Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis
Abstract
:1. Introduction
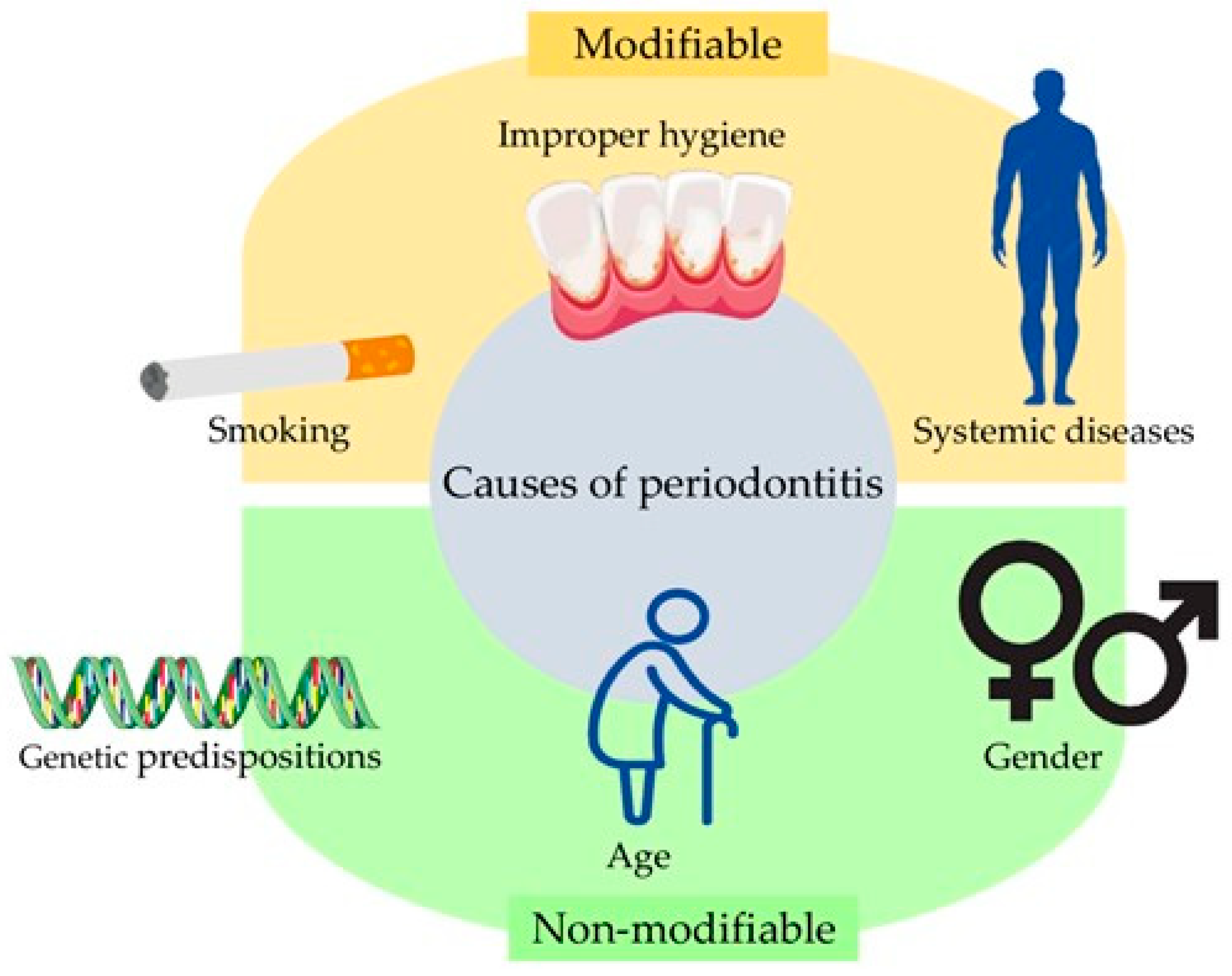
2. Symptoms and Risk Factors Associated with Periodontitis
3. Metalloproteinases and Their Role in the Human Body with Special Emphasis on the Stomatognathic Apparatus
4. Structure and Functions of Metalloproteinase 8 (MMP-8)
5. Importance of MMP-8 in Periodontitis
6. MMP-8 Gene Polymorphisms and Periodontitis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cope, G.; Cope, A. The Periodontium: An Anatomical Guide. Dent. Nurs. 2011, 7, 376–378. [Google Scholar] [CrossRef]
- Palumbo, A. The Anatomy and Physiology of the Healthy Periodontium. In Gingival Diseases—Their Aetiology, Prevention and Treatment; Panagakos, F., Ed.; InTech: London, UK, 2011; ISBN 9789533073767. [Google Scholar]
- Garg, A.; Bhickta, S.; Gupta, R.; Sharma, A. Aging and Periodontium. Dent. J. Adv. Stud. 2013, 1, 26–29. [Google Scholar] [CrossRef]
- Hasan, A.; Palmer, R.M. A Clinical Guide to Periodontology: Pathology of Periodontal Disease. Br. Dent. J. 2014, 216, 457–461. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef]
- Dubey, P.; Mittal, N. Periodontal Diseases—A Brief Review. Int. J. Oral Health Dent. 2020, 6, 177–187. [Google Scholar] [CrossRef]
- Lim, G.; Janu, U.; Chiou, L.-L.; Gandhi, K.K.; Palomo, L.; John, V. Periodontal Health and Systemic Conditions. Dent. J. 2020, 8, 130. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Bangalore Balaram, S.; Galgali, S.R.; Rajendra Santosh, A.B. Periodontal Epidemiology. Eur. Dent. Res. Biomater. J. 2020, 1, 20–26. [Google Scholar] [CrossRef]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Bräuninger, H.; Krüger, S.; Bacmeister, L.; Nyström, A.; Eyerich, K.; Westermann, D.; Lindner, D. Matrix Metalloproteinases in Coronary Artery Disease and Myocardial Infarction. Basic Res. Cardiol. 2023, 118, 18. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De La Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Lubowicka, E.; Zbucka-Kretowska, M.; Sidorkiewicz, I.; Zajkowska, M.; Gacuta, E.; Puchnarewicz, A.; Chrostek, L.; Szmitkowski, M.; Ławicki, S. Diagnostic Power of Cytokine M-CSF, Metalloproteinase 2 (MMP-2) and Tissue Inhibitor-2 (TIMP-2) in Cervical Cancer Patients Based on ROC Analysis. Pathol. Oncol. Res. 2020, 26, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Sidorkiewicz, I.; Piskór, B.; Dąbrowska, E.; Guzińska-Ustymowicz, K.; Pryczynicz, A.; Zbucka-Krętowska, M.; Ławicki, S. Plasma Levels and Tissue Expression of Selected Cytokines, Metalloproteinases and Tissue Inhibitors in Patients with Cervical Cancer. Anticancer Res. 2019, 39, 6403–6412. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, M.; Zbucka-Krętowska, M.; Sidorkiewicz, I.; Lubowicka, E.; Gacuta, E.; Szmitkowski, M.; Chrostek, L.; Ławicki, S. Plasma Levels and Diagnostic Utility of Macrophage-Colony Stimulating Factor, Matrix Metalloproteinase-9 and Tissue Inhibitor of Metalloproteinase-1 as Tumor Markers in Cervical Cancer Patients. Tumour Biol. 2018, 40, 101042831879036. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef]
- Gaurilcikaite, E.; Renton, T.; Grant, A. The Paradox of Painless Periodontal Disease. Oral Dis. 2017, 23, 451–463. [Google Scholar] [CrossRef]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute Periodontal Lesions (Periodontal Abscesses and Necrotizing Periodontal Diseases) and Endo-periodontal Lesions. J. Periodontol. 2018, 89, S85–S102. [Google Scholar] [CrossRef]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, Aging, and Periodontal Disease: Basic Biologic Considerations. Periodontology 2000 2021, 87, 143–156. [Google Scholar] [CrossRef]
- AlJehani, Y.A. Risk Factors of Periodontal Disease: Review of the Literature. Int. J. Dent. 2014, 2014, 182513. [Google Scholar] [CrossRef]
- López, R.; Smith, P.C.; Göstemeyer, G.; Schwendicke, F. Ageing, Dental Caries and Periodontal Diseases. J. Clin. Periodontol. 2017, 44, S145–S152. [Google Scholar] [CrossRef]
- Madiba, T.; Bhayat, A. Periodontal Disease—Risk Factors and Treatment Options. S. Afr. Dent. J. 2018, 73, 571–575. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk Factors for Periodontal Disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Nickel, J.C.; Iwasaki, L.R.; Duan, P.; Simmer-Beck, M.; Brown, L. Gender Differences in the Association of Periodontitis and Type 2 Diabetes. Int. Dent. J. 2018, 68, 433–440. [Google Scholar] [CrossRef]
- Ioannidou, E. The Sex and Gender Intersection in Chronic Periodontitis. Front. Public Health 2017, 5, 189. [Google Scholar] [CrossRef]
- Abe, M.; Mitani, A.; Hoshi, K.; Yanagimoto, S. Large Gender Gap in Oral Hygiene Behavior and Its Impact on Gingival Health in Late Adolescence. Int. J. Environ. Res. Public Health 2020, 17, 4394. [Google Scholar] [CrossRef]
- White, A. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res. Curr. Rev. 2020, 40, 1. [Google Scholar] [CrossRef]
- Sreeramulu, B.; Shyam, N.D.; Ajay, P.; Suman, P. Papillon-Lefèvre syndrome: Clinical Presentation and Management Options. Clin. Cosmet. Investig. Dent. 2015, 7, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Thota, E.; Veeravalli, J.J.; Manchala, S.K.; Lakkepuram, B.P.; Kodapaneni, J.; Chen, Y.-W.; Wang, L.-T.; Ma, K.S.-K. Age-Dependent Oral Manifestations of Neurofibromatosis Type 1: A Case–Control Study. Orphanet J. Rare Dis. 2022, 17, 93. [Google Scholar] [CrossRef]
- Wankhede, A.; Wankhede, S.; Wasu, S. Role of Genetic in Periodontal Disease. J. Int. Clin. Dent. Res. Organ. 2017, 9, 53. [Google Scholar] [CrossRef]
- Loos, B.G.; Van Dyke, T.E. The Role of Inflammation and Genetics in Periodontal Disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Brodzikowska, A.; Górski, B. Polymorphisms in Genes Involved in Inflammation and Periodontitis: A Narrative Review. Biomolecules 2022, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, D.; Altalhi, A.; Sambawa, Z.; Koppolu, P.; Alsinaidi, A.; Krishnan, P. The Association of Matrix Metalloproteinase Gene Polymorphisms and Periodontitis: An Overview. J. Pharm. Bioall. Sci. 2020, 12, 37. [Google Scholar] [CrossRef]
- Weng, H.; Yan, Y.; Jin, Y.-H.; Meng, X.-Y.; Mo, Y.-Y.; Zeng, X.-T. Matrix Metalloproteinase Gene Polymorphisms and Periodontitis Susceptibility: A Meta-Analysis Involving 6162 Individuals. Sci. Rep. 2016, 6, 24812. [Google Scholar] [CrossRef]
- Grover, H.S.; Bhardwaj, A.; Singh, Y. Smoking and periodontal disease. J. Pharm. Sci. Innov. 2013, 2, 7–13. [Google Scholar] [CrossRef]
- Borojevic, T. Smoking and Periodontal Disease. Mater. Sociomed. 2012, 24, 274. [Google Scholar] [CrossRef]
- Silva, H. Tobacco Use and Periodontal Disease—The Role of Microvascular Dysfunction. Biology 2021, 10, 441. [Google Scholar] [CrossRef]
- Chaffee, B.W.; Couch, E.T.; Vora, M.V.; Holliday, R.S. Oral and Periodontal Implications of Tobacco and Nicotine Products. Periodontology 2000 2021, 87, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Dukka, H.; Saxena, D. Potential Mechanisms Underlying Marijuana-Associated Periodontal Tissue Destruction. J. Dent. Res. 2022, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lazureanu, P.C.; Popescu, F.G.; Stef, L.; Focsa, M.; Vaida, M.A.; Mihaila, R. The Influence of Periodontal Disease on Oral Health Quality of Life in Patients with Cardiovascular Disease: A Cross-Sectional Observational Single-Center Study. Medicina 2022, 58, 584. [Google Scholar] [CrossRef]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The Association between Oral Hygiene and Periodontitis: A Systematic Review and Meta-Analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Grassi, R.; Nardi, G.; Mazur, M.; Di Giorgio, R.; Ottolenghi, L.; Guerra, F. The Dental-BIOfilm Detection TECHnique (D-BioTECH): A Proof of Concept of a Patient-Based Oral Hygiene. Medicina 2022, 58, 537. [Google Scholar] [CrossRef]
- Visentin, D.; Gobin, I.; Maglica, Ž. Periodontal Pathogens and Their Links to Neuroinflammation and Neurodegeneration. Microorganisms 2023, 11, 1832. [Google Scholar] [CrossRef]
- Carrouel, F.; Kanoute, A.; Lvovschi, V.-E.; Bourgeois, D. Periodontal Pathogens of the Interdental Microbiota in a 3 Months Pregnant Population with an Intact Periodontium. Front. Microbiol. 2023, 14, 1275180. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and Diabetes: A Two-Way Relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Pucher, J.; Stewart, J. Periodontal Disease and Diabetes Mellitus. Curr Diab Rep 2004, 4, 46–50. [Google Scholar] [CrossRef]
- Loffek, S.; Schilling, O.; Franzke, C.-W. Biological Role of Matrix Metalloproteinases: A Critical Balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Kicman, A.; Niczyporuk, M.; Kulesza, M.; Motyka, J.; Ławicki, S. Utility of Matrix Metalloproteinases in the Diagnosis, Monitoring and Prognosis of Ovarian Cancer Patients. Cancer Manag. Res. 2022, 14, 3359–3382. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 147, pp. 1–73. ISBN 9780128116371. [Google Scholar]
- Liu, J.; Khalil, R.A. Matrix Metalloproteinase Inhibitors as Investigational and Therapeutic Tools in Unrestrained Tissue Remodeling and Pathological Disorders. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 148, pp. 355–420. ISBN 9780128127766. [Google Scholar]
- Xie, Y.; Mustafa, A.; Yerzhan, A.; Merzhakupova, D.; Yerlan, P.; Orakov, A.N.; Wang, X.; Huang, Y.; Miao, L. Nuclear Matrix Metalloproteinases: Functions Resemble the Evolution from the Intracellular to the Extracellular Compartment. Cell Death Discov. 2017, 3, 17036. [Google Scholar] [CrossRef]
- Palosaari, H.; Pennington, C.J.; Larmas, M.; Edwards, D.R.; Tjäderhane, L.; Salo, T. Expression Profile of Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of MMPs in Mature Human Odontoblasts and Pulp Tissue. Eur. J. Oral. Sci. 2003, 111, 117–127. [Google Scholar] [CrossRef]
- Guan, X.; Bartlett, J.D. MMP20 Modulates Cadherin Expression in Ameloblasts as Enamel Develops. J. Dent. Res. 2013, 92, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Bahuguna, R. Role of Matrix Metalloproteinases in Dental Caries, Pulp and Periapical Inflammation: An Overview. J. Oral Biol. Craniofacial Res. 2015, 5, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Pereira Prado, V.; Asquino, N.; Apellaniz, D.; Bueno Rossy, L.; Tapia, G.; Bologna Molina, R. Metalloproteinases (MMPs) of the Extracellular Matrix in Dentistry. Odontoestomatologia 2016, 18, 20–29. [Google Scholar]
- Shin, M.; Chavez, M.B.; Ikeda, A.; Foster, B.L.; Bartlett, J.D. MMP20 Overexpression Disrupts Molar Ameloblast Polarity and Migration. J. Dent. Res. 2018, 97, 820–827. [Google Scholar] [CrossRef]
- Boelen, G.-J.; Boute, L.; d’Hoop, J.; EzEldeen, M.; Lambrichts, I.; Opdenakker, G. Matrix Metalloproteinases and Inhibitors in Dentistry. Clin. Oral Investig. 2019, 23, 2823–2835. [Google Scholar] [CrossRef]
- Anshida, V.; Kumari, R.; Murthy, C.; Samuel, A. Extracellular Matrix Degradation by Host Matrix Metalloproteinases in Restorative Dentistry and Endodontics: An Overview. J. Oral Maxillofac. Pathol. 2020, 24, 352. [Google Scholar] [CrossRef]
- Elgezawi, M.; Haridy, R.; Almas, K.; Abdalla, M.A.; Omar, O.; Abuohashish, H.; Elembaby, A.; Christine Wölfle, U.; Siddiqui, Y.; Kaisarly, D. Matrix Metalloproteinases in Dental and Periodontal Tissues and Their Current Inhibitors: Developmental, Degradational and Pathological Aspects. Int. J. Mol. Sci. 2022, 23, 8929. [Google Scholar] [CrossRef]
- Sandoval, N.G.; Lima, N.S.C.; Bautz, W.G. Matrix Metalloproteinase 2: A Possible Role inTooth Development and Eruption. Odovtos-Int. J. Dent. Sci. 2019, 21, 41–51. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, J.; Seymen, F.; Koruyucu, M.; Gencay, K.; Shin, T.J.; Hyun, H.-K.; Lee, Z.H.; Hu, J.C.-C.; Simmer, J.P.; et al. Analyses of MMP20 Missense Mutations in Two Families with Hypomaturation Amelogenesis Imperfecta. Front. Physiol. 2017, 8, 229. [Google Scholar] [CrossRef]
- Nikolopoulos, G.; Smith, C.E.L.; Poulter, J.A.; Murillo, G.; Silva, S.; Lamb, T.; Berry, I.R.; Brown, C.J.; Day, P.F.; Soldani, F. Spectrum of Pathogenic Variants and Founder Effects in Amelogenesis Imperfecta Associated with MMP20. Hum. Mutat. 2021, 42, 567–576. [Google Scholar] [CrossRef]
- Pescetto, N.; Céspedes, A.; Bologna, M.; Pereira-Prado, V. Molecular mechanisms of amelogenesis imperfecta. A review of the ENAM, AMBN, FAM83H, MMP20, and KLK4 genes. Odontoestomatología 2021, 38, e306. [Google Scholar]
- Azevedo, A.; Prado, A.F.; Antonio, R.C.; Issa, J.P.; Gerlach, R.F. Matrix Metalloproteinases Are Involved in Cardiovascular Diseases. Basic Clin. Pharmacol. Toxicol. 2014, 115, 301–314. [Google Scholar] [CrossRef]
- Brkic, M.; Balusu, S.; Libert, C.; Vandenbroucke, R.E. Friends or Foes: Matrix Metalloproteinases and Their Multifaceted Roles in Neurodegenerative Diseases. Mediat. Inflamm. 2015, 2015, 620581. [Google Scholar] [CrossRef]
- Tokuhara, C.K.; Santesso, M.R.; Oliveira, G.S.N.D.; Ventura, T.M.D.S.; Doyama, J.T.; Zambuzzi, W.F.; Oliveira, R.C.D. Updating the Role of Matrix Metalloproteinases in Mineralized Tissue and Related Diseases. J. Appl. Oral Sci. 2019, 27, e20180596. [Google Scholar] [CrossRef]
- Zakiyanov, O.; Kalousová, M.; Zima, T.; Tesař, V. Matrix Metalloproteinases in Renal Diseases: A Critical Appraisal. Kidney Blood Press Res. 2019, 44, 298–330. [Google Scholar] [CrossRef]
- Hannas, A.R.; Pereira, J.C.; Granjeiro, J.M.; Tjäderhane, L. The Role of Matrix Metalloproteinases in the Oral Environment. Acta Odontol. Scand. 2007, 65, 1–13. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Kato, M.T.; Hannas, A.R. The Role of Matrix Metalloproteinases in Dental Erosion. Adv. Dent. Res. 2012, 24, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of Matrix Metalloproteinases in Cancer Progression and Their Pharmacological Targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Morad, G.; Jedinak, A.; Moses, M.A. Metalloproteinases and Their Roles in Human Cancer. Anat. Rec. 2020, 303, 1557–1572. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Mackay, A. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers 2014, 6, 240–296. [Google Scholar] [CrossRef]
- Takata, T.; Zhao, M.; Uchida, T.; Wang, T.; Aoki, T.; Bartlett, J.D.; Nikai, H. Immunohistochemical Detection and Distribution of Enamelysin (MMP-20) in Human Odontogenic Tumors. J. Dent. Res. 2000, 79, 1608–1613. [Google Scholar] [CrossRef]
- Farias, L.C.; Gomes, C.C.; Rodrigues, M.C.; De Castro, W.H.; Lacerda, J.C.T.; E Ferreira, E.F.; Gomez, R.S. Epigenetic Regulation of Matrix Metalloproteinase Expression in Ameloblastoma. BMC Clin. Pathol. 2012, 12, 11. [Google Scholar] [CrossRef]
- Ribeiro, B.F.; Ferreira De Araújo, C.R.; Dos Santos, B.R.M.; De Almeida Freitas, R. Immunohistochemical Expression of Matrix Metalloproteinases 1, 2, 7, 9, and 26 in the Calcifying Cystic Odontogenic Tumor. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, 609–615. [Google Scholar] [CrossRef]
- Zhou, Y.-M.; Zhong, Q.-B.; Ye, K.-N.; Wang, H.-Y.; Ren, Z.-H. Expression of Matrix Metalloproteinases in Ameloblastomas and Ameloblastic Carcinoma: Systematic Review and Meta-Analysis. Explor. Res. Hypothesis Med. 2019, 4, 19–28. [Google Scholar] [CrossRef]
- Anne, R.; Krisnuhoni, E.; Chotimah, C.; Latief, B.S. Matrix Metalloproteinase-9 (MMP-9) Expression in Different Subtypes of Ameloblastoma. J. Maxillofac. Oral Surg. 2014, 13, 281–285. [Google Scholar] [CrossRef]
- Atanasova, T.; Stankova, T.; Bivolarska, A.; Vlaykova, T. Matrix Metalloproteinases in Oral Health—Special Attention on MMP-8. Biomedicines 2023, 11, 1514. [Google Scholar] [CrossRef]
- Bertini, I.; Calderone, V.; Fragai, M.; Luchinat, C.; Maletta, M.; Yeo, K.J. Snapshots of the Reaction Mechanism of Matrix Metalloproteinases. Angew. Chem. Int. Ed. 2006, 45, 7952–7955. [Google Scholar] [CrossRef]
- Stawowczyk, M.; Wellenstein, M.D.; Lee, S.B.; Yomtoubian, S.; Durrans, A.; Choi, H.; Narula, N.; Altorki, N.K.; Gao, D.; Mittal, V. Matrix Metalloproteinase 14 Promotes Lung Cancer by Cleavage of Heparin-Binding EGF-like Growth Factor. Neoplasia 2016, 19, 55–64. [Google Scholar] [CrossRef]
- Hernandez-Rios, P.; Hernandez, M.; Garrido, M.; Tervahartiala, T.; Leppilahti, J.; Kuula, H.; Heikkinen, A.M.; Mäntylä, P.; Rathnayake, N.; Nwhator, S.; et al. Oral Fluid Matrix Metalloproteinase (MMP)-8 as a Diagnostic Tool in Chronic Periodontitis. Met. Med. 2016, 3, 11–18. [Google Scholar]
- Ala-aho, R.; Kähäri, V.-M. Collagenases in Cancer. Biochimie 2005, 87, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Sasano, Y.; Zhu, J.X.; Tsubota, M.; Takahashi, I.; Onodera, K.; Mizoguchi, I.; Kagayama, M. Gene expression of MMP8 and MMP13 during embryonic development of bone and cartilage in the rat mandible and hind limb. J. Histochem. Cytochem. 2002, 50, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Nwomeh, B.C.; Liang, H.X.; Diegelmann, R.F.; Cohen, I.K.; Yager, D.R. Dynamics of the Matrix Metalloproteinases MMP-1 and MMP-8 in Acute Open Human Dermal Wounds. Wound Repair Regen. 1998, 6, 127–134. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 714–770. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.G.; Cha, H.J.; Song, S.H.; Park, J.W.; Suh, J.Y.; Lee, J.M. MMP-2, MMP-8 Expression in Gingival Tissue of Chronic Periodontitis Associated to Type 2 Diabetes Mellitus. J. Korean Acad. Periodontol. 2005, 35, 661. [Google Scholar] [CrossRef]
- Räisänen, I.T.; Umeizudike, K.A.; Pärnänen, P.; Heikkilä, P.; Tervahartiala, T.; Nwhator, S.O.; Grigoriadis, A.; Sakellari, D.; Sorsa, T. Periodontal Disease and Targeted Prevention Using aMMP-8 Point-of-Care Oral Fluid Analytics in the COVID-19 Era. Med. Hypotheses 2020, 144, 110276. [Google Scholar] [CrossRef] [PubMed]
- Leppilahti, J.M.; Hernández-Ríos, P.A.; Gamonal, J.A.; Tervahartiala, T.; Brignardello-Petersen, R.; Mantyla, P.; Sorsa, T.; Hernández, M. Matrix Metalloproteinases and Myeloperoxidase in Gingival Crevicular Fluid Provide Site-specific Diagnostic Value for Chronic Periodontitis. J. Clin. Periodontol. 2014, 41, 348–356. [Google Scholar] [CrossRef]
- Hernández, M.; Baeza, M.; Räisänen, I.T.; Contreras, J.; Tervahartiala, T.; Chaparro, A.; Sorsa, T.; Hernández-Ríos, P. Active MMP-8 Quantitative Test as an Adjunctive Tool for Early Diagnosis of Periodontitis. Diagnostics 2021, 11, 1503. [Google Scholar] [CrossRef]
- Rahnama, M.; Czupkałło, Ł.; Kozicka-Czupkałło, M.; Łobacz, M. Gingival Crevicular Fluid—Composition and Clinical Importance in Gingivitis and Periodontitis. Pol. J. Public Health 2014, 124, 96–98. [Google Scholar] [CrossRef]
- Komala, O.N.; Lessang, R.; Sunarto, H.; Bachtiar, B.M.; Soeroso, Y. Effect of Scaling and Root Planing Based on MMP-8 mRNA Expression and Clinical Parameters in Periodontitis Patients. J. Int. Dent. Med. Res. 2019, 12, 1068–1073. [Google Scholar]
- Ramenzoni, L.L.; Hofer, D.; Solderer, A.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Origin of MMP-8 and Lactoferrin Levels from Gingival Crevicular Fluid, Salivary Glands and Whole Saliva. BMC Oral Health 2021, 21, 385. [Google Scholar] [CrossRef]
- Fatemi, K.; Rezaee, S.A.; Banihashemrad, S.A.; Keyvanfar, S.; Eslami, M. Importance of MMP-8 in Salivary and Gingival Crevicular Fluids of Periodontitis Patients. Iran. J. Immunol. 2020, 17, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Hernández, M.; Leppilahti, J.; Munjal, S.; Netuschil, L.; Mäntylä, P. Detection of Gingival Crevicular Fluid MMP-8 Levels with Different Laboratory and Chair-Side Methods: Detection of GCF MMP-8 with Different Methods. Oral Dis. 2010, 16, 39–45. [Google Scholar] [CrossRef]
- Mauramo, M.; Ramseier, A.; Mauramo, E.; Buser, A.; Tervahartiala, T.; Sorsa, T.; Waltimo, T. Associations of Oral Fluid MMP -8 with Periodontitis in Swiss Adult Subjects. Oral Dis. 2018, 24, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Nędzi-Góra, M.; Górska, R.; Kostrzewa-Janicka, J.; Kowalski, J. Concentration of MMP-8 and IL-1β in Gingival Crevicular Fluid in Patients with Chronic and Aggressive Periodontitis. Cent. Eur. J. Immunol. 2017, 42, 342–346. [Google Scholar] [CrossRef]
- Nędzi-Góra, M.; Górska, R.; Górski, B. The Utility of Gingival Crevicular Fluid Matrix Metalloproteinase-8 Provides Site-Specific Diagnostic Value for Periodontal Grading. Cent. Eur. J. Immunol. 2021, 46, 236–243. [Google Scholar] [CrossRef]
- Sopi, M.; Koçani, F.; Bardhoshi, M.; Meqa, K. The Effect of Periodontal Therapy on the Level of MMP-8 in Patients with Chronic Periodontitis. Eur. J. Dent. 2023, 17, 70–75. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, X.; Zheng, S. Matrix Metalloproteinase-8 Levels in Oral Samples as a Biomarker for Periodontitis in the Chinese Population: An Observational Study. BMC Oral Health 2018, 18, 51. [Google Scholar] [CrossRef]
- Konopka, Ł.; Pietrzak, A.; Brzezińska-Błaszczyk, E. Effect of Scaling and Root Planing on Interleukin-1β, Interleukin-8 and MMP-8 Levels in Gingival Crevicular Fluid from Chronic Periodontitis Patients. J. Periodontal Res. 2012, 47, 681–688. [Google Scholar] [CrossRef]
- Mc Crudden, M.T.C.; Irwin, C.R.; El Karim, I.; Linden, G.J.; Lundy, F.T. Matrix Metalloproteinase-8 Activity in Gingival Crevicular Fluid: Development of a Novel Assay. J. Periodontal. Res. 2017, 52, 556–561. [Google Scholar] [CrossRef]
- Marcaccini, A.M.; Meschiari, C.A.; Zuardi, L.R.; De Sousa, T.S.; Taba, M.; Teofilo, J.M.; Jacob-Ferreira, A.L.B.; Tanus-Santos, J.E.; Novaes, A.B.; Gerlach, R.F. Gingival Crevicular Fluid Levels of MMP-8, MMP-9, TIMP-2, and MPO Decrease after Periodontal Therapy. J. Clin. Periodontol. 2010, 37, 180–190. [Google Scholar] [CrossRef]
- Ingman, T.; Tervahartiala, T.; Ding, Y.; Tschesche, H.; Haerian, A.; Kinane, D.F.; Konttinen, Y.T.; Sorsa, T. Matrix Metalloproteinases and Their Inhibitors in Gingival Crevicular Fluid and Saliva of Periodontitis Patients. J. Clin. Periodontol. 1996, 23, 1127–1132. [Google Scholar] [CrossRef]
- Chen, H.Y.; Cox, S.W.; Eley, B.M.; Mäntylä, P.; Rönkä, H.; Sorsa, T. Matrix Metalloproteinase-8 Levels and Elastase Activities in Gingival Crevicular Fluid from Chronic Adult Periodontitis Patients. J. Clin. Periodontol. 2000, 27, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Leppilahti, J.M.; Kallio, M.A.; Tervahartiala, T.; Sorsa, T.; Mäntylä, P. Gingival Crevicular Fluid Matrix Metalloproteinase-8 Levels Predict Treatment Outcome Among Smokers with Chronic Periodontitis. J. Periodontol. 2014, 85, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Yakob, M.; Meurman, J.; Sorsa, T.; Söder, B. Treponema Denticola Associates with Increased Levels of MMP -8 and MMP -9 in Gingival Crevicular Fluid. Oral Dis. 2013, 19, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Nardi, G.M.; Cesarano, F.; Papa, G.; Chiavistelli, L.; Ardan, R.; Jedlinski, M.; Mazur, M.; Grassi, R.; Grassi, F.R. Evaluation of Salivary Matrix Metalloproteinase (MMP-8) in Periodontal Patients Undergoing Non-Surgical Periodontal Therapy and Mouthwash Based on Ozonated Olive Oil: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 6619. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Darby, I.B.; Said, S.; Luoto, H.; Sorsa, T.; Tikanoja, S.; Mäntylä, P. Changes in Gingival Crevicular Fluid Matrix Metalloproteinase-8 Levels during Periodontal Treatment and Maintenance. J. Periodontal Res. 2003, 38, 400–404. [Google Scholar] [CrossRef]
- Hernández, M.; Gamonal, J.; Tervahartiala, T.; Mäntylä, P.; Rivera, O.; Dezerega, A.; Dutzan, N.; Sorsa, T. Associations Between Matrix Metalloproteinase-8 and -14 and Myeloperoxidase in Gingival Crevicular Fluid from Subjects With Progressive Chronic Periodontitis: A Longitudinal Study. J. Periodontol. 2010, 81, 1644–1652. [Google Scholar] [CrossRef]
- Emingil, G.; Atilla, G.; Sorsa, T.; Luoto, H.; Kirilmaz, L.; Baylas, H. The Effect of Adjunctive Low-Dose Doxycycline Therapy on Clinical Parameters and Gingival Crevicular Fluid Matrix Metalloproteinase-8 Levels in Chronic Periodontitis. J. Periodontol. 2004, 75, 106–115. [Google Scholar] [CrossRef]
- Choi, D.; Moon, I.; Choi, B.; Paik, J.; Kim, Y.; Choi, S.; Kim, C. Effects of Sub-antimicrobial Dose Doxycycline Therapy on Crevicular Fluid MMP-8, and Gingival Tissue MMP-9, TIMP-1 and IL-6 Levels in Chronic Periodontitis. J. Periodontal Res. 2004, 39, 20–26. [Google Scholar] [CrossRef]
- Buduneli, N.; Vardar, S.; Atilla, G.; Sorsa, T.; Luoto, H.; Baylas, H. Gingival Crevicular Fluid Matrix Metalloproteinase-8 Levels Following Adjunctive Use of Meloxicam and Initial Phase of Periodontal Therapy. J. Periodontol. 2002, 73, 103–109. [Google Scholar] [CrossRef] [PubMed]
- AlMudaris, I.Z.; AlRawi, N.A. Salivary Matrix Metalloproteinase-8 (MMP-8) in Relation to Periodontal Health Status Among a Group of Hypertensive Patients. J. Bagh. Coll. Dent. 2018, 30, 48–53. [Google Scholar] [CrossRef]
- Banaz, J.A.; Hassan, B.K.; Athraa, A.M. The assessment of MMP-8 among different stages of periodontitis in the Iraqi population. J. Emerg. Med. 2023, 3, 1986–1999. [Google Scholar]
- Mohammed, H.A.; Abdulkareem, A.A.; Zardawi, F.M.; Gul, S.S. Determination of the Accuracy of Salivary Biomarkers for Periodontal Diagnosis. Diagnostics 2022, 12, 2485. [Google Scholar] [CrossRef]
- Gürsoy, U.K.; Könönen, E.; Tervahartiala, T.; Gürsoy, M.; Pitkänen, J.; Torvi, P.; Suominen, A.L.; Pussinen, P.; Sorsa, T. Molecular Forms and Fragments of Salivary MMP-8 in Relation to Periodontitis. J. Clin. Periodontol. 2018, 45, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Górska, R.; Nędzi-Góra, M. The Effects of the Initial Treatment Phase and of Adjunctive Low-Dose Doxycycline Therapy on Clinical Parameters and MMP-8, MMP-9, and TIMP-1 Levels in the Saliva and Peripheral Blood of Patients with Chronic Periodontitis. Arch. Immunol. Ther. Exp. 2006, 54, 419–426. [Google Scholar] [CrossRef]
- Alassiri, S.; Parnanen, P.; Rathnayake, N.; Johannsen, G.; Heikkinen, A.-M.; Lazzara, R.; Van Der Schoor, P.; Van Der Schoor, J.G.; Tervahartiala, T.; Gieselmann, D.; et al. The Ability of Quantitative, Specific, and Sensitive Point-of-Care/Chair-Side Oral Fluid Immunotests for aMMP-8 to Detect Periodontal and Peri-Implant Diseases. Dis. Markers 2018, 2018, 1306396. [Google Scholar] [CrossRef]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J. Analysis of Matrix Metalloproteinases, Especially MMP-8, in Gingival Crevicular Fluid, Mouthrinse and Saliva for Monitoring Periodontal Diseases. Periodontology 2000 2016, 70, 142–163. [Google Scholar] [CrossRef]
- Sorsa, T.; Alassiri, S.; Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Nwhator, S.O.; Gieselmann, D.-R.; Sakellari, D. Active MMP-8 (aMMP-8) as a Grading and Staging Biomarker in the Periodontitis Classification. Diagnostics 2020, 10, 61. [Google Scholar] [CrossRef]
- Räisänen, I.; Sorsa, T.; Van Der Schoor, G.-J.; Tervahartiala, T.; Van Der Schoor, P.; Gieselmann, D.-R.; Heikkinen, A. Active Matrix Metalloproteinase-8 Point-of-Care (PoC)/Chairside Mouthrinse Test vs. Bleeding on Probing in Diagnosing Subclinical Periodontitis in Adolescents. Diagnostics 2019, 9, 34. [Google Scholar] [CrossRef]
- Heikkinen, A.M.; Raivisto, T.; Kettunen, K.; Kovanen, L.; Haukka, J.; Pakbaznejad Esmaeili, E.; Elg, J.; Gieselmann, D.; Rathnayake, N.; Ruokonen, H.; et al. Pilot Study on the Genetic Background of an Active Matrix Metalloproteinase-8 Test in Finnish Adolescents. J. Periodontol. 2017, 88, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, I.; Heikkinen, A.; Siren, E.; Tervahartiala, T.; Gieselmann, D.-R.; Van Der Schoor, G.-J.; Van Der Schoor, P.; Sorsa, T. Point-of-Care/Chairside aMMP-8 Analytics of Periodontal Diseases’ Activity and Episodic Progression. Diagnostics 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Hübscher, A.E.; Angermann, H.; Schmidt, J.; Schmickler, J.; Legler, T.J.; Ziebolz, D. Associations of Chairside Salivary aMMP-8 Findings with Periodontal Parameters, Potentially Periodontal Pathogenic Bacteria and Selected Blood Parameters in Systemically Healthy Adults. Diagn. Microbiol. Infect. Dis. 2019, 95, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Raivisto, T.; Sorsa, T.; Räisänen, I.; Kauppila, T.; Ruokonen, H.; Tervahartiala, T.; Haukka, J.; Heikkinen, A.M. Active Matrix Metalloproteinase-8 Chair Side Mouth Rinse Test, Health Behaviour and Oral Health in Finnish Adolescent Cohort. J. Clin. Diagn. Res. 2020, 14, ZC35–ZC39. [Google Scholar] [CrossRef]
- Lähteenmäki, H.; Pätilä, T.; Pärnänen, P.; Räisänen, I.; Tervahartiala, T.; Gupta, S.; Sorsa, T. aMMP-8 Point-of-Care—Diagnostic Methods and Treatment Modalities in Periodontitis and Peri-Implantitis. Expert Opin. Ther. Targets 2023, 27, 627–637. [Google Scholar] [CrossRef]
- Öztürk, V.Ö.; Emingil, G.; Umeizudike, K.; Tervahartiala, T.; Gieselmann, D.-R.; Maier, K.; Köse, T.; Sorsa, T.; Alassiri, S. Evaluation of Active Matrix Metalloproteinase-8 (aMMP-8) Chair-Side Test as a Diagnostic Biomarker in the Staging of Periodontal Diseases. Arch. Oral Biol. 2021, 124, 104955. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, N.D.; Gupta, A.; Khan, S.; Bansal, N. Role of Salivary Matrix Metalloproteinase-8 (MMP-8) in Chronic Periodontitis Diagnosis. Front. Med. 2015, 9, 72–76. [Google Scholar] [CrossRef]
- Kim, H.-N. Changes in Salivary Matrix Metalloproteinase-3, -8, and -9 Concentrations after 6 Weeks of Non-Surgical Periodontal Therapy. BMC Oral Health 2022, 22, 175. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.H.; Ho, Y.P.; Lin, Y.C.; Hu, K.F.; Yang, Y.H.; Ho, K.Y.; Wu, W.M.; Hsi, E.; Tsai, C.C. MMP-8 -799 C>T genetic polymorphism is associated with the susceptibility to chronic and aggressive periodontitis in Taiwanese. J. Clin. Periodontol. 2011, 38, 1078–1084. [Google Scholar] [CrossRef]
- Emingil, G.; Han, B.; Gürkan, A.; Berdeli, A.; Tervahartiala, T.; Salo, T.; Pussinen, P.J.; Köse, T.; Atilla, G.; Sorsa, T. Matrix Metalloproteinase (MMP)-8 and Tissue Inhibitor of MMP-1 (TIMP-1) Gene Polymorphisms in Generalized Aggressive Periodontitis: Gingival Crevicular Fluid MMP-8 and TIMP-1 Levels and Outcome of Periodontal Therapy. J. Periodontol. 2014, 85, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Izakovicova Holla, L.; Hrdlickova, B.; Vokurka, J.; Fassmann, A. Matrix Metalloproteinase 8 (MMP8) Gene Polymorphisms in Chronic Periodontitis. Arch. Oral Biol. 2012, 57, 188–196. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Singh, P.; Ajmera, D.H.; Song, J.; Ji, P. Association of Common Variants in MMPs with Periodontitis Risk. Dis. Markers 2016, 2016, 1545974. [Google Scholar] [CrossRef]
- Heikkinen, A.M.; Kettunen, K.; Kovanen, L.; Haukka, J.; Elg, J.; Husu, H.; Tervahartiala, T.; Pussinen, P.; Meurman, J.; Sorsa, T. Inflammatory Mediator Polymorphisms Associate with Initial Periodontitis in Adolescents. Clin. Exp Dent. Res 2016, 2, 208–215. [Google Scholar] [CrossRef]
- Putri, H.; Sulijaya, B.; Hartomo, B.T.; Suhartono, A.W.; Auerkari, E.I. +17 C/G Polymorphism in Matrix Metalloproteinase (MMP)-8 Gene and Its Association with Periodontitis. J. Stomatol. 2020, 73, 154–158. [Google Scholar] [CrossRef]
- Majumder, P.; Ghosh, S.; Dey, S.K. Matrix metalloproteinase gene polymorphisms in chronic periodontitis: A case-control study in the Indian population. J. Genet. 2019, 98, 32. [Google Scholar] [CrossRef] [PubMed]


| Summary of the Usefulness of MMP-8 in Different Types of Biological Material in Patients with Periodontitis |
|---|
| Gingival fluid |
|
| Saliva |
|
| Oral rinse fluid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewska, E.A.; Ławicka, R.; Grygorczuk, P.; Nowosielska, M.; Kicman, A.; Ławicki, S. Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis. Int. J. Mol. Sci. 2024, 25, 2721. https://doi.org/10.3390/ijms25052721
Zalewska EA, Ławicka R, Grygorczuk P, Nowosielska M, Kicman A, Ławicki S. Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis. International Journal of Molecular Sciences. 2024; 25(5):2721. https://doi.org/10.3390/ijms25052721
Chicago/Turabian StyleZalewska, Emilia Anna, Renata Ławicka, Piotr Grygorczuk, Magdalena Nowosielska, Aleksandra Kicman, and Sławomir Ławicki. 2024. "Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis" International Journal of Molecular Sciences 25, no. 5: 2721. https://doi.org/10.3390/ijms25052721
APA StyleZalewska, E. A., Ławicka, R., Grygorczuk, P., Nowosielska, M., Kicman, A., & Ławicki, S. (2024). Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis. International Journal of Molecular Sciences, 25(5), 2721. https://doi.org/10.3390/ijms25052721





