Elucidating the Role of OXPHOS Variants in Asthenozoospermia: Insights from Whole Genome Sequencing and an In Silico Analysis
Abstract
1. Introduction
2. Results
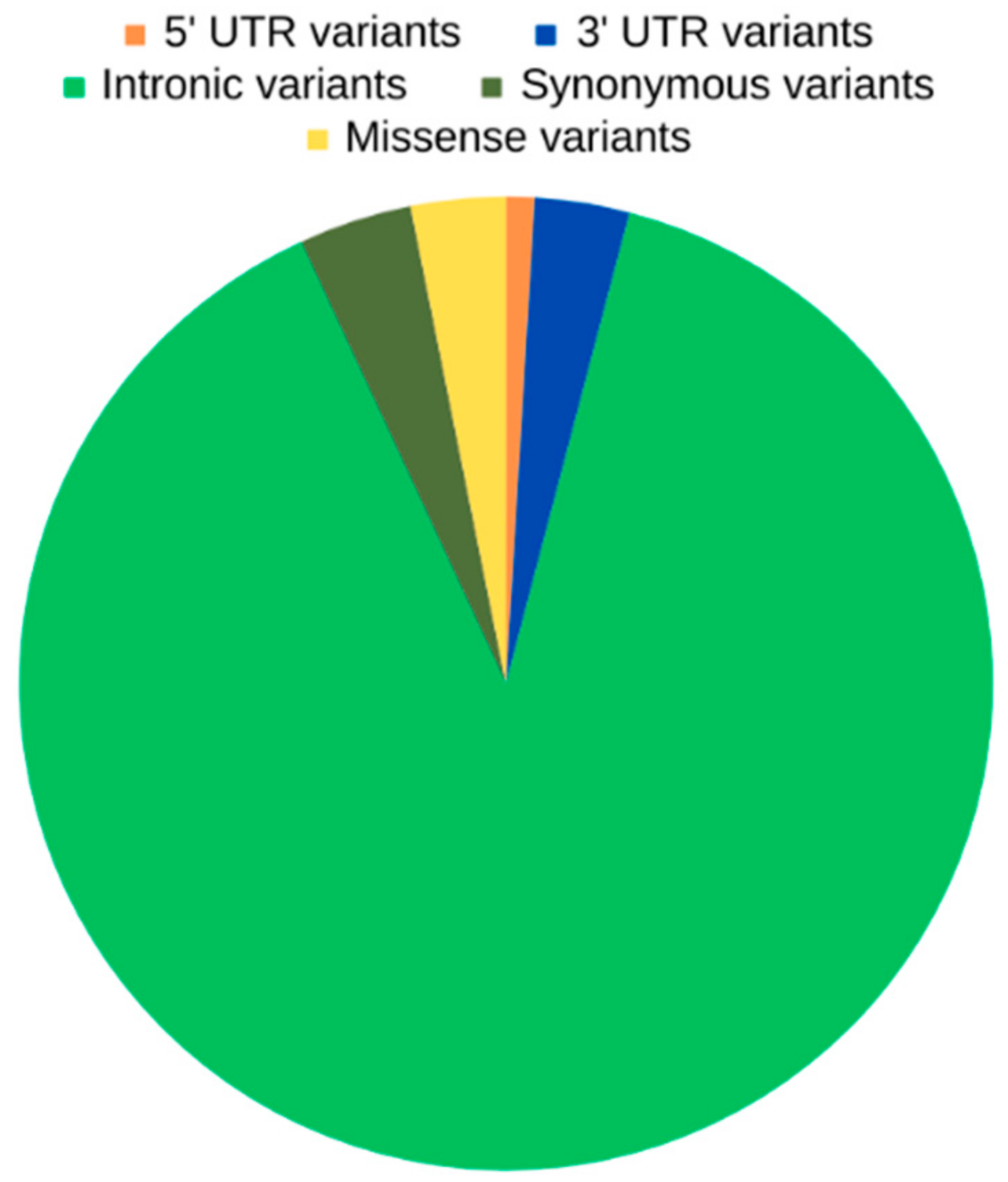
2.1. WGS Results—Variant Calling and Annotation
2.2. Unique OXPHOS Variants in Asthenozoospermic Men
2.3. Unique OXPHOS Variants in Asthenozoospermic Men—Genomic Consequences and Missense Variants
2.4. Unique OXPHOS Variants in Asthenozoospermic Men—Variants with Potential Functional Effect
2.5. Unique OXPHOS Variants in Asthenozoospermic Men—Expression Quantitative Trait Loci (eQTL) and Splicing Quantitative Trait Loci (sQTL)
2.6. Unique OXPHOS Variants in Asthenozoospermic Men—Association with Diseases
2.7. Unique OXPHOS Variants in Asthenozoospermic Men—Interactions with miRNAs
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. DNA Extraction and Sample Preparation
4.3. Whole Genome Sequencing (WGS)
4.4. Investigation of Unique Mutations in OXPHOS Genes—Bioinformatics Approach and Tools
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fainberg, J.; Kashanian, J.A. Recent Advances in Understanding and Managing Male Infertility. F1000Research 2019, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.-S. Male Infertility. Nat. Rev. Dis. Prim. 2023, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Kothandaraman, N.; Agarwal, A.; Abu-Elmagd, M.; Al-Qahtani, M.H. Pathogenic Landscape of Idiopathic Male Infertility: New Insight towards Its Regulatory Networks. NPJ Genom. Med. 2016, 1, 16023. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, D.V.S.; Shah, R.; Gajbhiye, R.K. Genetics of Male Infertility—Present and Future: A Narrative Review. J. Hum. Reprod. Sci. 2021, 14, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.R.; Meyers, S. The Sperm Mitochondrion: Organelle of Many Functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ford, W.C.L. Glycolysis and Sperm Motility: Does a Spoonful of Sugar Help the Flagellum Go Round? Hum. Reprod. Update 2006, 12, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Bioenergetics of Mammalian Sperm Capacitation. BioMed Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.; Kesari, K.K.; Roychoudhury, S.; Kolleboyina, J.; Jha, N.K.; Jha, S.K.; Sharma, A.; Kumar, A.; Rathi, B.; Kumar, D. Metabolic Dysregulation and Sperm Motility in Male Infertility. Adv. Exp. Med. Biol. 2022, 1358, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; Van Der Linde, M. Oxidative Phosphorylation versus Glycolysis: What Fuel Do Spermatozoa Use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Signes, A.; Fernandez-Vizarra, E. Assembly of Mammalian Oxidative Phosphorylation Complexes I–V and Supercomplexes. Essays Biochem. 2018, 62, 255–270. [Google Scholar] [PubMed]
- Vaser, R.; Adusumalli, S.; Ngak Leng, S.; Sikic, M.; Ng, P.C. SIFT Missense Predictions for Genomes. Nat. Protoc. 2015, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of Functional Variation in Personal Genomes Using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Quan, C.; Chen, H.; Bo, X.; Zhang, C. 3DSNP: A Database for Linking Human Noncoding SNPs to Their Three-Dimensional Interacting Genes. Nucleic Acids Res. 2017, 45, D643–D649. [Google Scholar] [CrossRef] [PubMed]
- Westra, H.J.; Peters, M.J.; Esko, T.; Yaghootkar, H.; Schurmann, C.; Kettunen, J.; Christiansen, M.W.; Fairfax, B.P.; Schramm, K.; Powell, J.E.; et al. Systematic Identification of Trans EQTLs as Putative Drivers of Known Disease Associations. Nat. Genet. 2013, 45, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Ardlie, K.G.; DeLuca, D.S.; Segrè, A.V.; Sullivan, T.J.; Young, T.R.; Gelfand, E.T.; Trowbridge, C.A.; Maller, J.B.; Tukiainen, T.; Lek, M.; et al. Human Genomics. The Genotype-Tissue Expression (GTEx) Pilot Analysis: Multitissue Gene Regulation in Humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ishigaki, K.; Suzuki, A.; Tsuchida, Y.; Tsuchiya, H.; Sumitomo, S.; Nagafuchi, Y.; Miya, F.; Tsunoda, T.; Shoda, H.; et al. Splicing QTL Analysis Focusing on Coding Sequences Reveals Mechanisms for Disease Susceptibility Loci. Nat. Commun. 2022, 13, 4659. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) Project. Nat. Genet. 2013, 45, 580. [Google Scholar] [CrossRef] [PubMed]
- Oscanoa, J.; Sivapalan, L.; Gadaleta, E.; Dayem Ullah, A.Z.; Lemoine, N.R.; Chelala, C. SNPnexus: A Web Server for Functional Annotation of Human Genome Sequence Variation (2020 Update). Nucleic Acids Res. 2020, 48, W185–W192. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The Intricate Balance between MicroRNA-Induced MRNA Decay and Translational Repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Xi, E.; Bai, J.; Zhang, K.; Yu, H.; Guo, Y. Genomic Variants Disrupt MiRNA-MRNA Regulation. Chem. Biodivers. 2022, 19, e202200623. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Fu, X.; Xia, M.; Zhang, Q.; Gu, Z.; Guo, A.Y. MiRNASNP-v3: A Comprehensive Database for SNPs and Disease-Related Variations in MiRNAs and MiRNA Targets. Nucleic Acids Res. 2021, 49, D1276–D1281. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Holyoake, A.J.; McHugh, P.; Wu, M.; O’Carroll, S.; Benny, P.; Sin, I.L.; Sin, F.Y.T. High Incidence of Single Nucleotide Substitutions in the Mitochondrial Genome Is Associated with Poor Semen Parameters in Men. Int. J. Androl. 2001, 24, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.B.; Kumar, R.; Bhatt, A.; Bamezai, R.N.K.; Kumar, R.; Gupta, N.P.; Das, T.K.; Dada, R. Mitochondrial DNA Mutations in Etiopathogenesis of Male Infertility. Indian J. Urol. 2008, 24, 150–154. [Google Scholar] [PubMed]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary Evidence on the Physiological Role of Reactive Oxygen Species in Human Sperm Function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Lake, N.J.; Bird, M.J.; Isohanni, P.; Paetau, A. Leigh Syndrome: Neuropathology and Pathogenesis. J. Neuropathol. Exp. Neurol. 2015, 74, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Kwon, W.S.; Karmakar, P.C.; Yoon, S.J.; Ryu, B.Y.; Pang, M.G. Gestational Exposure to Bisphenol A Affects the Function and Proteome Profile of F1 Spermatozoa in Adult Mice. Environ. Health Perspect. 2017, 125, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cui, H.; Chai, Z.; Zou, P.; Shi, F.; Yang, B.; Zhang, G.; Yang, H.; Chen, Q.; Liu, J.; et al. Benzo[a]Pyrene Inhibits Testosterone Biosynthesis via NDUFA10-Mediated Mitochondrial Compromise in Mouse Leydig Cells: Integrating Experimental and in Silico Toxicological Approaches. Ecotoxicol. Environ. Saf. 2022, 244, 114075. [Google Scholar] [CrossRef]
- Ryu, D.Y.; Pang, W.K.; Adegoke, E.O.; Rahman, M.S.; Park, Y.J.; Pang, M.G. Bisphenol-A Disturbs Hormonal Levels and Testis Mitochondrial Activity, Reducing Male Fertility. Hum. Reprod. Open 2023, 2023, hoad044. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Xu, Y.; Phoon, C.K.L.; Erdjument-Bromage, H.; Neubert, T.A.; Rajan, S.; Hussain, M.M.; Schlame, M. Condensed Mitochondria Assemble Into the Acrosomal Matrix During Spermiogenesis. Front. Cell Dev. Biol. 2022, 10, 867175. [Google Scholar] [CrossRef]
- Liu, R.; Huang, X.; Sun, Q.; Hou, Z.; Yang, W.; Zhang, J.; Zhang, P.; Huang, L.; Lu, Y.; Fu, Q. Comparative Proteomic Analyses of Poorly Motile Swamp Buffalo Spermatozoa Reveal Low Energy Metabolism and Deficiencies in Motility-Related Proteins. Animals 2022, 12, 1706. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.-G.; He, Z.; Wang, F.-C.; Li, S.; Shang, Q.-B.; Yang, Q.-E. Transcription Factor E4F1 Dictates Spermatogonial Stem Cell Fate Decisions by Regulating Mitochondrial Functions and Cell Cycle Progression. Cell Biosci. 2023, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Lord, T.; Nixon, B. Metabolic Changes Accompanying Spermatogonial Stem Cell Differentiation. Dev. Cell 2020, 52, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Moszyńska, A.; Gebert, M.; Collawn, J.F.; Bartoszewski, R. SNPs in MicroRNA Target Sites and Their Potential Role in Human Disease. Open Biol. 2017, 7, 170019. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Qin, Y.; Li, Z.; Dong, J.; Dai, J.; Lu, C.; Guo, X.; Zhao, Y.; Zhu, Y.; Zhang, W.; et al. Genome-Wide MicroRNA Expression Profiling in Idiopathic Non-Obstructive Azoospermia: Significant up-Regulation of MiR-141, MiR-429 and MiR-7-1-3p. Hum. Reprod. 2013, 28, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Mokánszki, A.; Molnár, Z.; Varga Tóthné, E.; Bodnár, B.; Jakab, A.; Bálint, B.L.; Balogh, I. Altered MicroRNAs Expression Levels of Sperm and Seminal Plasma in Patients with Infertile Ejaculates Compared with Normozoospermic Males. Hum. Fertil. 2020, 23, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Andrabi, S.W.; Yadav, R.K.; Sankhwar, S.N.; Gupta, G.; Rajender, S. Qualitative and Quantitative Assessment of Sperm MiRNAs Identifies Hsa-MiR-9-3p, Hsa-MiR-30b-5p and Hsa-MiR-122-5p as Potential Biomarkers of Male Infertility and Sperm Quality. Reprod. Biol. Endocrinol. 2022, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, S.; Pashaiasl, M.; Ezzati, M.; Ahmadi AsrBadr, Y.; Mohammadi-Dehcheshmeh, M.; Mohammadi, S.A.; Ghaffari Novin, M. MicroRNA-Based Regulatory Circuit Involved in Sperm Infertility. Andrologia 2020, 52, e13453. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Hammadeh, M.; Schmitt, J.; Leidinger, P.; Keller, A.; Meese, E.; Backes, C. Altered MicroRNA Expression Profiles of Human Spermatozoa in Patients with Different Spermatogenic Impairments. Fertil. Steril. 2013, 99, P1249–P1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, Y.; Liu, J.; Zhao, Y.; Jiang, L.; Huang, Q.; Cheng, W.; Guo, L. MicroRNA-122 Influences the Development of Sperm Abnormalities from Human Induced Pluripotent Stem Cells by Regulating TNP2 Expression. Stem Cells Dev. 2013, 22, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Hammadeh, M.; Backes, C.; Fischer, U.; Leidinger, P.; Lubbad, A.M.; Keller, A.; Meese, E. Panel of Five MicroRNAs as Potential Biomarkers for the Diagnosis and Assessment of Male Infertility. Fertil. Steril. 2014, 102, 989–997.e1. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Goodyear, S.M.; Rao, S.; Wu, X.; Tobias, J.W.; Avarbock, M.R.; Brinster, R.L. MicroRNA-21 Regulates the Self-Renewal of Mouse Spermatogonial Stem Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 12740–12745. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xie, Y.; Zhou, C.; Hu, Q.; Gu, T.; Yang, J.; Zheng, E.; Huang, S.; Xu, Z.; Cai, G.; et al. Expression Pattern of Seminal Plasma Extracellular Vesicle Small RNAs in Boar Semen. Front. Vet. Sci. 2020, 7, 585276. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liao, M.Z.; Zhu, H.J.; Kang, K.; Mu, H.L.; Song, W.C.; Niu, Z.W.; He, X.; Bai, C.L.; Li, G.P.; et al. CD49f-Positive Testicular Cells in Saanen Dairy Goat Were Identified as Spermatogonia-Like Cells by MiRNA Profiling Analysis. J. Cell. Biochem. 2014, 115, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Ribas-Maynou, J.; Mateo-Otero, Y.; Tamargo, C.; Llavanera, M.; Yeste, M. Expression of MiR-138 in Cryopreserved Bovine Sperm Is Related to Their Fertility Potential. J. Anim. Sci. Biotechnol. 2023, 14, 129. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Tang, J.S.; Di, R.; Liu, Q.Y.; Wang, X.Y.; Gan, S.Q.; Zhang, X.S.; Zhang, J.L.; Chu, M.X.; Hu, W.P. Integrated Hypothalamic Transcriptome Profiling Reveals the Reproductive Roles of MRNAs and MiRNAs in Sheep. Front. Genet. 2020, 10, 1296. [Google Scholar] [CrossRef]
- Gebremedhn, S.; Salilew-Wondim, D.; Hoelker, M.; Held-Hoelker, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Tesfaye, D. Exploring Maternal Serum MicroRNAs during Early Pregnancy in Cattle. Theriogenology 2018, 121, 196–203. [Google Scholar] [CrossRef]
- Vashukova, E.S.; Kozyulina, P.Y.; Illarionov, R.A.; Yurkina, N.O.; Pachuliia, O.V.; Butenko, M.G.; Postnikova, T.B.; Ivanova, L.A.; Eremeeva, D.R.; Zainulina, M.S.; et al. High-Throughput Sequencing of Circulating MicroRNAs in Plasma and Serum during Pregnancy Progression. Life 2021, 11, 1055. [Google Scholar] [CrossRef]
- Miguel, V.; Ramos, R.; García-Bermejo, L.; Rodríguez-Puyol, D.; Lamas, S. The Program of Renal Fibrogenesis Is Controlled by MicroRNAs Regulating Oxidative Metabolism. Redox Biol. 2021, 40, 101851. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, X.; Xiao, X.; Ye, L.; Huang, Y.; Lu, C.; Su, Z. Identification and Functional Characterization of MicroRNAs in Rat Leydig Cells during Development from the Progenitor to the Adult Stage. Mol. Cell. Endocrinol. 2019, 493, 110453. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.W.; Wang, B.; Ding, C.H.; Li, T.; Gu, F.; Zhou, C. Differentially Expressed MicoRNAs in Human Oocytes. J. Assist. Reprod. Genet. 2011, 28, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Rooda, I.; Kaselt, B.; Liivrand, M.; Smolander, O.P.; Salumets, A.; Velthut-Meikas, A. Hsa-Mir-548 Family Expression in Human Reproductive Tissues. BMC Genom. Data 2021, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, W.; Wang, X.; Dang, Y.; Xu, L.; Lu, G.; Chan, W.Y.; Leung, P.C.K.; Zhao, S.; Qin, Y. LncRNA DDGC Participates in Premature Ovarian Insufficiency through Regulating RAD51 and WT1. Mol. Ther. Nucleic Acids 2021, 26, 1092–1106. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.C.; Zhang, Y.; Yu, K.; Li, Y.; Yu, H.; Zhou, S.J.; Wang, Y.P.; Deng, S.L.; Tian, L. LncRNAs Induce Oxidative Stress and Spermatogenesis by Regulating Endoplasmic Reticulum Genes and Pathways. Aging 2021, 13, 13764–13787. [Google Scholar] [CrossRef]
- Lü, M.; Tian, H.; Cao, Y.X.; He, X.; Chen, L.; Song, X.; Ping, P.; Huang, H.; Sun, F. Downregulation of MiR-320a/383-Sponge-like Long Non-Coding RNA NLC1-C (Narcolepsy Candidate-Region 1 Genes) Is Associated with Male Infertility and Promotes Testicular Embryonal Carcinoma Cell Proliferation. Cell Death Dis. 2015, 6, E1960. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiafini, M.A.; Giannoulis, T.; Chatziparasidou, A.; Christoforidis, N.; Mamuris, Z. Unveiling the Genetic Complexity of Teratozoospermia: Integrated Genomic Analysis Reveals Novel Insights into LncRNAs’ Role in Male Infertility. Int. J. Mol. Sci. 2023, 24, 15002. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J.; Amode, M.R.; Aneja, A.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2023. Nucleic Acids Res. 2023, 51, D933–D941. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve Years of SAMtools and BCFtools. Gigascience 2021, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Garrison, E.; Marth, G. Haplotype-Based Variant Detection from Short-Read Sequencing. arXiv 2012, arXiv:1207.3907. [Google Scholar]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Wang, Q.; Alföldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; Poterba, T.; et al. A Genome-Wide Mutational Constraint Map Quantified from Variation in 76,156 Human Genomes. bioRxiv 2022. [Google Scholar] [CrossRef]
- Phan, L.; Jin, Y.; Zhang, H.; Qiang, W.; Shekhtman, E.; Shao, D.; Revoe, D.; Villamarin, R.; Ivanchenko, E.; Kimura, M.; et al. ALFA: Allele Frequency Aggregator. Available online: www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/ (accessed on 5 March 2024).
| Genes | Variant Number | Length of Gene/ Variant Number (%) | OXPHOS Variants/Total Variants in Asthenozoospermic (%) |
|---|---|---|---|
| Mitochondrial Respiratory Complex I | |||
| NDUFS1 | 3 | 0.0067 | 0.0004 |
| NDUFS2 | 3 | 0.0170 | 0.0004 |
| NDUFS3 | 1 | 0.0052 | 0.0001 |
| NDUFS7 | 7 | 0.0580 | 0.0010 |
| NDUFV1 | 1 | 0.0145 | 0.0001 |
| NDUFV2 | 5 | 0.0580 | 0.0007 |
| MT-ND2 | 1 | 0.0961 | 0.0001 |
| MT-ND5 | 10 | 0.5522 | 0.0001 |
| MT-ND6 | 1 | 0.1908 | 0.0001 |
| NDUFAB1 | 3 | 0.0196 | 0.0004 |
| NDUFA5 | 3 | 0.0143 | 0.0004 |
| NDUFA8 | 3 | 0.0191 | 0.0004 |
| NDUFA9 | 8 | 0.0177 | 0.0012 |
| NDUFA10 | 42 | 0.3151 | 0.0062 |
| NDUFA11 | 1 | 0.0079 | 0.0001 |
| NDUFA12 | 30 | 0.0277 | 0.0044 |
| NDUFA13 | 2 | 0.0150 | 0.0003 |
| NDUFB1 | 5 | 0.0863 | 0.0007 |
| NDUFB2 | 4 | 0.0125 | 0.0006 |
| NDUFB3 | 4 | 0.0279 | 0.0006 |
| NDUFB4 | 2 | 0.0324 | 0.0003 |
| NDUFB5 | 2 | 0.0087 | 0.0003 |
| NDUFB6 | 3 | 0.0149 | 0.0004 |
| NDUFB8 | 2 | 0.0319 | 0.0003 |
| NDUFB9 | 10 | 0.0241 | 0.0015 |
| NDUFB10 | 1 | 0.0410 | 0.0001 |
| NDUFC1 | 2 | 0.0056 | 0.0003 |
| NDUFC2 | 2 | 0.0173 | 0.0003 |
| NDUFS4 | 11 | 0.0090 | 0.0016 |
| NDUFS5 | 5 | 0.0601 | 0.0007 |
| NDUFS6 | 1 | 0.0068 | 0.0001 |
| NDUFV3 | 5 | 0.0149 | 0.0007 |
| Mitochondrial Respiratory Complex II | |||
| SDHA | 3 | 0.0077 | 0.0004 |
| SDHB | 8 | 0.0226 | 0.0012 |
| SDHC | 11 | 0.0225 | 0.0016 |
| SDHD | 48 | 0.1446 | 0.0071 |
| Mitochondrial Respiratory Complex III | |||
| UQCRC2 | 4 | 0.0132 | 0.0006 |
| MT-CYB | 2 | 0.1754 | 0.0003 |
| Mitochondrial Respiratory Complex IV | |||
| COX5A | 1 | 0.0055 | 0.0001 |
| COX6B1 | 8 | 0.0765 | 0.0012 |
| COX6C | 4 | 0.0195 | 0.0006 |
| COX7B2 | 34 | 0.0195 | 0.0050 |
| MT-CO2 | 2 | 0.2928 | 0.0003 |
| MT-CO3 | 1 | 0.1277 | 0.0001 |
| Mitochondrial Respiratory Complex I | |
|---|---|
| Variants in mitochondrial-encoded genes | 12 |
| Variants in nuclear-encoded genes | 171 |
| Mitochondrial Respiratory Complex II | |
| Variants in mitochondrial-encoded genes | 0 |
| Variants in nuclear-encoded genes | 70 |
| Mitochondrial Respiratory Complex III | |
| Variants in mitochondrial-encoded genes | 2 |
| Variants in nuclear-encoded genes | 4 |
| Mitochondrial Respiratory Complex IV | |
| Variants in mitochondrial-encoded genes | 3 |
| Variants in nuclear-encoded genes | 47 |
| Genomic Coordinates | Allele | Allele Frequency (Europeans) | Variant | Gene | SIFT Score | Polyphen2 Score |
|---|---|---|---|---|---|---|
| MT:12406-12406 | A | 0.2% | rs28617389 | MT-ND5 | 0.45 (tolerated) | 0 (benign) |
| MT:13708-13708 | A | 11.6% | rs28359178 | MT-ND5 | 0.26 (tolerated) | 0 (benign) |
| MT:13780-13780 | G | 2.9% | rs41358152 | MT-ND5 | 0.01 (deleterious) | 0.003 (benign) |
| MT:13928-13928 | C | 0.2% | rs28359184 | MT-ND5 | 1 (tolerated) | 0.18 (benign) |
| MT:14178-14178 | C | 0.2% | rs28357671 | MT-ND6 | 0.4 (tolerated) | 0.023 (benign) |
| MT:14793-14793 | G | 3.7% | rs2853504 | MT-CYB | 0.04 (deleterious) | 0.003 (benign) |
| MT:9477-9477 | A | 8.5% | rs2853825 | MT-CO3 | 0.1 (tolerated) | 0 (benign) |
| 2:240951071-240951071 | T | 1.1% | rs35462421 | NDUFA10 | 0.01 (deleterious) | 0.995 (probably damaging) |
| 16:21976762-21976762 | A | 4.3% | rs4850 | UQCRC2 | 0.04 (deleterious) | 0.003 (benign) |
| 5:52942083-52942083 | C | 96% | rs31304 | NDUFS4 | - | 0 (unknown) |
| Genomic Coordinates | Allele | Allele Frequency (Europeans) | Variant | Gene | Genomic Consequences | RegulomeDB Rank | 3DSNP Score |
|---|---|---|---|---|---|---|---|
| 19:1394865-1394865 | C | 2.1% | rs73515054 | NDUFS7 | 3′ UTR variant, intron variant | 2b | 13.72 |
| 9:124897110-124897110 | T | 8.7% | rs11998959 | NDUFA8 | Intron variant | 1f | 36.76 |
| 9:124897088-124897088 | T | 8.3% | rs11998958 | NDUFA8 | Intron variant | 1f | 36.36 |
| 7:123197559-123197559 | C | 8.6% | rs17146099 | NDUFA5 | 5′ UTR variant, intron variant | 1f | 146.4 |
| 2:240897460-240897460 | C | 3.5% | rs7588974 | NDUFA10 | 3′ UTR variant, intron variant | 2b | 10.56 |
| 16:2011653-2011667 | CCCCCA | 0.03% | rs774819361 | NDUFB10 | Intron variant | 2a | 103.27 |
| 8:125551858-125551858 | G | 3.5% | rs72713101 | NDUFB9 | Intron variant | 1f | 108.59 |
| 8:125554452-125554452 | T | 3.3% | rs111795428 | NDUFB9 | Intron variant | 1f | 11.6 |
| 8:125552526-125552527 | - | 3.3% | rs112295879 | NDUFB9 | Intron variant | 1b | 116.14 |
| 11:77790158-77790158 | AAAAA | 0.1% | rs752264424 | NDUFC2 | Intron variant | 2b | 104.37 |
| 1:161175652-161175652 | A | 1.8% | rs145629160 | NDUFS2 | Intron variant | 1f | 13.25 |
| 1:161171736-161171736 | G | 1.8% | rs115518404 | NDUFS2 | Intron variant | 1b | 146.84 |
| 21:44313221-44313221 | C | 20.2% | rs35197797 | NDUFV3 | Intron variant | 1a | 211.4 |
| 8:100903890-100903890 | G | 14.1% | rs12544943 | COX6C | Intron variant | 1f | 66.33 |
| 11:67374581-67374581 | C | 38.2% | rs1871043 | NDUFV1 | Intron variant | 1f | 208.1 |
| 18:9119489-9119489 | T | 9.1% | rs41274300 | NDUFV2 | Synonymous variant | 1f | 28.68 |
| 14:92586558-92586558 | A | 16.3% | rs79507139 | NDUFB1 | Intron variant | 1f | 16.69 |
| 12:95376507-95376507 | T | 9.2% | rs4923659 | NDUFA12 | Intron variant | 1b | 16.37 |
| 12:95371804-95371806 | - | 9.2% | rs113060515 | NDUFA12 | Intron variant | 1f | 13.75 |
| 12:95374449-95374449 | C | 9.2% | rs76835653 | NDUFA12 | Intron variant | 1b | 59.71 |
| 12:95397275-95397275 | T | 10.1% | rs17321986 | NDUFA12 | Intron variant | 1b | 201 |
| 11:112044398-112044398 | C | 22.9% | rs12420476 | SDHD | Intron variant | 1f | 11.55 |
| 11:112034062-112034063 | AA | 22% | rs5744230 | SDHD | Intron variant | 1d | 33.1 |
| 11:112037730-112037730 | A | 10.8% | rs72992972 | SDHD | Intron variant | 1d | 14.26 |
| 11:112047061-112047061 | A | 12.2% | rs10431036 | SDHD | Intron variant | 1f | 20.65 |
| 11:112043614-112043614 | A | 12.2% | rs11214108 | SDHD | Intron variant | 1f | 12.25 |
| 11:112048051-112048051 | Τ | 22.7% | rs7121554 | SDHD | Intron variant | 1f | 12.03 |
| 11:111991866-111991868 | - | 0.3% | rs1453244355 | SDHD | Intron variant | 2b | 11.47 |
| Genomic Coordinates | Allele | Allele Frequency (Europeans) | Variant | Gene | Genomic Consequences | Function | p-Value |
|---|---|---|---|---|---|---|---|
| 7:123197559-123197559 | C | 8.6% | rs17146099 | NDUFA5 | 5′ UTR variant, intron variant | eQTL (Testis) | 0.000089 |
| 7:123197559-123197559 | C | 8.6% | rs17146099 | NDUFA5 | 5′ UTR variant, intron variant | sQTL (Testis) | 9.3 × 10−8 |
| 7:123190928-123190928 | T | 8.6% | rs34225533 | NDUFA5 | Intron variant | eQTL (Testis) | 0.000036 |
| 7:123190928-123190928 | T | 8.6% | rs34225533 | NDUFA5 | Intron variant | sQTL (Testis) | 9.8 × 10−7 |
| 2:240872465-240872465 | A | 14.7% | rs11684384 | NDUFA10 | Intron variant | eQTL (Testis) | 8.4 × 10−10 |
| 2:240872465-240872465 | A | 14.7% | rs11684384 | NDUFA10 | Intron variant | eQTL (Prostate) | 2.1 × 10−15 |
| 21:44325525-44325525 | T | 20.2% | rs8134542 | NDUFV3 | Intron variant | eQTL (Prostate) | 3.9 × 10−12 |
| 21:44328278-44328278 | A | 20.2% | rs35893787 | NDUFV3 | Intron variant | eQTL (Prostate) | 7.8 × 10−13 |
| 21:44313221-44313221 | C | 20.2% | rs35197797 | NDUFV3 | Intron variant | eQTL (Prostate) | 9.5 × 10−13 |
| 8:100894978-100894986 | AAAC | 18.2% | rs71274941 | COX6C | Intron variant | sQTL (Testis) | 1.1 × 10−59 |
| 8:100894978-100894986 | AAAC | 18.2% | rs71274941 | COX6C | Intron variant | sQTL (Prostate) | 1.4 × 10−28 |
| 8:100903890-100903890 | G | 14.1% | rs12544943 | COX6C | Intron variant | sQTL (Testis) | 9.1 × 10−36 |
| 8:100903890-100903890 | G | 14.1% | rs12544943 | COX6C | Intron variant | sQTL (Prostate) | 1.8 × 10−16 |
| 11:67374581-67374581 | C | 38.2% | rs1871043 | NDUFV1 | Intron variant | eQTL (Prostate) | 7.6 × 10−9 |
| 4:46775623-46775623 | G | 4.7% | rs78130313 | COX7B2 | Intron variant | eQTL (Testis) | 0.000032 |
| 4:46908004-46908004 | A | 5.4% | rs371114117 | COX7B2 | Intron variant | eQTL (Testis) | 0.00010 |
| 4:46908004-46908004 | A | 5.4% | rs371114117 | COX7B2 | Intron variant | sQTL (Testis) | 3.9 × 10−7 |
| 12:95387542-95387542 | Τ | 44.4% | rs4923660 | NDUFA12 | Intron variant | eQTL (Testis) | 0.000015 |
| 12:95387542-95387542 | Τ | 44.4% | rs4923660 | NDUFA12 | Intron variant | sQTL (Testis) | 0.0000037 |
| Genomic Coordinates | Allele | Allele Frequency (Europeans) | Variant | Gene | Genomic Consequence | Association with Diseases |
|---|---|---|---|---|---|---|
| 19:1391059-1391059 | T | 1.9% | rs2074896 | NDUFS7 | intron variant | Leigh syndrome, Mitochondrial complex I deficiency (Benign/Likely benign) |
| 2:240897460-240897460 | C | 3.5% | rs7588974 | NDUFA10 | 3′ UTR variant, intron variant | Leigh syndrome, Mitochondrial complex I deficiency |
| 2:240951071-240951071 | T | 1.1% | rs35462421 | NDUFA10 | Missense variant | Leigh syndrome (Benign/Likely benign) |
| 5:52942083-52942083 | C | 96% | rs31304 | NDUFS4 | Synonymous variant | Leigh syndrome, Mitochondrial complex I deficiency (Benign) |
| 18:9119489-9119489 | T | 9.1% | rs41274300 | NDUFV2 | Synonymous variant | Mitochondrial complex I deficiency (Benign/Likely benign) |
| Genomic Coordinates | Allele | Allele Frequency (Europeans) | Variant | Gene | miRNA Loss | miRNA Gain |
|---|---|---|---|---|---|---|
| 3:120320652-120320652 | C | 0.7% | rs190013694 | NDUFB4 | hsa-miR-1273h-3p, hsa-miR-1245b-3p, hsa-miR-5700, hsa-miR-3678-3p | hsa-miR-1193, hsa-miR-105-3p, hsa-miR-4754, hsa-miR-6850-5p |
| 19:1394865-1394865 | C | 2.1% | rs73515054 | NDUFS7 | hsa-miR-495-3p, hsa-miR-5688, hsa-miR-7-2-3p, hsa-miR-589-3p, hsa-miR-7-1-3p, hsa-miR-4773 | hsa-miR-2278, hsa-miR-548p, hsa-miR-6501-3p |
| 7:123180937-123180942 | GCG | 0.6% | rs201784621 | NDUFA5 | hsa-miR-4536-3p, hsa-miR-4787-3p | hsa-miR-8064, hsa-miR-6821-5p, hsa-miR-4783-5p |
| 2:240897460-240897460 | C | 3.5% | rs7588974 | NDUFA10 | hsa-miR-3155b, hsa-miR-3155a, hsa-miR-4518, hsa-miR-1266-5p, hsa-miR-484, hsa-miR-3664-3p | hsa-miR-6829-3p, hsa-miR-6741-3p, hsa-miR-6778-3p, hsa-miR-6791-3p |
| 12:4798415-4798415 | Τ | 0.1% | rs181096156 | NDUFA9 | hsa-miR-4712-3p, hsa-miR-580-3p, hsa-miR-539-5p | hsa-miR-577 |
| 4:46736853-46736853 | Τ | 13.4% | rs11736008 | COX7B2 | - | hsa-miR-12135, hsa-miR-4748, hsa-miR-299-5p, hsa-miR-548m, hsa-miR-4464, hsa-miR-548at-5p, hsa-miR-561-3p, hsa-miR-329-5p |
| 11:111966122-111966122 | G | 0.7% | rs184654032 | SDHD | hsa-miR-3120-5p, hsa-miR-200a-3p, hsa-miR-1208, hsa-miR-6757-3p, hsa-miR-141-3p, hsa-miR-6760-3p | hsa-miR-340-3p, hsa-miR-122b-3p, hsa-miR-6827-3p, hsa-miR-21-3p |
| Variant | Gene | Allele Frequency (Europeans) | Missense Variant | Functional Significance | Association with Diseases | eQTLs/sQTLs | miRNA Interactions |
|---|---|---|---|---|---|---|---|
| rs35462421 | NDUFA10 | 1.1% | Damaging according to both databases | - | ✓ | - | - |
| rs31304 | NDUFS4 | 96% | Unknown impact | - | ✓ | - | - |
| rs73515054 | NDUFS7 | 2.1% | - | ✓ | - | - | ✓ |
| rs17146099 | NDUFA5 | 8.6% | - | ✓ | - | ✓ | - |
| rs7588974 | NDUFA10 | 3.5% | - | ✓ | ✓ | - | ✓ |
| rs35197797 | NDUFV3 | 20.2% | - | ✓ | - | ✓ | - |
| rs12544943 | COX6C | 14.1% | - | ✓ | - | ✓ | - |
| rs1871043 | NDUFV1 | 38.2% | - | ✓ | - | ✓ | - |
| rs41274300 | NDUFV2 | 9.1% | - | ✓ | ✓ | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyrgiafini, M.-A.; Giannoulis, T.; Chatziparasidou, A.; Mamuris, Z. Elucidating the Role of OXPHOS Variants in Asthenozoospermia: Insights from Whole Genome Sequencing and an In Silico Analysis. Int. J. Mol. Sci. 2024, 25, 4121. https://doi.org/10.3390/ijms25074121
Kyrgiafini M-A, Giannoulis T, Chatziparasidou A, Mamuris Z. Elucidating the Role of OXPHOS Variants in Asthenozoospermia: Insights from Whole Genome Sequencing and an In Silico Analysis. International Journal of Molecular Sciences. 2024; 25(7):4121. https://doi.org/10.3390/ijms25074121
Chicago/Turabian StyleKyrgiafini, Maria-Anna, Themistoklis Giannoulis, Alexia Chatziparasidou, and Zissis Mamuris. 2024. "Elucidating the Role of OXPHOS Variants in Asthenozoospermia: Insights from Whole Genome Sequencing and an In Silico Analysis" International Journal of Molecular Sciences 25, no. 7: 4121. https://doi.org/10.3390/ijms25074121
APA StyleKyrgiafini, M.-A., Giannoulis, T., Chatziparasidou, A., & Mamuris, Z. (2024). Elucidating the Role of OXPHOS Variants in Asthenozoospermia: Insights from Whole Genome Sequencing and an In Silico Analysis. International Journal of Molecular Sciences, 25(7), 4121. https://doi.org/10.3390/ijms25074121






