Tough Hydrogels with Different Toughening Mechanisms and Applications
Abstract
:1. Introduction
2. Strategies and Properties of Tough Hydrogels
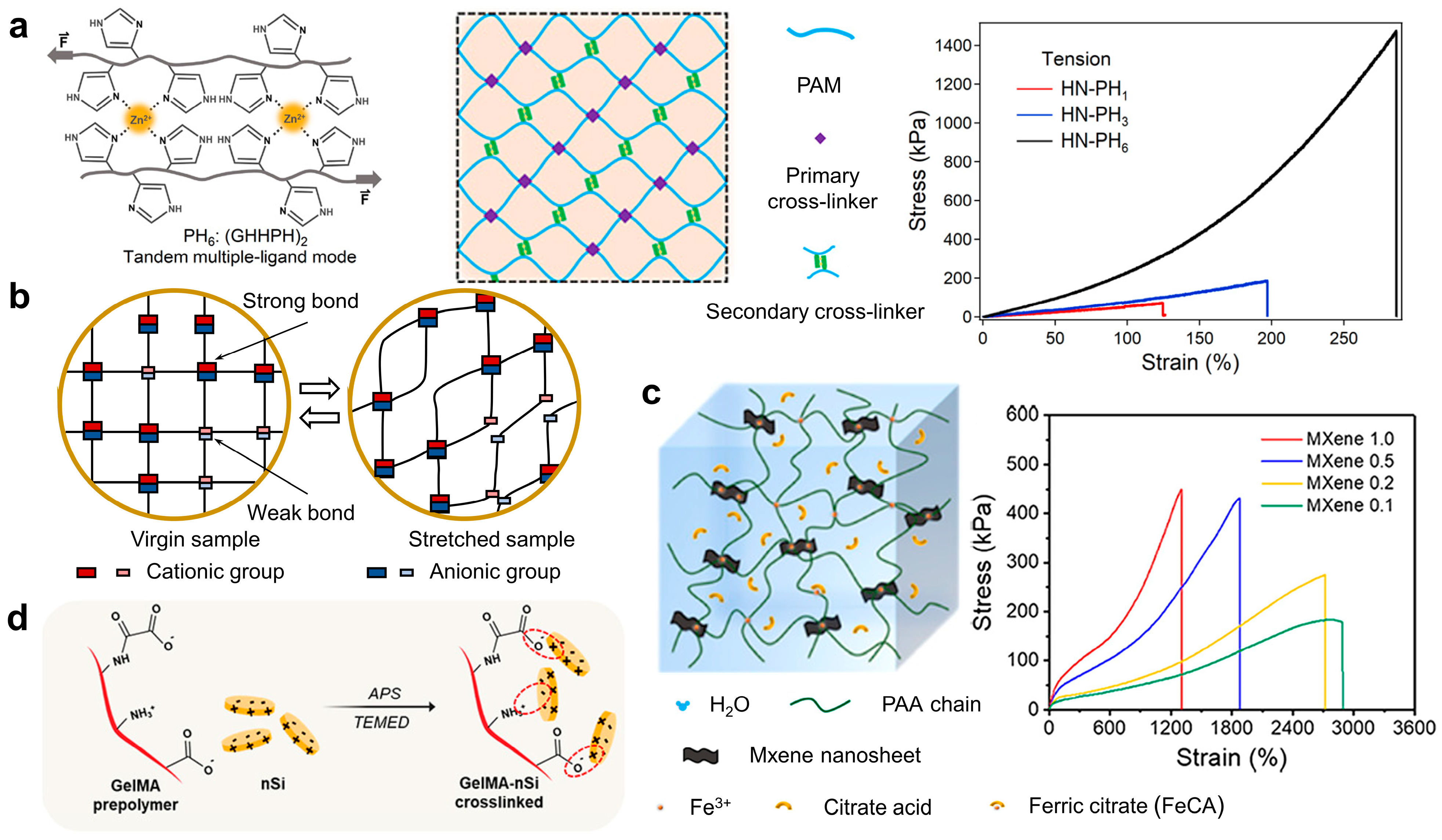
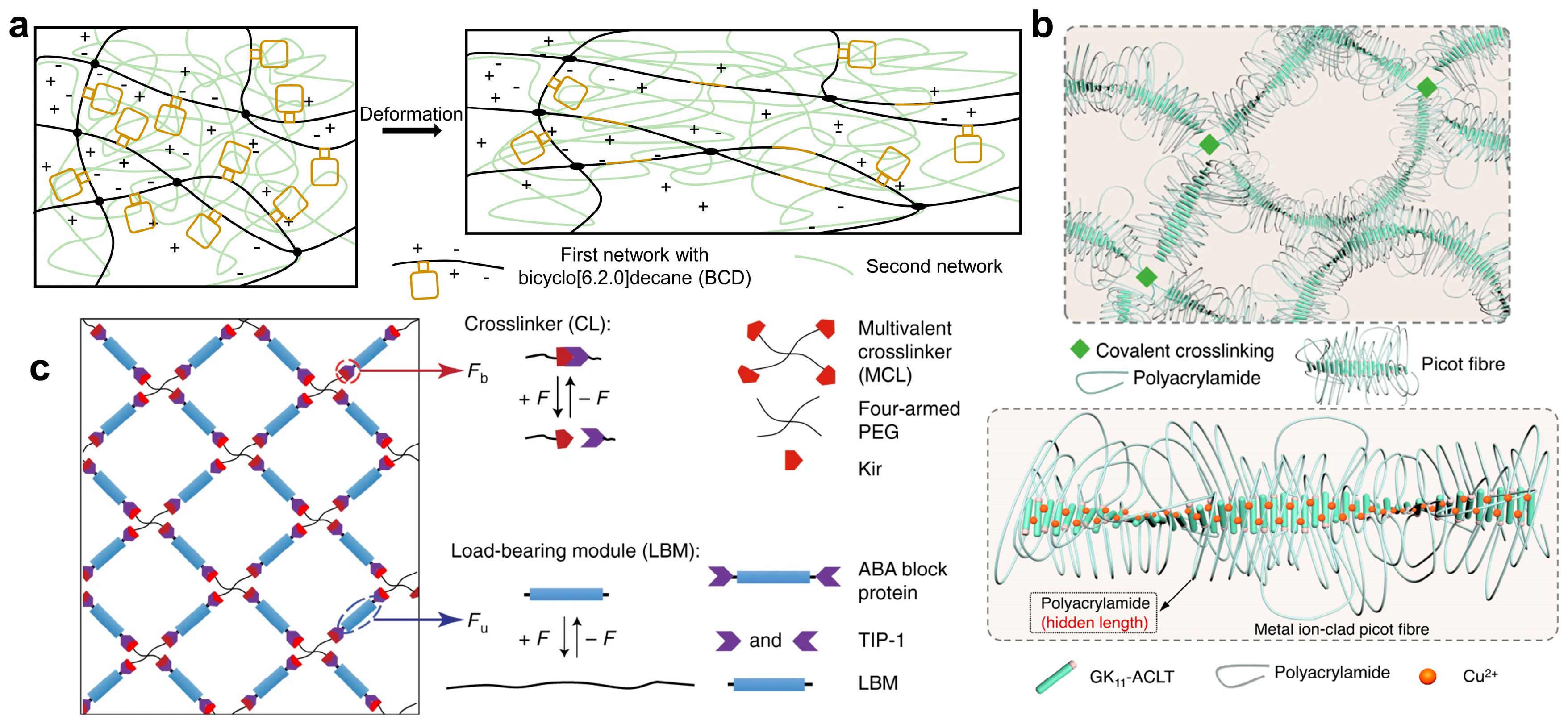
2.1. Toughening Hydrogel with Sacrificial Bonds
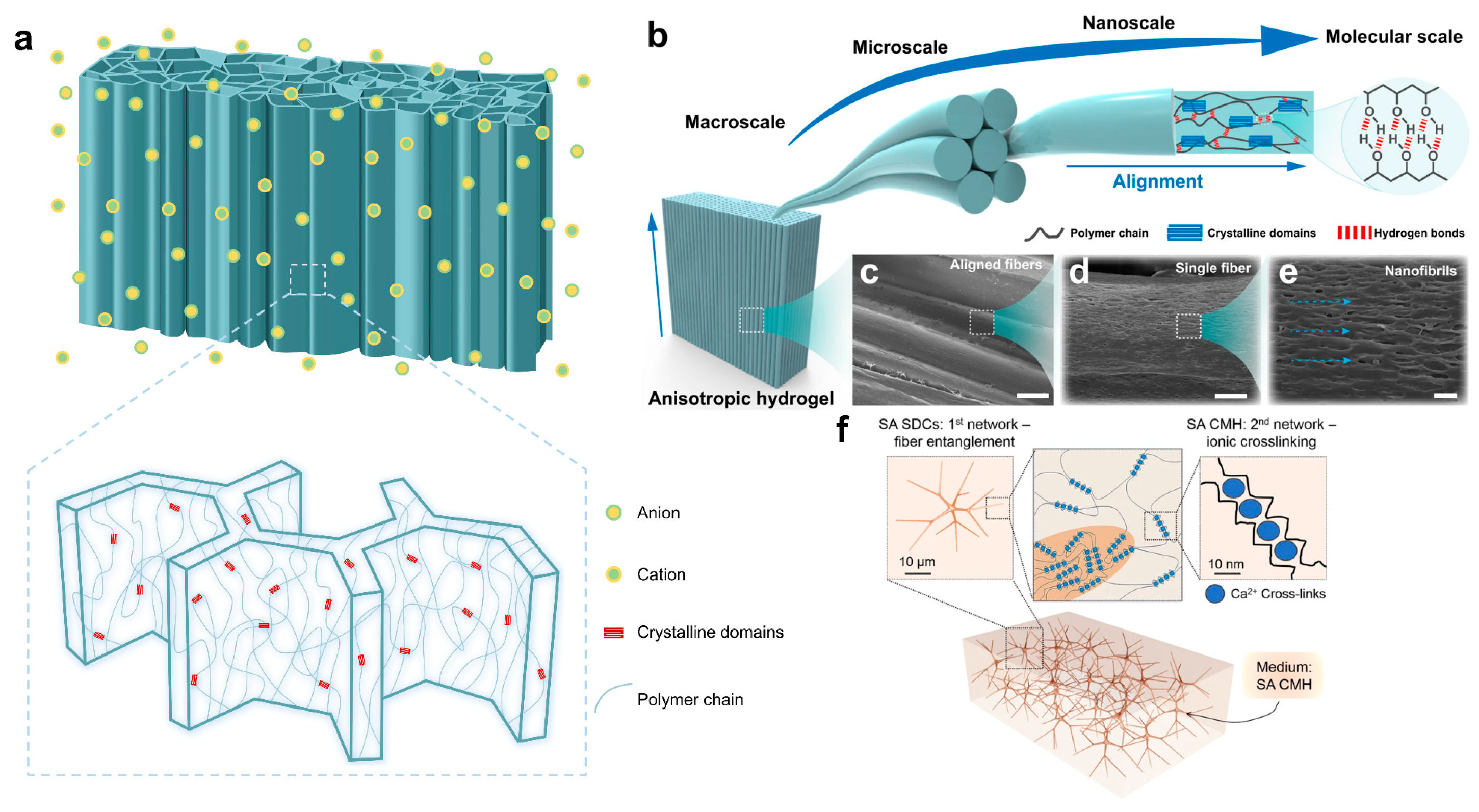
2.2. Toughening Hydrogel with Hierarchical Architecture
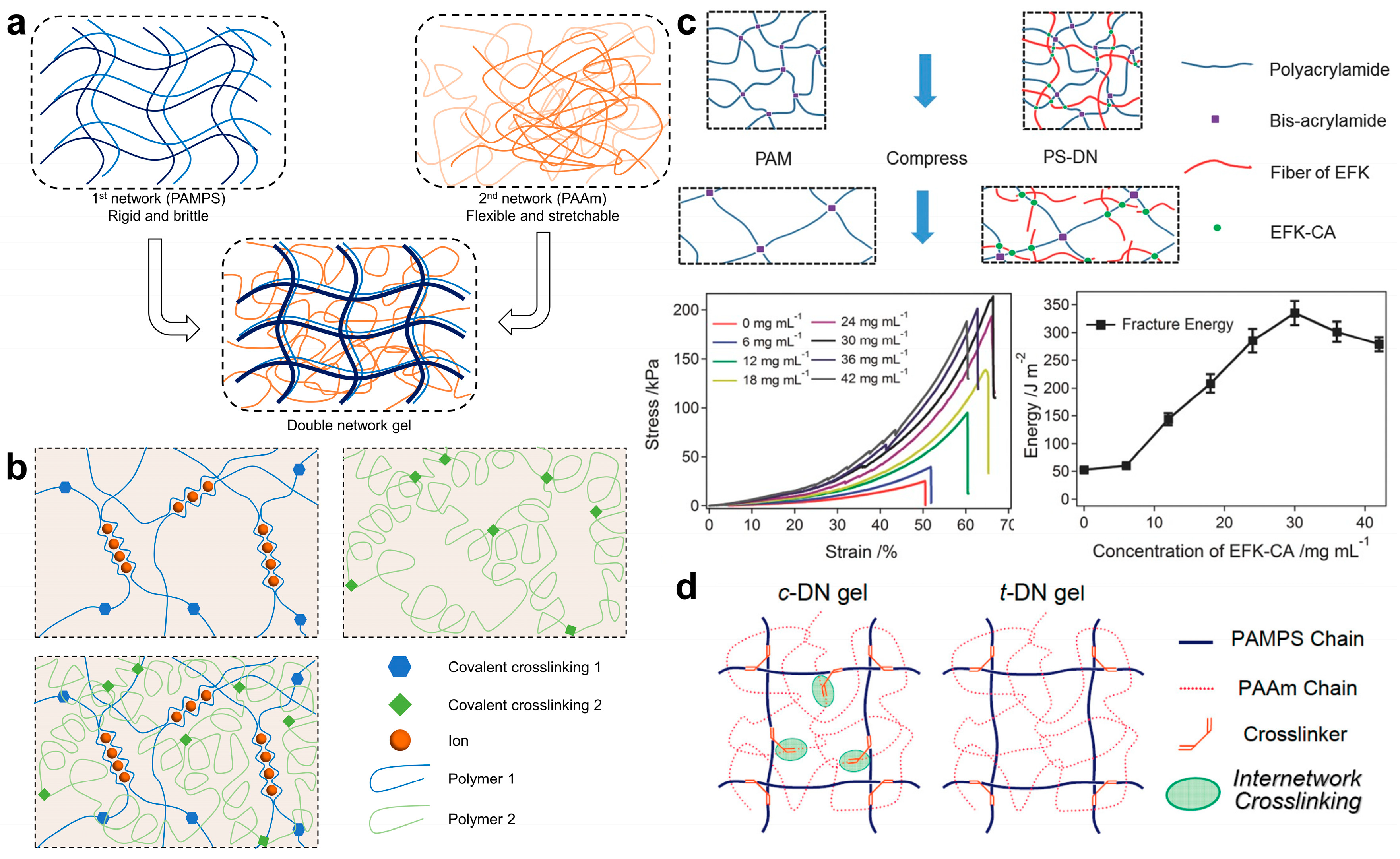
2.3. Toughening Hydrogel with Network Topology
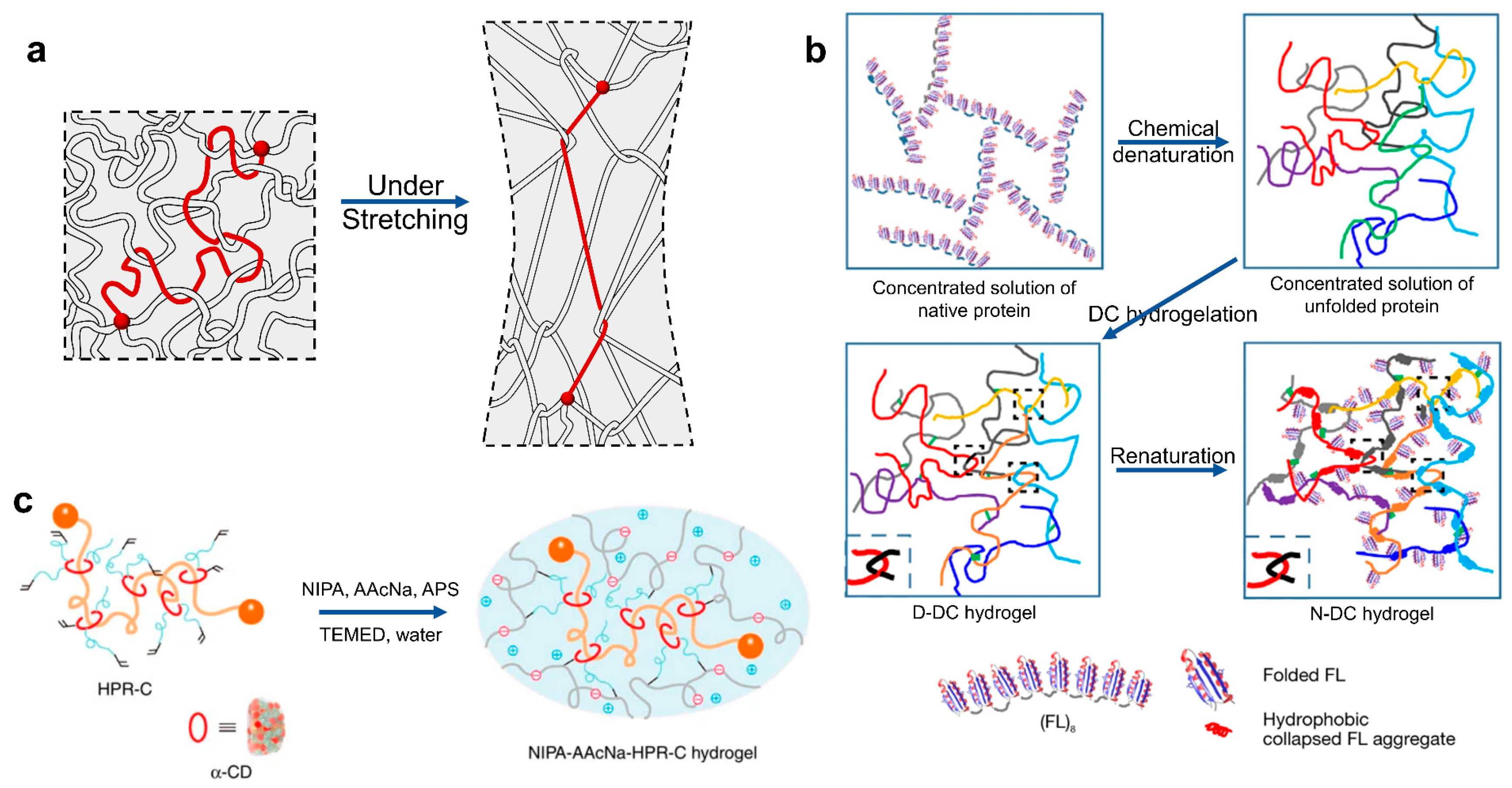
2.4. Toughening Hydrogel with Force-Triggered Length Release
3. Applications of Tough Hydrogels
3.1. Tissue Engineering
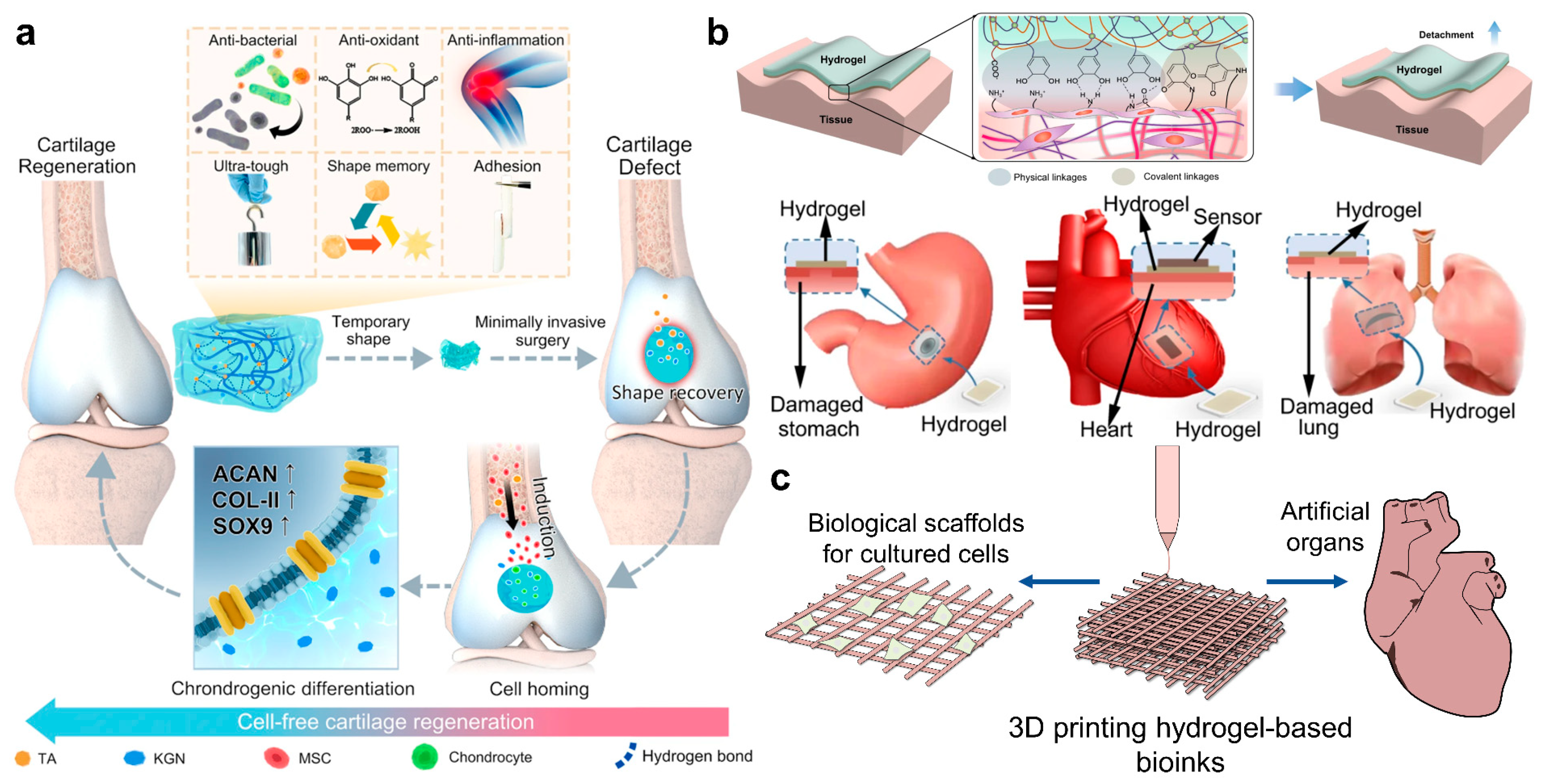
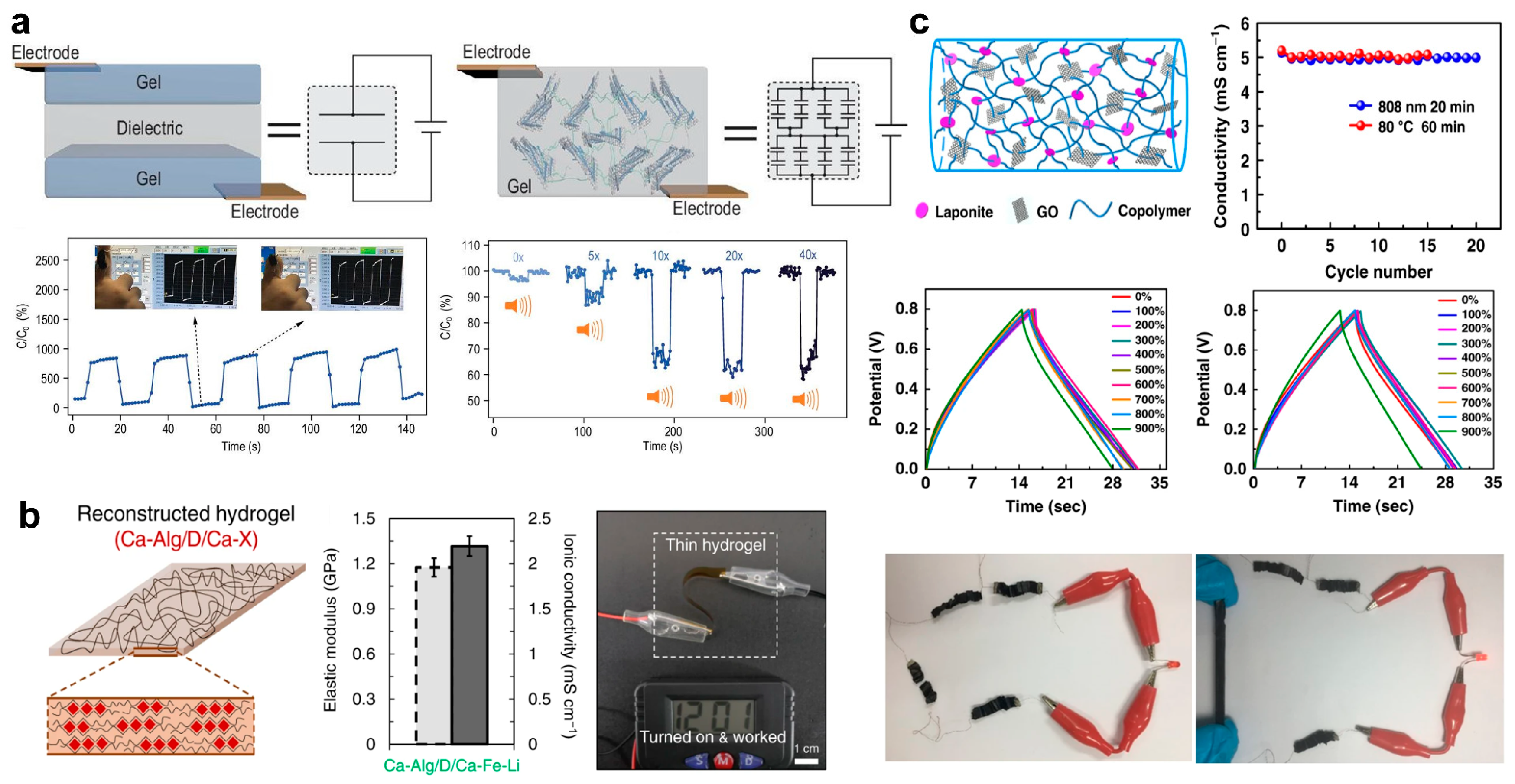
3.2. Flexible Electronics
4. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and Properties of Silk Hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef]
- Griffith, L.G.; Naughton, G. Tissue Engineering–Current Challenges and Expanding Opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, K.; Suo, Z. Photodetachable Adhesion. Adv. Mater. 2019, 31, 1806948. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, A.; Qin, M.; Huang, R.; Zhang, G.; Xue, B.; Wei, J.; Li, Y.; Cao, Y.; Wang, W. Hierarchical construction of a mechanically stable peptide–graphene oxide hybrid hydrogel for drug delivery and pulsatile triggered release in vivo. Nanoscale 2015, 7, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Bassil, M.; Davenas, J.; El Tahchi, M. Electrochemical properties and actuation mechanisms of polyacrylamide hydrogel for artificial muscle application. Sens. Actuators B Chem. 2008, 134, 496–501. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Qian, K.; Chen, J.; Li, S.; Lee, P.S. Extremely Stretchable Strain Sensors Based on Conductive Self-Healing Dynamic Cross-Links Hydrogels for Human-Motion Detection. Adv. Sci. 2017, 4, 1600190. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, F.; Cai, P.; Wang, M.; He, K.; Luo, Y.; Li, Z.; Chen, G.; Ji, S.; Liu, Z.; et al. Mechanically Interlocked Hydrogel–Elastomer Hybrids for On-Skin Electronics. Adv. Funct. Mater. 2020, 30, 1909540. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Banerjee, H.; Suhail, M.; Ren, H. Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef]
- Banerjee, H.; Ren, H. Optimizing Double-Network Hydrogel for Biomedical Soft Robots. Soft Robot. 2017, 4, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ware, T.; Simon, D.; Rennaker, R.L.; Voit, W. Smart Polymers for Neural Interfaces. Polym. Rev. 2013, 53, 108–129. [Google Scholar] [CrossRef]
- Guo, J.; Liu, M.; Zehnder, A.T.; Zhao, J.; Narita, T.; Creton, C.; Hui, C.-Y. Fracture mechanics of a self-healing hydrogel with covalent and physical crosslinks: A numerical study. J. Mech. Phys. Solids 2018, 120, 79–95. [Google Scholar] [CrossRef]
- Lucantonio, A.; Noselli, G.; Trepat, X.; DeSimone, A.; Arroyo, M. Hydraulic Fracture and Toughening of a Brittle Layer Bonded to a Hydrogel. Phys. Rev. Lett. 2015, 115, 188105. [Google Scholar] [CrossRef]
- Li, X.; Gong, J.P. Role of dynamic bonds on fatigue threshold of tough hydrogels. Proc. Natl. Acad. Sci. USA 2022, 119, e2200678119. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.-K.; Zhong, M.; Zhang, L.-Q.; Liu, X.-Y.; Xie, X.-M. Robust and self-healable nanocomposite physical hydrogel facilitated by the synergy of ternary crosslinking points in a single network. J. Mater. Chem. B 2016, 4, 6221–6227. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X.; Shao, Y.; Chen, P.; Xing, Y.; Yang, Z.; Li, Z.; Liu, D. A supramolecular hydrogel with identical cross-linking point density but distinctive rheological properties. Mater. Chem. Front. 2017, 1, 654–659. [Google Scholar] [CrossRef]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U.-I. Design and Fabrication of a High-Strength Hydrogel with Ideally Homogeneous Network Structure from Tetrahedron-like Macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]
- Pan, W.; Wen, H.; Niu, L.; Su, C.; Liu, C.; Zhao, J.; Mao, C.; Liang, D. Effects of chain flexibility on the properties of DNA hydrogels. Soft Matter 2016, 12, 5537–5541. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Q.; Hu, R.; Wang, H.; Newby, B.-M.Z.; Chang, Y.; Zheng, J. Mechanically strong hybrid double network hydrogels with antifouling properties. J. Mater. Chem. B 2015, 3, 5426–5435. [Google Scholar] [CrossRef]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High Mechanical Strength Double-Network Hydrogel with Bacterial Cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Creton, C. 50th Anniversary Perspective: Networks and Gels: Soft but Dynamic and Tough. Macromolecules 2017, 50, 8297–8316. [Google Scholar] [CrossRef]
- Shao, C.; Chang, H.; Wang, M.; Xu, F.; Yang, J. High-Strength, Tough, and Self-Healing Nanocomposite Physical Hydrogels Based on the Synergistic Effects of Dynamic Hydrogen Bond and Dual Coordination Bonds. ACS Appl. Mater. Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, G.; Jiang, H.; Li, F.; Liu, H.; Xia, Y.; Zhang, Y.; Xin, F.; Zhang, X.; Li, H. Synergistic toughening of nanocomposite hydrogel based on ultrasmall aluminum hydroxide nanoparticles and hydroxyapatite nanoparticles. Polym. Compos. 2019, 40, 942–951. [Google Scholar] [CrossRef]
- Ishii, S.; Kokubo, H.; Hashimoto, K.; Imaizumi, S.; Watanabe, M. Tetra-PEG Network Containing Ionic Liquid Synthesized via Michael Addition Reaction and Its Application to Polymer Actuator. Macromolecules 2017, 50, 2906–2915. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Liu, C.; Jiang, L.; Lin, J.; Qian, J.; Mayumi, K.; Wu, Z.L.; Ito, K.; Zheng, Q. Slide-Ring Cross-Links Mediated Tough Metallosupramolecular Hydrogels with Superior Self-Recoverability. Macromolecules 2019, 52, 6748–6755. [Google Scholar] [CrossRef]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Charaya, H.; Liu, L.; Li, X. Tough Hydrogels: Toughening Mechanisms and Their Utilization in Stretchable Electronics and in Regenerative Medicines. In Hybrid Organic-Inorganic Interfaces; John Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 535–580. [Google Scholar]
- Kuang, X.; Arıcan, M.O.; Zhou, T.; Zhao, X.; Zhang, Y.S. Functional Tough Hydrogels: Design, Processing, and Biomedical Applications. Acc. Mater. Res. 2023, 4, 101–114. [Google Scholar] [CrossRef]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, W.; Wu, W.-H.; Xue, B.; Xiang, D.; Li, Y.; Qin, M.; Sun, F.; Wang, W.; Zhang, W.-B.; et al. Reversible hydrogels with tunable mechanical properties for optically controlling cell migration. Nano Res. 2018, 11, 5556–5565. [Google Scholar] [CrossRef]
- Xiang, D.; Wu, X.; Cao, W.; Xue, B.; Qin, M.; Cao, Y.; Wang, W. Hydrogels With Tunable Mechanical Properties Based on Photocleavable Proteins. Front. Chem. 2020, 8, 7. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, T.; Wang, X.; Yu, B.; Zhou, F. Freezing Molecular Orientation under Stretch for High Mechanical Strength but Anisotropic Hydrogels. Small 2016, 12, 4386–4392. [Google Scholar] [CrossRef]
- Wu, Q.; Wei, J.; Xu, B.; Liu, X.; Wang, H.; Wang, W.; Wang, Q.; Liu, W. A robust, highly stretchable supramolecular polymer conductive hydrogel with self-healability and thermo-processability. Sci. Rep. 2017, 7, 41566. [Google Scholar] [CrossRef]
- Lian, W.Z.; Fan, Z.W.; Cui, K.; Yin, P.; Yang, J.; Jiang, H.; Tang, L.; Sun, T. Tough Hydrogels with Dynamic H-Bonds: Structural Heterogeneities and Mechanical Performances. Macromolecules 2021, 54, 8996–9006. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, X.N.; Song, Y.; Zhao, Y.; Chen, L.; Su, F.; Li, L.; Wu, Z.L.; Zheng, Q. Ultrastiff and Tough Supramolecular Hydrogels with a Dense and Robust Hydrogen Bond Network. Chem. Mater. 2019, 31, 1430–1440. [Google Scholar] [CrossRef]
- Sun, W.; Xue, B.; Fan, Q.; Tao, R.; Wang, C.; Wang, X.; Li, Y.; Qin, M.; Wang, W.; Chen, B.; et al. Molecular engineering of metal coordination interactions for strong, tough, and fast-recovery hydrogels. Sci. Adv. 2020, 6, eaaz9531. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef]
- Hu, X.; Vatankhah-Varnoosfaderani, M.; Zhou, J.; Li, Q.; Sheiko, S.S. Weak Hydrogen Bonding Enables Hard, Strong, Tough, and Elastic Hydrogels. Adv. Mater. 2015, 27, 6899–6905. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Y.; Gao, L.; Bai, T.; Wang, W.; Cui, Y.; Liu, W. A Mechanically Strong, Highly Stable, Thermoplastic, and Self-Healable Supramolecular Polymer Hydrogel. Adv. Mater. 2015, 27, 3566–3571. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Li, Y.; Fang, X.; Sun, J. Salt-Mediated Polyampholyte Hydrogels with High Mechanical Strength, Excellent Self-Healing Property, and Satisfactory Electrical Conductivity. Adv. Funct. Mater. 2018, 28, 1804416. [Google Scholar] [CrossRef]
- Xue, B.; Qin, M.; Wang, T.; Wu, J.; Luo, D.; Jiang, Q.; Li, Y.; Cao, Y.; Wang, W. Electrically Controllable Actuators Based on Supramolecular Peptide Hydrogels. Adv. Funct. Mater. 2016, 26, 9053–9062. [Google Scholar] [CrossRef]
- Xia, Y.; Xue, B.; Qin, M.; Cao, Y.; Li, Y.; Wang, W. Printable Fluorescent Hydrogels Based on Self-Assembling Peptides. Sci. Rep. 2017, 7, 9691. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Liu, Z.Q.; Xu, F.; Zhou, J.X.; Zrínyi, M.; Osada, Y.; Chen, Y.M. Novel Biocompatible Polysaccharide-Based Self-Healing Hydrogel. Adv. Funct. Mater. 2015, 25, 1352–1359. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel Mussel-Inspired Injectable Self-Healing Hydrogel with Anti-Biofouling Property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Yu, Z.; Li, N.; Abell, C.; Scherman, O.A. Tough Supramolecular Polymer Networks with Extreme Stretchability and Fast Room-Temperature Self-Healing. Adv. Mater. 2017, 29, 1605325. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, J.; Liu, Y.; Xie, X.-M. Super Tough and Intelligent Multibond Network Physical Hydrogels Facilitated by Ti3C2Tx MXene Nanosheets. ACS Nano 2022, 16, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Pacelli, S.; Alexander, S.; Huayamares, S.; Rosenkrans, Z.; Vergel, F.E.; Wu, Y.; Chakravorty, A.; Paul, A. Nanoparticle-Reinforced Tough Hydrogel as a Versatile Platform for Pharmaceutical Drug Delivery: Preparation and in Vitro Characterization. Mol. Pharm. 2023, 20, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zhang, D.; Tang, Z.; Wu, Q.; Wang, Q. HRP-mediated polymerization forms tough nanocomposite hydrogels with high biocatalytic performance. Chem. Commun. 2013, 49, 8033–8035. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Li, Z.; Hou, Z.; Fan, H.; Li, H. Organic–Inorganic Hierarchical Self-Assembly into Robust Luminescent Supramolecular Hydrogel. Adv. Funct. Mater. 2017, 27, 1604379. [Google Scholar] [CrossRef]
- Sun, G.; Li, Z.; Liang, R.; Weng, L.-T.; Zhang, L. Super stretchable hydrogel achieved by non-aggregated spherulites with diameters <5 nm. Nat. Commun. 2016, 7, 12095. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, X.; Li, L.; Li, Q.; Zheng, S.; Liu, Z.; Li, W.; Yan, F. Low-Hysteresis and High-Toughness Hydrogels Regulated by Porous Cationic Polymers: The Effect of Counteranions. Angew. Chem. Int. Ed. 2024, 63, e202316375. [Google Scholar] [CrossRef] [PubMed]
- Schütt, F.; Signetti, S.; Krüger, H.; Röder, S.; Smazna, D.; Kaps, S.; Gorb, S.N.; Mishra, Y.K.; Pugno, N.M.; Adelung, R. Hierarchical self-entangled carbon nanotube tube networks. Nat. Commun. 2017, 8, 1215. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Rehman, H.U.; Chen, Y.; Guo, Y.; Du, Q.; Zhou, J.; Guo, Y.; Duan, H.; Li, H.; Liu, H. Stretchable, strong and self-healing hydrogel by oxidized CNT-polymer composite. Compos. Part A Appl. Sci. Manuf. 2016, 90, 250–260. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic–Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-swelling Properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef]
- Luo, C.; Wang, W.; Yang, W.; Liu, X.; Lin, J.; Zhang, L.; He, S. High-Strength and Multi-Recyclable Epoxy Vitrimer Containing Dual-Dynamic Covalent Bonds Based on the Disulfide and Imine Bond Metathesis. ACS Sustain. Chem. Eng. 2023, 11, 14591–14600. [Google Scholar] [CrossRef]
- Hammer, L.; Van Zee, N.J.; Nicolaÿ, R. Dually Crosslinked Polymer Networks Incorporating Dynamic Covalent Bonds. Polymers 2021, 13, 396. [Google Scholar] [CrossRef]
- Bui, K.; Nguyen, G.T.; Vancaeyzeele, C.; Vidal, F.; Hu, X.; Wan, C.; Plesse, C. Stretchable, healable, and weldable vitrimer ionogel for ionotronic applications. Chem. Eng. J. 2023, 474, 145533. [Google Scholar] [CrossRef]
- Gong, J.P. Materials both Tough and Soft. Science 2014, 344, 161–162. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Chen, H.; Yan, H.; Huang, L.; Yang, J.; Zheng, J. A Novel Design Strategy for Fully Physically Linked Double Network Hydrogels with Tough, Fatigue Resistant, and Self-Healing Properties. Adv. Funct. Mater. 2015, 25, 1598–1607. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef]
- Sun, W.; Xue, B.; Li, Y.; Qin, M.; Wu, J.; Lu, K.; Wu, J.; Cao, Y.; Jiang, Q.; Wang, W. Polymer-Supramolecular Polymer Double-Network Hydrogel. Adv. Funct. Mater. 2016, 26, 9044–9052. [Google Scholar] [CrossRef]
- Nakajima, T.; Furukawa, H.; Tanaka, Y.; Kurokawa, T.; Osada, Y.; Gong, J.P. True chemical structure of double network hydrogels. Macromolecules 2009, 42, 2184–2189. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Wang, L.; Wu, D. Highly Elastic and Ultratough Hybrid Ionic–Covalent Hydrogels with Tunable Structures and Mechanics. Adv. Mater. 2018, 30, 1707071. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Simon, J.R.; Ghoorchian, A.; Scholl, Z.; Lin, S.; Rubinstein, M.; Marszalek, P.; Chilkoti, A.; López, G.P.; Zhao, X. Strong, Tough, Stretchable, and Self-Adhesive Hydrogels from Intrinsically Unstructured Proteins. Adv. Mater. 2017, 29, 1604743. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, J.; Daniel, W.F.M.; Vatankhah-Varnoosfaderani, M.; Dobrynin, A.V.; Sheiko, S.S. Dynamics of Dual Networks: Strain Rate and Temperature Effects in Hydrogels with Reversible H-Bonds. Macromolecules 2017, 50, 652–659. [Google Scholar] [CrossRef]
- Lin, P.; Ma, S.; Wang, X.; Zhou, F. Molecularly Engineered Dual-Crosslinked Hydrogel with Ultrahigh Mechanical Strength, Toughness, and Good Self-Recovery. Adv. Mater. 2015, 27, 2054–2059. [Google Scholar] [CrossRef]
- Grindy, S.C.; Learsch, R.; Mozhdehi, D.; Cheng, J.; Barrett, D.G.; Guan, Z.; Messersmith, P.B.; Holten-Andersen, N. Control of hierarchical polymer mechanics with bioinspired metal-coordination dynamics. Nat. Mater. 2015, 14, 1210–1216. [Google Scholar] [CrossRef]
- Fullenkamp, D.E.; He, L.; Barrett, D.G.; Burghardt, W.R.; Messersmith, P.B. Mussel-Inspired Histidine-Based Transient Network Metal Coordination Hydrogels. Macromolecules 2013, 46, 1167–1174. [Google Scholar] [CrossRef]
- Bai, R.; Yang, Q.; Tang, J.; Morelle, X.P.; Vlassak, J.; Suo, Z. Fatigue fracture of tough hydrogels. Extrem. Mech. Lett. 2017, 15, 91–96. [Google Scholar] [CrossRef]
- Mow, V.C.; Lai, W.M. Recent Developments in Synovial Joint Biomechanics. SIAM Rev. 1980, 22, 275–317. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Gu, Z.; Cheng, W.; Lei, H.; Qin, M.; Xue, B.; Wang, W.; Cao, Y. Regulating Mechanical Properties of Polymer-Supramolecular Double-Network Hydrogel by Supramolecular Self-assembling Structures. Chin. J. Chem. 2021, 39, 2711–2717. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough Physical Double-Network Hydrogels Based on Amphiphilic Triblock Copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, L.; Zhao, C.; Wang, Q.; Zheng, J. A Robust, One-Pot Synthesis of Highly Mechanical and Recoverable Double Network Hydrogels Using Thermoreversible Sol-Gel Polysaccharide. Adv. Mater. 2013, 25, 4171–4176. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, L.; Zhang, X.; Chen, L.; Cai, Q.; Yang, X. Hierarchical and heterogeneous hydrogel system as a promising strategy for diversified interfacial tissue regeneration. Biomater. Sci. 2021, 9, 1547–1573. [Google Scholar] [CrossRef]
- An, Y.; Gao, L.; Wang, T. Graphene Oxide/Alginate Hydrogel Fibers with Hierarchically Arranged Helical Structures for Soft Actuator Application. ACS Appl. Nano Mater. 2020, 3, 5079–5087. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Tan, R.; Zhang, S.; Zhang, K.; Hu, J. Hierarchically Structured Hydrogel Composites with Ultra-High Conductivity for Soft Electronics. Adv. Funct. Mater. 2023, 2312667. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Y.; Liu, J.; Wang, Y.; Cui, Q.; Song, P.; Zhang, X.; Zhang, C. Hierarchically Structured Hydrogel Actuator for Microplastic Pollutant Detection and Removal. Chem. Mater. 2022, 34, 5165–5175. [Google Scholar] [CrossRef]
- Liu, K.; Zang, S.; Xue, R.; Yang, J.; Wang, L.; Huang, J.; Yan, Y. Coordination-Triggered Hierarchical Folate/Zinc Supramolecular Hydrogels Leading to Printable Biomaterials. ACS Appl. Mater. Interfaces 2018, 10, 4530–4539. [Google Scholar] [CrossRef]
- Garg, V.; Gupta, T.; Rani, S.; Bandyopadhyay-Ghosh, S.; Ghosh, S.B.; Qiao, L.; Liu, G. A hierarchically designed nanocomposite hydrogel with multisensory capabilities towards wearable devices for human-body motion and glucose concentration detection. Compos. Sci. Technol. 2021, 213, 108894. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Guo, Y.Z.; Nonoyama, T.; Nakajima, T.; Kurokawa, T.; Gong, J.P. A Facile Method to Fabricate Anisotropic Hydrogels with Perfectly Aligned Hierarchical Fibrous Structures. Adv. Mater. 2018, 30, 1704937. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, A.M.; Ford, E.M.; Guo, C.; Sloppy, J.D.; Kloxin, A.M. Hierarchically structured hydrogels utilizing multifunctional assembling peptides for 3D cell culture. Biomater. Sci. 2020, 8, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, G.; Lei, I.M.; Zhang, P.; Wang, Z.; Chen, X.; Lu, M.; Zhang, J.; Wang, Z.; Sun, T.; et al. Impact-Resistant Hydrogels by Harnessing 2D Hierarchical Structures. Adv. Mater. 2023, 35, 2207587. [Google Scholar] [CrossRef]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, B.; Yao, B.; Qiu, Y.; Peng, Z.; Zhang, Y.; Alsaid, Y.; Frenkel, I.; Youssef, K.; Pei, Q.; et al. Hierarchically Structured Stretchable Conductive Hydrogels for High-Performance Wearable Strain Sensors and Supercapacitors. Matter 2020, 3, 1196–1210. [Google Scholar] [CrossRef]
- Guo, X.; Dong, X.; Zou, G.; Gao, H.; Zhai, W. Strong and tough fibrous hydrogels reinforced by multiscale hierarchical structures with multimechanisms. Sci. Adv. 2023, 9, eadf7075. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, S.; Huang, Y.; Tang, Q.; Fu, T.; Su, R.; Fan, C.; Xia, S.; Lee, P.S.; Lin, Y. Bioinspired structural hydrogels with highly ordered hierarchical orientations by flow-induced alignment of nanofibrils. Nat. Commun. 2024, 15, 118. [Google Scholar] [CrossRef]
- Williams, A.H.; Roh, S.; Jacob, A.R.; Stoyanov, S.D.; Hsiao, L.; Velev, O.D. Printable homocomposite hydrogels with synergistically reinforced molecular-colloidal networks. Nat. Commun. 2021, 12, 2834. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-P.; Zhu, Y.-J. Bioinspired flexible, high-strength, and versatile hydrogel with the fiberboard-and-mortar hierarchically ordered structure. Nano Res. 2021, 14, 3643–3652. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Le, H.H.; Tran, V.T.; Trtik, P.; Cui, J.; Jeon, I. Anisotropic tough multilayer hydrogels with programmable orientation. Mater. Horiz. 2019, 6, 1504–1511. [Google Scholar] [CrossRef]
- Puza, F.; Zheng, Y.; Han, L.; Xue, L.; Cui, J. Physical entanglement hydrogels: Ultrahigh water content but good toughness and stretchability. Polym. Chem. 2020, 11, 2339–2345. [Google Scholar] [CrossRef]
- Sugimura, A.; Asai, M.; Matsunaga, T.; Akagi, Y.; Sakai, T.; Noguchi, H.; Shibayama, M. Mechanical properties of a polymer network of Tetra-PEG gel. Polym. J. 2013, 45, 300–306. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sakai, T.; Akagi, Y.; Chung, U.-I.; Shibayama, M. Structure Characterization of Tetra-PEG Gel by Small-Angle Neutron Scattering. Macromolecules 2009, 42, 1344–1351. [Google Scholar] [CrossRef]
- Sakai, T. Gelation mechanism and mechanical properties of Tetra-PEG gel. React. Funct. Polym. 2013, 73, 898–903. [Google Scholar] [CrossRef]
- Xu, K.; Liang, X.; Li, P.; Deng, Y.; Pei, X.; Tan, Y.; Zhai, K.; Wang, P. Tough, stretchable chemically cross-linked hydrogel using core–shell polymer microspheres as cross-linking junctions. Polymer 2017, 118, 58–67. [Google Scholar] [CrossRef]
- Fukasawa, M.; Sakai, T.; Chung, U.-I.; Haraguchi, K. Synthesis and Mechanical Properties of a Nanocomposite Gel Consisting of a Tetra-PEG/Clay Network. Macromolecules 2010, 43, 4370–4378. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Butcher, A.L.; Oyen, M.L. Strong and tough nanofibrous hydrogel composites based on biomimetic principles. Mater. Sci. Eng. C 2017, 72, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, G.; Shi, M.; Suo, Z. Fracture, fatigue, and friction of polymers in which entanglements greatly outnumber cross-links. Science 2021, 374, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Tsukeshiba, H.; Huang, M.; Na, Y.-H.; Kurokawa, T.; Kuwabara, R.; Tanaka, Y.; Furukawa, H.; Osada, Y.; Gong, J.P. Effect of Polymer Entanglement on the Toughening of Double Network Hydrogels. J. Phys. Chem. B 2005, 109, 16304–16309. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Li, Z.; Li, Q.; Song, G.; Huo, M. Strong and Tough Nanostructured Hydrogels and Organogels Prepared by Polymerization-Induced Self-Assembly. Small Methods 2023, 7, 2201592. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, L.; Bian, Q.; Xue, B.; Jin, J.; Li, J.; Cao, Y.; Jiang, Q.; Li, H. Cartilage-like protein hydrogels engineered via entanglement. Nature 2023, 618, 740–747. [Google Scholar] [CrossRef]
- Kubota, R.; Naritomi, M.; Fujimoto, I. Synthesis of a stretchable polymer crosslinker for reinforced atelocollagen threads. React. Funct. Polym. 2023, 182, 105462. [Google Scholar] [CrossRef]
- Liu, C.; Morimoto, N.; Jiang, L.; Kawahara, S.; Noritomi, T.; Yokoyama, H.; Mayumi, K.; Ito, K. Tough hydrogels with rapid self-reinforcement. Science 2021, 372, 1078–1081. [Google Scholar] [CrossRef]
- Lu, X.; Chan, C.Y.; Lee, K.I.; Ng, P.F.; Fei, B.; Xin, J.H.; Fu, J. Super-tough and thermo-healable hydrogel—Promising for shape-memory absorbent fiber. J. Mater. Chem. B 2014, 2, 7631–7638. [Google Scholar] [CrossRef]
- Du, C.; Zhang, X.N.; Sun, T.L.; Du, M.; Zheng, Q.; Wu, Z.L. Hydrogen-Bond Association-Mediated Dynamics and Viscoelastic Properties of Tough Supramolecular Hydrogels. Macromolecules 2021, 54, 4313–4325. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Q.; Ma, L.; Zhao, Y.; Ran, J. Rational Design of a High-Strength Tough Hydrogel from Fundamental Principles. Macromol. Chem. Phys. 2021, 222, 2100064. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, X.; Ouchi, T.; Kouznetsova, T.B.; Beech, H.K.; Av-Ron, S.; Matsuda, T.; Bowser, B.H.; Wang, S.; Johnson, J.A.; et al. Toughening hydrogels through force-triggered chemical reactions that lengthen polymer strands. Science 2021, 374, 193–196. [Google Scholar] [CrossRef]
- Xue, B.; Bashir, Z.; Guo, Y.; Yu, W.; Sun, W.; Li, Y.; Zhang, Y.; Qin, M.; Wang, W.; Cao, Y. Strong, tough, rapid-recovery, and fatigue-resistant hydrogels made of picot peptide fibres. Nat. Commun. 2023, 14, 2583. [Google Scholar] [CrossRef]
- Hua, J.; Liu, C.; Ng, P.F.; Fei, B. Bacterial cellulose reinforced double-network hydrogels for shape memory strand. Carbohydr. Polym. 2021, 259, 117737. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Dong, C.; Jiang, H.; Bin, X.; Gao, X.; Qin, M.; Wang, W.; Bin, C.; Cao, Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018, 9, 620. [Google Scholar] [CrossRef]
- Zhu, F.; Cheng, L.; Wang, Z.J.; Hong, W.; Wu, Z.L.; Yin, J.; Qian, J.; Zheng, Q. 3D-Printed Ultratough Hydrogel Structures with Titin-like Domains. ACS Appl. Mater. Interfaces 2017, 9, 11363–11367. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Meng, L.; Wang, M.; Cui, C.; Wang, B.; Han, C.-R.; Xu, F.; Yang, J. Mimicking Dynamic Adhesiveness and Strain-Stiffening Behavior of Biological Tissues in Tough and Self-Healable Cellulose Nanocomposite Hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 5885–5895. [Google Scholar] [CrossRef] [PubMed]
- Sabzi, M.; Samadi, N.; Abbasi, F.; Mahdavinia, G.R.; Babaahmadi, M. Bioinspired fully physically cross-linked double network hydrogels with a robust, tough and self-healing structure. Mater. Sci. Eng. C 2017, 74, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liao, S.; Tao, X.; Xu, X.-Q.; Hong, Q.; Wu, D.; Wang, Y. Spider-Inspired Multicomponent 3D Printing Technique for Next-Generation Complex Biofabrication. ACS Appl. Bio Mater. 2018, 1, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gui, Q.; Yu, W.; Liao, S.; He, Y.; Tao, X.; Yu, Y.; Wang, Y. Interfacial Diffusion Printing: An Efficient Manufacturing Technique for Artificial Tubular Grafts. ACS Biomater. Sci. Eng. 2019, 5, 6311–6318. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-Printed High Strength Bioactive Supramolecular Polymer/Clay Nanocomposite Hydrogel Scaffold for Bone Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Parks, J.; Choi, P.; Ji, H.-F. Applications of Highly Stretchable and Tough Hydrogels. Polymers 2019, 11, 1773. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Bodugoz-Senturk, H.; Macias, C.E.; Kung, J.H.; Muratoglu, O.K. Poly(vinyl alcohol)–acrylamide hydrogels as load-bearing cartilage substitute. Biomaterials 2009, 30, 589–596. [Google Scholar] [CrossRef]
- Moutos, F.T.; Freed, L.E.; Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007, 6, 162–167. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Xue, B.; Tang, D.; Wu, X.; Xu, Z.; Gu, J.; Han, Y.; Zhu, Z.; Qin, M.; Zou, X.; Wang, W.; et al. Engineering hydrogels with homogeneous mechanical properties for controlling stem cell lineage specification. Proc. Natl. Acad. Sci. USA 2021, 118, e2110961118. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakajima, T.; Yang, J.J.; Kurokawa, T.; Liu, J.; Lu, J.; Mizumoto, S.; Sugahara, K.; Kitamura, N.; Yasuda, K.; et al. Proteoglycans and Glycosaminoglycans Improve Toughness of Biocompatible Double Network Hydrogels. Adv. Mater. 2014, 26, 436–442. [Google Scholar] [CrossRef]
- Yasuda, K.; Ping Gong, J.; Katsuyama, Y.; Nakayama, A.; Tanabe, Y.; Kondo, E.; Ueno, M.; Osada, Y. Biomechanical properties of high-toughness double network hydrogels. Biomaterials 2005, 26, 4468–4475. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Zhou, L.; Wang, Z.; Dai, C.; Ning, C.; Tan, G. A Dual-Bonded Approach for Improving Hydrogel Implant Stability in Cartilage Defects. Materials 2017, 10, 191. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Wang, S.; Zhang, Y.; Yang, A.; Cheng, Y.; Chen, X. Ultra-durable cell-free bioactive hydrogel with fast shape memory and on-demand drug release for cartilage regeneration. Nat. Commun. 2023, 14, 7771. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, H.; Zhou, J.; Zhao, Z.; Huang, J.; Chen, L.; Ru, Y.; Liu, M. High-Performance Organohydrogel Artificial Muscle with Compartmentalized Anisotropic Actuation Under Microdomain Confinement. Adv. Mater. 2023, 35, 2202193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, J.; Su, S.; Qiu, J.; Wang, S. Ion-linked double-network hydrogel with high toughness and stiffness. J. Mater. Sci. 2015, 50, 5458–5465. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Zhang, H.; Wang, G.; Wei, H.; Zhang, X.; Ma, N. Bioinspired Conductive Hydrogel with Ultrahigh Toughness and Stable Antiswelling Properties for Articular Cartilage Replacement. ACS Mater. Lett. 2021, 3, 807–814. [Google Scholar] [CrossRef]
- Xue, B.; Gu, J.; Li, L.; Yu, W.; Yin, S.; Qin, M.; Jiang, Q.; Wang, W.; Cao, Y. Hydrogel tapes for fault-tolerant strong wet adhesion. Nat. Commun. 2021, 12, 7156. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Uzun, O.; Luna, N.M.M.; Rock, A.; Clifford, C.; Stoler, E.; Östlund-Sholars, G.; Johnson, C.; Mooney, D.J. Degradable and Removable Tough Adhesive Hydrogels. Adv. Mater. 2021, 33, 2008553. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Moon, J.R.; Park, N.; Im, J.; Kim, Y.E.; Kim, J.-H.; Kim, J. Bone-Adhesive Anisotropic Tough Hydrogel Mimicking Tendon Enthesis. Adv. Mater. 2023, 35, 2206207. [Google Scholar] [CrossRef] [PubMed]
- Means, A.K.; Shrode, C.S.; Whitney, L.V.; Ehrhardt, D.A.; Grunlan, M.A. Double Network Hydrogels that Mimic the Modulus, Strength, and Lubricity of Cartilage. Biomacromolecules 2019, 20, 2034–2042. [Google Scholar] [CrossRef]
- Zhao, Y.; Lo, C.-Y.; Ruan, L.; Pi, C.-H.; Kim, C.; Alsaid, Y.; Frenkel, I.; Rico, R.; Tsao, T.-C.; He, X. Somatosensory actuator based on stretchable conductive photothermally responsive hydrogel. Sci. Robot. 2021, 6, eabd5483. [Google Scholar] [CrossRef]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, K.; He, J.; Yang, H.; Zhang, X. A novel 3D-printable hydrogel with high mechanical strength and shape memory properties. J. Mater. Chem. C 2019, 7, 14913–14922. [Google Scholar] [CrossRef]
- Yang, F.; Tadepalli, V.; Wiley, B.J. 3D Printing of a Double Network Hydrogel with a Compression Strength and Elastic Modulus Greater than those of Cartilage. ACS Biomater. Sci. Eng. 2017, 3, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Hu, J.; Wu, X.; Shi, H.; Yu, H.C.; Qian, J.; Yin, J.; Gao, C.; Wu, Z.L.; Zheng, Q. 3D printing of a tough double-network hydrogel and its use as a scaffold to construct a tissue-like hydrogel composite. J. Mater. Chem. B 2022, 10, 468–476. [Google Scholar] [CrossRef] [PubMed]
- 3D-Printed Hydrogel Technologies for Tissue-Engineered Heart Valves. 3D Print. Addit. Manuf. 2014, 1, 122–136. [CrossRef]
- Heo, D.N.; Castro, N.J.; Lee, S.-J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale 2017, 9, 5055–5062. [Google Scholar] [CrossRef] [PubMed]
- Wallin, T.J.; Simonsen, L.-E.; Pan, W.; Wang, K.; Giannelis, E.; Shepherd, R.F.; Mengüç, Y. 3D printable tough silicone double networks. Nat. Commun. 2020, 11, 4000. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Huang, L.; Hao, W.; Zhao, T.; Zhao, H.; Yang, W.; Xie, X.; Qian, L.; Chen, Y.; Dai, J. Long-term stability, high strength, and 3D printable alginate hydrogel for cartilage tissue engineering application. Biomed. Mater. 2021, 16, 064102. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, K.Y.; Kim, S.L.; MacQueen, L.A.; Chang, H.; Zimmerman, J.F.; Jin, Q.; Peters, M.M.; Ardoña, H.A.M.; Liu, X.; et al. Fibre-infused gel scaffolds guide cardiomyocyte alignment in 3D-printed ventricles. Nat. Mater. 2023, 22, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent advances in conductive hydrogels: Classifications, properties, and applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef]
- Zhang, L.M.; He, Y.; Cheng, S.; Sheng, H.; Dai, K.; Zheng, W.J.; Wang, M.X.; Chen, Z.S.; Chen, Y.M.; Suo, Z. Self-Healing, Adhesive, and Highly Stretchable Ionogel as a Strain Sensor for Extremely Large Deformation. Small 2019, 15, 1804651. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.; Li, S.; Liu, X.; Sun, J. Mechanically Robust, Elastic, and Healable Ionogels for Highly Sensitive Ultra-Durable Ionic Skins. Adv. Mater. 2020, 32, 2002706. [Google Scholar] [CrossRef] [PubMed]
- Yiming, B.; Guo, X.; Ali, N.; Zhang, N.; Zhang, X.; Han, Z.; Lu, Y.; Wu, Z.; Fan, X.; Jia, Z.; et al. Ambiently and Mechanically Stable Ionogels for Soft Ionotronics. Adv. Funct. Mater. 2021, 31, 2102773. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, G.; Wang, S.; Zhang, Y.; Jian, Y.; He, L.; Yu, T.; Luo, H.; Kong, D.; Xianyu, Y.; et al. Stretchable graphene–hydrogel interfaces for wearable and implantable bioelectronics. Nat. Electron. 2023, 7, 51–65. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, P.; Wang, X.; Wang, Y.; Li, P.; Huang, W.; Li, B.; Jin, R.; Han, N.; Wu, J.; et al. Sebum-Membrane-Inspired Protein-Based Bioprotonic Hydrogel for Artificial Skin and Human-Machine Merging Interface. Adv. Funct. Mater. 2023, 33, 2211056. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.-L.; Huang, L.-Z.; Guo, W.-Y.; Zhao, J.; Ma, M.-G. A stretchable, environmentally tolerant, and photoactive liquid metal/MXene hydrogel for high performance temperature monitoring, human motion detection and self-powered application. Nano Energy 2023, 117, 108875. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, X.; Zheng, M.; Yue, O.; Huang, M.; Zou, X.; Cui, B.; Xie, L.; Dong, S.; Shang, J.; et al. Mechanically Robust and Transparent Organohydrogel-Based E-Skin Nanoengineered from Natural Skin. Adv. Funct. Mater. 2023, 33, 2212856. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H.N. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4, eaat0098. [Google Scholar] [CrossRef]
- Xue, B.; Sheng, H.; Li, Y.; Li, L.; Di, W.; Xu, Z.; Ma, L.; Wang, X.; Jiang, H.; Qin, M.; et al. Stretchable and self-healable hydrogel artificial skin. Natl. Sci. Rev. 2022, 9, nwab147. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Li, H.; Lv, T.; Sun, H.; Qian, G.; Li, N.; Yao, Y.; Chen, T. Ultrastretchable and superior healable supercapacitors based on a double cross-linked hydrogel electrolyte. Nat. Commun. 2019, 10, 536. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 2022, 7, 935–952. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, Y.; Liu, F.; Wang, H.; Yu, H.; Dai, K.; Zheng, G.; Feng, W. Carbon Dots-Based Ultrastretchable and Conductive Hydrogels for High-Performance Tactile Sensors and Self-Powered Electronic Skin. Small 2023, 19, 2204365. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 2023, 14, 759. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Sung, C.; Nam, K.S.; Kang, T.; Kim, H.; Lee, H.; Park, H.; Park, S.; Kang, J. Highly conductive tissue-like hydrogel interface through template-directed assembly. Nat. Commun. 2023, 14, 2206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B.; et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Dorval Courchesne, N.-M.; Hyder, M.N.; Qi, J.; Belcher, A.M.; Hammond, P.T. Carbon nanotube–polyaniline core–shell nanostructured hydrogel for electrochemical energy storage. RSC Adv. 2015, 5, 37970–37977. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Yu, G. Nanostructured conducting polymer hydrogels for energy storage applications. Nanoscale 2015, 7, 12796–12806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 1804560. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Jia, H.; Ng, P.F.; Chow, L.; Fei, B. Recent advances of hydrogel electrolytes in flexible energy storage devices. J. Mater. Chem. A 2021, 9, 2043–2069. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, G.; Zhan, Y.; Li, H.; Wang, Z.; Ma, L.; Wang, Y.; Niu, X.; Zhi, C. A soft yet device-level dynamically super-tough supercapacitor enabled by an energy-dissipative dual-crosslinked hydrogel electrolyte. Nano Energy 2019, 58, 732–742. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lu, H.; Wang, Y.; Xu, J.; Zhu, J.; Zhang, C.; Liu, T. Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage. Nat. Commun. 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Dong, L.; Sha, W.; Wei, L.; Guo, X. Highly stretchable, compressible and arbitrarily deformable all-hydrogel soft supercapacitors. Chem. Eng. J. 2020, 383, 123098. [Google Scholar] [CrossRef]
- Fang, L.; Cai, Z.; Ding, Z.; Chen, T.; Zhang, J.; Chen, F.; Shen, J.; Chen, F.; Li, R.; Zhou, X.; et al. Skin-Inspired Surface-Microstructured Tough Hydrogel Electrolytes for Stretchable Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 21895–21903. [Google Scholar] [CrossRef] [PubMed]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.S.; Katti, D.R. Why is nacre so tough and strong? Mater. Sci. Eng. C 2006, 26, 1317–1324. [Google Scholar] [CrossRef]
- Lewis, R.V. Spider Silk: Ancient Ideas for New Biomaterials. Chem. Rev. 2006, 106, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.; Guan, J.; Vollrath, F. Spider Silk: Super Material or Thin Fibre? Adv. Mater. 2013, 25, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, N.K.; Goel, G.; Hawi, S.; Goel, S. Nature-inspired materials: Emerging trends and prospects. NPG Asia Mater. 2021, 13, 56. [Google Scholar] [CrossRef]
- Chan, N.J.-A.; Gu, D.; Tan, S.; Fu, Q.; Pattison, T.G.; O’Connor, A.J.; Qiao, G.G. Spider-silk inspired polymeric networks by harnessing the mechanical potential of β-sheets through network guided assembly. Nat. Commun. 2020, 11, 1630. [Google Scholar] [CrossRef]
- Gu, L.; Jiang, Y.; Hu, J. Scalable Spider-Silk-Like Supertough Fibers using a Pseudoprotein Polymer. Adv. Mater. 2019, 31, 1904311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, Q.; Tang, Z. Layered nanocomposites inspired by the structure and mechanical properties of nacre. Chem. Soc. Rev. 2012, 41, 1111–1129. [Google Scholar] [CrossRef]
- Dou, Y.; Wang, Z.-P.; He, W.; Jia, T.; Liu, Z.; Sun, P.; Wen, K.; Gao, E.; Zhou, X.; Hu, X.; et al. Artificial spider silk from ion-doped and twisted core-sheath hydrogel fibres. Nat. Commun. 2019, 10, 5293. [Google Scholar] [CrossRef]
- Fu, F.; Chen, Z.; Zhao, Z.; Wang, H.; Shang, L.; Gu, Z.; Zhao, Y. Bio-inspired self-healing structural color hydrogel. Proc. Natl. Acad. Sci. USA 2017, 114, 5900–5905. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Huang, R.; Lin, R.; Liu, Z. A phase field solution for modelling hyperelastic material and hydrogel fracture in ABAQUS. Eng. Fract. Mech. 2022, 276, 108894. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, D.; Chen, H.; Zhang, Y.; Liu, Y.; Ren, B.; Zheng, J. A multiscale polymerization framework towards network structure and fracture of double-network hydrogels. NPJ Comput. Mater. 2021, 7, 39. [Google Scholar] [CrossRef]
- Liu, H.; Du, C.; Liao, L.; Zhang, H.; Zhou, H.; Zhou, W.; Ren, T.; Sun, Z.; Lu, Y.; Nie, Z.; et al. Approaching intrinsic dynamics of MXenes hybrid hydrogel for 3D printed multimodal intelligent devices with ultrahigh superelasticity and temperature sensitivity. Nat. Commun. 2022, 13, 3420. [Google Scholar] [CrossRef]
- Castro, A.P.G.; Yao, J.; Battisti, T.; Lacroix, D. Poroelastic Modeling of Highly Hydrated Collagen Hydrogels: Experimental Results vs. Numerical Simulation With Custom and Commercial Finite Element Solvers. Front. Bioeng. Biotechnol. 2018, 6, 142. [Google Scholar] [CrossRef]
- Roy, J.K.; Pinto, H.P.; Leszczynski, J. Interaction of epoxy-based hydrogels and water: A molecular dynamics simulation study. J. Mol. Graph. Model. 2021, 106, 107915. [Google Scholar] [CrossRef]
- Angelerou, M.G.F.; Frederix, P.W.J.M.; Wallace, M.; Yang, B.; Rodger, A.; Adams, D.J.; Marlow, M.; Zelzer, M. Supramolecular Nucleoside-Based Gel: Molecular Dynamics Simulation and Characterization of Its Nanoarchitecture and Self-Assembly Mechanism. Langmuir 2018, 34, 6912–6921. [Google Scholar] [CrossRef]
- Ou, X.; Xue, B.; Lao, Y.; Wutthinitikornkit, Y.; Tian, R.; Zou, A.; Yang, L.; Wang, W.; Cao, Y.; Li, J. Structure and sequence features of mussel adhesive protein lead to its salt-tolerant adhesion ability. Sci. Adv. 2020, 6, eabb7620. [Google Scholar] [CrossRef]
- Guo, K.; Yang, Z.; Yu, C.-H.; Buehler, M.J. Artificial intelligence and machine learning in design of mechanical materials. Mater. Horiz. 2021, 8, 1153–1172. [Google Scholar] [CrossRef]
- Sha, W.; Guo, Y.; Yuan, Q.; Tang, S.; Zhang, X.; Lu, S.; Guo, X.; Cao, Y.-C.; Cheng, S. Artificial Intelligence to Power the Future of Materials Science and Engineering. Adv. Intell. Syst. 2020, 2, 1900143. [Google Scholar] [CrossRef]
- Huang, J.S.; Liew, J.X.; Ademiloye, A.S.; Liew, K.M. Artificial Intelligence in Materials Modeling and Design. Arch. Comput. Methods Eng. 2021, 28, 3399–3413. [Google Scholar] [CrossRef]
- Nagahama, K.; Kimura, Y.; Takemoto, A. Living functional hydrogels generated by bioorthogonal cross-linking reactions of azide-modified cells with alkyne-modified polymers. Nat. Commun. 2018, 9, 2195. [Google Scholar] [CrossRef]
- Liu, X.; Inda, M.E.; Lai, Y.; Lu, T.K.; Zhao, X. Engineered living hydrogels. Adv. Mater. 2022, 34, 2201326. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Shang, L.; Chen, Z.; Yu, Y.; Zhao, Y. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3, eaar8580. [Google Scholar] [CrossRef]
- Liu, X.; Tang, T.-C.; Tham, E.; Yuk, H.; Lin, S.; Lu, T.K.; Zhao, X. Stretchable living materials and devices with hydrogel–elastomer hybrids hosting programmed cells. Proc. Natl. Acad. Sci. USA 2017, 114, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, F.; Zhang, X.; Shang, Y.; Zhao, Y. Living microecological hydrogels for wound healing. Sci. Adv. 2023, 9, eadg3478. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Chen, Y.; Cao, Y.; Xue, B. Tough Hydrogels with Different Toughening Mechanisms and Applications. Int. J. Mol. Sci. 2024, 25, 2675. https://doi.org/10.3390/ijms25052675
Xu Z, Chen Y, Cao Y, Xue B. Tough Hydrogels with Different Toughening Mechanisms and Applications. International Journal of Molecular Sciences. 2024; 25(5):2675. https://doi.org/10.3390/ijms25052675
Chicago/Turabian StyleXu, Zhengyu, Yanru Chen, Yi Cao, and Bin Xue. 2024. "Tough Hydrogels with Different Toughening Mechanisms and Applications" International Journal of Molecular Sciences 25, no. 5: 2675. https://doi.org/10.3390/ijms25052675
APA StyleXu, Z., Chen, Y., Cao, Y., & Xue, B. (2024). Tough Hydrogels with Different Toughening Mechanisms and Applications. International Journal of Molecular Sciences, 25(5), 2675. https://doi.org/10.3390/ijms25052675







