Exogenous SNP Alleviates Drought Stress in Wheat During the Grain-Filling Stage by Modulating TaP5CS Gene Transcription
Abstract
:1. Introduction
2. Results
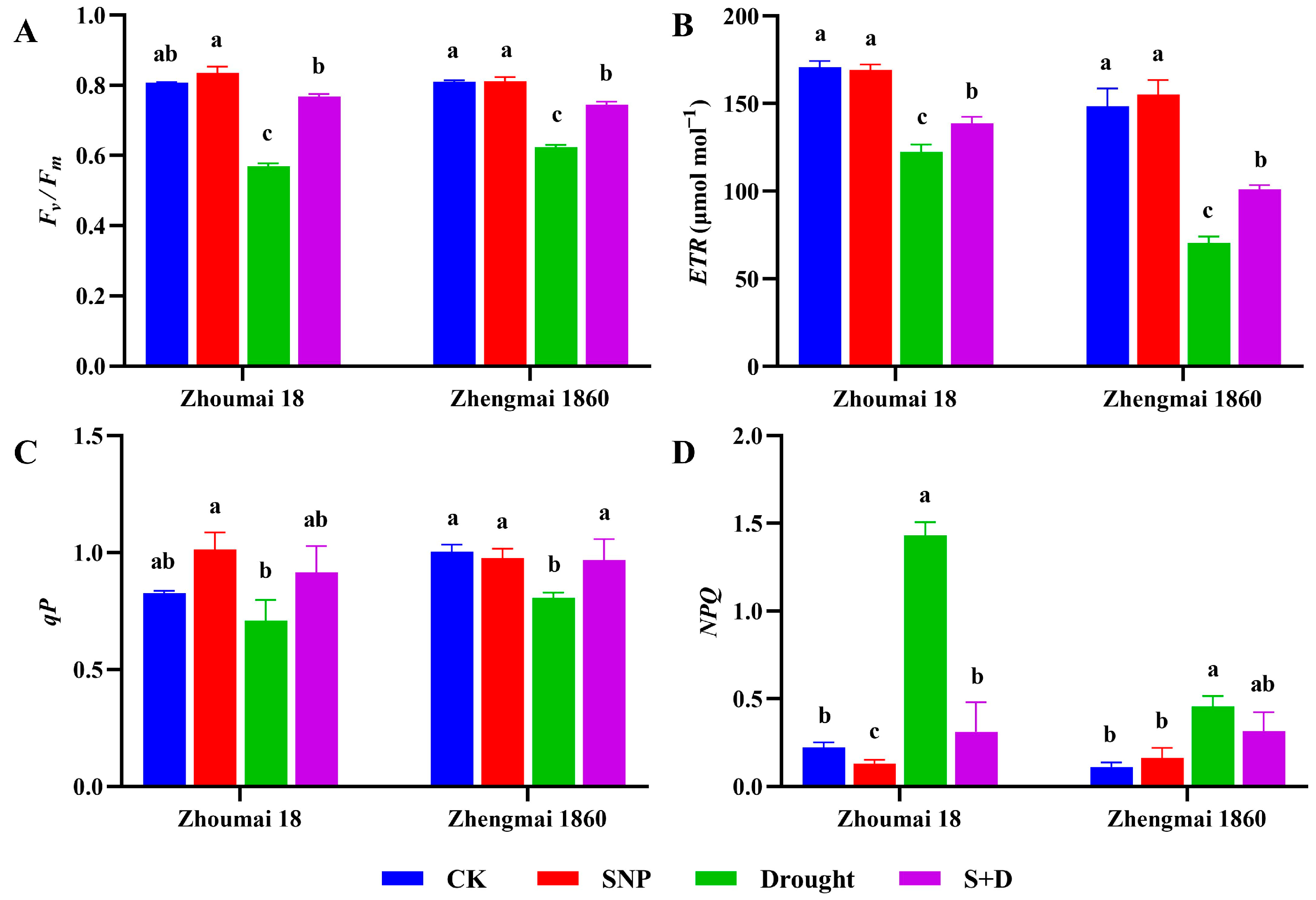
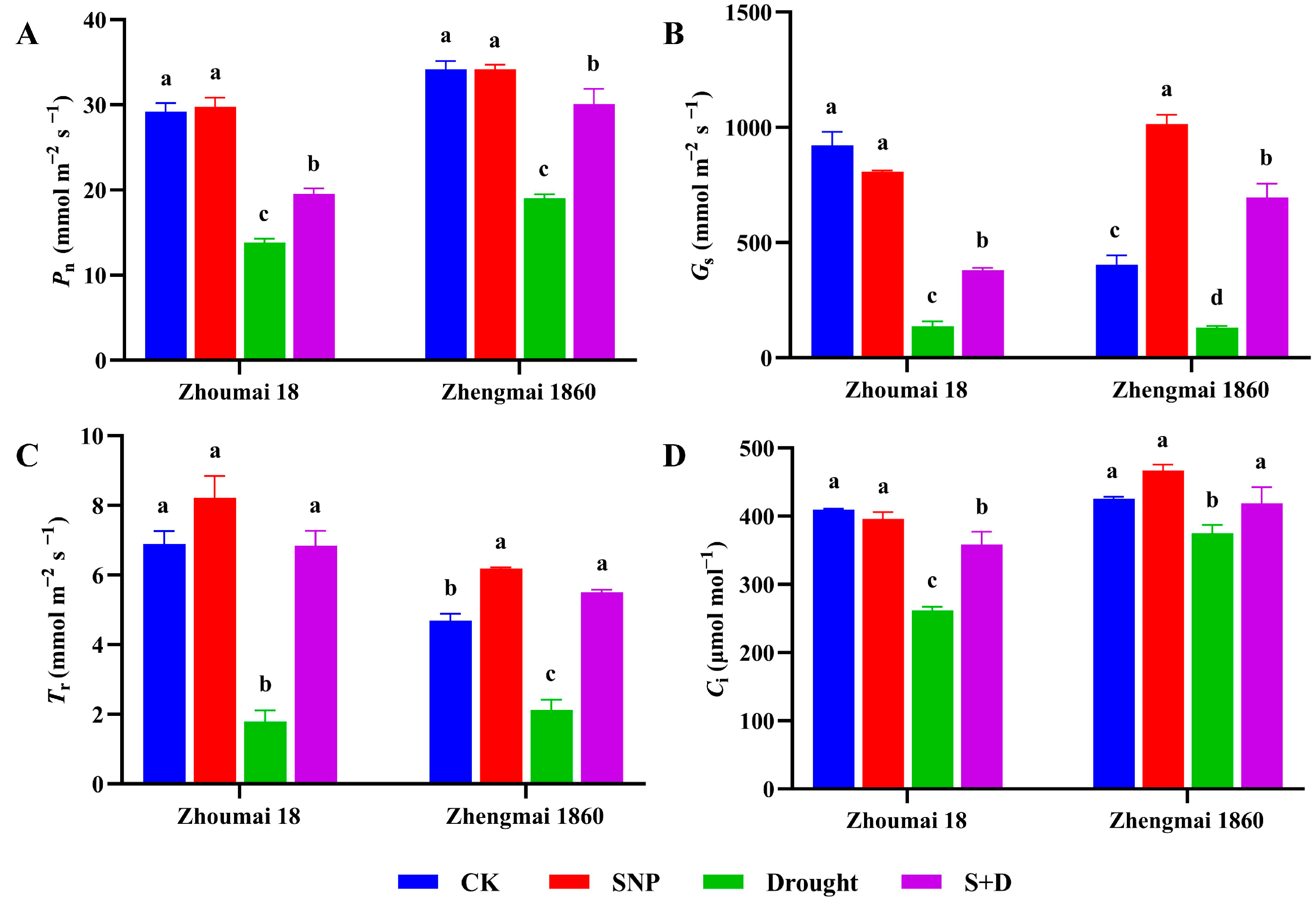
2.1. Effects of Exogenous SNP on Phenotype and Physiological Characters of Flag Leaves of Wheat Under Drought Stress
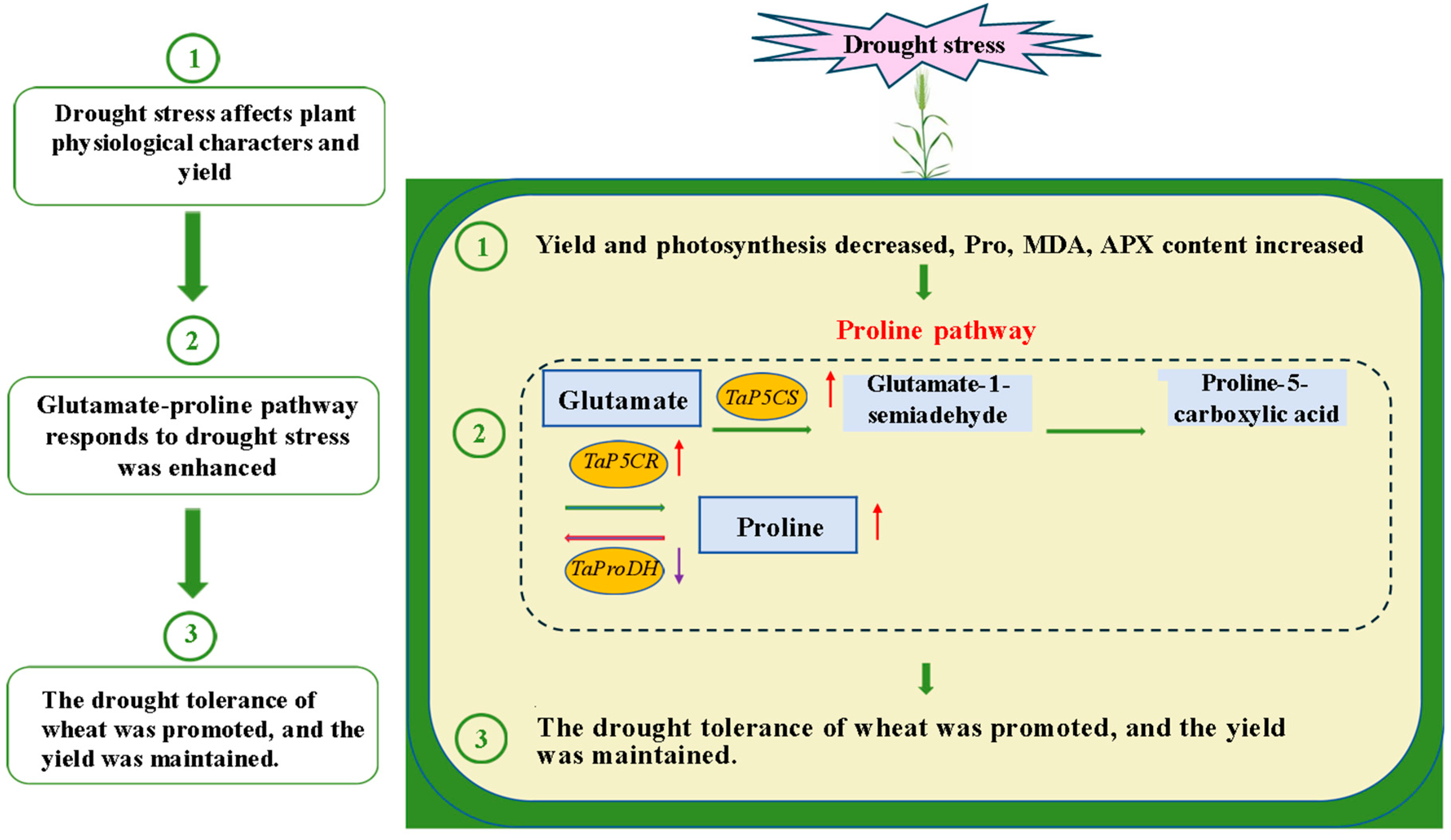
2.2. Regulation of Proline Pathway Gene Transcription in Flag Leaves of Wheat at Filling Stage Under Drought Stress
3. Discussion
4. Materials and Methods
4.1. Wheat Plant Materials, Growth Conditions, SNP Pretreatment, and Drought Stress Treatment
4.2. Measurement of the MDA Content and SPAD
4.3. Determination of Ascorbate Peroxidase (APX) Activity
4.4. Determination of Chlorophyll Fluorescence Parameters
4.5. Determination of Photosynthetic Gas Exchange Parameters
4.6. Determination of Proline Content
4.7. Reverse Transcription Real-Time PCR Analysis
4.8. Wheat Yield Trait Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Aslam, Z.; Javed, T.; Hussain, S.; Raza, A.; Shabbir, R.; Mora-Poblete, F.; Saeed, T.; Zulfiqar, F.; Ali, M.M.; et al. Screening of wheat (Triticum aestivum L.) genotypes for drought tolerance through agronomic and physiological response. Agronomy 2022, 12, 287. [Google Scholar] [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought stress in wheat during flowering and grain-filling periods. Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Arnab, M.; Shubha, V.; Akhilesh, K.T. Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc. Natl. Acad. Sci. USA 2004, 101, 6309–6314. [Google Scholar] [CrossRef]
- Han, D.; Ding, H.; Chai, L.; Liu, W.; Zhang, Z.; Hou, Y.; Yang, G.; Willenborg, C. Isolation and characterization of MbWRKY1, a WRKY transcription factor gene from Malus baccata (L.) Borkh involved in drought tolerance. Can. J. Plant Sci. 2018, 98, 1023–1034. [Google Scholar] [CrossRef]
- Han, D.; Hou, Y.; Ding, H.; Zhou, Z.; Li, H.; Yang, G. Isolation and preliminary functional analysis of MbWRKY4 gene involved in salt tolerance in transgenic tobacco. Agric. Biol. 2018, 20, 433–441. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Wang, Y.; Liang, X.; Han, J.; Hou, R.; Han, D. The Transcription Factor MbWRKY46 in Malus baccata (L.) Borkh Mediate Cold and Drought Stress Responses. Int. J. Mol. Sci. 2023, 24, 12468. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, T.; Liang, X.; Ye, Q.; Wang, Y.; Han, J.; Han, D. MbWRKY53, a M. baccata WRKY Transcription Factor, Contributes to Cold and Drought Stress Tolerance in Transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 7626. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, X.; Li, W.; Yao, A.; Liu, W.; Wang, Y.; Yang, G.; Han, D. Isolation and Functional Analysis of MbCBF2, a Malus baccata (L.) Borkh CBF Transcription Factor Gene, with Functions in Tolerance to Cold and Salt Stress in Transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 9827. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, B.; He, H.; Zhang, Y.; Tian, H.; Wang, B. Current Understanding of bHLH Transcription Factors in Plant Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 4921. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Xu, J.; Lu, X.; Liu, Y. Anthocyanin Biosynthesis Induced by MYB Transcription Factors in Plants. Int. J. Mol. Sci. 2022, 23, 11701. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhang, Y.; Guo, J. Identification and Characterization of AP2/ERF Transcription Factors in Yellow Horn. Int. J. Mol. Sci. 2022, 23, 14991. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Li, H.; Zhang, S.; Zhang, X.; Yan, X.; Wang, Z.; Yang, Y.; Zhang, S. Overexpression of NtGCN2 improves drought tolerance in tobacco by regulating proline accumulation, ROS scavenging ability, and stomatal closure. Plant Physiol. Biochem. 2023, 198, 107665. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Niazi, A.; Pessarakli, M.; Arvin, M.J. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J. Plant Interact. 2018, 13, 461–471. [Google Scholar] [CrossRef]
- Niu, S.; Gao, Y.; Zi, H.; Liu, Y.; Liu, X.; Xiong, X.; Yao, Q.; Qin, Z.; Chen, N.; Guo, L. The osmolyte-producing endophyte Streptomyces albidoflavus OsiLf-2 induces drought and salt tolerance in rice via a multi-level mechanism. Crop J. 2022, 10, 375–386. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A.; Chaudhry, M.A.M.; Indrasti, R.; Rehman, A. Improving resistance against terminal drought in bread wheat by exogenous application of proline and gamma-aminobutyric acid. J. Agron. Crop Sci. 2017, 203, 464–472. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Ding, L.; Wang, Z.; Tang, D.; Chen, B.; Ao, L.; Liu, Y.; Kang, Z.; Mao, H. TaERF87 and TaAKS1 synergistically regulate TaP5CS1/TaP5CR1-mediated proline biosynthesis to enhance drought tolerance in wheat. New Phytol. 2023, 237, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Su, J.C.; Zhang, Y.H.; Nie, Y.; Cheng, D.; Wang, R.; Hu, H.L.; Chen, J.; Zhang, J.F.; Du, Y.W.; Shen, W.B. Hydrogen-induced osmotic tolerance is associated with nitric oxide-mediated proline accumulation and reestablishment of redox balance in alfalfa seedlings. Environ. Exp. Bot. 2018, 147, 249–260. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, X.; Chen, C.; Zhang, J. Overexpression of the VyP5CR gene increases drought tolerance in transgenic grapevine (V. vinifera L.). Sci. Hortic. 2023, 316, 112019. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Zarea, M.J.; Karimi, N. Zinc-Regulated P5CS and Sucrose Transporters SUT1B Expression to Enhance Drought Stress Tolerance in Wheat. J. Plant Growth Regul. 2023, 42, 5831–5841. [Google Scholar] [CrossRef]
- Zhou, R.; Song, Y.; Xue, X.; Xue, R.; Jiang, H.; Zhou, Y.; Qi, X.; Wang, Y. Differential transcription profiling reveals the microRNAs involved in alleviating damage to photosynthesis under drought stress during the grain filling stage in wheat. Int. J. Mol. Sci. 2024, 25, 5518. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A. Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 2015, 6, 503. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric oxide: A multitasked signaling gas in plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef]
- Sun, C.L.; Lu, L.L.; Liu, L.J.; Liu, W.J.; Yu, Y.; Liu, X.X.; Hu, Y.; Jin, C.W.; Lin, X.Y. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol. 2014, 201, 1240–1250. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, X.; Long, Y.; Ji, X. Transcriptional analysis reveals sodium nitroprusside affects alfalfa in response to PEG-induced osmotic stress at germination stage. Protoplasma 2020, 257, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Saqib, S.; Khan, W.; Ayaz, A.; Batool, A.; Wang, W.-Y.; Xiong, Y.-C. The multifaceted role of sodium nitroprusside in plants: Crosstalk with phytohormones under normal and stressful conditions. Plant Growth Regul. 2024, 103, 453–470. [Google Scholar] [CrossRef]
- Yavuz, D.; Seymen, M.; Kal, Ü.; Atakul, Z.; Tanrıverdi, Ö.B.; Türkmen, Ö.; Yavuz, N. Agronomic and physio-biochemical responses of lettuce to exogenous sodium nitroprusside (SNP) applied under different irrigation regimes. Agric. Water Manag. 2023, 277, 108127. [Google Scholar] [CrossRef]
- Sayyadi, G.; Niknezhad, Y.; Fallah, H. Sodium nitroprusside ameliorates lead toxicity in rice (Oryza sativa L.) by modulating the antioxidant scavenging system, nitrogen metabolism, lead sequestration mechanism, and proline metabolism. Environ. Sci. Pollut. Res. Int. 2023, 30, 24408–24423. [Google Scholar] [CrossRef] [PubMed]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef] [PubMed]
- Jiantang, Z.; Pengchao, H.; Guanxing, C.; Caixia, H.; Xiaohui, L.; Friedrich, J.Z.; Sai LK, H.; Yingkao, H.; Yueming, Y. Molecular cloning, phylogenetic analysis, and expression profiling of endoplasmic reticulum molecular chaperone BiP genes from bread wheat (Triticum aestivum L.). BMC Plant Biol. 2014, 14, 1–16. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Tang, D.; Chen, Y.; Chen, G.; Zou, J.; Tan, L.; Tang, Q.; Chen, W. Effects of Brassinosteroid on the Physiological Changes on Two Varieties of Tea Plants Under Salt Stress. Int. J. Mol. Sci. 2024, 25, 13445. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, F.; Duan, Z.; Wang, S.; Qu, Y.; Ao, B.; Sun, X.; Zhang, J. Comparative Transcriptome Analysis Revealing the Potential Salt Tolerance Mechanism of Exogenous Abscisic Acid Application in Melilotus albus. Int. J. Mol. Sci. 2024, 25, 13261. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Hussain, I.; Akhtar, G.; Ahmad, K.S.; Nawaz, F.; Faried, H.N.; Mehmood, A. Insights into physiological and metabolic modulations instigated by exogenous sodium nitroprusside and spermidine reveals drought tolerance in Helianthus annuus L. Plant Physiol. Biochem. 2023, 202, 107935. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Feng, X.-H.; Feng, Y.-X. Regulation of enzymatic and non-enzymatic antioxidants in rice seedlings against chromium stress through sodium hydrosulfide and sodium nitroprusside. Environ. Sci. Pollut. Res. 2023, 30, 25851–25862. [Google Scholar] [CrossRef]
- Wang, Y.X.; Suo, B.; Zhao, T.F.; Qu, X.F.; Yuan, L.G.; Zhao, X.J.; Zhao, H.J. Effect of nitric oxide treatment on antioxidant responses and psbA gene expression in two wheat cultivars during grain filling stage under drought stress and rewatering. Acta Physiol. Plant. 2011, 33, 1923–1932. [Google Scholar] [CrossRef]
- Altaf, M.A.; Hao, Y.; Shu, H.; Mumtaz, M.A.; Cheng, S.; Alyemeni, M.N.; Ahmad, P.; Wang, Z. Melatonin enhanced the heavy metal-stress tolerance of pepper by mitigating the oxidative damage and reducing the heavy metal accumulation. J. Hazard. Mater. 2023, 454, 131468. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Cui, L.; Tang, Z.; Ci, D.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. The multifaceted roles of Arbuscular Mycorrhizal Fungi in peanut responses to salt, drought, and cold stress. BMC Plant Biol. 2023, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Abd Elhady, S.A.; El-Gawad, H.G.A.; Ibrahim, M.F.; Mukherjee, S.; Elkelish, A.; Azab, E.; Gobouri, A.A.; Farag, R.; Ibrahim, H.A.; El-Azm, N.A. Hydrogen peroxide supplementation in irrigation water alleviates drought stress and boosts growth and productivity of potato plants. Sustainability 2021, 13, 899. [Google Scholar] [CrossRef]
- Xia, H.; Liu, X.; Wang, Y.; Lin, Z.; Deng, H.; Wang, J.; Lin, L.; Deng, Q.; Lv, X.; Xu, K. 24-Epibrassinolide and nitric oxide combined to improve the drought tolerance in kiwifruit seedlings by proline pathway and nitrogen metabolism. Sci. Hortic. 2022, 297, 110929. [Google Scholar] [CrossRef]
- Ballottari, M.; Dall’Osto, L.; Morosinotto, T.; Bassi, R. Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J. Biol. Chem. 2007, 282, 8947–8958. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Azmat, A.; Tanveer, Y.; Yasmin, H.; Hassan, M.N.; Shahzad, A.; Reddy, M.; Ahmad, A. Coactive role of zinc oxide nanoparticles and plant growth promoting rhizobacteria for mitigation of synchronized effects of heat and drought stress in wheat plants. Chemosphere 2022, 297, 133982. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi Goharrizi, K.; Baghizadeh, A.; Karami, S.; Nazari, M.; Afroushteh, M. Expression of the W36, P5CS, P5CR, MAPK3, and MAPK6 genes and proline content in bread wheat genotypes under drought stress. Cereal Res. Commun. 2023, 51, 545–556. [Google Scholar] [CrossRef]
- Soda, K. Polyamine metabolism and gene methylation in conjunction with one-carbon metabolism. Int. J. Mol. Sci. 2018, 19, 3106. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, W.; Wang, G.; Hu, Y.; Zhong, X.; Tang, G. Physiological regulation of photosynthetic-related indices, antioxidant defense, and proline anabolism on drought tolerance of wild soybean (Glycine soja L.). Plants 2024, 13, 880. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Yang, M.; Zhang, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Wollenweber, B.; Jiang, D. Crosstalk between hydrogen peroxide and nitric oxide mediates priming-induced drought tolerance in wheat. J. Agron. Crop Sci. 2021, 207, 224–235. [Google Scholar] [CrossRef]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol. Biochem. 2020, 155, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Palombi, N.; Funck, D.; Trovato, M. Proline accumulation in pollen grains as potential target for improved yield stability under salt stress. Front. Plant Sci. 2020, 11, 582877. [Google Scholar] [CrossRef] [PubMed]
- Urmi, T.A.; Islam, M.M.; Zumur, K.N.; Abedin, M.A.; Haque, M.M.; Siddiqui, M.H.; Murata, Y.; Hoque, M.A. Combined effect of salicylic acid and proline mitigates drought stress in rice (Oryza sativa L.) through the modulation of physiological attributes and antioxidant enzymes. Antioxidants 2023, 12, 1438. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Ditta, A.; Suleman, M.; Ullah, M. Zinc-induced anti-oxidative defense and osmotic adjustments to enhance drought stress tolerance in sunflower (Helianthus annuus L.). Environ. Exp. Bot. 2022, 193, 104682. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Kooten, O.; Snel, J.F. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Wei, S.M.; Wang, J.N.; Su, X.Y.; Suo, B.; Qin, F.J.; Zhao, H.J. Exogenous application of 5-aminolevulinic acid on wheat seedlings under drought stress enhances the transcription of psbA and psbD genes and improves photosynthesis. Braz. J. Bot. 2018, 41, 275–285. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liang, W.; Cui, W.; Ma, X.; Wang, G.; Huang, Z. Function of wheat Ta-UnP gene in enhancing salt tolerance in transgenic Arabidopsis and rice. Biochem. Biophys. Res. Commun. 2014, 450, 794–801. [Google Scholar] [CrossRef]



| Primer Name | Sequnce (5′-3′) | Purpose | Reference |
|---|---|---|---|
| Actin-F | TGCTATCCTTCGTTTGGACCTT | Internal reference | [61] |
| Actin-R | AGCGGTTGTTGTGAGGGAGT | ||
| TaP5CS-F | GTCCCGACCTGATGCCTT | RT–qPCR | Own design |
| TaP5CS-R | GGAATCCTTACCACGCCA | ||
| TaP5CR-F | TGTTCAATCGTCAGCCTCCG | RT–qPCR | Own design |
| TaP5CR-R | GCGAGGGCGTTTTAGGAGTA | ||
| TaProDH-F | GCGACGGAGTTAGGAGTTGT | RT–qPCR | Own design |
| TaProDH-R | AGGTGTCTGGTCCTTTCCGT | ||
| TaSAMDC-F | TCTGGCGGCAATGCTTATGT | RT–qPCR | Own design |
| TaSAMDC-R | CGCAGGAAACGTGGCTATCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Li, R.; Zhang, M.; Jin, S.; Jiang, H.; Wang, C.; Pang, Y.; Xue, R.; Wang, Y. Exogenous SNP Alleviates Drought Stress in Wheat During the Grain-Filling Stage by Modulating TaP5CS Gene Transcription. Int. J. Mol. Sci. 2025, 26, 618. https://doi.org/10.3390/ijms26020618
Xue X, Li R, Zhang M, Jin S, Jiang H, Wang C, Pang Y, Xue R, Wang Y. Exogenous SNP Alleviates Drought Stress in Wheat During the Grain-Filling Stage by Modulating TaP5CS Gene Transcription. International Journal of Molecular Sciences. 2025; 26(2):618. https://doi.org/10.3390/ijms26020618
Chicago/Turabian StyleXue, Xinyu, Ruqing Li, Menghan Zhang, Sixu Jin, Haifang Jiang, Chongju Wang, Yifei Pang, Ruili Xue, and Yuexia Wang. 2025. "Exogenous SNP Alleviates Drought Stress in Wheat During the Grain-Filling Stage by Modulating TaP5CS Gene Transcription" International Journal of Molecular Sciences 26, no. 2: 618. https://doi.org/10.3390/ijms26020618
APA StyleXue, X., Li, R., Zhang, M., Jin, S., Jiang, H., Wang, C., Pang, Y., Xue, R., & Wang, Y. (2025). Exogenous SNP Alleviates Drought Stress in Wheat During the Grain-Filling Stage by Modulating TaP5CS Gene Transcription. International Journal of Molecular Sciences, 26(2), 618. https://doi.org/10.3390/ijms26020618





