Overexpression of a Fragaria vesca NAM, ATAF, and CUC (NAC) Transcription Factor Gene (FvNAC29) Increases Salt and Cold Tolerance in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
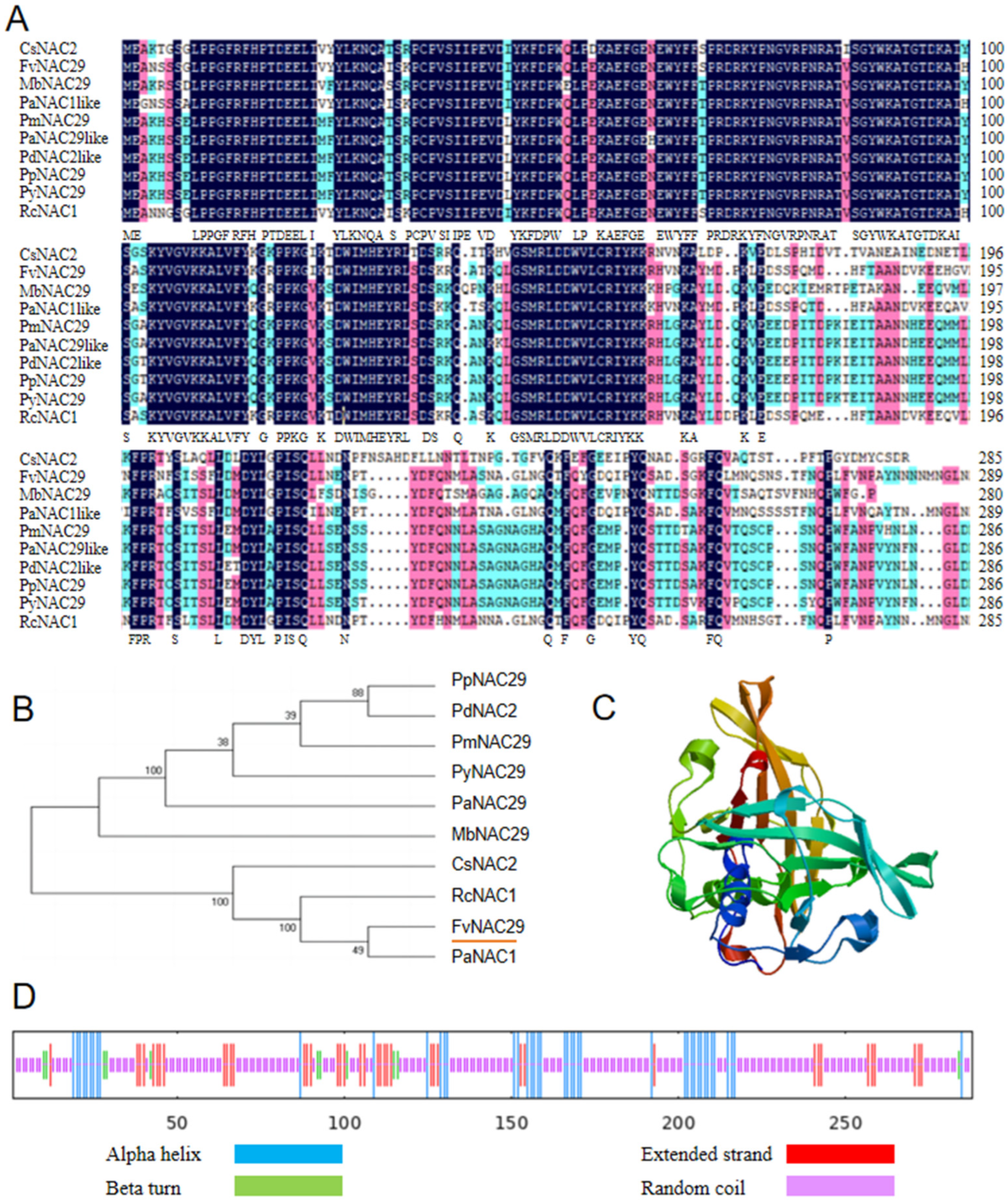
2.1. Cloning and Bioinformatic Analysis of FvNAC29
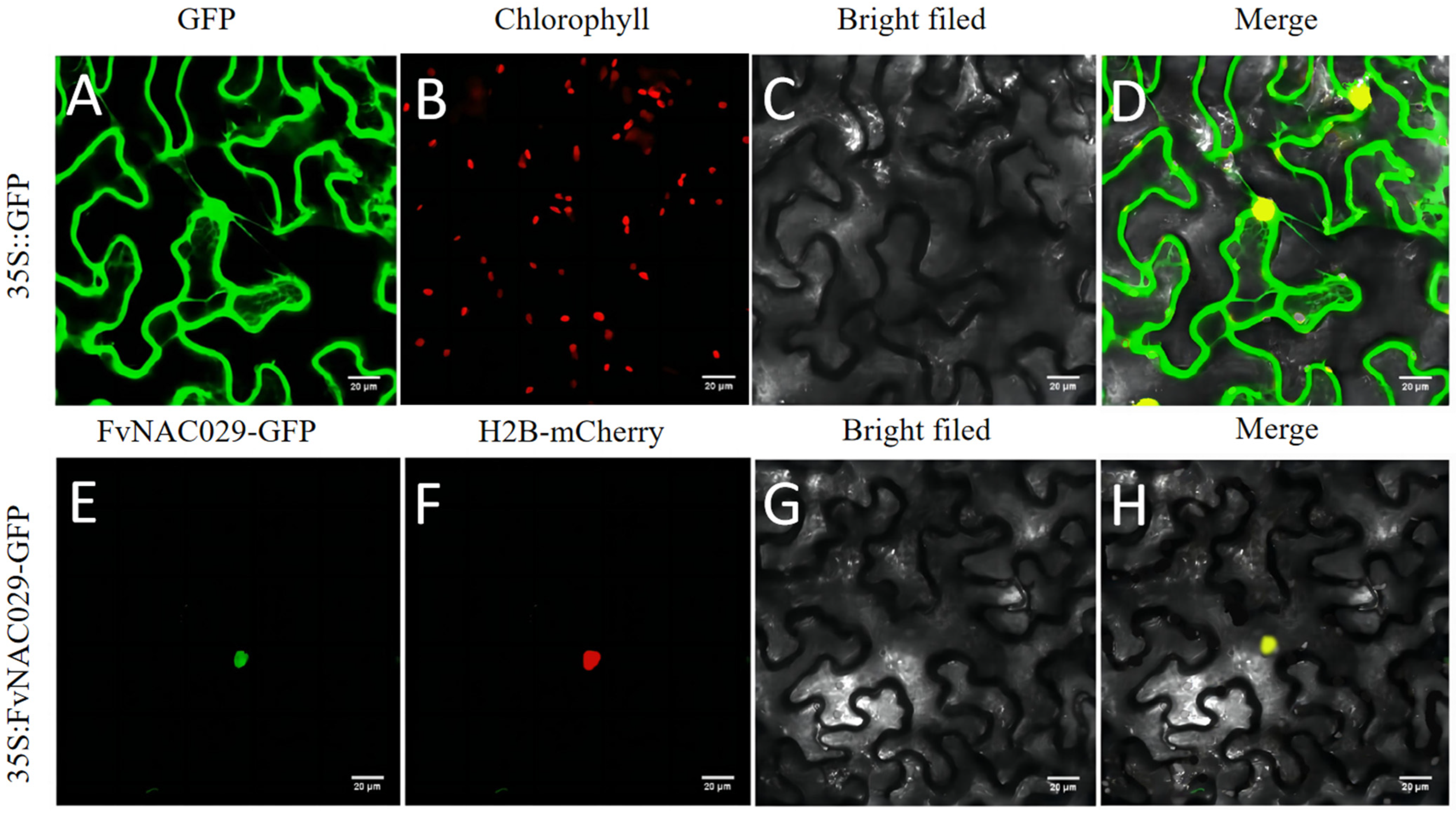
2.2. FvNAC29 Was Localizated onto Nucleus
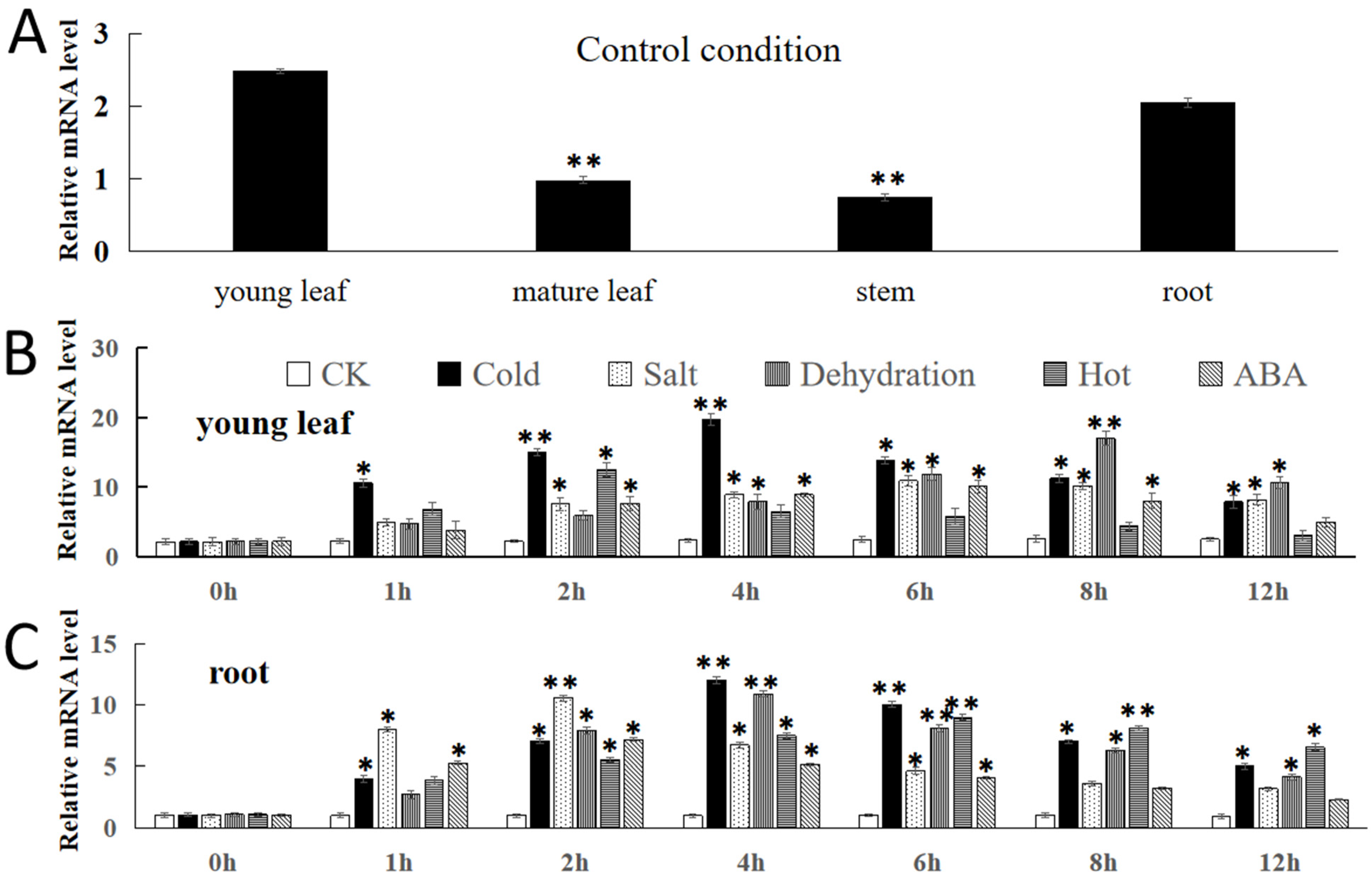
2.3. Expression Analysis of FvNAC29
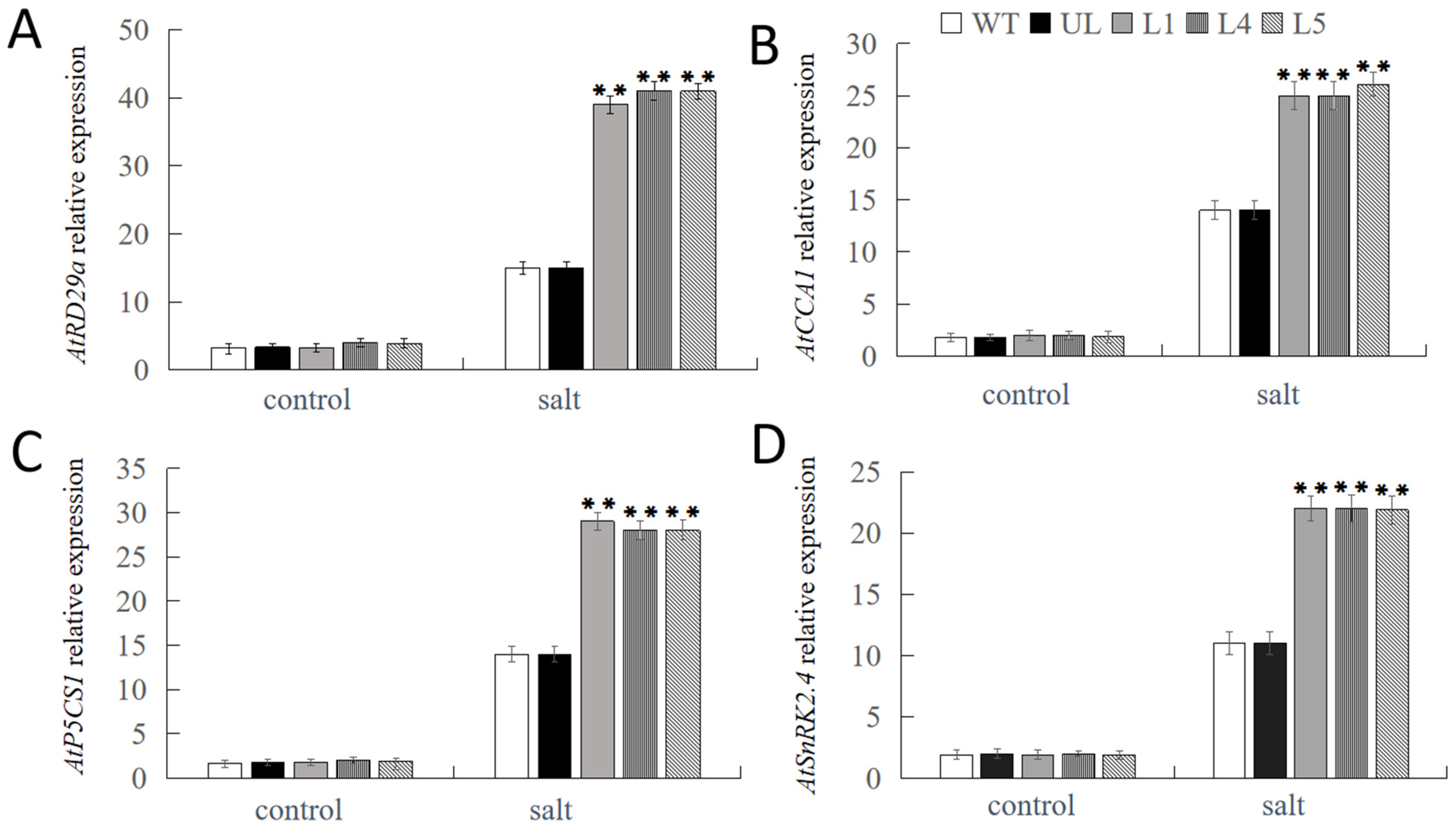
2.4. Overexpression of FvNAC29 Increases Tolerance to Salt Stress
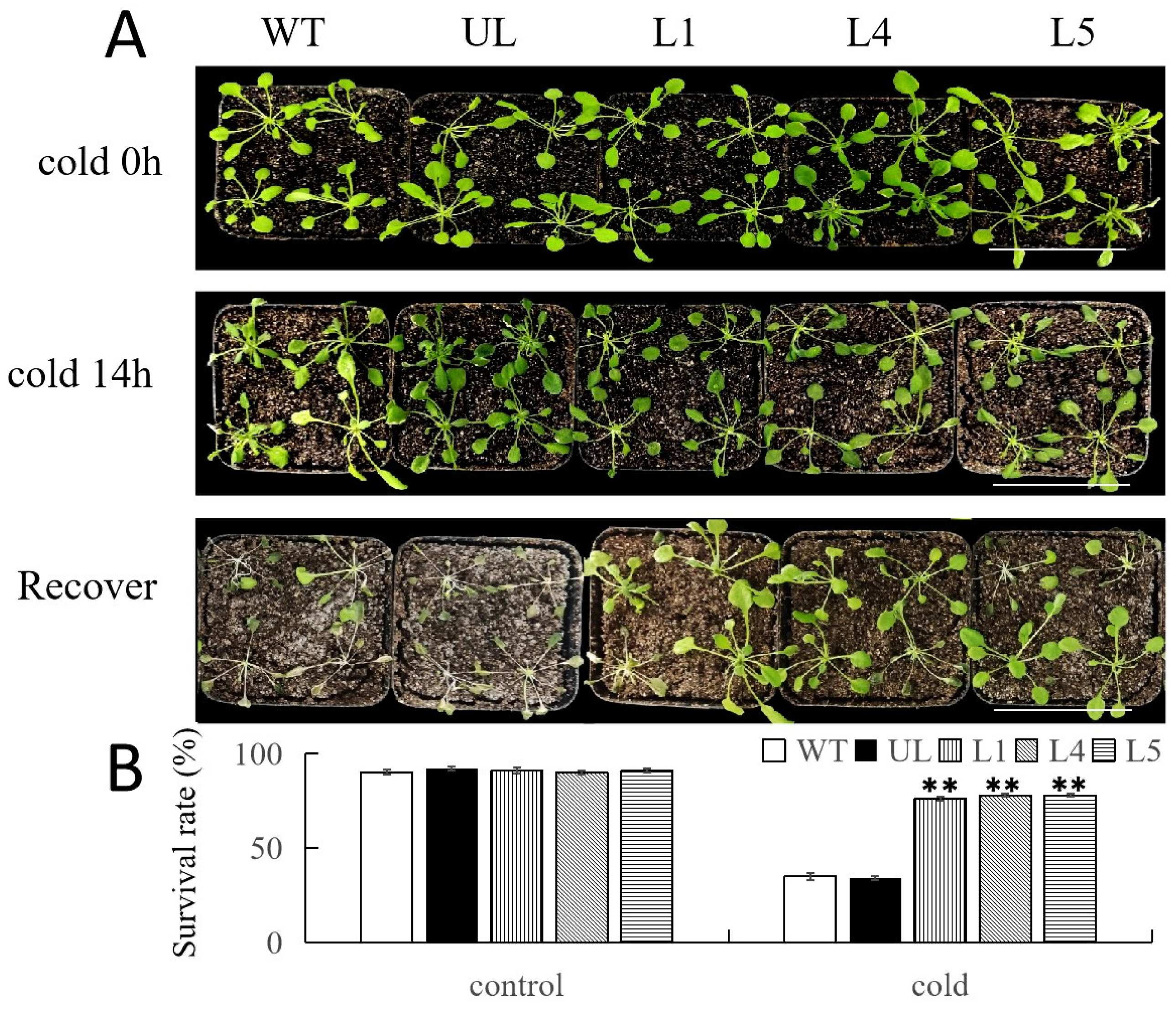
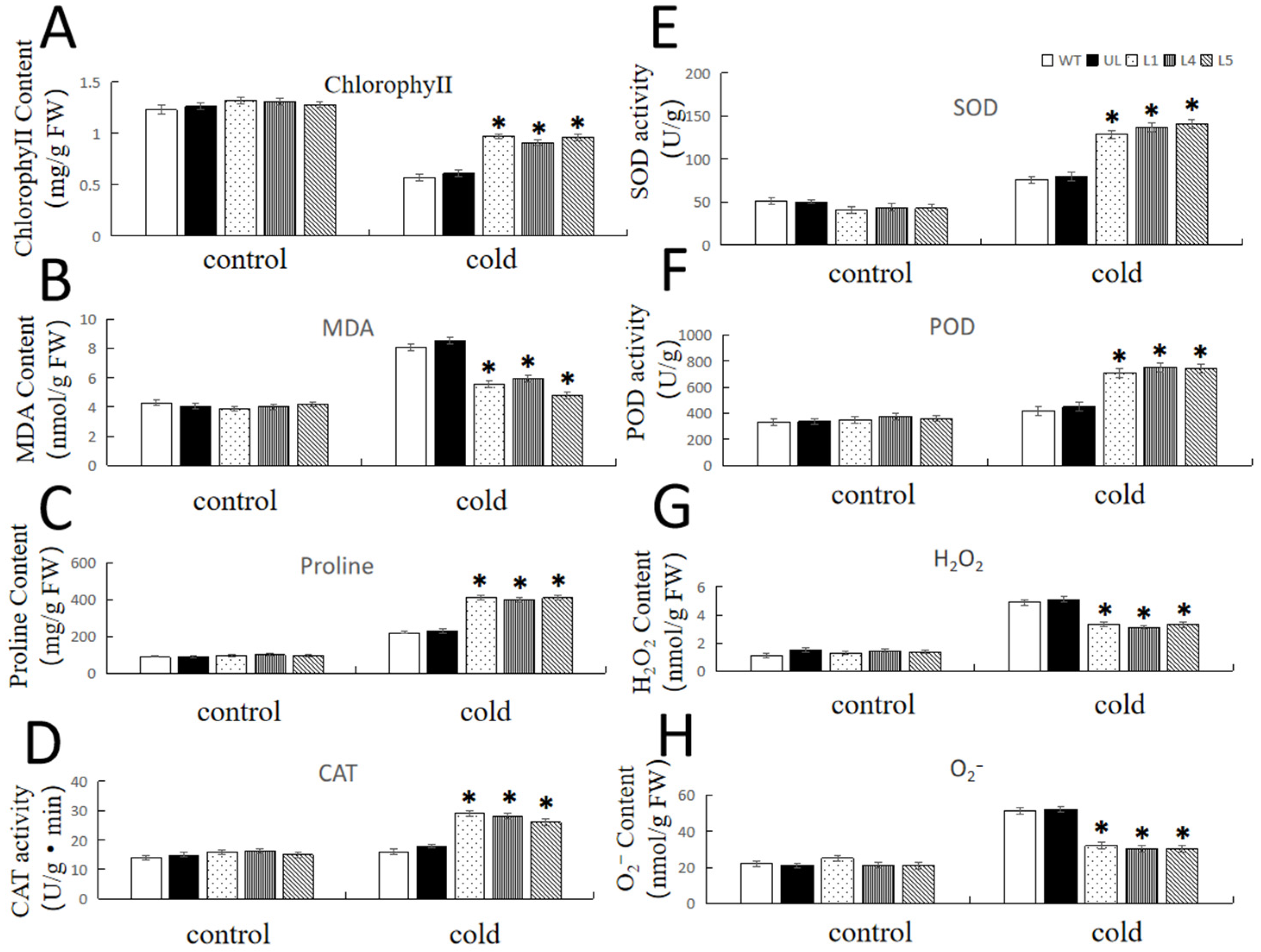
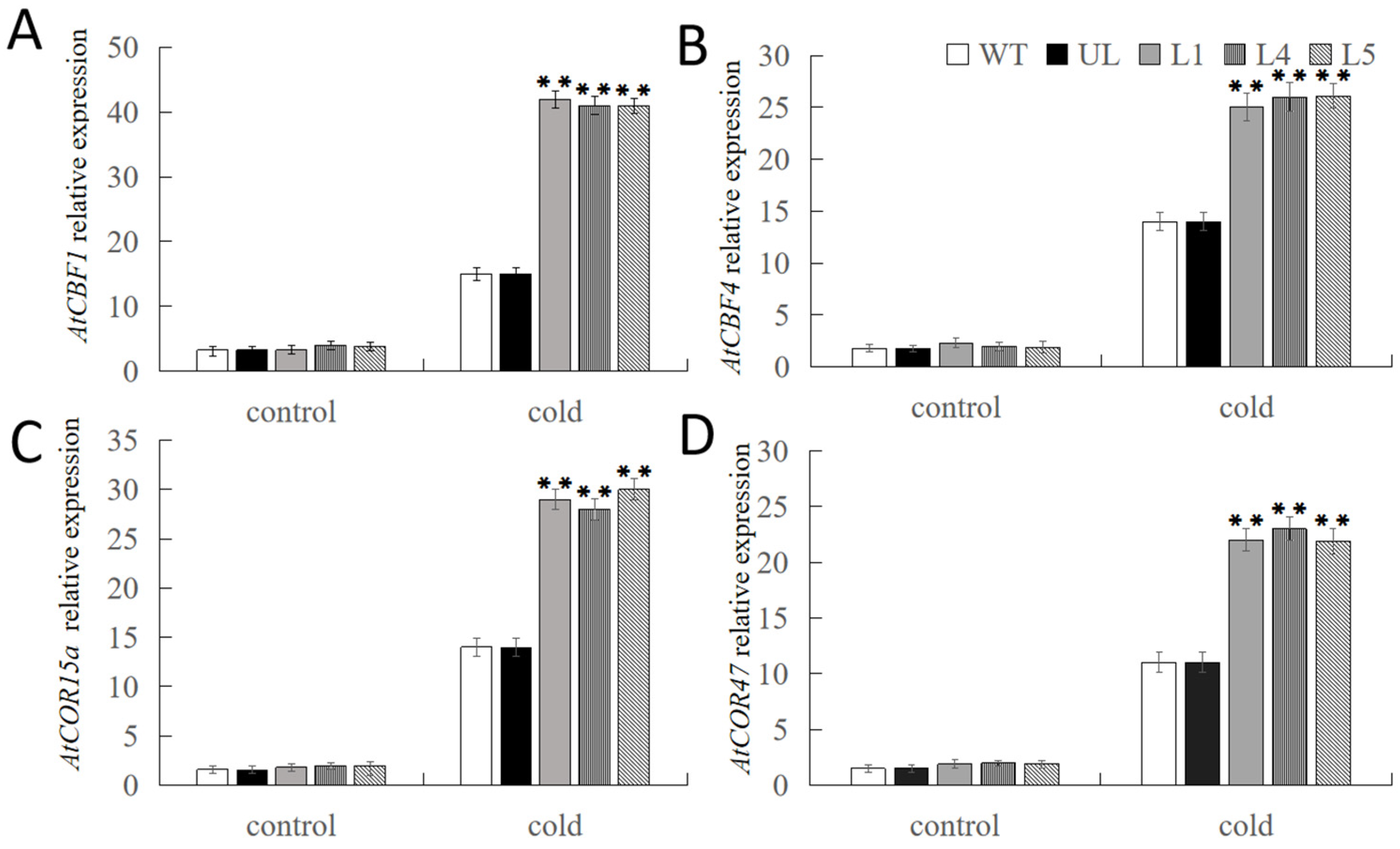
2.5. Overexpression of FvNAC29 Increases Tolerance to Cold Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Expression Patterns of FvNAC29
4.3. RNA Extraction and Cloning of FvNAC29
4.4. Subcellular Localization Analysis of FvNAC29
4.5. Sequence Analysis and Structure Prediction of FvNAC29
4.6. Expression Analysis of FvNAC29
4.7. Generation of A. thaliana Lines Overexpressing FvNAC29
4.8. Stress Treatment
4.9. Determination of Physiological Indexes
4.10. Expression Analysis of Salt- and Cold-Stress-Related Response Genes
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, D.; Zhou, Z.; Du, M.; Li, T.; Wu, X.; Yu, J.; Zhang, P.; Yang, G. Overexpression of a Malus xiaojinensis WRKY transcription factor gene (MxWRKY55) increased iron and high salinity stress tolerance in Arabidopsis thaliana. In Vitro Cell. Dev. Biol. Plant 2020, 56, 600–609. [Google Scholar] [CrossRef]
- Zandalinas, S.; Fritschi, F.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yang, G.; Xu, K.; Shao, Q.; Yu, Z.; Wang, B.; Ge, Q.; Yu, Y. Overexpression of a Malus xiaojinensis Nas1 gene influences flower development and tolerance to iron stress in transgenic tobacco. Plant Mol. Biol. Rep. 2013, 31, 802–809. [Google Scholar] [CrossRef]
- Han, D.; Shi, Y.; Yu, Z.; Liu, W.; Lv, B.; Wang, B.; Yang, G. Isolation and functional analysis of MdCS1: A gene encoding a citrate synthase in Malus domestica (L.) Borkh. Plant Growth Regul. 2015, 75, 209–218. [Google Scholar] [CrossRef]
- Thakur, P.; Nayyar, H. Facing the cold stress by plants in the changing environment: Sensing, signaling, and defending mechanisms. In Plant Acclimation to Environmental Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–69. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Guo, H.; Wu, T.; Li, S.; He, Q.; Yang, Z.; Zhang, W.; Gan, Y.; Sun, P.; Xiang, G.; Zhang, H. The methylation patterns and transcriptional responses to chilling stress at the seedling stage in rice. Int. J. Mol. Sci. 2019, 20, 5089. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants. Int. J. Mol. Sci. 2022, 23, 1995. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Takeda, S.; Nick, P. Life and death under salt stress: Same players, different timing? J. Exp. Bot. 2014, 65, 2963–2979. [Google Scholar] [CrossRef] [PubMed]
- Julkowska, M.; Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, M.; Liu, L.; Meng, F. Physiological and proteomic responses of diploid and tetraploid black locust (Robinia pseudoacacia L.) subjected to salt stress. Int. J. Mol. Sci. 2013, 14, 20299–20325. [Google Scholar] [CrossRef] [PubMed]
- Bazihizina, N.; Colmer, T.; Cuin, T.; Mancuso, S.; Shabala, S. Friend or foe? Chloride patterning in halophytes. Trends Plant Sci. 2019, 24, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Mulet, J.; Porcel, R.; Yenush, L. Modulation of potassium transport to increase abiotic stress tolerance in plants. J. Exp. Bot. 2023, 74, 5989–6005. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, K.; Wu, J.; Liu, L.; Sun, G.; He, Y.; Chen, F.; Yu, D. Identification and characterization of a novel NAC-like gene in chrysanthemum (Dendranthema lavandulifolium). Plant Cell Rep. 2016, 35, 1783–1798. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, T.; Zhang, Y.; Zhang, K.; Feng, K.; Wu, P.; Li, L. Identification of the NAC Transcription Factors and Their Function in ABA and Salinity Response in Nelumbo nucifera. Int. J. Mol. Sci. 2022, 23, 12394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, F.; Wang, X.; Liu, S.; Saeed, U.; Hou, X.; Zhang, Y.; Luo, D.; Meng, Y.; Zhang, W.; et al. Molecular and Functional Characterization of CaNAC035, an NAC Transcription Factor From Pepper (Capsicum annuum L.). Front. Plant Sci. 2020, 11, 479015. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Su, M.; Jiao, Y.; Xu, S.; Song, J.; Wang, H.; Li, Q. A Transcription Factor SlNAC10 Gene of Suaeda liaotungensis Regulates Proline Synthesis and Enhances Salt and Drought Tolerance. Int. J. Mol. Sci. 2022, 23, 9625. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Oh, N.; Chung, P.; Kim, Y.; Do Choi, Y.; Kim, J. Overexpression of OsNAC14 Improves Drought Tolerance in Rice. Front. Plant Sci. 2018, 9, 326760. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.; Dennis, E.; Llewellyn, D.; Wilson, I. ATAF NAC transcription factors: Regulators of plant stress signaling. Plant Signal Behav. 2010, 5, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Duval, M.; Hsieh, T.; Kim, S.; Thomas, T. Molecular characterization of AtNAM: A member of the Arabidopsis NAC domain superfamily. Plant Mol. Biol. 2002, 50, 237–248. [Google Scholar] [CrossRef]
- Olsen, A.; Ernst, H.; Leggio, L.; Skriver, K. NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 2005, 10, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, J.; Xu, P.; Zhang, R.; Li, L. Intron-mediated alternative splicing of wood-associated nac transcription factor1b regulates cell wall thickening during fiber development in Populus species. Plant Physiol. 2014, 164, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Huang, J.; Guo, Y.; Yang, M.; Guo, Y.; Li, J.; Zhang, J.; Xu, W. A cotton NAC domain transcription factor, GhFSN5, negatively regulates secondary cell wall biosynthesis and anther development in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 146, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Wang, H.; Tang, X. NAC transcription factors in plant multiple abiotic stress responses: Progress and prospects. Front. Plant Sci. 2015, 6, 902. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Koyama, H.; Bhati, K.; Alok, A. The biotechnological importance of the plant-specific NAC transcription factor family in crop improvement. J. Plant Res. 2021, 134, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Kobayashi, Y.; Koyama, H.; Sahoo, L. Cowpea NAC1/NAC2 transcription factors improve growth and tolerance to drought and heat in transgenic cowpea through combined activation of photosynthetic and antioxidant mechanisms. J. Integr. Plant Biol. 2023, 65, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Kuang, J.; Lu, W.; Chen, J. Banana fruit NAC transcription factor MaNAC 1 is a direct target of MaICE 1 and involved in cold stress through interacting with MaCBF 1. Plant Cell Environ. 2014, 37, 2116–2127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Wu, Y.; Mu, M.; Jiang, J.; Nie, W.; Zhao, S.; Cui, G.; Yin, X. Genome-wide identification and characterization of NAC transcription factor family members in Trifolium pratense and expression analysis under lead stress. BMC Genom. 2024, 25, 128. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Li, M.; Chen, N.; Xie, X.; Han, Z.; Ren, G.; Yin, Y.; Jiang, H. Genome-Wide Identification of NAC Gene Family and Expression Analysis under Abiotic Stresses in Avena sativa. Genes 2023, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, G.; Chen, S.; Jia, Z.; Zhang, S.; He, F.; Ren, M. Genome-wide analysis of the Tritipyrum NAC gene family and the response of TtNAC477 in salt tolerance. BMC Plant Biol. 2024, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Zheng, H.; Jiang, Q.; Sun, X.; Yuan, G.; Qi, J.; Zhang, H. GmNAC15 overexpression in hairy roots enhances salt tolerance in soybean. J. Integr. Agric. 2018, 17, 530–538. [Google Scholar] [CrossRef]
- An, J.; Yao, J.; Xu, R.; You, C.; Wang, X.; Hao, Y. An apple NAC transcription factor enhances salt stress tolerance by modulating the ethylene response. Physiol. Plant. 2018, 164, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K.; Seki, M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 2003, 6, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhao, X.; Gong, X.; Zhao, W.; Sun, M.; Chen, X.; Li, D.; Li, L.; Xiao, W. The NAC transcription factor MdNAC4 positively regulates nitrogen deficiency-induced leaf senescence by enhancing ABA biosynthesis in apple. Mol. Hortic. 2023, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, M.; Daszkowska-Golec, A. Molecular mechanisms of SNAC1 (Stress-responsive NAC1) in conferring the abiotic stress tolerance. Plant Sci. 2023, 337, 111894. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Q.; Shang, X.; Chen, C.; Xu, N.; Guan, J.; Meng, Q. Overexpression of transcription factor SlNAC35 enhances the chilling tolerance of transgenic tomato. Biol. Plant. 2018, 62, 479–488. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Q.; Wu, H.; Wang, A.; Wang, X.; Li, C.; Zhao, H.; Wu, Q. FtNAC31, a Tartary buckwheat NAC transcription factor, enhances salt and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2022, 191, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, Z.; Gu, H.; Cheng, D.; Guo, X.; Li, L.; Shi, C.; Xu, G.; Gu, S.; Abid, M. AvNAC030, a NAC domain transcription factor, enhances salt stress tolerance in Kiwifruit. Int. J. Mol. Sci. 2021, 22, 11897. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Huang, F.; Cheng, H.; Song, H.; Yu, D. Overexpression of the GmNAC2 Gene, an NAC Transcription Factor, Reduces Abiotic Stress Tolerance in Tobacco. Plant Mol. Biol. Rep. 2013, 31, 435–442. [Google Scholar] [CrossRef]
- Han, D.; Han, J.; Xu, T.; Li, X.; Yao, C.; Li, T.; Sun, X.; Wang, X.; Yang, G. Overexpression of MbERF12, an ERF gene from Malus baccata (L.) Borkh increases cold and salt tolerance in Arabidopsis thaliana associated with the ROS scavenging through ethylene signal transduction. In Vitro Cell. Dev. Biol. Plant 2021, 57, 760–770. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, H.; Liu, S.; Wang, X.; Zhang, Y.; Meng, Y.; Luo, D.; Chen, R. The NAC transcription factor CaNAC064 is a regulator of cold stress tolerance in peppers. Plant Sci. 2020, 291, 110346. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xie, F.; Liang, W.; Liang, Y.; Zhang, Z.; Zhao, J.; Hu, G.; Qin, Y. HuNAC20 and HuNAC25, Two Novel NAC Genes from Pitaya, Confer Cold Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2022, 23, 2189. [Google Scholar] [CrossRef] [PubMed]
- Moyano, E.; Martínez-Rivas, F.; Blanco-Portales, R.; Molina-Hidalgo, F.; Ric-Varas, P.; Matas-Arroyo, A.; Caballero, J.; Muñoz-Blanco, J.; Rodríguez-Franco, A. Genome-wide analysis of the NAC transcription factor family and their expression during the development and ripening of the Fragaria× ananassa fruits. PLoS ONE 2018, 13, e0196953. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Zhang, B.; Li, C.; Nagawa, S. FvNST1b NAC protein induces secondary cell wall formation in strawberry. Int. J. Mol. Sci. 2022, 23, 13212. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Orellana, C.; Stappung, Y.; Mendez-Yanez, A.; Allan, A.; Espley, R.; Plunkett, B.; Moya-Leon, M.A.; Herrera, R. Characterization of a ripening-related transcription factor FcNAC1 from fragaria chiloensis fruit. Sci. Rep. 2018, 8, 10524. [Google Scholar] [CrossRef]
- Martín-Pizarro, C.; Vallarino, J.; Osorio, S.; Meco, V.; Urrutia, M.; Pillet, J.; Casanal, A.; Merchante, C.; Amaya, I.; Willmitzer, L. The NAC transcription factor FaRIF controls fruit ripening in strawberry. Plant Cell. 2021, 33, 1574–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dang, X.; Chen, H.; Li, T.; Zhu, F.; Nagawa, S. Ectopic Expression of FvVND4c Promotes Secondary Cell Wall Thickening and Flavonoid Accumulation in Fragaria vesca. Int. J. Mol. Sci. 2023, 24, 8110. [Google Scholar] [CrossRef]
- Chen, T.; Wei, W.; Shan, W.; Kuang, J.; Chen, J.; Lu, W.; Yang, Y. MaHDA6-MaNAC154 module regulates the transcription of cell wall modification genes during banana fruit ripening. Postharvest Biol. Technol. 2023, 198, 112237. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Wang, S.; Tao, Y.; Wang, Z.; Shu, Y.; Peng, H.; Mijiti, A.; Wang, Z.; Zhang, H.; et al. CarNAC4, a NAC-type chickpea transcription factor conferring enhanced drought and salt stress tolerances in Arabidopsis. Plant Cell Rep. 2016, 35, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Wang, H.; Yuan, X.; Yan, Y.; Li, D.; Song, F. The NAC transcription factor ONAC083 negatively regulates rice immunity against Magnaporthe oryzae by directly activating transcription of the RING-H2 gene OsRFPH2-6. J. Integr. Plant Biol. 2023, 65, 854–875. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.; Kim, S.; Park, C. Membrane-bound transcription factors in plants. Trends Plant Sci. 2008, 13, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Huh, S.; Paek, K.; Ha, J.; Park, C. The Arabidopsis NAC transcription factor NTL4 participates in a positive feedback loop that induces programmed cell death under heat stress conditions. Plant Sci. 2014, 227, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, M.; Seo, P.; Song, J.; Kim, H.; Park, C. Controlled nuclear import of the transcription factor NTL6 reveals a cytoplasmic role of SnRK2. 8 in the drought-stress response. Biochem. J. 2012, 448, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lu, S.; Wang, M.; Bi, D.; Sun, L.; Zhou, S.; Song, Z.; Liu, J. A plasma membrane-tethered transcription factor, NAC 062/ANAC 062/NTL 6, mediates the unfolded protein response in Arabidopsis. Plant J. 2014, 79, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, I.; Vermeirssen, V.; Van Aken, O.; Vandepoele, K.; Murcha, M.; Law, S.; Inzé, A.; Ng, S.; Ivanova, A.; Rombaut, D. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell. 2013, 25, 3472–3490. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, M.; Sun, L.; Lu, S.; Bi, D.; Sun, L.; Song, Z.; Zhang, S.-S.; Zhou, S.; Liu, J. The membrane-associated transcription factor NAC089 controls ER-stress-induced programmed cell death in plants. PLoS Genet. 2014, 10, e1004243. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, X.; Duan, H.; Lian, C.; Liu, C.; Yin, W.; Xia, X. Three stress-responsive NAC transcription factors from Populus euphratica differentially regulate salt and drought tolerance in transgenic plants. Physiol. Plant. 2018, 162, 73–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, T.; Ren, Y.; Song, L.; Liu, Z.; Kang, X.; Li, Y. The NAC transcription factor family in Eucommia ulmoides: Genome-wide identification, characterization, and network analysis in relation to the rubber biosynthetic genes. Front. Plant Sci. 2023, 14, 1030298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, J.; Yang, Q.; Huang, M.; Song, Y.; Li, M.; Sui, S.; Liu, D. Functional Characterization of the CpNAC1 Promoter and Gene from Chimonanthus praecox in Arabidopsis. Int. J. Mol. Sci. 2023, 24, 542. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C. Ascorbate and Glutathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Matysik, J.; Alia; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 5, 525–532. [Google Scholar]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. CRC Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Dai, F.; Zhou, M.; Zhang, G. The change of chlorophyll fluorescence parameters in winter barley during recovery after freezing shock and as affected by cold acclimation and irradiance. Plant Physiol. Biochem. 2007, 45, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Tak, H.; Negi, S.; Ganapathi, T. Banana NAC transcription factor MusaNAC042 is positively associated with drought and salinity tolerance. Protoplasma 2017, 254, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Chen, S.; Li, A.; Zhai, C.; Jing, R. Novel NAC transcription factor TaNAC67 confers enhanced multi-abiotic stress tolerances in Arabidopsis. PLoS ONE 2014, 9, e84359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Zhang, X.; Long, Y.; Huang, J.; Xia, J. OsNAC45 is Involved in ABA Response and Salt Tolerance in Rice. Rice 2020, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, S.; Zhang, C.; He, J.; Ma, D.; Wang, X.; Dong, T.; Guo, F.; Cai, J.; Long, T.; et al. The unique sweet potato NAC transcription factor IbNAC3 modulates combined salt and drought stresses. Plant Physiol. 2022, 191, 747–771. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Su, M.; Li, Q.; Xu, H.; Song, J.; Li, C.; Li, Q. A Transcription Factor SlNAC4 Gene of Suaeda liaotungensis Enhances Salt and Drought Tolerance through Regulating ABA Synthesis. Plants 2023, 12, 2951. [Google Scholar] [CrossRef]
- Xu, Z.; Kim, S.; Dai, J.; Hyeon, D.; Kim, D.; Dong, T.; Park, Y.; Jin, J.; Joo, S.; Kim, S.; et al. The Arabidopsis NAC Transcription Factor ANAC096 Cooperates with bZIP-Type Transcription Factors in Dehydration and Osmotic Stress Responses. Plant Cell. 2013, 25, 4708–4724. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, R.; Jiang, Q.; Sun, X.; Zhang, H.; Hu, Z. GmNAC06, a NAC domain transcription factor enhances salt stress tolerance in soybean. Plant Mol. Biol. 2021, 105, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xiong, A.; Peng, R.; Jin, X.; Xu, J.; Zhu, B.; Chen, J.; Yao, Q. OsNAC52, a rice NAC transcription factor, potentially responds to ABA and confers drought tolerance in transgenic plants. Plant Cell Tiss. Organ. Cult. 2010, 100, 255–262. [Google Scholar] [CrossRef]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Yu, S.; Pan, Y.; Wang, J.; Kay, S. The interplay between the circadian clock and abiotic stress responses mediated by ABF3 and CCA1/LHY. Proc. Natl. Acad. Sci. USA 2024, 121, e2316825121. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, Y.; Nanjo, T.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Stress-responsive and developmental regulation of Δ1-pyrroline-5-carboxylate synthetase 1 (P5CS1) gene expression in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 1999, 261, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Soon, F.; Ng, L.; Zhou, X.; West, G.; Kovach, A.; Tan, M.; Suino-Powell, K.; He, Y.; Xu, Y.; Chalmers, M.J.; et al. Molecular Mimicry Regulates ABA Signaling by SnRK2 Kinases and PP2C Phosphatases. Science 2012, 335, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dang, P.; Liu, L.; He, C. Cold acclimation by the CBF–COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shen, C.; Wu, L.; Tang, K.; Lin, J. CBF-dependent signaling pathway: A key responder to low temperature stress in plants. Crit. Rev. Biotechnol. 2011, 31, 186–192. [Google Scholar] [CrossRef]
- Haake, V.; Cook, K.; Riechmann, J.; Pineda, O.; Thomashow, M.; Zhang, J. Transcription Factor CBF4 Is a Regulator of Drought Adaptation in Arabidopsis. Plant Physiol. 2002, 130, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, N.; Xin, H.; Li, S. Overexpression of VaCBF4, a Transcription Factor from Vitis amurensis, Improves Cold Tolerance Accompanying Increased Resistance to Drought and Salinity in Arabidopsis. Plant Mol. Biol. Rep. 2013, 31, 1518–1528. [Google Scholar] [CrossRef]
- Mei, C.; Yang, J.; Mei, Q.; Jia, D.; Yan, P.; Feng, B.; Mamat, A.; Gong, X.Q.; Guan, Q.M.; Mao, K.; et al. MdNAC104 positively regulates apple cold tolerance via CBF-dependent and CBF-independent pathways. Plant Biotechnol. J. 2023, 21, 2057–2073. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Zhang, Y.; Lyu, Y. A Stress-Responsive NAC Transcription Factor from Tiger Lily (LlNAC2) Interacts with LlDREB1 and LlZHFD4 and Enhances Various Abiotic Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 3225. [Google Scholar] [CrossRef] [PubMed]
- Darwish, O.; Shahan, R.; Liu, Z.; Slovin, J.; Alkharouf, N. Re-annotation of the woodland strawberry (Fragaria vesca) genome. BMC Genom. 2015, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhang, Z.; Ni, B.; Ding, H.; Liu, W.; Li, W.; Chai, L.; Yang, G. Isolation and functional analysis of MxNAS3 involved in enhanced iron stress tolerance and abnormal flower in transgenic Arabidopsis. J. Plant Interact. 2018, 13, 433–441. [Google Scholar] [CrossRef]
- Li, X.; Liang, X.; Li, W.; Yao, A.; Liu, W.; Wang, Y.; Yang, G.; Han, D. Isolation and functional analysis of MbCBF2, a Malus baccata (L.) Borkh CBF transcription factor gene, with functions in tolerance to cold and salt stress in transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 9827. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Shi, Y.; Wang, B.; Liu, W.; Yu, Z.; Lv, B.; Yang, G. Isolation and preliminary functional analysis of MxCS2: A gene encoding a citrate synthase in Malus xiaojinensis. Plant Mol. Biol. Rep. 2015, 33, 133–142. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, L.; Xu, Y.; Lv, L.; Li, X.; Li, W.; Liu, W.; Ma, F.; Li, M.; Han, D. Genome-wide identiffcation and function analysis of the sucrose phosphate synthase MdSPS gene family in apple. J. Integr. Agric. 2023, 22, 2080–2093. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, J.; Huang, P.; Shao, B.; Li, W.; Liu, W.; Wang, Y.; Xie, L.; Han, M.; Han, D. Overexpression of MxFRO6, a FRO gene from Malus xiaojinensis, increases iron and salt tolerance in Arabidopsis thaliana. In Vitro Cell. Dev. Biol. Plant 2022, 58, 189–199. [Google Scholar] [CrossRef]
- Han, D.; Wang, L.; Wang, Y.; Yang, G.; Gao, C.; Yu, Z.; Li, T.; Zhang, X.; Ma, L.; Xu, X.; et al. Overexpression of Malus xiaojinensis CS1 gene in tobacco affects plant development and increases iron stress tolerance. Sci. Hortic. 2013, 150, 65–72. [Google Scholar] [CrossRef]
- Han, D.; Hou, Y.; Ding, H.; Zhou, Z.; Li, H.; Yang, G. Isolation and preliminary functional analysis of MbWRKY4 gene involved in salt tolerance in transgenic tobacco. Int. J. Agric. Biol. 2018, 20, 2045–2052. [Google Scholar] [CrossRef]
- Han, J.; Li, X.; Li, W.; Yang, Q.; Li, Z.; Cheng, Z.; Lv, L.; Zhang, L.; Han, D. Isolation and preliminary functional analysis of FvICE1, involved in cold and drought tolerance in Fragaria vesca through overexpression and CRISPR/Cas9 technologies. Plant Physiol. Biochem. 2023, 196, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Luo, G.; Li, X.; Yao, A.; Liu, W.; Zhang, L.; Wang, Y.; Li, W.; Han, D. MxFRO4 confers iron and salt tolerance through up-regulating antioxidant capacity associated with the ROS scavenging. J. Plant Physiol. 2023, 285, 154001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kirkham, M. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.; Singh, P.; Sardar, M.; Sarin, N. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- Liu, W.; Liang, X.; Cai, W.; Wang, H.; Liu, X.; Cheng, L.; Song, P.; Luo, G.; Han, D. Isolation and functional analysis of VvWRKY28, a Vitis Vinifera WRKY transcription factor gene, with functions in tolerance to cold and salt stress in transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 13418. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhong, J.; Zhang, L.; Wang, Y.; Song, P.; Liu, W.; Li, X.; Han, D. Overexpression of a Fragaria vesca MYB transcription factor gene (FvMYB82) increases salt and cold tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 10538. [Google Scholar] [CrossRef] [PubMed]
- Malnoy, M.; Jin, Q.; Borejsza-Wysocka, E.; He, S.; Aldwinckle, H. Overexpression of the Apple MpNPR1 Gene Confers Increased Disease Resistance in Malus × domestica. Mol. Plant. Microbe Interact. 2007, 20, 1568–1580. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Li, H.; Wei, Y.; Han, J.; Wang, Y.; Li, X.; Zhang, L.; Han, D. Overexpression of a Fragaria vesca NAM, ATAF, and CUC (NAC) Transcription Factor Gene (FvNAC29) Increases Salt and Cold Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 4088. https://doi.org/10.3390/ijms25074088
Li W, Li H, Wei Y, Han J, Wang Y, Li X, Zhang L, Han D. Overexpression of a Fragaria vesca NAM, ATAF, and CUC (NAC) Transcription Factor Gene (FvNAC29) Increases Salt and Cold Tolerance in Arabidopsis thaliana. International Journal of Molecular Sciences. 2024; 25(7):4088. https://doi.org/10.3390/ijms25074088
Chicago/Turabian StyleLi, Wenhui, Huiwen Li, Yangfan Wei, Jiaxin Han, Yu Wang, Xingguo Li, Lihua Zhang, and Deguo Han. 2024. "Overexpression of a Fragaria vesca NAM, ATAF, and CUC (NAC) Transcription Factor Gene (FvNAC29) Increases Salt and Cold Tolerance in Arabidopsis thaliana" International Journal of Molecular Sciences 25, no. 7: 4088. https://doi.org/10.3390/ijms25074088




