children. Rheumatology 2024, 63, SI122–SI128. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.M.; Page, J.; Uribe, A.G.; Espinosa, V.; Cabral, D.A. Establishment of a pilot pediatric registry for chronic vasculitis is both essential and feasible: A Childhood Arthritis and Rheumatology Alliance (CARRA) survey. J. Rheumatol. 2007, 34, 224–226. [Google Scholar] [PubMed]
- Bohm, M.; Gonzalez Fernandez, M.I.; Ozen, S.; Pistorio, A.; Dolezalova, P.; Brogan, P.; Barbano, G.; Sengler, C.; Klein-Gitelman, M.; Quartier, P.; et al. Clinical features of childhood granulomatosis with polyangiitis (wegener’s granulomatosis). Pediatr. Rheumatol. Online J. 2014, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Pistorio, A.; Iusan, S.M.; Bakkaloglu, A.; Herlin, T.; Brik, R.; Buoncompagni, A.; Lazar, C.; Bilge, I.; Uziel, Y.; et al. EULAR/PRINTO/PRES criteria for Henoch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann. Rheum. Dis. 2010, 69, 798–806. [Google Scholar] [CrossRef]
- Iudici, M.; Quartier, P.; Terrier, B.; Mouthon, L.; Guillevin, L.; Puechal, X. Childhood-onset granulomatosis with polyangiitis and microscopic polyangiitis: Systematic review and meta-analysis. Orphanet J. Rare Dis. 2016, 11, 141. [Google Scholar] [CrossRef]
- Iudici, M.; Pagnoux, C.; Quartier, P.; Buchler, M.; Cevallos, R.; Cohen, P.; de Moreuil, C.; Guilpain, P.; Le Quellec, A.; Serratrice, J.; et al. Childhood- versus adult-onset ANCA-associated vasculitides: A nested, matched case-control study from the French Vasculitis Study Group Registry. Autoimmun. Rev. 2018, 17, 108–114. [Google Scholar] [CrossRef]
- von Vigier, R.O.; Trummler, S.A.; Laux-End, R.; Sauvain, M.J.; Truttmann, A.C.; Bianchetti, M.G. Pulmonary renal syndrome in childhood: A report of twenty-one cases and a review of the literature. Pediatr. Pulmonol. 2000, 29, 382–388. [Google Scholar] [CrossRef]
- Bakkaloglu, A.; Ozen, S.; Baskin, E.; Besbas, N.; Gur-Guven, A.; Kasapcopur, O.; Tinaztepe, K. The significance of antineutrophil cytoplasmic antibody in microscopic polyangitis and classic polyarteritis nodosa. Arch. Dis. Child. 2001, 85, 427–430. [Google Scholar] [CrossRef]
- Mahr, A.; Moosig, F.; Neumann, T.; Szczeklik, W.; Taille, C.; Vaglio, A.; Zwerina, J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Evolutions in classification, etiopathogenesis, assessment and management. Curr. Opin. Rheumatol. 2014, 26, 16–23. [Google Scholar] [CrossRef]
- Barnini, C.; Oni, L.; Kronbichler, A. Course of paediatric ANCA-associated glomerulonephritis: Advocating for an age-inclusive approach. RMD Open 2024, 10, e004481. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.K.; Bone, J.N.; Bosman, E.S.; Cabral, D.A.; Morishita, K.A.; Brown, K.L.; PedVas Investigator, N. Predictive utility of ANCA positivity and antigen specificity in the assessment of kidney disease in paediatric-onset small vessel vasculitis. RMD Open 2024, 10, e004315. [Google Scholar] [CrossRef] [PubMed]
- Calatroni, M.; Consonni, F.; Allinovi, M.; Bettiol, A.; Jawa, N.; Fiasella, S.; Curi, D.; Abu Rumeileh, S.; Tomei, L.; Fortunato, L.; et al. Prognostic Factors and Long-Term Outcome with ANCA-Associated Kidney Vasculitis in Childhood. Clin. J. Am. Soc. Nephrol. 2021, 16, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Mahi, S.L.; Bahram, S.; Harambat, J.; Allard, L.; Merlin, E.; Belot, A.; Ranchin, B.; Tenenbaum, J.; Magnavacca, M.; Haumesser, L.; et al. Pediatric ANCA vasculitis: Clinical presentation, treatment, and outcomes in a French retrospective study. Pediatr. Nephrol. 2023, 38, 2649–2658. [Google Scholar] [CrossRef]
- Marlais, M.; Wlodkowski, T.; Printza, N.; Kronsteiner, D.; Krisam, R.; Sauer, L.; Aksenova, M.; Ashoor, I.; Awan, A.; Bacchetta, J.; et al. Clinical Factors and Adverse Kidney Outcomes in Children With Antineutrophil Cytoplasmic Antibody-Associated Glomerulonephritis. Am. J. Kidney Dis. 2023, 81, 119–122. [Google Scholar] [CrossRef]
- Morishita, K.A.; Moorthy, L.N.; Lubieniecka, J.M.; Twilt, M.; Yeung, R.S.M.; Toth, M.B.; Shenoi, S.; Ristic, G.; Nielsen, S.M.; Luqmani, R.A.; et al. Early Outcomes in Children With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2017, 69, 1470–1479. [Google Scholar] [CrossRef]
- Sayad, E.; Vogel, T.P.; Guillerman, R.P.; Spielberg, D.; McNeill, D.M.; De Guzman, M.; Orman, G.; Silva-Carmona, M. Pulmonary manifestations and outcomes in paediatric ANCA-associated vasculitis: A single-centre experience. Rheumatology 2021, 60, 3199–3208. [Google Scholar] [CrossRef]
- Guillevin, L. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine 1999, 78, 26. [Google Scholar] [CrossRef]
- Moosig, F.; Bremer, J.P.; Hellmich, B.; Holle, J.U.; Holl-Ulrich, K.; Laudien, M.; Matthis, C.; Metzler, C.; Nölle, B.; Richardt, G.; et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): Monocentric experiences in 150 patients. Ann. Rheum. Dis. 2013, 72, 1011–1017. [Google Scholar] [CrossRef]
- Sablé-Fourtassou, R.; Cohen, P.; Mahr, A.; Pagnoux, C.; Mouthon, L.; Jayne, D.; Blockmans, D.; Cordier, J.F.; Delaval, P.; Puechal, X.; et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann. Intern. Med. 2005, 143, 632–638. [Google Scholar] [CrossRef]
- Sinico, R.A.; Di Toma, L.; Maggiore, U.; Bottero, P.; Radice, A.; Tosoni, C.; Grasselli, C.; Pavone, L.; Gregorini, G.; Monti, S.; et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheumatol. 2005, 52, 2926–2935. [Google Scholar] [CrossRef] [PubMed]
- Comarmond, C.; Pagnoux, C.; Khellaf, M.; Cordier, J.F.; Hamidou, M.; Viallard, J.F.; Maurier, F.; Jouneau, S.; Bienvenu, B.; Puéchal, X.; et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheumatol. 2013, 65, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, S.; Bossuyt, X.; Arimura, Y.; Blockmans, D.; Csernok, E.; Damoiseaux, J.; Emmi, G.; Flores-Suárez, L.F.; Hellmich, B.; Jayne, D.; et al. International Consensus on ANCA Testing in Eosinophilic Granulomatosis with Polyangiitis. Am. J. Respir. Crit. Care Med. 2020. ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Zwerina, J.; Eger, G.; Englbrecht, M.; Manger, B.; Schett, G. Churg-Strauss syndrome in childhood: A systematic literature review and clinical comparison with adult patients. Semin. Arthritis Rheumatol. 2009, 39, 108–115. [Google Scholar] [CrossRef]
- Nakazawa, D.; Masuda, S.; Tomaru, U.; Ishizu, A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 2019, 15, 91–101. [Google Scholar] [CrossRef]
- Little, M.A.; Smyth, L.; Salama, A.D.; Mukherjee, S.; Smith, J.; Haskard, D.; Nourshargh, S.; Cook, H.T.; Pusey, C.D. Experimental autoimmune vasculitis: An animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am. J. Pathol. 2009, 174, 1212–1220. [Google Scholar] [CrossRef]
- Little, M.A.; Al-Ani, B.; Ren, S.; Al-Nuaimi, H.; Leite, M., Jr.; Alpers, C.E.; Savage, C.O.; Duffield, J.S. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS ONE 2012, 7, e28626. [Google Scholar] [CrossRef]
- Walulik, A.; Łysak, K.; Błaszkiewicz, M.; Górecki, I.; Gomułka, K. The Role of Neutrophils in ANCA-Associated Vasculitis: The Pathogenic Role and Diagnostic Utility of Autoantibodies. Int. J. Mol. Sci. 2023, 24, 17217. [Google Scholar] [CrossRef]
- Suzuki, K.; Nagao, T.; Itabashi, M.; Hamano, Y.; Sugamata, R.; Yamazaki, Y.; Yumura, W.; Tsukita, S.; Wang, P.C.; Nakayama, T.; et al. A novel autoantibody against moesin in the serum of patients with MPO-ANCA-associated vasculitis. Nephrol. Dial. Transplant. 2014, 29, 1168–1177. [Google Scholar] [CrossRef][Green Version]
- Gibson, K.M.; Kain, R.; Luqmani, R.A.; Ross, C.J.; Cabral, D.A.; Brown, K.L. Autoantibodies Against Lysosome Associated Membrane Protein-2 (LAMP-2) in Pediatric Chronic Primary Systemic Vasculitis. Front. Immunol. 2020, 11, 624758. [Google Scholar] [CrossRef]
- Yang, J.; Bautz, D.J.; Lionaki, S.; Hogan, S.L.; Chin, H.; Tisch, R.M.; Schmitz, J.L.; Pressler, B.M.; Jennette, J.C.; Falk, R.J.; et al. ANCA patients have T cells responsive to complementary PR-3 antigen. Kidney Int. 2008, 74, 1159–1169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bautz, D.J.; Preston, G.A.; Lionaki, S.; Hewins, P.; Wolberg, A.S.; Yang, J.J.; Hogan, S.L.; Chin, H.; Moll, S.; Jennette, J.C.; et al. Antibodies with dual reactivity to plasminogen and complementary PR3 in PR3-ANCA vasculitis. J. Am. Soc. Nephrol. 2008, 19, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Subra, J.-F.; Guilpain, P.; Jeannin, P.; Pignon, P.; Blanchard, S.; Garo, E.; Jaillon, S.; Chevailler, A.; Renier, G.; et al. Detection of Anti-Pentraxin-3 Autoantibodies in ANCA-Associated Vasculitis. PLoS ONE 2016, 11, e0147091. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Kamiya, M.; Urano, Y.; Nishi, H.; Herter, J.M.; Mayadas, T.; Hirohama, D.; Suzuki, K.; Kawakami, H.; Tanaka, M.; et al. Lactoferrin Suppresses Neutrophil Extracellular Traps Release in Inflammation. EBioMedicine 2016, 10, 204–215. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Serafinelli, J.; Giani, M.; Montini, G. Clinical and Pathophysiological Insights Into Immunological Mediated Glomerular Diseases in Childhood. Front. Pediatr. 2020, 8, 205. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, Q.; Qiu, Y.; Huang, F. Clinical and pathological characteristics of ANA/anti-dsDNA positive patients with antineutrophil cytoplasmic autoantibody-associated vasculitis. Rheumatol. Int. 2021, 41, 455–462. [Google Scholar] [CrossRef]
- Hunder, G.G.; Arend, W.P.; Bloch, D.A.; Calabrese, L.H.; Fauci, A.S.; Fries, J.F.; Leavitt, R.Y.; Lie, J.T.; Lightfoot, R.W., Jr.; Masi, A.T.; et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis: Introduction. Arthritis Rheumatol. 1990, 33, 1065–1067. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Andrassy, K.; Bacon, P.A.; Churg, J.; Gross, W.L.; Hagen, E.C.; Hoffman, G.S.; Hunder, G.G.; Kallenberg, C.G.; et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheumatol. 1994, 37, 187–192. [Google Scholar] [CrossRef]
- Watts, R.A.; Suppiah, R.; Merkel, P.A.; Luqmani, R. Systemic vasculitis—Is it time to reclassify? Rheumatology 2011, 50, 643–645. [Google Scholar] [CrossRef][Green Version]
- Ozen, S.; Ruperto, N.; Dillon, M.J.; Bagga, A.; Barron, K.; Davin, J.C.; Kawasaki, T.; Lindsley, C.; Petty, R.E.; Prieur, A.M.; et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann. Rheum. Dis. 2006, 65, 936–941. [Google Scholar] [CrossRef]
- Robson, J.C.; Grayson, P.C.; Ponte, C.; Suppiah, R.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Watts, R.A.; Merkel, P.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Granulomatosis With Polyangiitis. Arthritis Rheumatol. 2022, 74, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Wallace, Z.S.; Stone, J.H. Personalized Medicine in ANCA-Associated Vasculitis ANCA Specificity as the Guide? Front. Immunol. 2019, 10, 2855. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, D.; Brogan, P.A. Vasculitis in children. Best. Pract. Res. Clin. Rheumatol. 2009, 23, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Ntatsaki, E.; Watts, R.A.; Scott, D.G. Epidemiology of ANCA-associated vasculitis. Rheum. Dis. Clin. N. Am. 2010, 36, 447–461. [Google Scholar] [CrossRef]
- Gibson, K.M.; Drögemöller, B.I.; Foell, D.; Benseler, S.M.; Graham, J.; Hancock, R.E.W.; Luqmani, R.A.; Cabral, D.A.; Brown, K.L.; Ross, C.J. Association Between HLA-DPB1 and Antineutrophil Cytoplasmic Antibody-Associated Vasculitis in Children. Arthritis Rheumatol. 2023, 75, 1048–1057. [Google Scholar] [CrossRef]
- Stone, J.H.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.; St Clair, E.W.; Turkiewicz, A.; Tchao, N.K.; et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2010, 363, 221–232. [Google Scholar] [CrossRef]
- Koldingsnes, W.; Nossent, H. Epidemiology of Wegener’s granulomatosis in northern Norway. Arthritis Rheumatol. 2000, 43, 2481–2487. [Google Scholar] [CrossRef]
- Ortiz-Fernandez, L.; Carmona, E.G.; Kerick, M.; Lyons, P.; Carmona, F.D.; Lopez Mejias, R.; Khor, C.C.; Grayson, P.C.; Tombetti, E.; Jiang, L.; et al. Identification of new risk loci shared across systemic vasculitides points towards potential target genes for drug repurposing. Ann. Rheum. Dis. 2023, 82, 837–847. [Google Scholar] [CrossRef]
- Trivioli, G.; Marquez, A.; Martorana, D.; Tesi, M.; Kronbichler, A.; Lyons, P.A.; Vaglio, A. Genetics of ANCA-associated vasculitis: Role in pathogenesis, classification and management. Nat. Rev. Rheumatol. 2022, 18, 559–574. [Google Scholar] [CrossRef]
- Jagiello, P.; Gencik, M.; Arning, L.; Wieczorek, S.; Kunstmann, E.; Csernok, E.; Gross, W.L.; Epplen, J.T. New genomic region for Wegener’s granulomatosis as revealed by an extended association screen with 202 apoptosis-related genes. Hum. Genet. 2004, 114, 468–477. [Google Scholar] [CrossRef]
- Vaglio, A.; Martorana, D.; Maggiore, U.; Grasselli, C.; Zanetti, A.; Pesci, A.; Garini, G.; Manganelli, P.; Bottero, P.; Tumiati, B.; et al. HLA-DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheumatol. 2007, 56, 3159–3166. [Google Scholar] [CrossRef] [PubMed]
- Mahr, A.D.; Edberg, J.C.; Stone, J.H.; Hoffman, G.S.; St Clair, E.W.; Specks, U.; Dellaripa, P.F.; Seo, P.; Spiera, R.F.; Rouhani, F.N.; et al. Alpha₁-antitrypsin deficiency-related alleles Z and S and the risk of Wegener’s granulomatosis. Arthritis Rheumatol. 2010, 62, 3760–3767. [Google Scholar] [CrossRef] [PubMed]
- Gencik, M.; Meller, S.; Borgmann, S.; Fricke, H. Proteinase 3 gene polymorphisms and Wegener’s granulomatosis. Kidney Int. 2000, 58, 2473–2477. [Google Scholar] [CrossRef]
- Jagiello, P.; Aries, P.; Arning, L.; Wagenleiter, S.E.; Csernok, E.; Hellmich, B.; Gross, W.L.; Epplen, J.T. The PTPN22 620W allele is a risk factor for Wegener’s granulomatosis. Arthritis Rheumatol. 2005, 52, 4039–4043. [Google Scholar] [CrossRef]
- Willcocks, L.C.; Lyons, P.A.; Clatworthy, M.R.; Robinson, J.I.; Yang, W.; Newland, S.A.; Plagnol, V.; McGovern, N.N.; Condliffe, A.M.; Chilvers, E.R.; et al. Copy number of FCGR3B, which is associated with systemic lupus erythematosus, correlates with protein expression and immune complex uptake. J. Exp. Med. 2008, 205, 1573–1582. [Google Scholar] [CrossRef]
- Martorana, D.; Bonatti, F.; Alberici, F.; Gioffredi, A.; Reina, M.; Urban, M.L.; Maritati, F.; Adorni, A.; Radice, A.; Pizzolato, S.; et al. Fcγ-receptor 3B (FCGR3B) copy number variations in patients with eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. 2016, 137, 1597–1599.e1598. [Google Scholar] [CrossRef]
- Husmann, C.A.; Holle, J.U.; Moosig, F.; Mueller, S.; Wilde, B.; Cohen Tervaert, J.W.; Harper, L.; Assmann, G.; Gross, W.L.; Epplen, J.T.; et al. Genetics of toll like receptor 9 in ANCA associated vasculitides. Ann. Rheum. Dis. 2014, 73, 890–896. [Google Scholar] [CrossRef]
- Lyons, P.A.; Rayner, T.F.; Trivedi, S.; Holle, J.U.; Watts, R.A.; Jayne, D.R.; Baslund, B.; Brenchley, P.; Bruchfeld, A.; Chaudhry, A.N.; et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 2012, 367, 214–223. [Google Scholar] [CrossRef]
- Xie, G.; Roshandel, D.; Sherva, R.; Monach, P.A.; Lu, E.Y.; Kung, T.; Carrington, K.; Zhang, S.S.; Pulit, S.L.; Ripke, S.; et al. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1*04 and SEMA6A gene variants: Evidence from genome-wide analysis. Arthritis Rheumatol. 2013, 65, 2457–2468. [Google Scholar] [CrossRef]
- Merkel, P.A.; Xie, G.; Monach, P.A.; Ji, X.; Ciavatta, D.J.; Byun, J.; Pinder, B.D.; Zhao, A.; Zhang, J.; Tadesse, Y.; et al. Identification of Functional and Expression Polymorphisms Associated With Risk for Antineutrophil Cytoplasmic Autoantibody-Associated Vasculitis. Arthritis Rheumatol. 2017, 69, 1054–1066. [Google Scholar] [CrossRef]
- Lyons, P.A.; Peters, J.E.; Alberici, F.; Liley, J.; Coulson, R.M.R.; Astle, W.; Baldini, C.; Bonatti, F.; Cid, M.C.; Elding, H.; et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat. Commun. 2019, 10, 5120. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, J.; Wu, M.; Liu, Q. Causal relationship between immune cells and the risk of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis: A Mendelian randomization study. FASEB J. 2024, 38, e23821. [Google Scholar] [CrossRef] [PubMed]
- Calatroni, M.; Oliva, E.; Gianfreda, D.; Gregorini, G.; Allinovi, M.; Ramirez, G.A.; Bozzolo, E.P.; Monti, S.; Bracaglia, C.; Marucci, G.; et al. ANCA-associated vasculitis in childhood: Recent advances. Ital. J. Pediatr. 2017, 43, 46. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.S.; Guan, Y.J.; Yuan, Z.L.; Albina, J.E.; Chin, Y.E. Ankyrin repeat and SOCS box 3 (ASB3) mediates ubiquitination and degradation of tumor necrosis factor receptor II. Mol. Cell Biol. 2005, 25, 4716–4726. [Google Scholar] [CrossRef] [PubMed]
- Ernandez, T.; Mayadas, T.N. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009, 76, 262–276. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Liu, B.; Xie, X. Low LINC02147 expression promotes the malignant progression of oral submucous fibrosis. BMC Oral Health 2022, 22, 316. [Google Scholar] [CrossRef]
- Quintana, F.J.; Zaltzman, R.; Fernandez-Montesinos, R.; Herrera, J.L.; Gozes, I.; Cohen, I.R.; Pozo, D. NAP, a peptide derived from the activity-dependent neuroprotective protein, modulates macrophage function. Ann. N. Y. Acad. Sci. 2006, 1070, 500–506. [Google Scholar] [CrossRef]
- Gill, E.E.; Smith, M.L.; Gibson, K.M.; Morishita, K.A.; Lee, A.H.Y.; Falsafi, R.; Graham, J.; Foell, D.; Benseler, S.M.; Ross, C.J.; et al. Different Disease Endotypes in Phenotypically Similar Vasculitides Affecting Small-to-Medium Sized Blood Vessels. Front. Immunol. 2021, 12, 638571. [Google Scholar] [CrossRef]
- Li, W.; Huang, H.; Cai, M.; Yuan, T.; Sheng, Y. Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Update: Genetic Pathogenesis. Front. Immunol. 2021, 12, 624848. [Google Scholar] [CrossRef]
- Heckmann, M.; Holle, J.U.; Arning, L.; Knaup, S.; Hellmich, B.; Nothnagel, M.; Jagiello, P.; Gross, W.L.; Epplen, J.T.; Wieczorek, S. The Wegener’s granulomatosis quantitative trait locus on chromosome 6p21.3 as characterised by tagSNP genotyping. Ann. Rheum. Dis. 2008, 67, 972–979. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Kobayashi, S.; Hashimoto, H.; Ozaki, S.; Tokunaga, K. Association of HLA-DRB1*0901-DQB1*0303 haplotype with microscopic polyangiitis in Japanese. Genes. Immun. 2006, 7, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Kobayashi, S.; Kawasaki, A.; Kyogoku, C.; Arimura, Y.; Yoshida, M.; Tokunaga, K.; Hashimoto, H. Genetic background of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis: Association of HLA-DRB1*0901 with microscopic polyangiitis. J. Rheumatol. 2003, 30, 1534–1540. [Google Scholar] [PubMed]
- Kawasaki, A.; Hasebe, N.; Hidaka, M.; Hirano, F.; Sada, K.E.; Kobayashi, S.; Yamada, H.; Furukawa, H.; Yamagata, K.; Sumida, T.; et al. Protective Role of HLA-DRB1*13:02 against Microscopic Polyangiitis and MPO-ANCA-Positive Vasculitides in a Japanese Population: A Case-Control Study. PLoS ONE 2016, 11, e0154393. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Cui, Z.; Pei, Z.Y.; Fang, S.B.; Chen, S.F.; Zhu, L.; Chen, M.; Chen, N.; Zhao, M.H. Risk HLA class II alleles and amino acid residues in myeloperoxidase-ANCA-associated vasculitis. Kidney Int. 2019, 96, 1010–1019. [Google Scholar] [CrossRef]
- Chang, D.Y.; Luo, H.; Zhou, X.J.; Chen, M.; Zhao, M.H. Association of HLA genes with clinical outcomes of ANCA-associated vasculitis. Clin. J. Am. Soc. Nephrol. 2012, 7, 1293–1299. [Google Scholar] [CrossRef]
- Hilhorst, M.; Arndt, F.; Joseph Kemna, M.; Wieczorek, S.; Donner, Y.; Wilde, B.; Thomas Epplen, J.; van Paassen, P.; Cohen Tervaert, J.W. HLA-DPB1 as a Risk Factor for Relapse in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Cohort Study. Arthritis Rheumatol. 2016, 68, 1721–1730. [Google Scholar] [CrossRef]
- Lee, K.S.; Kronbichler, A.; Pereira Vasconcelos, D.F.; Pereira da Silva, F.R.; Ko, Y.; Oh, Y.S.; Eisenhut, M.; Merkel, P.A.; Jayne, D.; Amos, C.I.; et al. Genetic Variants in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Bayesian Approach and Systematic Review. J. Clin. Med. 2019, 8, 266. [Google Scholar] [CrossRef]
- Campbell, E.J.; Campbell, M.A.; Owen, C.A. Bioactive proteinase 3 on the cell surface of human neutrophils: Quantification, catalytic activity, and susceptibility to inhibition. J. Immunol. 2000, 165, 3366–3374. [Google Scholar] [CrossRef]
- Elzouki, A.N.; Segelmark, M.; Wieslander, J.; Eriksson, S. Strong link between the alpha 1-antitrypsin PiZ allele and Wegener’s granulomatosis. J. Intern. Med. 1994, 236, 543–548. [Google Scholar] [CrossRef]
- Stanford, S.M.; Bottini, N. PTPN22: The archetypal non-HLA autoimmunity gene. Nat. Rev. Rheumatol. 2014, 10, 602–611. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, K.; Tian, Z.; Hogan, S.L.; Yang, J.; Poulton, C.J.; Falk, R.J.; Li, W. PTPN22 R620W polymorphism and ANCA disease risk in white populations: A metaanalysis. J. Rheumatol. 2015, 42, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Langford, C.A.; Monach, P.A.; Specks, U.; Seo, P.; Cuthbertson, D.; McAlear, C.A.; Ytterberg, S.R.; Hoffman, G.S.; Krischer, J.P.; Merkel, P.A. An open-label trial of abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener’s). Ann. Rheum. Dis. 2014, 73, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.B.; Tebbutt, N.C.; Buchert, M.; Putoczki, T.L.; Doggett, K.; Bao, S.; Johnstone, C.N.; Masson, F.; Hollande, F.; Burgess, A.W.; et al. Glycoprotein A33 deficiency: A new mouse model of impaired intestinal epithelial barrier function and inflammatory disease. Dis. Model. Mech. 2015, 8, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Wieczorek, S.; Hellmich, B.; Gross, W.L.; Epplen, J.T. Associations of Churg-Strauss syndrome with the HLA-DRB1 locus, and relationship to the genetics of antineutrophil cytoplasmic antibody-associated vasculitides: Comment on the article by Vaglio et al. Arthritis Rheumatol. 2008, 58, 329–330. [Google Scholar] [CrossRef]
- McKinney, C.; Merriman, T.R. Meta-analysis confirms a role for deletion in FCGR3B in autoimmune phenotypes. Hum. Mol. Genet. 2012, 21, 2370–2376. [Google Scholar] [CrossRef]
- Wieczorek, S.; Hellmich, B.; Arning, L.; Moosig, F.; Lamprecht, P.; Gross, W.L.; Epplen, J.T. Functionally relevant variations of the interleukin-10 gene associated with antineutrophil cytoplasmic antibody-negative Churg-Strauss syndrome, but not with Wegener’s granulomatosis. Arthritis Rheumatol. 2008, 58, 1839–1848. [Google Scholar] [CrossRef]
- Zheng, Y.; Du, Y.; Wu, Y.; Li, F.; Gu, W.; Zhao, C. COPA syndrome caused by a novel p.Arg227Cys COPA gene variant. Mol. Genet. Genomic Med. 2024, 12, e2309. [Google Scholar] [CrossRef]
- Zhang, D.; Su, G.; Hao, S.; Lai, J.; Feng, S. Paediatric autoimmune diseases with ELANE mutations associated with neutropenia. Pediatr. Rheumatol. Online J. 2023, 21, 41. [Google Scholar] [CrossRef]
- Ochfeld, E.; Curran, M.L.; Chiarella, S.E.; Ardalan, K.; Khojah, A. A Case Report of SAVI Mimicking Early-Onset ANCA Vasculitis. J. Clin. Immunol. 2021, 41, 1652–1655. [Google Scholar] [CrossRef]
- Liu, Y.; Jesus, A.A.; Marrero, B.; Yang, D.; Ramsey, S.E.; Sanchez, G.A.M.; Tenbrock, K.; Wittkowski, H.; Jones, O.Y.; Kuehn, H.S.; et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 2014, 371, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Staels, F.; Betrains, A.; Doubel, P.; Willemsen, M.; Cleemput, V.; Vanderschueren, S.; Corveleyn, A.; Meyts, I.; Sprangers, B.; Crow, Y.J.; et al. Adult-Onset ANCA-Associated Vasculitis in SAVI: Extension of the Phenotypic Spectrum, Case Report and Review of the Literature. Front. Immunol. 2020, 11, 575219. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.K.; Francis, O.; Schworer, S.A.; Johnson, S.M.; Smith, B.D.; Googe, P.B.; Wu, E.Y. ANCA vasculitis expands the spectrum of autoimmune manifestations of activated PI3 kinase δ syndrome. Front. Pediatr. 2023, 11, 1179788. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Gu, W.; Sheng, Y.; Wang, J.; Xu, X. Case Report: Activating PIK3CD Mutation in Patients Presenting With Granulomatosis With Polyangiitis. Front. Immunol. 2021, 12, 670312. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhu, K.; Jin, Y.; Fu, H.; Mao, J. Activated phosphoinositide 3-kinase delta syndrome misdiagnosed as anti-neutrophil cytoplasmic antibody-associated vasculitis: A case report. J. Int. Med. Res. 2021, 49, 3000605211013222. [Google Scholar] [CrossRef]
- Scott, J.; Hartnett, J.; Mockler, D.; Little, M.A. Environmental risk factors associated with ANCA associated vasculitis: A systematic mapping review. Autoimmun. Rev. 2020, 19, 102660. [Google Scholar] [CrossRef]
- Ciavatta, D.J.; Yang, J.; Preston, G.A.; Badhwar, A.K.; Xiao, H.; Hewins, P.; Nester, C.M.; Pendergraft, W.F., 3rd; Magnuson, T.R.; Jennette, J.C.; et al. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J. Clin. Investig. 2010, 120, 3209–3219. [Google Scholar] [CrossRef]
- Yang, J.; Ge, H.; Poulton, C.J.; Hogan, S.L.; Hu, Y.; Jones, B.E.; Henderson, C.D.; McInnis, E.A.; Pendergraft, W.F., 3rd; Jennette, J.C.; et al. Histone modification signature at myeloperoxidase and proteinase 3 in patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Clin. Epigenetics 2016, 8, 85. [Google Scholar] [CrossRef]
- Jones, B.E.; Yang, J.; Muthigi, A.; Hogan, S.L.; Hu, Y.; Starmer, J.; Henderson, C.D.; Poulton, C.J.; Brant, E.J.; Pendergraft, W.F., 3rd; et al. Gene-Specific DNA Methylation Changes Predict Remission in Patients with ANCA-Associated Vasculitis. J. Am. Soc. Nephrol. 2017, 28, 1175–1187. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J. B cell-mediated pathogenesis of ANCA-mediated vasculitis. Semin. Immunopathol. 2014, 36, 327–338. [Google Scholar] [CrossRef]
- Kronbichler, A.; Lee, K.H.; Denicolò, S.; Choi, D.; Lee, H.; Ahn, D.; Kim, K.H.; Lee, J.H.; Kim, H.; Hwang, M.; et al. Immunopathogenesis of ANCA-Associated Vasculitis. Int. J. Mol. Sci. 2020, 21, 7319. [Google Scholar] [CrossRef] [PubMed]
- Sangaletti, S.; Tripodo, C.; Chiodoni, C.; Guarnotta, C.; Cappetti, B.; Casalini, P.; Piconese, S.; Parenza, M.; Guiducci, C.; Vitali, C.; et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 2012, 120, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, D.; Tomaru, U.; Ishizu, A. Possible implication of disordered neutrophil extracellular traps in the pathogenesis of MPO-ANCA-associated vasculitis. Clin. Exp. Nephrol. 2013, 17, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Manenti, L.; Vaglio, A.; Gnappi, E.; Maggiore, U.; Allegri, L.; Allinovi, M.; Urban, M.L.; Delsante, M.; Galetti, M.; Nicastro, M.; et al. Association of Serum C3 Concentration and Histologic Signs of Thrombotic Microangiopathy with Outcomes among Patients with ANCA-Associated Renal Vasculitis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2143–2151. [Google Scholar] [CrossRef]
- Berden, A.E.; Jones, R.B.; Erasmus, D.D.; Walsh, M.; Noël, L.H.; Ferrario, F.; Waldherr, R.; Bruijn, J.A.; Jayne, D.R.; Bajema, I.M. Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J. Am. Soc. Nephrol. 2012, 23, 313–321. [Google Scholar] [CrossRef]
- Kanzaki, G.; Nagasaka, S.; Higo, S.; Kajimoto, Y.; Kanemitsu, T.; Aoki, M.; Nagahama, K.; Natori, Y.; Tsuboi, N.; Yokoo, T.; et al. Impact of anti-glomerular basement membrane antibodies and glomerular neutrophil activation on glomerulonephritis in experimental myeloperoxidase-antineutrophil cytoplasmic antibody vasculitis. Nephrol. Dial. Transplant. 2016, 31, 574–585. [Google Scholar] [CrossRef]
- Horn, M.P.; Peter, A.M.; Righini Grunder, F.; Leichtle, A.B.; Spalinger, J.; Schibli, S.; Sokollik, C. PR3-ANCA and panel diagnostics in pediatric inflammatory bowel disease to distinguish ulcerative colitis from Crohn’s disease. PLoS ONE 2018, 13, e0208974. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Morishita, K.A.; Wagner-Weiner, L.; Yen, E.Y.; Sivaraman, V.; James, K.E.; Gerstbacher, D.; Szymanski, A.M.; O’Neil, K.M.; Cabral, D.A.; Childhood, A.; et al. Consensus Treatment Plans for Severe Pediatric Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Care Res. 2022, 74, 1550–1558. [Google Scholar] [CrossRef]
- Westwell-Roper, C.; Lubieniecka, J.M.; Brown, K.L.; Morishita, K.A.; Mammen, C.; Wagner-Weiner, L.; Yen, E.; Li, S.C.; O’Neil, K.M.; Lapidus, S.K.; et al. Clinical practice variation and need for pediatric-specific treatment guidelines among rheumatologists caring for children with ANCA-associated vasculitis: An international clinician survey. Pediatr. Rheumatol. Online J. 2017, 15, 61. [Google Scholar] [CrossRef]
- Chung, S.A.; Langford, C.A.; Maz, M.; Abril, A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2021, 73, 1366–1383. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes, A.V.W.G. KDIGO 2024 Clinical Practice Guideline for the Management of Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis. Kidney Int. 2024, 105, S71–S116. [Google Scholar] [CrossRef]
- Jayne, D.; Walsh, M.; Merkel, P.A.; Peh, C.A.; Szpirt, W.; Puechal, X.; Fujimoto, S.; Hawley, C.; Khalidi, N.; Jones, R.; et al. Plasma exchange and glucocorticoids to delay death or end-stage renal disease in anti-neutrophil cytoplasm antibody-associated vasculitis: PEXIVAS non-inferiority factorial RCT. Health Technol. Assess. 2022, 26, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Brogan, P.; Yeung, R.S.M.; Cleary, G.; Rangaraj, S.; Kasapcopur, O.; Hersh, A.O.; Li, S.; Paripovic, D.; Schikler, K.; Zeft, A.; et al. Phase IIa Global Study Evaluating Rituximab for the Treatment of Pediatric Patients With Granulomatosis With Polyangiitis or Microscopic Polyangiitis. Arthritis Rheumatol. 2022, 74, 124–133. [Google Scholar] [CrossRef]
- Delestre, F.; Charles, P.; Karras, A.; Pagnoux, C.; Neel, A.; Cohen, P.; Aumaitre, O.; Faguer, S.; Gobert, P.; Maurier, F.; et al. Rituximab as maintenance therapy for ANCA-associated vasculitides: Pooled analysis and long-term outcome of 277 patients included in the MAINRITSAN trials. Ann. Rheum. Dis. 2024, 83, 233–241. [Google Scholar] [CrossRef]
- Mendel, A.; Ennis, D.; Go, E.; Bakowsky, V.; Baldwin, C.; Benseler, S.M.; Cabral, D.A.; Carette, S.; Clements-Baker, M.; Clifford, A.H.; et al. CanVasc Consensus Recommendations for the Management of Antineutrophil Cytoplasm Antibody-associated Vasculitis: 2020 Update. J. Rheumatol. 2021, 48, 555–566. [Google Scholar] [CrossRef]
- O’Neil, E.R.; Quinn, R.E.; Olson, T.L.; Rycus, P.T.; Anders, M.M.; Chartan, C.A.; Vogel, T.P.; Silva-Carmona, M.; Coleman, R.D. Extracorporeal Membrane Oxygenation Support for Antineutrophil Cytoplasmic Antibody-associated Vasculitides: An ELSO Registry Analysis. ASAIO J. 2022, 68, 553–560. [Google Scholar] [CrossRef]
- Turgeon, D.; Bakowsky, V.; Baldwin, C.; Cabral, D.A.; Clements-Baker, M.; Clifford, A.; Cohen Tervaert, J.W.; Dehghan, N.; Ennis, D.; Famorca, L.; et al. CanVasc consensus recommendations for the use of avacopan in antineutrophil cytoplasm antibody-associated vasculitis: 2022 addendum. Rheumatology 2023, 62, 2646–2651. [Google Scholar] [CrossRef]
- Jayne, D.R.W.; Merkel, P.A.; Schall, T.J.; Bekker, P.; Group, A.S. Avacopan for the Treatment of ANCA-Associated Vasculitis. N. Engl. J. Med. 2021, 384, 599–609. [Google Scholar] [CrossRef]
- Chalkia, A.; Jayne, D. ANCA-associated vasculitis-treatment standard. Nephrol. Dial. Transplant. 2024, 39, 944–955. [Google Scholar] [CrossRef]
- Hellmich, B.; Sanchez-Alamo, B.; Schirmer, J.H.; Berti, A.; Blockmans, D.; Cid, M.C.; Holle, J.U.; Hollinger, N.; Karadag, O.; Kronbichler, A.; et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann. Rheum. Dis. 2024, 83, 30–47. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.E.; Gopaluni, S.; Wason, J.; Henderson, R.B.; Van Maurik, A.; Savage, C.C.O.; Pusey, C.D.; Salama, A.D.; Lyons, P.A.; Lee, J.; et al. A randomised study of rituximab and belimumab sequential therapy in PR3 ANCA-associated vasculitis (COMBIVAS): Design of the study protocol. Trials 2023, 24, 180. [Google Scholar] [CrossRef] [PubMed]
- Genga, E.; Oyoo, O.; Adebajo, A. Vasculitis in Africa. Curr. Rheumatol. Rep. 2018, 20, 4. [Google Scholar] [CrossRef] [PubMed]
| Genes/ Polymorphism | Geographical Area | Type of AAV | OR | Ref. |
|---|---|---|---|---|
| HLA-DPB1*04:01 | European | GPA, Unclassified, MPA | 3.5 | Gibson et al., 2023 [54] |
| ASB3 (Ankyrin Repeat And SOCS Box Containing 3) | European | GPA, Unclassified, MPA | 2.9 | Gibson et al., 2023 [54] |
| LINC02147 (long intergenic non-protein coding RNA 2147) | European | GPA, Unclassified, MPA | 5.7 | Gibson et al., 2023 [54] |
| ADNP (activity-dependent neuroprotector homeobox) | European | GPA, Unclassified, MPA | 6.6 | Gibson et al., 2023 [54] |
| Genes | Clinical Features | Paediatric Cases | References |
|---|---|---|---|
| COPA (coatomer protein subunit alpha) | Inflammatory lung disease, arthritis, renal disease | 5-year-old girl with maternally inherited COPA variant c.679C>T (p.Arg227Cys): arthritis, AAV, progressive renal failure, minimal lung involvement | Zheng Y. et al., 2024 [98] |
| ELANE (Elastase, Neutrophil Expressed) | Severe congenital neutropenia, cyclic neutropenia, and rarely autoimmune manifestations |
| Zhang D. et al., 2023 [99] |
| STING1 (Stimulator of Interferon Response CGAMP Interactor 1) or TMEM173 (transmembrane protein 173) | SAVI (STING-associated vasculopathy with onset in infancy)—type I interferonopathy caused by dominant gain-of-function mutations in STING1. Early onset systemic inflammation with cutaneous vasculitis and interstitial lung disease |
| Ochfeld E. et al., 2021 [100] Liu Y. et al., 2014 [101] Staels F. et al., 2020 [102] |
| APDS Activated phosphoinositide 3-kinase δ syndrome (APDS) 1 or 2 caused by gain/loss of function mutations in PIK3CD or PIK3R1 genes, respectively | primary immunodeficiency causing recurrent infections (particularly sinopulmonary and herpes), immune dysregulation, lymphoproliferation, autoimmunity and malignancy |
| Sood A.K. et al., 2023 [103] Lu M. et al., 2021 [104] Zhang X. et al., 2021 [105] |
| Early onset in childhood |
| Family history of relatives with childhood-onset autoimmune conditions |
| Acutely unwell |
| Recurrent infections or association with lymphoproliferative conditions |
| Systemic inflammation associated with severe skin and internal organ manifestations |
| Laboratory tests suggestive of immunodeficiency and/or immune dysregulation: e.g., T, B, or NK cytopenias, neutropaenia, low complement levels or immunoglobulins |
| Overlapping clinical phenotypes |
| Refractory to treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, L.; Naheed, A.; Richards, C.; Ciurtin, C. Childhood-Onset ANCA-Associated Vasculitis: From Genetic Studies to Advances in Pathogenesis, Classification and Novel Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 13704. https://doi.org/10.3390/ijms252413704
Yeo L, Naheed A, Richards C, Ciurtin C. Childhood-Onset ANCA-Associated Vasculitis: From Genetic Studies to Advances in Pathogenesis, Classification and Novel Therapeutic Approaches. International Journal of Molecular Sciences. 2024; 25(24):13704. https://doi.org/10.3390/ijms252413704
Chicago/Turabian StyleYeo, Liching, Asma Naheed, Chantelle Richards, and Coziana Ciurtin. 2024. "Childhood-Onset ANCA-Associated Vasculitis: From Genetic Studies to Advances in Pathogenesis, Classification and Novel Therapeutic Approaches" International Journal of Molecular Sciences 25, no. 24: 13704. https://doi.org/10.3390/ijms252413704
APA StyleYeo, L., Naheed, A., Richards, C., & Ciurtin, C. (2024). Childhood-Onset ANCA-Associated Vasculitis: From Genetic Studies to Advances in Pathogenesis, Classification and Novel Therapeutic Approaches. International Journal of Molecular Sciences, 25(24), 13704. https://doi.org/10.3390/ijms252413704





