Calcium and Neural Stem Cell Proliferation
Abstract
:1. Introduction
2. Calcium and Cell Cycle Regulation
3. G-Protein-Coupled Receptors and Calcium Signaling
4. Transitory Receptor Potential Channels and NSC Proliferation
4.1. TRPCs
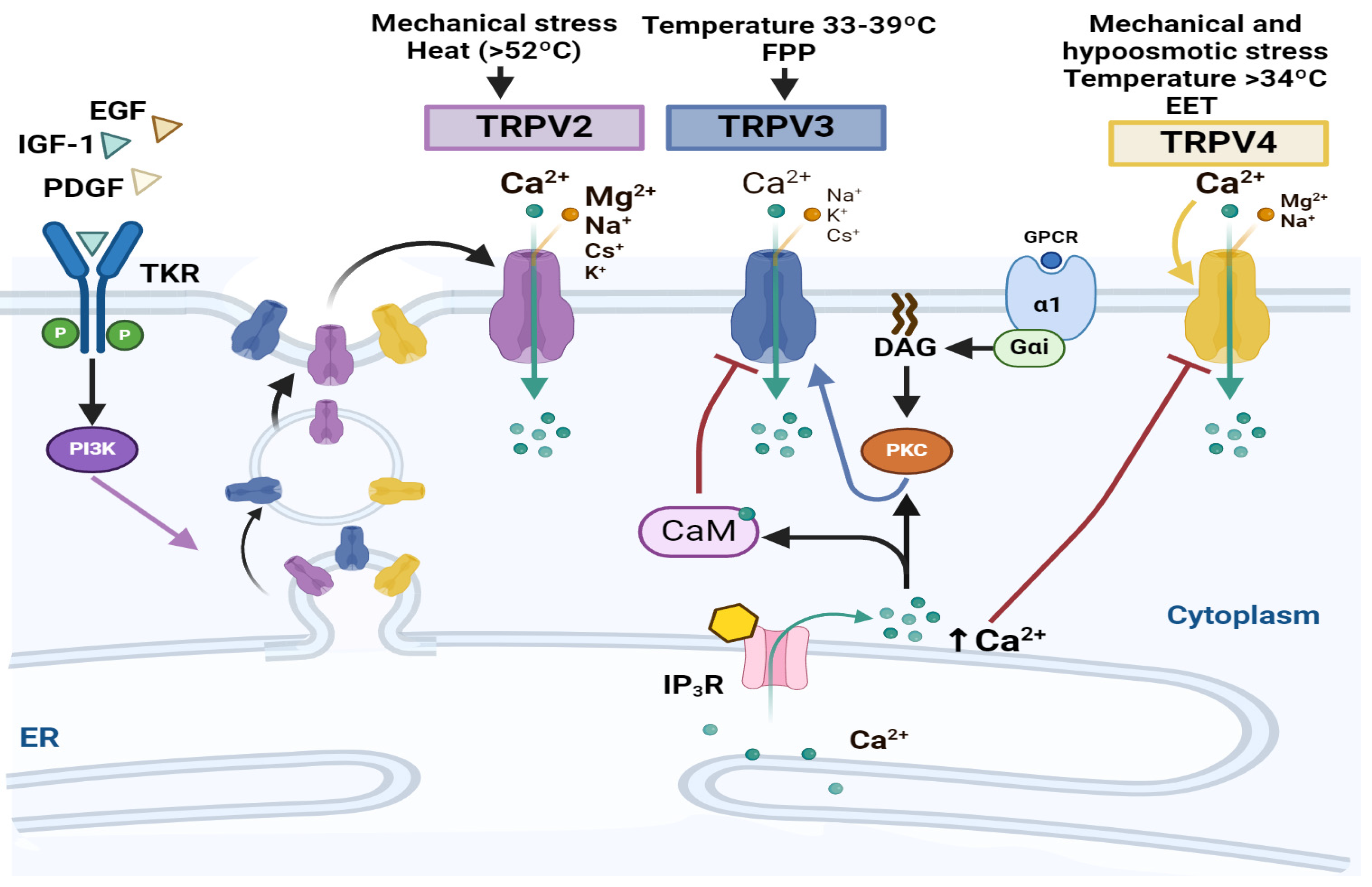
4.2. TRPVs
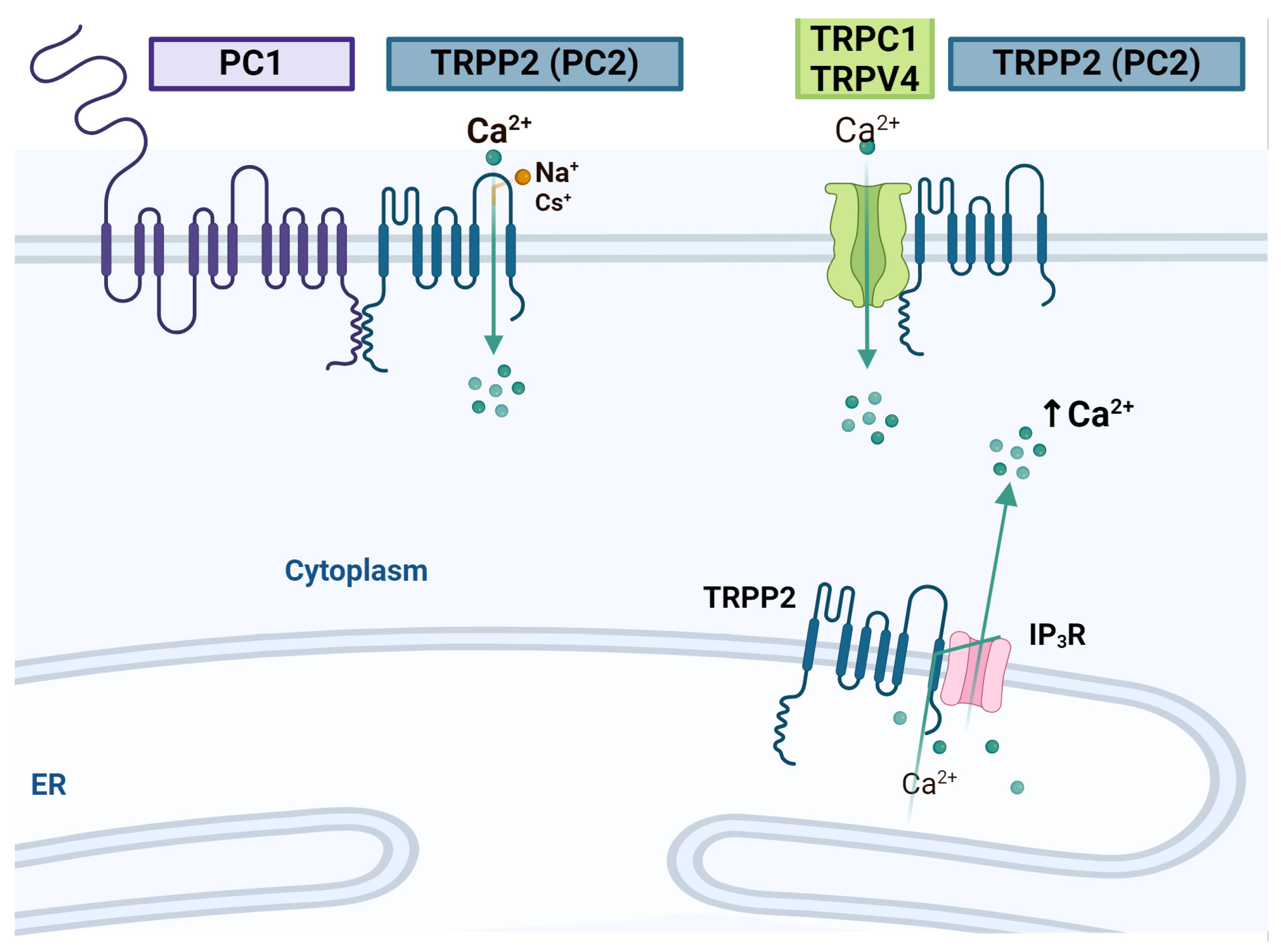
4.3. TRPPs
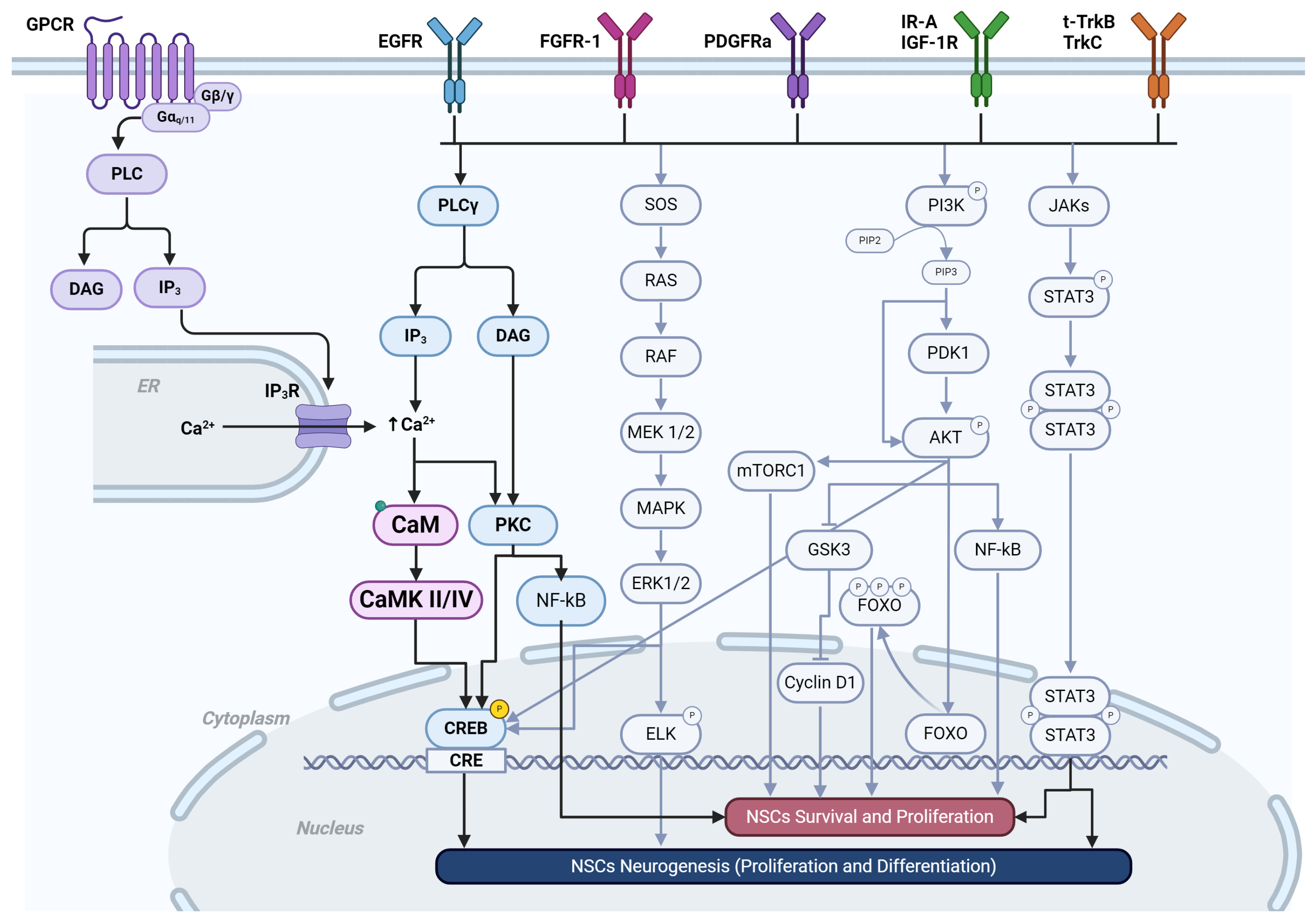
5. Receptor Tyrosine Kinases
| RTK | Role | Ligands/Agonists | Antagonists |
|---|---|---|---|
| EGFR (ErbB) | Increases NSC proliferation, migration, and survival in vivo and in vitro [209,210,211]. Promotes oligodendrocyte differentiation of NSCs in vivo [198,212]. The knockout mice show the atrophy of the anterior cerebral cortex in vivo [213] | EGF, TGF-α [209], Amphiregulin, Betacellulin, Heparin-binding EGF-like growth factor [214] Epiregulin, Epigen [215], Neuroregulins [211] | Cetuximab, Panitumumab [214], Trastuzumab, Pertuzumab, ABX-EGF, EMD-7200, h-R3, ICR-62, ZD1839 (Gefitinib Iressa), OSI-774 (Erlotinib), Lapatinib (GW572016, GW2016), EKB-569, AEE788, BMS-599626, AZD 9291, Dacomitinib, Afatinib, CO-1686, Neratinib, Canertinib, AC-480, AZD 8931, AST 1306 [216] |
| FGFR1 | Maintains the self-renewal of NSCs [217]. NSC proliferation and neurogenesis in the developing cerebral cortex [169] Its deletion, together with FGFR2 and FGFR3, leads to Foxg1-positive precursors telencephalic cell death, resulting in the loss of the basal ganglia and cortex in vivo [218]. | FGF-1, -2, -4, -6, -7, -8, -10, -16, -17, -18, -22 [219] | Derazantinib (ARQ087, ASP5878, AZD4547), Infigratinib (BGJ398), Debio-1347, Dovitinib [220,221], Brivanib (BMS-582664), BMS-540215, E-3810 (AL3810), NP603, LY2874455, Fisogatinib (CH518328, Debio 1347, E7090), Rogaratinib (BAY1163877), Futibatinib (TAS-120), Pemigatinib (INCB054828), Erdafitinib (JNJ-42756493) [221] |
| PDGFRα | The knocking down or blocking antibodies of PDGFRα suppresses the proliferation of NSCs and increases the cell death rate [222]. | PDGF-A, PDGF-B, PDGF-AB, PDGF-C [223] | Dasatinib, Masitinib [224], Axitinib [225], Sorafenib, Pazopanib, Cediranib [225,226] Imatinib, Sunitinib, Nilotinib [224,225,226] |
| IGF-1R C | The knocking down of the receptor reduces NSC proliferation, stunts brain growth, and decreases the neuronal number [227]. | IGF-II, Insulin, IGF-I (Somatomedin C) [228] | NVP-ADW742, α-IR3, JB-1 [229] NVP-AEW541 [230], MAB391, OSI-906 [231] |
| TrkB (Ntrk2) | Promotes NSC survival and proliferation [232] in cortical precursors in vivo and promotes proliferation and enhanced neurogenesis [233]. | BDNF [232,233], NT-4, NT-3 [234], L-783,281 [235] Amitriptyline, 7,8-Dihydroxyflavone, Deoxygedunin, Paecilomycine A [236] | Larotrectinib, Entrectinib, Selitrectinib (LOXO-195), Altiratinib, DS-6051b, Lestaurtinib, Merestinib, MGCD516, PLX7486, ONO-5390556, TPX-0005, Repotrectinib [237] |
| TrkC (Ntrk3) | Increases neuron differentiation in vitro [238] and NSC proliferation in vitro and in vivo [233]. | NT-3 [234,238], L-783,281 [235] | Larotrectinib, Entrectinib, Selitrectinib (LOXO-195), Repotrectinib, Altiratinib, Crizotinib, DS-6051b, Lestaurtinib, Merestinib, MGCD516, TSR-011, ONO-5390556, TPX-0005 [237] |
6. Gap Junctions
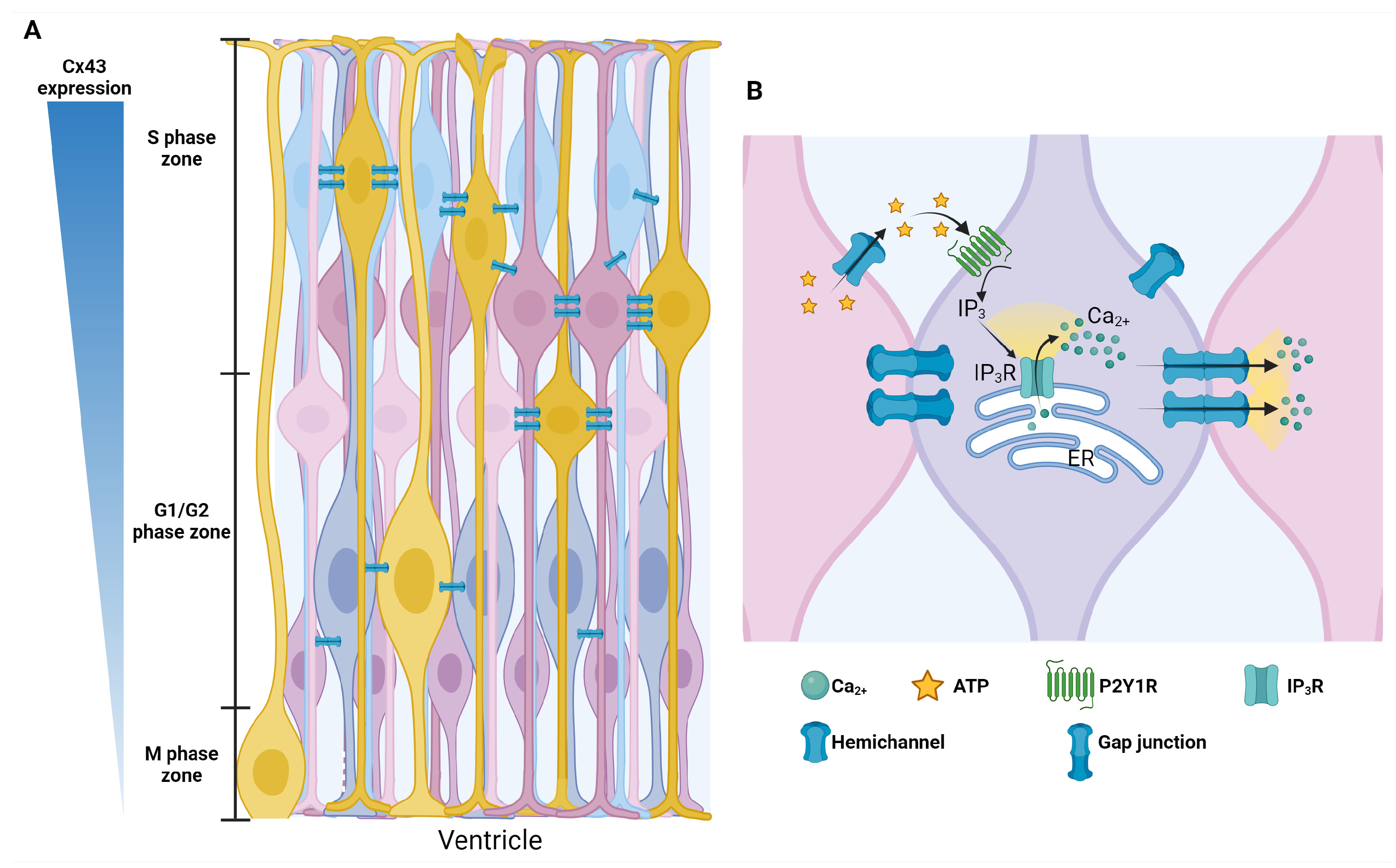
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noctor, S.C.; Martinez-Cerdeno, V.; Ivic, L.; Kriegstein, A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004, 7, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Noctor, S.C.; Martinez-Cerdeno, V.; Kriegstein, A.R. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J. Comp. Neurol. 2008, 508, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Lodato, S.; Arlotta, P. Generating neuronal diversity in the mammalian cerebral cortex. Annu. Rev. Cell Dev. Biol. 2015, 31, 699–720. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Nardelli, J. Cellular and molecular introduction to brain development. Neurobiol. Dis. 2016, 92, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A.R.; Gotz, M. Radial glia diversity: A matter of cell fate. Glia 2003, 43, 37–43. [Google Scholar] [CrossRef]
- Tan, X.; Shi, S.H. Neocortical neurogenesis and neuronal migration. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 443–459. [Google Scholar] [CrossRef]
- Gotz, M.; Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 777–788. [Google Scholar] [CrossRef]
- Huttner, W.B.; Kosodo, Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr. Opin. Cell Biol. 2005, 17, 648–657. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Leibovitz, Z.; Lerman-Sagie, T.; Haddad, L. Fetal Brain Development: Regulating Processes and Related Malformations. Life 2022, 12, 809. [Google Scholar] [CrossRef]
- Owens, D.F.; Flint, A.C.; Dammerman, R.S.; Kriegstein, A.R. Calcium dynamics of neocortical ventricular zone cells. Dev. Neurosci. 2000, 22, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.F.; Kriegstein, A.R. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J. Neurosci. 1998, 18, 5374–5388. [Google Scholar] [CrossRef]
- Bayer, S.A.; Altman, J. Development of the endopiriform nucleus and the claustrum in the rat brain. Neuroscience 1991, 45, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nowakowski, R.S.; Caviness, V.S., Jr. Mode of cell proliferation in the developing mouse neocortex. Proc. Natl. Acad. Sci. USA 1994, 91, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nowakowski, R.S.; Caviness, V.S., Jr. The leaving or Q fraction of the murine cerebral proliferative epithelium: A general model of neocortical neuronogenesis. J. Neurosci. 1996, 16, 6183–6196. [Google Scholar] [CrossRef]
- Barrack, D.S.; Thul, R.; Owen, M.R. Modelling the coupling between intracellular calcium release and the cell cycle during cortical brain development. J. Theor. Biol. 2014, 347, 17–32. [Google Scholar] [CrossRef]
- Saran, S. Calcium levels during cell cycle correlate with cell fate of Dictyostelium discoideum. Cell Biol. Int. 1999, 23, 399–405. [Google Scholar] [CrossRef]
- Humeau, J.; Bravo-San Pedro, J.M.; Vitale, I.; Nunez, L.; Villalobos, C.; Kroemer, G.; Senovilla, L. Calcium signaling and cell cycle: Progression or death. Cell Calcium 2018, 70, 3–15. [Google Scholar] [CrossRef]
- MacKrill, J.J. Protein-protein interactions in intracellular Ca2+-release channel function. Biochem. J. 1999, 337 Pt 3, 345–361. [Google Scholar] [CrossRef]
- Guirao, B.; Meunier, A.; Mortaud, S.; Aguilar, A.; Corsi, J.M.; Strehl, L.; Hirota, Y.; Desoeuvre, A.; Boutin, C.; Han, Y.G.; et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 2010, 12, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Kahl, C.R.; Means, A.R. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr. Rev. 2003, 24, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Weissman, T.A.; Riquelme, P.A.; Ivic, L.; Flint, A.C.; Kriegstein, A.R. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 2004, 43, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; Alkon, D.L.; Ma, W. c-Src protein tyrosine kinase activity is required for muscarinic receptor-mediated DNA synthesis and neurogenesis via ERK1/2 and c-AMP-responsive element-binding protein signaling in neural precursor cells. J. Neurosci. Res. 2003, 72, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.R.; Gomes, K.N.; Adhikari, A.; Britto, L.R.; Ulrich, H. Mechanism of acetylcholine-induced calcium signaling during neuronal differentiation of P19 embryonal carcinoma cells in vitro. Cell Calcium 2008, 43, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, P.; Fritz, N.; Smedler, E.; Malmersjo, S.; Kanatani, S. Calcium signaling in neocortical development. Dev. Neurobiol. 2015, 75, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nowakowski, R.S.; Caviness, V.S., Jr. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J. Neurosci. 1995, 15, 6046–6057. [Google Scholar] [CrossRef] [PubMed]
- Calegari, F.; Huttner, W.B. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J. Cell Sci. 2003, 116, 4947–4955. [Google Scholar] [CrossRef]
- Molina-Hernandez, A.; Rodriguez-Martinez, G.; Escobedo-Avila, I.; Velasco, I. Histamine up-regulates fibroblast growth factor receptor 1 and increases FOXP2 neurons in cultured neural precursors by histamine type 1 receptor activation: Conceivable role of histamine in neurogenesis during cortical development in vivo. Neural Dev. 2013, 8, 4. [Google Scholar] [CrossRef]
- Marquez-Valadez, B.; Aquino-Miranda, G.; Quintero-Romero, M.O.; Papacostas-Quintanilla, H.; Bueno-Nava, A.; Lopez-Rubalcava, C.; Diaz, N.F.; Arias-Montano, J.A.; Molina-Hernandez, A. The Systemic Administration of the Histamine H1 Receptor Antagonist/Inverse Agonist Chlorpheniramine to Pregnant Rats Impairs the Development of Nigro-Striatal Dopaminergic Neurons. Front. Neurosci. 2019, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hernandez, A.; Velasco, I. Histamine induces neural stem cell proliferation and neuronal differentiation by activation of distinct histamine receptors. J. Neurochem. 2008, 106, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Chau, K.F.; Springel, M.W.; Broadbelt, K.G.; Park, H.Y.; Topal, S.; Lun, M.P.; Mullan, H.; Maynard, T.; Steen, H.; LaMantia, A.S.; et al. Progressive Differentiation and Instructive Capacities of Amniotic Fluid and Cerebrospinal Fluid Proteomes following Neural Tube Closure. Dev. Cell 2015, 35, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Delling, M.; DeCaen, P.G.; Doerner, J.F.; Febvay, S.; Clapham, D.E. Primary cilia are specialized calcium signalling organelles. Nature 2013, 504, 311–314. [Google Scholar] [CrossRef]
- Desmond, M.E.; Levitan, M.L. Brain expansion in the chick embryo initiated by experimentally produced occlusion of the spinal neurocoel. Anat. Rec. 2002, 268, 147–159. [Google Scholar] [CrossRef]
- Park, M.G.; Jang, H.; Lee, S.H.; Lee, C.J. Flow Shear Stress Enhances the Proliferative Potential of Cultured Radial Glial Cells Possibly Via an Activation of Mechanosensitive Calcium Channel. Exp. Neurobiol. 2017, 26, 71–81. [Google Scholar] [CrossRef]
- Rasmussen, C.D.; Means, A.R. Calmodulin is involved in regulation of cell proliferation. EMBO J. 1987, 6, 3961–3968. [Google Scholar] [CrossRef]
- Malatesta, P.; Hartfuss, E.; Gotz, M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 2000, 127, 5253–5263. [Google Scholar] [CrossRef]
- Eze, U.C.; Bhaduri, A.; Haeussler, M.; Nowakowski, T.J.; Kriegstein, A.R. Single-cell atlas of early human brain development highlights heterogeneity of human neuroepithelial cells and early radial glia. Nat. Neurosci. 2021, 24, 584–594. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Rasmussen, C.D.; Means, A.R. Calmodulin is required for cell-cycle progression during G1 and mitosis. EMBO J. 1989, 8, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Chafouleas, J.G.; Bolton, W.E.; Hidaka, H.; Boyd, A.E., 3rd; Means, A.R. Calmodulin and the cell cycle: Involvement in regulation of cell-cycle progression. Cell 1982, 28, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Chafouleas, J.G.; Lagace, L.; Bolton, W.E.; Boyd, A.E., 3rd; Means, A.R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell 1984, 36, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Hidaka, H. Calmodulin and cell proliferation. Biochem. Biophys. Res. Commun. 1982, 104, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, N.; Zhou, W.; Kumada, M.; Takuwa, Y. Ca2+-dependent stimulation of retinoblastoma gene product phosphorylation and p34cdc2 kinase activation in serum-stimulated human fibroblasts. J. Biol. Chem. 1993, 268, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, N.; Zhou, W.; Takuwa, Y. Calcium, calmodulin and cell cycle progression. Cell. Signal. 1995, 7, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, N.; Zhou, W.; Kumada, M.; Takuwa, Y. Ca2+/calmodulin is involved in growth factor-induced retinoblastoma gene product phosphorylation in human vascular endothelial cells. FEBS Lett. 1992, 306, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Enslen, H.; Myung, P.S.; Maurer, R.A. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994, 8, 2527–2539. [Google Scholar] [CrossRef]
- Ma, H.; Groth, R.D.; Cohen, S.M.; Emery, J.F.; Li, B.; Hoedt, E.; Zhang, G.; Neubert, T.A.; Tsien, R.W. γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 2014, 159, 281–294. [Google Scholar] [CrossRef]
- Tiu, S.C.; Chan, W.Y.; Heizmann, C.W.; Schafer, B.W.; Shu, S.Y.; Yew, D.T. Differential expression of S100B and S100A61 in the human fetal and aged cerebral cortex. Brain Res. Dev. Brain Res. 2000, 119, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Kosaka, K.; Ilg, E.C.; Schafer, B.W.; Heizmann, C.W.; Kosaka, T. Selective association of S100A6 (calcyclin)-immunoreactive astrocytes with the tangential migration pathway of subventricular zone cells in the rat. Brain Res. 1997, 778, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Ilg, E.C.; Schafer, B.W.; Heizmann, C.W.; Kosaka, T. Distribution of a specific calcium-binding protein of the S100 protein family, S100A6 (calcyclin), in subpopulations of neurons and glial cells of the adult rat nervous system. J. Comp. Neurol. 1999, 404, 235–257. [Google Scholar] [CrossRef]
- Yamada, J.; Jinno, S. S100A6 (calcyclin) is a novel marker of neural stem cells and astrocyte precursors in the subgranular zone of the adult mouse hippocampus. Hippocampus 2014, 24, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kuznicki, J.; Kordowska, J.; Puzianowska, M.; Wozniewicz, B.M. Calcyclin as a marker of human epithelial cells and fibroblasts. Exp. Cell Res. 1992, 200, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Weterman, M.A.; Stoopen, G.M.; van Muijen, G.N.; Kuznicki, J.; Ruiter, D.J.; Bloemers, H.P. Expression of calcyclin in human melanoma cell lines correlates with metastatic behavior in nude mice. Cancer Res. 1992, 52, 1291–1296. [Google Scholar] [PubMed]
- Weterman, M.A.; van Muijen, G.N.; Bloemers, H.P.; Ruiter, D.J. Expression of calcyclin in human melanocytic lesions. Cancer Res. 1993, 53, 6061–6066. [Google Scholar] [PubMed]
- Hirschhorn, R.R.; Aller, P.; Yuan, Z.A.; Gibson, C.W.; Baserga, R. Cell-cycle-specific cDNAs from mammalian cells temperature sensitive for growth. Proc. Natl. Acad. Sci. USA 1984, 81, 6004–6008. [Google Scholar] [CrossRef] [PubMed]
- Slomnicki, L.P.; Lesniak, W. S100A6 (calcyclin) deficiency induces senescence-like changes in cell cycle, morphology and functional characteristics of mouse NIH 3T3 fibroblasts. J. Cell. Biochem. 2010, 109, 576–584. [Google Scholar] [CrossRef]
- Santella, L.; Ercolano, E.; Nusco, G.A. The cell cycle: A new entry in the field of Ca2+ signaling. Cell. Mol. Life Sci. 2005, 62, 2405–2413. [Google Scholar] [CrossRef]
- Li, Z.; McKercher, S.R.; Cui, J.; Nie, Z.; Soussou, W.; Roberts, A.J.; Sallmen, T.; Lipton, J.H.; Talantova, M.; Okamoto, S.; et al. Myocyte enhancer factor 2C as a neurogenic and antiapoptotic transcription factor in murine embryonic stem cells. J. Neurosci. 2008, 28, 6557–6568. [Google Scholar] [CrossRef]
- Urso, K.; Fernandez, A.; Velasco, P.; Cotrina, J.; de Andres, B.; Sanchez-Gomez, P.; Hernandez-Lain, A.; Hortelano, S.; Redondo, J.M.; Cano, E. NFATc3 controls tumour growth by regulating proliferation and migration of human astroglioma cells. Sci. Rep. 2019, 9, 9361. [Google Scholar] [CrossRef]
- Gonzalez, L.; Domingo-Muelas, A.; Duart-Abadia, P.; Nunez, M.; Mikolcevic, P.; Llonch, E.; Cubillos-Rojas, M.; Canovas, B.; Forrow, S.M.A.; Morante-Redolat, J.M.; et al. The atypical CDK activator RingoA/Spy1 regulates exit from quiescence in neural stem cells. iScience 2023, 26, 106202. [Google Scholar] [CrossRef]
- Lynch, J.R.; Wang, J.Y. G Protein-Coupled Receptor Signaling in Stem Cells and Cancer. Int. J. Mol. Sci. 2016, 17, 707. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.W. Store-operated Ca2+ entry: A STIMulating stOrai. Trends Biochem. Sci. 2006, 31, 597–601. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 2009, 1793, 933–940. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Black, A.R.; Black, J.D. Protein kinase C signaling and cell cycle regulation. Front. Immunol. 2012, 3, 423. [Google Scholar] [CrossRef]
- Xing, L.; Kalebic, N.; Namba, T.; Vaid, S.; Wimberger, P.; Huttner, W.B. Serotonin Receptor 2A Activation Promotes Evolutionarily Relevant Basal Progenitor Proliferation in the Developing Neocortex. Neuron 2020, 108, 1113–1129.e6. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, V.; Mancini, F.; Mangano, G.; Salem, R.; Xia, E.; Del Grosso, E.; Bianchi, M.; Canonico, P.L.; Polenzani, L.; Grilli, M. Proneurogenic Effects of Trazodone in Murine and Human Neural Progenitor Cells. ACS Chem. Neurosci. 2017, 8, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.B.; Cui, M.; Booth, R.G.; Canal, C.E. “Selective” serotonin 5-HT2A receptor antagonists. Biochem. Pharmacol. 2022, 200, 115028. [Google Scholar] [CrossRef]
- Nelson, D.L.; Lucaites, V.L.; Wainscott, D.B.; Glennon, R.A. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1999, 359, 1–6. [Google Scholar] [CrossRef]
- Jensen, A.A.; McCorvy, J.D.; Leth-Petersen, S.; Bundgaard, C.; Liebscher, G.; Kenakin, T.P.; Brauner-Osborne, H.; Kehler, J.; Kristensen, J.L. Detailed Characterization of the In Vitro Pharmacological and Pharmacokinetic Properties of N-(2-Hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH), a Highly Selective and Brain-Penetrant 5-HT2A Receptor Agonist. J. Pharmacol. Exp. Ther. 2017, 361, 441–453. [Google Scholar] [CrossRef]
- Vamvakopoulou, I.A.; Narine, K.A.D.; Campbell, I.; Dyck, J.R.B.; Nutt, D.J. Mescaline: The forgotten psychedelic. Neuropharmacology 2023, 222, 109294. [Google Scholar] [CrossRef]
- Gardell, L.R.; Vanover, K.E.; Pounds, L.; Johnson, R.W.; Barido, R.; Anderson, G.T.; Veinbergs, I.; Dyssegaard, A.; Brunmark, P.; Tabatabaei, A.; et al. ACP-103, a 5-hydroxytryptamine 2A receptor inverse agonist, improves the antipsychotic efficacy and side-effect profile of haloperidol and risperidone in experimental models. J. Pharmacol. Exp. Ther. 2007, 322, 862–870. [Google Scholar] [CrossRef]
- Millan, M.J.; Schreiber, R.; Dekeyne, A.; Rivet, J.M.; Bervoets, K.; Mavridis, M.; Sebban, C.; Maurel-Remy, S.; Newman-Tancredi, A.; Spedding, M.; et al. S 16924 ((R)-2-[1-[2-(2,3-dihydro-benzo[1,4] dioxin-5-yloxy)-ethyl]-pyrrolidin-3yl]-1-(4-fluoro-phenyl)-ethanone), a novel, potential antipsychotic with marked serotonin (5-HT)1A agonist properties: II. Functional profile in comparison to clozapine and haloperidol. J. Pharmacol. Exp. Ther. 1998, 286, 1356–1373. [Google Scholar]
- Shapiro, D.A.; Kristiansen, K.; Kroeze, W.K.; Roth, B.L. Differential modes of agonist binding to 5-hydroxytryptamine2A serotonin receptors revealed by mutation and molecular modeling of conserved residues in transmembrane region 5. Mol. Pharmacol. 2000, 58, 877–886. [Google Scholar] [CrossRef]
- Fiorino, F.; Magli, E.; Kedzierska, E.; Ciano, A.; Corvino, A.; Severino, B.; Perissutti, E.; Frecentese, F.; Di Vaio, P.; Saccone, I.; et al. New 5-HT1A, 5HT2A and 5HT2C receptor ligands containing a picolinic nucleus: Synthesis, in vitro and in vivo pharmacological evaluation. Bioorg Med. Chem. 2017, 25, 5820–5837. [Google Scholar] [CrossRef]
- Canal, C.E.; Morgan, D.; Felsing, D.; Kondabolu, K.; Rowland, N.E.; Robertson, K.L.; Sakhuja, R.; Booth, R.G. A novel aminotetralin-type serotonin (5-HT) 2C receptor-specific agonist and 5-HT2A competitive antagonist/5-HT2B inverse agonist with preclinical efficacy for psychoses. J. Pharmacol. Exp. Ther. 2014, 349, 310–318. [Google Scholar] [CrossRef]
- Sinh, V.; Ootsuka, Y. Blockade of 5-HT2A receptors inhibits emotional hyperthermia in mice. J. Physiol. Sci. 2019, 69, 1097–1102. [Google Scholar] [CrossRef]
- Aly, S.A.; Hossain, M.; Bhuiyan, M.A.; Nakamura, T.; Nagatomo, T. Assessment of binding affinity to 5-hydroxytryptamine 2A (5-HT2A) receptor and inverse agonist activity of naftidrofuryl: Comparison with those of sarpogrelate. J. Pharmacol. Sci. 2009, 110, 445–450. [Google Scholar] [CrossRef]
- Schotte, A.; Janssen, P.F.; Gommeren, W.; Luyten, W.H.; Van Gompel, P.; Lesage, A.S.; De Loore, K.; Leysen, J.E. Risperidone compared with new and reference antipsychotic drugs: In vitro and in vivo receptor binding. Psychopharmacology 1996, 124, 57–73. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Massey, B.W. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011, 11, 59–67. [Google Scholar] [CrossRef]
- Kennett, G.A.; Pittaway, K.; Blackburn, T.P. Evidence that 5-HT2c receptor antagonists are anxiolytic in the rat Geller-Seifter model of anxiety. Psychopharmacology 1994, 114, 90–96. [Google Scholar] [CrossRef]
- Martin, J.R.; Bos, M.; Jenck, F.; Moreau, J.; Mutel, V.; Sleight, A.J.; Wichmann, J.; Andrews, J.S.; Berendsen, H.H.; Broekkamp, C.L.; et al. 5-HT2C receptor agonists: Pharmacological characteristics and therapeutic potential. J. Pharmacol. Exp. Ther. 1998, 286, 913–924. [Google Scholar]
- Smith, B.M.; Smith, J.M.; Tsai, J.H.; Schultz, J.A.; Gilson, C.A.; Estrada, S.A.; Chen, R.R.; Park, D.M.; Prieto, E.B.; Gallardo, C.S.; et al. Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J. Med. Chem. 2008, 51, 305–313. [Google Scholar] [CrossRef]
- do Carmo Silva, R.X.; do Nascimento, B.G.; Gomes, G.C.V.; da Silva, N.A.H.; Pinheiro, J.S.; da Silva Chaves, S.N.; Pimentel, A.F.N.; Costa, B.P.D.; Herculano, A.M.; Lima-Maximino, M.; et al. 5-HT2C agonists and antagonists block different components of behavioral responses to potential, distal, and proximal threat in zebrafish. Pharmacol. Biochem. Behav. 2021, 210, 173276. [Google Scholar] [CrossRef] [PubMed]
- Demireva, E.Y.; Suri, D.; Morelli, E.; Mahadevia, D.; Chuhma, N.; Teixeira, C.M.; Ziolkowski, A.; Hersh, M.; Fifer, J.; Bagchi, S.; et al. 5-HT2C receptor blockade reverses SSRI-associated basal ganglia dysfunction and potentiates therapeutic efficacy. Mol. Psychiatry 2020, 25, 3304–3321. [Google Scholar] [CrossRef]
- Millan, M.J. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: Focus on novel therapeutic strategies. Therapie 2005, 60, 441–460. [Google Scholar] [CrossRef]
- Cervo, L.; Samanin, R. 5-HT1A receptor full and partial agonists and 5-HT2C (but not 5-HT3) receptor antagonists increase rates of punished responding in rats. Pharmacol. Biochem. Behav. 1995, 52, 671–676. [Google Scholar] [CrossRef]
- Williams, B.P.; Milligan, C.J.; Street, M.; Hornby, F.M.; Deuchars, J.; Buckley, N.J. Transcription of the M1 muscarinic receptor gene in neurons and neuronal progenitors of the embryonic rat forebrain. J. Neurochem. 2004, 88, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, J.; Rauh, W.; Brantl, V.; Schloesser, R.J.; Moessner, R.; Moller, H.J.; Rujescu, D. Cholinergic impact on neuroplasticity drives muscarinic M1 receptor mediated differentiation into neurons. World J. Biol. Psychiatry 2013, 14, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Heldman, E.; Gurwitz, D.; Haring, R.; Barak, D.; Meshulam, H.; Marciano, D.; Brandeis, R.; Pittel, Z.; Segal, M.; et al. Selective signaling via unique M1 muscarinic agonists. Ann. N. Y. Acad. Sci. 1993, 695, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Bymaster, F.P.; Wong, D.T.; Mitch, C.H.; Ward, J.S.; Calligaro, D.O.; Schoepp, D.D.; Shannon, H.E.; Sheardown, M.J.; Olesen, P.H.; Suzdak, P.D.; et al. Neurochemical effects of the M1 muscarinic agonist xanomeline (LY246708/NNC11-0232). J. Pharmacol. Exp. Ther. 1994, 269, 282–289. [Google Scholar] [PubMed]
- Harries, M.H.; Samson, N.A.; Cilia, J.; Hunter, A.J. The profile of sabcomeline (SB-202026), a functionally selective M1 receptor partial agonist, in the marmoset. Br. J. Pharmacol. 1998, 124, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.A.; Kreatsoulas, C.; Pascarella, D.M.; O’Brien, J.A.; Sur, C. The M1 muscarinic receptor allosteric agonists AC-42 and 1-[1’-(2-methylbenzyl)-1,4’-bipiperidin-4-yl]-1,3-dihydro-2H-benzimidazol-2-one bind to a unique site distinct from the acetylcholine orthosteric site. Mol. Pharmacol. 2010, 78, 648–657. [Google Scholar] [CrossRef]
- Vanderheyden, P.; Gies, J.P.; Ebinger, G.; De Keyser, J.; Landry, Y.; Vauquelin, G. Human M1-, M2- and M3-muscarinic cholinergic receptors: Binding characteristics of agonists and antagonists. J. Neurol. Sci. 1990, 97, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Langmead, C.J.; Austin, N.E.; Branch, C.L.; Brown, J.T.; Buchanan, K.A.; Davies, C.H.; Forbes, I.T.; Fry, V.A.; Hagan, J.J.; Herdon, H.J.; et al. Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-1. Br. J. Pharmacol. 2008, 154, 1104–1115. [Google Scholar] [CrossRef]
- Del Tacca, M.; Danesi, R.; Blandizzi, C.; Bernardini, M.C. A selective antimuscarinic agent: Pirenzepine. Review of its pharmacologic and clinical properties. Minerva Dietol. Gastroenterol. 1989, 35, 175–189. [Google Scholar] [PubMed]
- Eltze, M. Assessment of selectivity of muscarinic antagonists for M1 and M2 receptors in rabbit isolated vas deferens. Pharmacology 1988, 37 (Suppl. 1), 40–47. [Google Scholar] [CrossRef]
- Salin-Pascual, R.J.; Granados-Fuentes, D.; Galicia-Polo, L.; Nieves, E.; Gillin, J.C. Development of tolerance after repeated administration of a selective muscarinic M1 antagonist biperiden in healthy human volunteers. Biol. Psychiatry 1993, 33, 188–193. [Google Scholar] [CrossRef]
- Palma, A.; Chara, J.C.; Montilla, A.; Otxoa-de-Amezaga, A.; Ruiz-Jaen, F.; Planas, A.M.; Matute, C.; Perez-Samartin, A.; Domercq, M. Clemastine Induces an Impairment in Developmental Myelination. Front. Cell Dev. Biol. 2022, 10, 841548. [Google Scholar] [CrossRef]
- Wu, G.Y.; Han, X.H.; Zhuang, Q.X.; Zhang, J.; Yung, W.H.; Chan, Y.S.; Zhu, J.N.; Wang, J.J. Excitatory effect of histamine on rat spinal motoneurons by activation of both H1 and H2 receptors in vitro. J. Neurosci. Res. 2012, 90, 132–142. [Google Scholar] [CrossRef]
- Lacour, M.; Sterkers, O. Histamine and betahistine in the treatment of vertigo: Elucidation of mechanisms of action. CNS Drugs 2001, 15, 853–870. [Google Scholar] [CrossRef]
- Carman-Krzan, M.; Bavec, A.; Zorko, M.; Schunack, W. Molecular characterization of specific H1-receptor agonists histaprodifen and its Nα-substituted analogues on bovine aortic H1-receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 367, 538–546. [Google Scholar] [CrossRef]
- Monti, J.M.; Pellejero, T.; Jantos, H. Effects of H1- and H2-histamine receptor agonists and antagonists on sleep and wakefulness in the rat. J. Neural Transm. 1986, 66, 1–11. [Google Scholar] [CrossRef]
- Diaz, P.; Jones, D.G.; Kay, A.B. Histamine receptors on guinea-pig alveolar macrophages: Chemical specificity and the effects of H1- and H2-receptor agonists and antagonists. Clin. Exp. Immunol. 1979, 35, 462–469. [Google Scholar]
- Reinhardt, D.; Wiemann, H.M.; Schumann, H.J. Effects of the H1-antagonist promethazine and the H2-antagonist burimamide on chronotropic, inotropic and coronary vascular responses to histamine in isolated perfused guinea-pig hearts. Agents Actions 1976, 6, 683–688. [Google Scholar] [CrossRef]
- Moguilevsky, N.; Varsalona, F.; Noyer, M.; Gillard, M.; Guillaume, J.P.; Garcia, L.; Szpirer, C.; Szpirer, J.; Bollen, A. Stable expression of human H1-histamine-receptor cDNA in Chinese hamster ovary cells. Pharmacological characterisation of the protein, tissue distribution of messenger RNA and chromosomal localisation of the gene. Eur. J. Biochem. 1994, 224, 489–495. [Google Scholar] [CrossRef]
- Gillard, M.; Van Der Perren, C.; Moguilevsky, N.; Massingham, R.; Chatelain, P. Binding characteristics of cetirizine and levocetirizine to human H1 histamine receptors: Contribution of Lys191 and Thr194. Mol. Pharmacol. 2002, 61, 391–399. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, R.; Li, G. Drug repurposing: Clemastine fumarate and neurodegeneration. Biomed. Pharmacother. 2023, 157, 113904. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hiraoka, K.; Harada, R.; Matsuzawa, T.; Ishikawa, Y.; Funaki, Y.; Yoshikawa, T.; Tashiro, M.; Yanai, K.; Okamura, N. Brain histamine H1 receptor occupancy after oral administration of desloratadine and loratadine. Pharmacol. Res. Perspect. 2019, 7, e00499. [Google Scholar] [CrossRef] [PubMed]
- Devillier, P.; Roche, N.; Faisy, C. Clinical pharmacokinetics and pharmacodynamics of desloratadine, fexofenadine and levocetirizine: A comparative review. Clin. Pharmacokinet. 2008, 47, 217–230. [Google Scholar] [CrossRef]
- Slater, J.W.; Zechnich, A.D.; Haxby, D.G. Second-generation antihistamines: A comparative review. Drugs 1999, 57, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, T.; Ihara, Y.; Watanabe, Y. α-1 Adrenergic receptors stimulation induces the proliferation of neural progenitor cells in vitro. Neurosci. Lett. 2006, 408, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.K.; Souza-Formigoni, M.L. Alpha1-adrenergic drugs affect the development and expression of ethanol-induced behavioral sensitization. Behav. Brain Res. 2013, 256, 646–654. [Google Scholar] [CrossRef]
- Thiele, R.H.; Nemergut, E.C.; Lynch, C., 3rd. The physiologic implications of isolated alpha1 adrenergic stimulation. Anesth. Analg. 2011, 113, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A. Structure and function of alpha-adrenergic receptors. Am. J. Med. 1989, 87, 12S–18S. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.J. Recent advances in the discovery of alpha1-adrenoceptor agonists. Current topics in medicinal chemistry. Curr. Top. Med. Chem. 2007, 7, 135–145. [Google Scholar] [CrossRef]
- Frishman, W.H.; Eisen, G.; Lapsker, J. Terazosin: A new long-acting α1-adrenergic antagonist for hypertension. Med. Clin. North Am. 1988, 72, 441–448. [Google Scholar] [CrossRef]
- Goldstein, I.I. Oral phentolamine: An alpha-1, alpha-2 adrenergic antagonist for the treatment of erectile dysfunction. Int. J. Impot. Res. 2000, 12, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ohtani, H.; Sawada, Y. Assessment of α1-adrenoceptor antagonists in benign prostatic hyperplasia based on the receptor occupancy theory. Br. J. Clin. Pharmacol. 2007, 63, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Lowe, F.C. Role of the newer alpha, -adrenergic-receptor antagonists in the treatment of benign prostatic hyperplasia-related lower urinary tract symptoms. Clin. Ther. 2004, 26, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Stanic, D.; Paratcha, G.; Ledda, F.; Herzog, H.; Kopin, A.S.; Hokfelt, T. Peptidergic influences on proliferation, migration, and placement of neural progenitors in the adult mouse forebrain. Proc. Natl. Acad. Sci. USA 2008, 105, 3610–3615. [Google Scholar] [CrossRef] [PubMed]
- Asin, K.E.; Bednarz, L.; Nikkel, A.L.; Gore, P.A., Jr.; Nadzan, A.M. A-71623, a selective CCK-A receptor agonist, suppresses food intake in the mouse, dog, and monkey. Pharmacol. Biochem. Behav. 1992, 42, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Gouldson, P.; Legoux, P.; Carillon, C.; Delpech, B.; Le Fur, G.; Ferrara, P.; Shire, D. The agonist SR 146131 and the antagonist SR 27897 occupy different sites on the human CCK1 receptor. Eur. J. Pharmacol. 2000, 400, 185–194, Erratum in Eur. J. Pharmacol. 2000, 407, 333. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, J.R.; Laudeman, C. CCK1R agonists: A promising target for the pharmacological treatment of obesity. Curr. Top. Med. Chem. 2003, 3, 837–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Portincasa, P.; Wang, D.Q. The cholecystokinin-1 receptor antagonist devazepide increases cholesterol cholelithogenesis in mice. Eur. J. Clin. Investig. 2016, 46, 158–169. [Google Scholar] [CrossRef]
- Makovec, F.; Bani, M.; Cereda, R.; Chiste, R.; Pacini, M.A.; Revel, L.; Rovati, L.A.; Rovati, L.C.; Setnikar, I. Pharmacological properties of lorglumide as a member of a new class of cholecystokinin antagonists. Arzneimittelforschung 1987, 37, 1265–1268. [Google Scholar]
- Bandyopadhyay, S.; Tfelt-Hansen, J.; Chattopadhyay, N. Diverse roles of extracellular calcium-sensing receptor in the central nervous system. J. Neurosci. Res. 2010, 88, 2073–2082. [Google Scholar] [CrossRef]
- Gregory, K.J.; Kufareva, I.; Keller, A.N.; Khajehali, E.; Mun, H.C.; Goolam, M.A.; Mason, R.S.; Capuano, B.; Conigrave, A.D.; Christopoulos, A.; et al. Dual Action Calcium-Sensing Receptor Modulator Unmasks Novel Mode-Switching Mechanism. ACS Pharmacol. Transl. Sci. 2018, 1, 96–109. [Google Scholar] [CrossRef]
- McLarnon, S.J.; Riccardi, D. Physiological and pharmacological agonists of the extracellular Ca2+-sensing receptor. Eur. J. Pharmacol. 2002, 447, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.J.; Bell, G.; Pickthorn, K.; Huang, S.; Vick, A.; Hodsman, P.; Peacock, M. Velcalcetide (AMG 416), a novel peptide agonist of the calcium-sensing receptor, reduces serum parathyroid hormone and FGF23 levels in healthy male subjects. Nephrol. Dial. Transplant. 2014, 29, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Centeno, P.P.; Herberger, A.; Mun, H.C.; Tu, C.; Nemeth, E.F.; Chang, W.; Conigrave, A.D.; Ward, D.T. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat. Commun. 2019, 10, 4693. [Google Scholar] [CrossRef] [PubMed]
- Filopanti, M.; Corbetta, S.; Barbieri, A.M.; Spada, A. Pharmacology of the calcium sensing receptor. Clin. Cases Miner. Bone Metab. 2013, 10, 162–165. [Google Scholar] [PubMed]
- Shcherbakova, I.; Huang, G.; Geoffroy, O.J.; Nair, S.K.; Swierczek, K.; Balandrin, M.F.; Fox, J.; Heaton, W.L.; Conklin, R.L. Design, new synthesis, and calcilytic activity of substituted 3H-pyrimidin-4-ones. Bioorg. Med. Chem. Lett. 2005, 15, 2537–2540. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Y.; Roberge, J.Y.; Ma, Z.; Liu, Y.; Michael Lawrence, R.; Rotella, D.P.; Seethala, R.; Feyen, J.H.; Dickson, J.K., Jr. Discovery and structure-activity relationships of 2-benzylpyrrolidine-substituted aryloxypropanols as calcium-sensing receptor antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Faure, H.; Roussanne, M.C.; Ferry, S.; Ruat, M.; Dauban, P.; Dodd, R.H. N1-Arylsulfonyl-N2-(1-(1-naphthyl)ethyl)-1,2-diaminocyclohexanes: A new class of calcilytic agents acting at the calcium-sensing receptor. Chembiochem 2004, 5, 1131–1136. [Google Scholar] [CrossRef]
- Gavai, A.V.; Vaz, R.J.; Mikkilineni, A.B.; Roberge, J.Y.; Liu, Y.; Lawrence, R.M.; Corte, J.R.; Yang, W.; Bednarz, M.; Dickson, J.K., Jr.; et al. Discovery of novel 1-arylmethyl pyrrolidin-2-yl ethanol amines as calcium-sensing receptor antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 5478–5482. [Google Scholar] [CrossRef]
- Montell, C. The TRP superfamily of cation channels. Sci. STKE 2005, 2005, re3. [Google Scholar] [CrossRef]
- Gaudet, R. Divide and conquer: High resolution structural information on TRP channel fragments. J. Gen. Physiol. 2009, 133, 231–237. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Kalia, J.; Swartz, K.J. Exploring structure-function relationships between TRP and Kv channels. Sci. Rep. 2013, 3, 1523. [Google Scholar] [CrossRef] [PubMed]
- Minke, B.; Wu, C.; Pak, W.L. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 1975, 258, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Bruford, E.A.; Caterina, M.J.; Clapham, D.E.; Harteneck, C.; Heller, S.; Julius, D.; et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E.; Montell, C.; Schultz, G.; Julius, D. International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: Transient receptor potential channels. Pharmacol. Rev. 2003, 55, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Sun, C.; Zheng, J. Heteromerization of TRP channel subunits: Extending functional diversity. Protein Cell 2010, 1, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Vannier, B.; Peyton, M.; Boulay, G.; Brown, D.; Qin, N.; Jiang, M.; Zhu, X.; Birnbaumer, L. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ entry channel. Proc. Natl. Acad. Sci. USA 1999, 96, 2060–2064. [Google Scholar] [CrossRef]
- Zeng, C.; Tian, F.; Xiao, B. TRPC Channels: Prominent Candidates of Underlying Mechanism in Neuropsychiatric Diseases. Mol. Neurobiol. 2016, 53, 631–647. [Google Scholar] [CrossRef]
- Hao, B.; Lu, Y.; Wang, Q.; Guo, W.; Cheung, K.H.; Yue, J. Role of STIM1 in survival and neural differentiation of mouse embryonic stem cells independent of Orai1-mediated Ca2+ entry. Stem Cell Res. 2014, 12, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Webb, S.E.; Miller, A.L.; Yue, J. The role of Ca2+ signaling on the self-renewal and neural differentiation of embryonic stem cells (ESCs). Cell Calcium 2016, 59, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.B.; Webb, S.E.; Yue, J.; Moreau, M.; Leclerc, C.; Miller, A.L. TRPC3 is required for the survival, pluripotency and neural differentiation of mouse embryonic stem cells (mESCs). Sci. China Life Sci. 2018, 61, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Hong, Y.H.; Jang, S.S.; Chae, H.G.; Paek, S.L.; Moon, H.E.; Kim, D.G.; Kim, J.; Paek, S.H.; Kim, S.J. A role of canonical transient receptor potential 5 channel in neuronal differentiation from A2B5 neural progenitor cells. PLoS ONE 2010, 5, e10359. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Feng, S.; Du, W.; Wang, Y. Functional roles of TRPC channels in the developing brain. Pflugers Arch. 2009, 458, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Jia, Y. TRPC Channels and Neuron Development, Plasticity, and Activities. Adv. Exp. Med. Biol. 2017, 976, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.P.; Sen, N.; Bayliss, D.A. TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J. Neurosci. 2007, 27, 8845–8856. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Nakamura, S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007, 581, 2047–2054. [Google Scholar] [CrossRef]
- Lemonnier, L.; Trebak, M.; Putney, J.W., Jr. Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium 2008, 43, 506–514. [Google Scholar] [CrossRef]
- Trebak, M.; Lemonnier, L.; DeHaven, W.I.; Wedel, B.J.; Bird, G.S.; Putney, J.W., Jr. Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 2009, 457, 757–769. [Google Scholar] [CrossRef]
- Tang, J.; Lin, Y.; Zhang, Z.; Tikunova, S.; Birnbaumer, L.; Zhu, M.X. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J. Biol. Chem. 2001, 276, 21303–21310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, J.; Tikunova, S.; Johnson, J.D.; Chen, Z.; Qin, N.; Dietrich, A.; Stefani, E.; Birnbaumer, L.; Zhu, M.X. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc. Natl. Acad. Sci. USA 2001, 98, 3168–3173. [Google Scholar] [CrossRef] [PubMed]
- Numaga, T.; Wakamori, M.; Mori, Y. Trpc7. In Transient Receptor Potential (TRP) Chanels; Handbook of Expermental Pharmacology Book Series; Springer: Berlin/Heidelberg, Germany, 2007; Volume 179, pp. 143–151. [Google Scholar] [CrossRef]
- Strubing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 2003, 278, 39014–39019. [Google Scholar] [CrossRef] [PubMed]
- Golovina, V.A.; Platoshyn, O.; Bailey, C.L.; Wang, J.; Limsuwan, A.; Sweeney, M.; Rubin, L.J.; Yuan, J.X. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H746–H755. [Google Scholar] [CrossRef] [PubMed]
- Antoniotti, S.; Lovisolo, D.; Fiorio Pla, A.; Munaron, L. Expression and functional role of bTRPC1 channels in native endothelial cells. FEBS Lett. 2002, 510, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, F.M.; Schwartz, M.L.; Raballo, R.; Nilsen, J.; Rhee, J.; Zhou, M.; Doetschman, T.; Coffin, J.D.; Wyland, J.J.; Hung, Y.T. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat. Neurosci. 1999, 2, 848. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, F.M.; Schwartz, M.L.; Raballo, R.; Rhee, J.; Lyn-Cook, R. Fibroblast growth factor signaling regulates growth and morphogenesis at multiple steps during brain development. Curr. Top. Dev. Biol. 1999, 46, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Raballo, R.; Rhee, J.; Lyn-Cook, R.; Leckman, J.F.; Schwartz, M.L.; Vaccarino, F.M. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J. Neurosci. 2000, 20, 5012–5023. [Google Scholar] [CrossRef]
- Fiorio Pla, A.; Maric, D.; Brazer, S.C.; Giacobini, P.; Liu, X.; Chang, Y.H.; Ambudkar, I.S.; Barker, J.L. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J. Neurosci. 2005, 25, 2687–2701. [Google Scholar] [CrossRef]
- Li, M.; Chen, C.; Zhou, Z.; Xu, S.; Yu, Z. A TRPC1-mediated increase in store-operated Ca2+ entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium 2012, 51, 486–496. [Google Scholar] [CrossRef]
- She, Y.J.; Pan, J.; Peng, L.M.; Ma, L.; Guo, X.; Lei, D.X.; Wang, H.Z. Ketamine modulates neural stem cell differentiation by regulating TRPC3 expression through the GSK3β/β-catenin pathway. Neurotoxicology 2023, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zagranichnaya, T.K.; Gurda, G.T.; Eves, E.M.; Villereal, M.L. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. J. Biol. Chem. 2004, 279, 43392–43402. [Google Scholar] [CrossRef] [PubMed]
- Seebohm, G.; Schreiber, J.A. Beyond Hot and Spicy: TRPV Channels and their Pharmacological Modulation. Cell Physiol. Biochem. 2021, 55, 108–130. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanz, N.; Valente, P.; Gomis, A.; Fernandez-Carvajal, A.; Fernandez-Ballester, G.; Viana, F.; Belmonte, C.; Ferrer-Montiel, A. A role of the transient receptor potential domain of vanilloid receptor I in channel gating. J. Neurosci. 2007, 27, 11641–11650. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Sasase, T.; Fatchiyah, F.; Ohta, T. Transient receptor potential vanilloid (TRPV) channels: Basal properties and physiological potential. Gen. Physiol. Biophys. 2022, 41, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Kanzaki, M.; Zhang, Y.Q.; Mashima, H.; Li, L.; Shibata, H.; Kojima, I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat. Cell Biol. 1999, 1, 165–170. [Google Scholar] [CrossRef]
- Penna, A.; Juvin, V.; Chemin, J.; Compan, V.; Monet, M.; Rassendren, F.A. PI3-kinase promotes TRPV2 activity independently of channel translocation to the plasma membrane. Cell Calcium 2006, 39, 495–507. [Google Scholar] [CrossRef]
- Morgan, P.J.; Hubner, R.; Rolfs, A.; Frech, M.J. Spontaneous calcium transients in human neural progenitor cells mediated by transient receptor potential channels. Stem Cells Dev. 2013, 22, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Maggi, F.; Morelli, M.B.; Santoni, M.; Marinelli, O. Transient Receptor Potential Cation Channels in Cancer Therapy. Med. Sci. 2019, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Deyashiki, A.; Miyake, T.; Nagayasu, K.; Shibasaki, K.; Shirakawa, H.; Kaneko, S. TRPV4 is functionally expressed in oligodendrocyte precursor cells and increases their proliferation. Pflugers Arch. 2018, 470, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C. Polycystic kidney disease: Genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J. Intern. Med. 2007, 261, 17–31. [Google Scholar] [CrossRef]
- Giamarchi, A.; Padilla, F.; Coste, B.; Raoux, M.; Crest, M.; Honore, E.; Delmas, P. The versatile nature of the calcium-permeable cation channel TRPP2. EMBO Rep. 2006, 7, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Franco, S.J.; Muller, U. Shaping our minds: Stem and progenitor cell diversity in the mammalian neocortex. Neuron 2013, 77, 19–34. [Google Scholar] [CrossRef]
- Li, Y.; Wright, J.M.; Qian, F.; Germino, G.G.; Guggino, W.B. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J. Biol. Chem. 2005, 280, 41298–41306. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Santoso, N.G.; Yu, S.; Woodward, O.M.; Qian, F.; Guggino, W.B. Polycystin-1 interacts with inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling with implications for polycystic kidney disease. J. Biol. Chem. 2009, 284, 36431–36441. [Google Scholar] [CrossRef]
- Tian, P.F.; Sun, M.M.; Hu, X.Y.; Du, J.; He, W. TRPP2 ion channels: The roles in various subcellular locations. Biochimie 2022, 201, 116–127. [Google Scholar] [CrossRef]
- Winokurow, N.; Schumacher, S. A role for polycystin-1 and polycystin-2 in neural progenitor cell differentiation. Cell Mol. Life Sci. 2019, 76, 2851–2869. [Google Scholar] [CrossRef]
- Gaiano, N.; Nye, J.S.; Fishell, G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 2000, 26, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, M.R. Signal transducer and activator of transcription-3 maintains the stemness of radial glia at mid-neurogenesis. J. Neurosci. 2015, 35, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Trenker, R.; Jura, N. Receptor tyrosine kinase activation: From the ligand perspective. Curr. Opin. Cell Biol. 2020, 63, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors (review). Int. J. Mol. Med. 2002, 10, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Diliberto, P.A.; Hubbert, T.; Herman, B. Early PDGF-induced alterations in cytosolic free calcium are required for mitogenesis. Res. Commun. Chem. Pathol. Pharmacol. 1991, 72, 3–12. [Google Scholar] [PubMed]
- Moolenaar, W.H.; Aerts, R.J.; Tertoolen, L.G.; de Laat, S.W. The epidermal growth factor-induced calcium signal in A431 cells. J. Biol. Chem. 1986, 261, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Merlet, E.; Lipskaia, L.; Marchand, A.; Hadri, L.; Mougenot, N.; Atassi, F.; Liang, L.; Hatem, S.N.; Hajjar, R.J.; Lompre, A.M. A calcium-sensitive promoter construct for gene therapy. Gene Ther. 2013, 20, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gomez, T.M.; Zheng, J.Q. The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Neurosci. 2006, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zheng, J.Q. Directional guidance of nerve growth cones. Curr. Opin. Neurobiol. 2006, 16, 52–58. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Poo, M.M. Calcium signaling in neuronal motility. Annu. Rev. Cell Dev. Biol. 2007, 23, 375–404. [Google Scholar] [CrossRef]
- Nelson, W.J. Remodeling epithelial cell organization: Transitions between front-rear and apical-basal polarity. Cold Spring Harb. Perspect. Biol. 2009, 1, a000513. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.S.; Yang, Y.R.; Lee, C.; Park, B.; Park, K.I.; Seo, J.K.; Seo, Y.K.; Cho, H.; Cocco, L.; Suh, P.G. Netrin-1/DCC-mediated PLCγ1 activation is required for axon guidance and brain structure development. EMBO Rep. 2019, 20, e48117. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xie, H.; Sekar, M.C.; Gupta, K.; Wells, A. Epidermal growth factor receptor-mediated cell motility: Phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J. Cell Biol. 1994, 127, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Suh, P.G.; Park, J.I.; Manzoli, L.; Cocco, L.; Peak, J.C.; Katan, M.; Fukami, K.; Kataoka, T.; Yun, S.; Ryu, S.H. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008, 41, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Fietz, S.A.; Huttner, W.B. Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Curr. Opin. Neurobiol. 2011, 21, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Fietz, S.A.; Kelava, I.; Vogt, J.; Wilsch-Brauninger, M.; Stenzel, D.; Fish, J.L.; Corbeil, D.; Riehn, A.; Distler, W.; Nitsch, R.; et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 2010, 13, 690–699. [Google Scholar] [CrossRef]
- Pollard, T.D. Mechanics of cytokinesis in eukaryotes. Curr. Opin. Cell Biol. 2010, 22, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Goderie, S.K.; Temple, S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron 2005, 45, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Reinchisi, G.; Parada, M.; Lois, P.; Oyanadel, C.; Shaughnessy, R.; Gonzalez, A.; Palma, V. Sonic Hedgehog modulates EGFR dependent proliferation of neural stem cells during late mouse embryogenesis through EGFR transactivation. Front. Cell Neurosci. 2013, 7, 166. [Google Scholar] [CrossRef]
- Ayuso-Sacido, A.; Graham, C.; Greenfield, J.P.; Boockvar, J.A. The duality of epidermal growth factor receptor (EGFR) signaling and neural stem cell phenotype: Cell enhancer or cell transformer? Curr. Stem Cell Res. Ther. 2006, 1, 387–394. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Johnson, C.; Cai, Y.; Horowitz, Z.K.; Mennicke, C.; Coffey, R.; Haider, M.; Threadgill, D.; Eliscu, R.; et al. Bulk and mosaic deletions of Egfr reveal regionally defined gliogenesis in the developing mouse forebrain. iScience 2023, 26, 106242. [Google Scholar] [CrossRef] [PubMed]
- Sibilia, M.; Steinbach, J.P.; Stingl, L.; Aguzzi, A.; Wagner, E.F. A strain-independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO J. 1998, 17, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr. Top. Med. Chem. 2020, 20, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Contreras, A.Y.; Quinones-Hinojosa, A.; Gonzalez-Perez, O. The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Front. Cell Neurosci. 2013, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Emdad, L.; Das, S.K.; Fisher, P.B. EGFR: An essential receptor tyrosine kinase-regulator of cancer stem cells. Adv. Cancer Res. 2020, 147, 161–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Jang, D.J.; Kaang, B.K. Two major gate-keepers in the self-renewal of neural stem cells: Erk1/2 and PLCγ1 in FGFR signaling. Mol. Brain 2009, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Paek, H.; Gutin, G.; Hebert, J.M. FGF signaling is strictly required to maintain early telencephalic precursor cell survival. Development 2009, 136, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Goetz, R.; Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 2013, 14, 166–180. [Google Scholar] [CrossRef]
- Katoh, M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int. J. Mol. Med. 2016, 38, 3–15. [Google Scholar] [CrossRef]
- Repetto, M.; Crimini, E.; Giugliano, F.; Morganti, S.; Belli, C.; Curigliano, G. Selective FGFR/FGF pathway inhibitors: Inhibition strategies, clinical activities, resistance mutations, and future directions. Expert. Rev. Clin. Pharmacol. 2021, 14, 1233–1252. [Google Scholar] [CrossRef]
- Son, D.I.; Hong, S.; Shin, K.S.; Kang, S.J. PARP-1 regulates mouse embryonic neural stem cell proliferation by regulating PDGFRα expression. Biochem. Biophys. Res. Commun. 2020, 526, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Guerit, E.; Arts, F.; Dachy, G.; Boulouadnine, B.; Demoulin, J.B. PDGF receptor mutations in human diseases. Cell Mol. Life Sci. 2021, 78, 3867–3881. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, S.A.; Fredriksson, L.; Lawrence, D.A.; Eriksson, U. Pharmacological targeting of the PDGF-CC signaling pathway for blood-brain barrier restoration in neurological disorders. Pharmacol. Ther. 2016, 167, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H. Targeting the PDGF signaling pathway in the treatment of non-malignant diseases. J. Neuroimmune Pharmacol. 2014, 9, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. 2013, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lee, S.Y.; O’Kusky, J.R.; Ye, P. Signalling through the type 1 insulin-like growth factor receptor (IGF1R) interacts with canonical Wnt signalling to promote neural proliferation in developing brain. ASN Neuro 2012, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, F.; Takahashi, S.I. IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef] [PubMed]
- Mitsiades, C.S.; Mitsiades, N.S.; McMullan, C.J.; Poulaki, V.; Shringarpure, R.; Akiyama, M.; Hideshima, T.; Chauhan, D.; Joseph, M.; Libermann, T.A.; et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004, 5, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lazaro, R.S.; Lamdan, H.; Caligiuri, L.G.; Lorenzo, N.; Berengeno, A.L.; Ortega, H.H.; Alonso, D.F.; Farina, H.G. In vitro and in vivo antitumor activity of Yerba Mate extract in colon cancer models. J. Food Sci. 2020, 85, 2186–2197. [Google Scholar] [CrossRef]
- Buck, E.; Gokhale, P.C.; Koujak, S.; Brown, E.; Eyzaguirre, A.; Tao, N.; Rosenfeld-Franklin, M.; Lerner, L.; Chiu, M.I.; Wild, R.; et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): Rationale for cotargeting IGF-1R and IR in cancer. Mol. Cancer Ther. 2010, 9, 2652–2664. [Google Scholar] [CrossRef]
- Islam, O.; Loo, T.X.; Heese, K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr. Neurovasc. Res. 2009, 6, 42–53. [Google Scholar] [CrossRef]
- Bartkowska, K.; Paquin, A.; Gauthier, A.S.; Kaplan, D.R.; Miller, F.D. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development 2007, 134, 4369–4380. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef]
- Wilkie, N.; Wingrove, P.B.; Bilsland, J.G.; Young, L.; Harper, S.J.; Hefti, F.; Ellis, S.; Pollack, S.J. The non-peptidyl fungal metabolite L-783,281 activates TRK neurotrophin receptors. J. Neurochem. 2001, 78, 1135–1145. [Google Scholar] [CrossRef]
- Josephy-Hernandez, S.; Jmaeff, S.; Pirvulescu, I.; Aboulkassim, T.; Saragovi, H.U. Neurotrophin receptor agonists and antagonists as therapeutic agents: An evolving paradigm. Neurobiol. Dis. 2017, 97, 139–155. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Wang, J.M.; Zeng, Y.S.; Liu, R.Y.; Huang, W.L.; Xiong, Y.; Wang, Y.H.; Chen, S.J.; Teng, Y.D. Recombinant adenovirus vector-mediated functional expression of neurotropin-3 receptor (TrkC) in neural stem cells. Exp. Neurol. 2007, 203, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Axelsen, L.N.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N.H. Gap junctions. Compr. Physiol. 2012, 2, 1981–2035. [Google Scholar] [CrossRef]
- Sohl, G.; Willecke, K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 2003, 10, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cina, C.; Bechberger, J.F.; Ozog, M.A.; Naus, C.C. Expression of connexins in embryonic mouse neocortical development. J. Comp. Neurol. 2007, 504, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Bittman, K.; Owens, D.F.; Kriegstein, A.R.; LoTurco, J.J. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J. Neurosci. 1997, 17, 7037–7044. [Google Scholar] [CrossRef]
- Nadarajah, B.; Jones, A.M.; Evans, W.H.; Parnavelas, J.G. Differential expression of connexins during neocortical development and neuronal circuit formation. J. Neurosci. 1997, 17, 3096–3111. [Google Scholar] [CrossRef]
- Oyamada, M.; Oyamada, Y.; Takamatsu, T. Regulation of connexin expression. Biochim. Biophys. Acta 2005, 1719, 6–23. [Google Scholar] [CrossRef]
- Liu, X.; Hashimoto-Torii, K.; Torii, M.; Ding, C.; Rakic, P. Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J. Neurosci. 2010, 30, 4197–4209. [Google Scholar] [CrossRef]
- Malmersjo, S.; Rebellato, P.; Smedler, E.; Planert, H.; Kanatani, S.; Liste, I.; Nanou, E.; Sunner, H.; Abdelhady, S.; Zhang, S.; et al. Neural progenitors organize in small-world networks to promote cell proliferation. Proc. Natl. Acad. Sci. USA 2013, 110, E1524–E1532. [Google Scholar] [CrossRef] [PubMed]
- Lo Turco, J.J.; Kriegstein, A.R. Clusters of coupled neuroblasts in embryonic neocortex. Science 1991, 252, 563–566. [Google Scholar] [CrossRef]
- Ulrich, H.; Abbracchio, M.P.; Burnstock, G. Extrinsic purinergic regulation of neural stem/progenitor cells: Implications for CNS development and repair. Stem Cell Rev. Rep. 2012, 8, 755–767. [Google Scholar] [CrossRef]
- Elias, L.A.; Kriegstein, A.R. Gap junctions: Multifaceted regulators of embryonic cortical development. Trends Neurosci. 2008, 31, 243–250. [Google Scholar] [CrossRef]
- Wiencken-Barger, A.E.; Djukic, B.; Casper, K.B.; McCarthy, K.D. A role for Connexin43 during neurodevelopment. Glia 2007, 55, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Khodosevich, K.; Zuccotti, A.; Kreuzberg, M.M.; Le Magueresse, C.; Frank, M.; Willecke, K.; Monyer, H. Connexin45 modulates the proliferation of transit-amplifying precursor cells in the mouse subventricular zone. Proc. Natl. Acad. Sci. USA 2012, 109, 20107–20112. [Google Scholar] [CrossRef]
- Bittman, K.S.; LoTurco, J.J. Differential regulation of connexin 26 and 43 in murine neocortical precursors. Cereb. Cortex 1999, 9, 188–195. [Google Scholar] [CrossRef] [PubMed]
 ) to increase their pool or asymmetrically (black) to give rise, directly or indirectly, in a time-dependent manner, to neurons (green arrows), astrocytes (blue arrows), and oligodendrocytes (red arrow). NSCs = neural stem cells; IPCs = intermediate progenitor cells. Blue doted arrow = repressed gliogenic signals; Blunt green arrow = pro-neuronal genes repressing gliogenic signals; Blunt blue arrow = gliogenic signals repressing neuronal genes. Created using BioRender.com.
) to increase their pool or asymmetrically (black) to give rise, directly or indirectly, in a time-dependent manner, to neurons (green arrows), astrocytes (blue arrows), and oligodendrocytes (red arrow). NSCs = neural stem cells; IPCs = intermediate progenitor cells. Blue doted arrow = repressed gliogenic signals; Blunt green arrow = pro-neuronal genes repressing gliogenic signals; Blunt blue arrow = gliogenic signals repressing neuronal genes. Created using BioRender.com.
 ) to increase their pool or asymmetrically (black) to give rise, directly or indirectly, in a time-dependent manner, to neurons (green arrows), astrocytes (blue arrows), and oligodendrocytes (red arrow). NSCs = neural stem cells; IPCs = intermediate progenitor cells. Blue doted arrow = repressed gliogenic signals; Blunt green arrow = pro-neuronal genes repressing gliogenic signals; Blunt blue arrow = gliogenic signals repressing neuronal genes. Created using BioRender.com.
) to increase their pool or asymmetrically (black) to give rise, directly or indirectly, in a time-dependent manner, to neurons (green arrows), astrocytes (blue arrows), and oligodendrocytes (red arrow). NSCs = neural stem cells; IPCs = intermediate progenitor cells. Blue doted arrow = repressed gliogenic signals; Blunt green arrow = pro-neuronal genes repressing gliogenic signals; Blunt blue arrow = gliogenic signals repressing neuronal genes. Created using BioRender.com.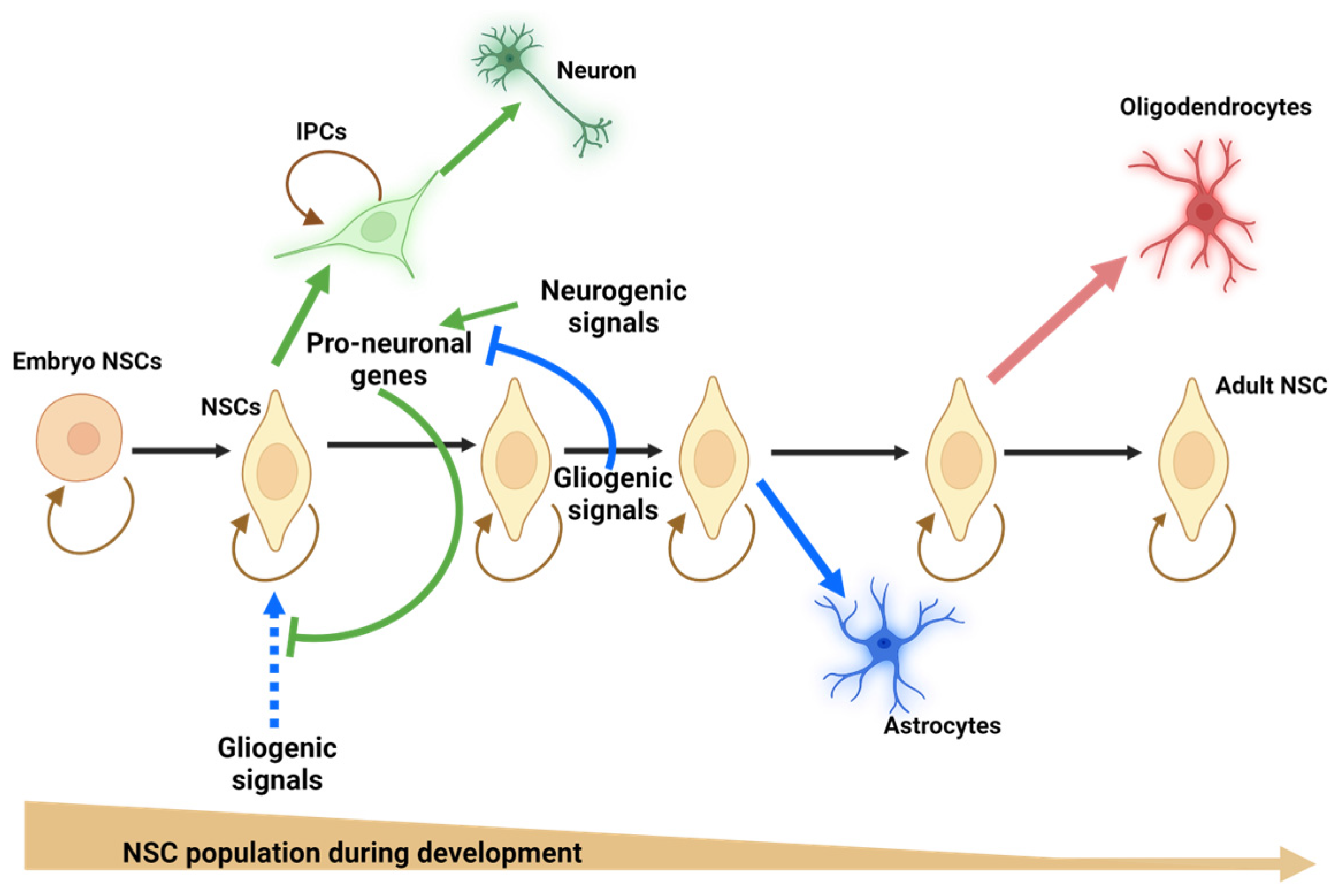


| Receptor | Function | Ligand | Agonists | Antagonist |
|---|---|---|---|---|
| 5-HT2AR (serotonin 2A receptor) | Promotes NSC proliferation ex vivo, in vitro, and in vivo [69] Its antagonism increases NSC differentiation in vitro [70] | Serotonin | LSD [71], DOI [72], 25CN-NBOH [73], Mescaline [74], Pimavanserin [75], S 16924 [76] | Spiperone [71,77], compound 3b [PMID: 28943244] [78], M100,907 (volinanserin), Pirenperone [71], (−)-MBP (meta-bromo-phenylisopropylamine) [79], Eplivanserin hemi fumarate (SR-46349B) [80], Sarpogrelate, Naftidrofuryl [81], Risperidone, Pipamperone [82], Olanzapine, Ketanserin, Clozapine, Zotepine, Ziprasidone, ORG5222, Tiaspirone, Ocaperidone [83], Ketanserin, Altanserin [84] |
| 5-HT2CR (serotonin 2C receptor) | Its antagonism increases NSC differentiation in vitro [70]. | Serotonin | LSD [71], DOI [72], Mescaline [74], (−)-MBP (meta-bromo-phenylisopropylamine) [79], RO 60-0175 [85], Lorcaserin [86], MK-212, WAY-161503 [87] | Spiperone [71], RS-102221[87], Mianserin, 1-NP, ICI 169,369, LY 53857 [84], SB206553, SB242084 [88], Nefazodone, Mirtazapine [89], Ritanserin, Mesulergine [90] |
| M1R (muscarinic type 1 receptor) | Promotes NSC differentiation in vitro [91,92]. | Acetylcholine | AF102B, AF150 (S), AF267B [93], Xanomeline [94], Sabcomeline [95], AC-42, TBPB, N-desmethylclozapine [96], Pirenzepine, Carbachol [97], 77-LH-28-1 [98] | Pirenzepin [99], Telenzepine [100], Biperiden [101], Clemastine [102] |
| H1R (Histamine type 1 receptor) | Increases NSC neuron differentiation in vitro. Its antagonism reduces neurogenesis in vivo [32]. | Histamine | PEA [103], Beta-histine [104], Histaprodifen [105], 2-TEA [106] | Diphenhydramine, Pyrilamine [106], Chlorpheniramine, Mepyramine [107], Promethazine [108], Cetirizine [109], Hydroxyzine [110], Clemastine [111], Loratadine, Desloratadine [112], Fexofenadine, Levocetirizine [113], Azelastine, Acrivastine, Astemizole, Ebastine, Fexodenadine, Ketotifen, Mizolastine, Terfenadine [114] |
| ADRα-1AR (adrenergic α1-receptor) | Increases NSC proliferation in vitro [115]. | Norepinephrine (noradrenaline) Epinephrine (adrenaline) | Phenylephrine [116], Methoxamine [117], Metaraminol, Midodrine, Xylometazoline, Oxymetazoline, Naphazoline, Tetrahydrozoline [118], Clonidine, Cirazoline, Sgd 101/75, St 587, Amidephrine, SKF89748, SDZ NVI 085, SK&F 102652, ST-1059, A-61603, A-204176, NS-49, ABT-866, BMY 7378 [119] | Prazosin [116], Terazosin [117], Tamsulosin [120], Phentolamine [121], Doxazosin [122], Alfuzosin [123] |
| CCK1R (Cholecystokinin type 1 receptor) | Increases NSC proliferation and differentiation into neurons in vitro [124]. | CCK-8 | A-71623 [125], SR-146131 [126], FPL 14294, AR-R 15849, A-71623, PD149164, PD170292, PD151932, GI 18177 [127] | SR 27897 [126], L-364,718, Devazepide [128], Dexloxiglumide, Lorglumide, Proglumide [129], and MK 329 (devazepide) [127] |
| CaSR (Ca2+-sensing receptor) | NSCs differentiate to the oligodendrocyte [130]. | Ca2+, Mg2+, L-tryptophan [130], spermine [131] | Neomycin [132], Vitamin D, Velcalcetide [133] | Calcilytics, Phosphate [134], Ronacaleret [135], 2-methyl-3-phenethyl-3H-pyrimidin-4-one [136], compound (S)-3h [PMID: 15686947] [137], compound 17 [PMID: 15300839] [138], 1-arylmethylpyrrolidin-2-yl ethanol amine [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Piña, D.A.; Rivera-Ramírez, N.; García-López, G.; Díaz, N.F.; Molina-Hernández, A. Calcium and Neural Stem Cell Proliferation. Int. J. Mol. Sci. 2024, 25, 4073. https://doi.org/10.3390/ijms25074073
Díaz-Piña DA, Rivera-Ramírez N, García-López G, Díaz NF, Molina-Hernández A. Calcium and Neural Stem Cell Proliferation. International Journal of Molecular Sciences. 2024; 25(7):4073. https://doi.org/10.3390/ijms25074073
Chicago/Turabian StyleDíaz-Piña, Dafne Astrid, Nayeli Rivera-Ramírez, Guadalupe García-López, Néstor Fabián Díaz, and Anayansi Molina-Hernández. 2024. "Calcium and Neural Stem Cell Proliferation" International Journal of Molecular Sciences 25, no. 7: 4073. https://doi.org/10.3390/ijms25074073
APA StyleDíaz-Piña, D. A., Rivera-Ramírez, N., García-López, G., Díaz, N. F., & Molina-Hernández, A. (2024). Calcium and Neural Stem Cell Proliferation. International Journal of Molecular Sciences, 25(7), 4073. https://doi.org/10.3390/ijms25074073




