Cytokines and Brain-Derived Neurotrophic Factor as Biomarkers of Cognitive Impairment Related to Breast Cancer and Its Treatments: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search
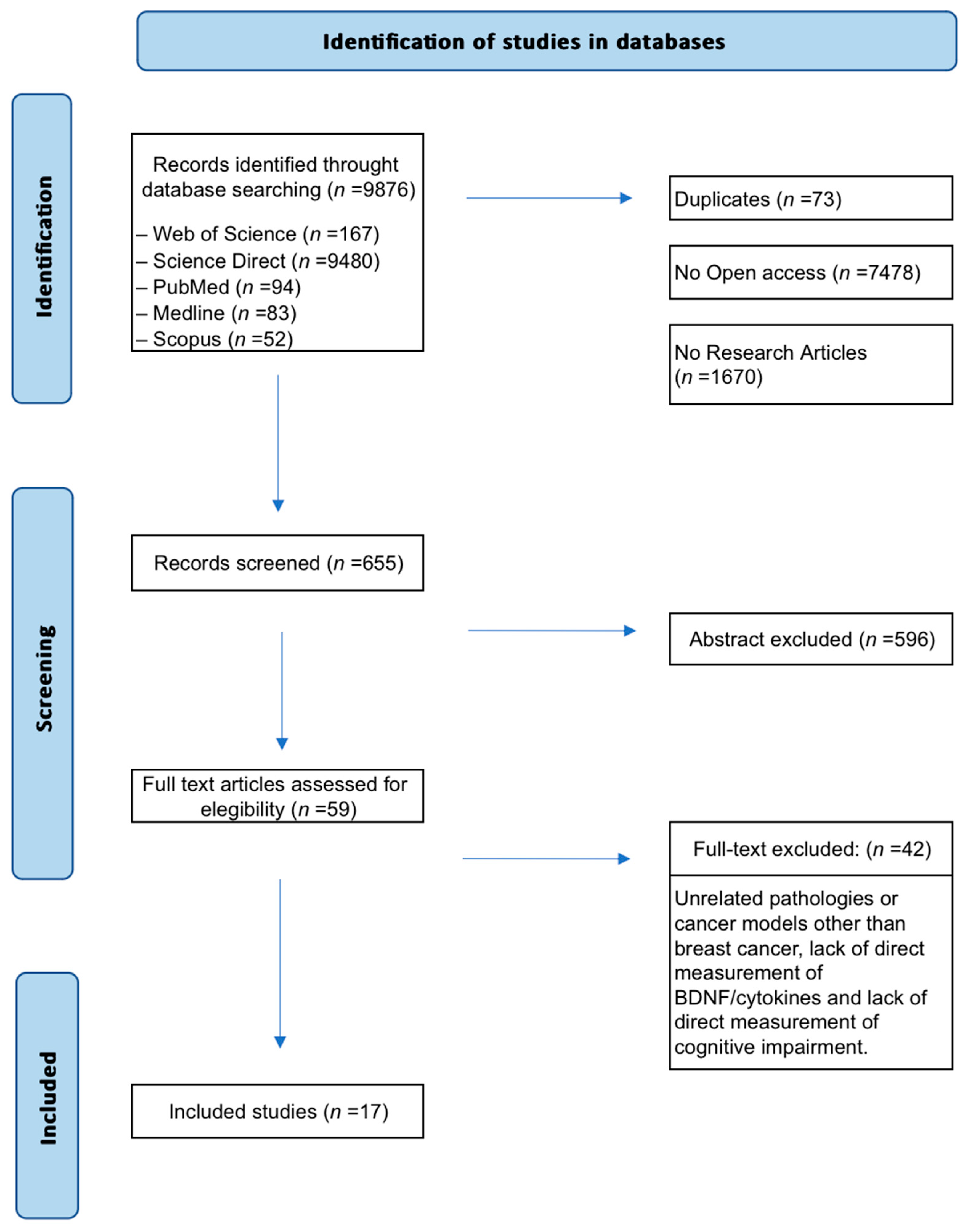
2.3. Study Selection
3. Results
3.1. Data Extraction
- Species and strain, and animal model used (i.e., breast cancer, ovariectomy, healthy animals).
- Cancer treatment, dosage and duration.
- Therapeutic interventions aimed at mitigating the tumor/treatment effects, their dosage and duration.
- Cognitive function and paradigm used.
- Analyzed biomarkers (pro- and anti-inflammatory cytokines, BDNF) and biological sample.
- Main findings related to the effects of tumor, cancer treatment, and/or interventions on cognitive functions and biomarkers.
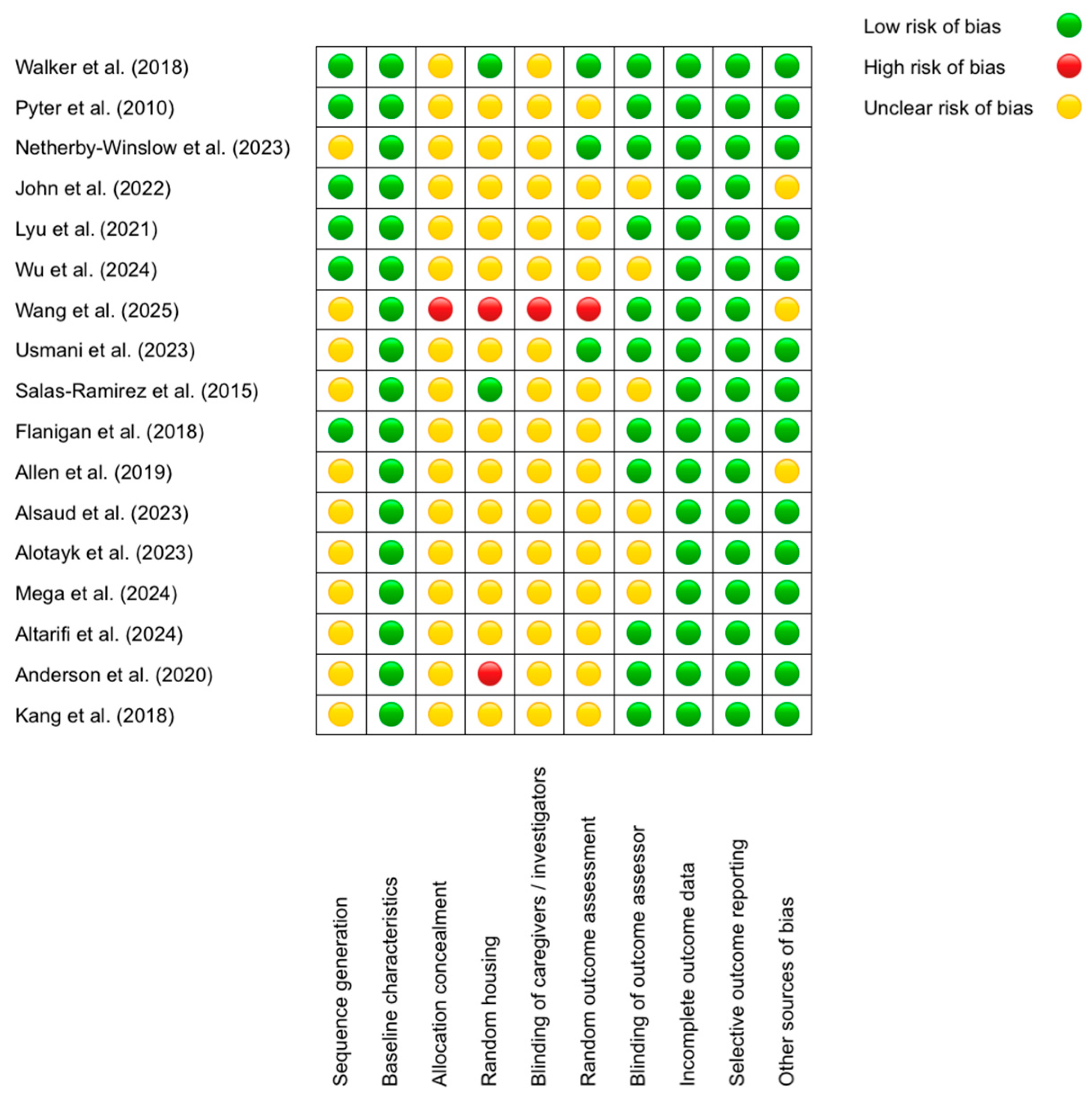
3.2. Quality of Included Studies
3.3. Tumor Effects
3.4. Effects of Chemotherapy
3.4.1. Chemotherapy in Tumor-Bearing Models: Amplifying the Pro-Inflammatory State
3.4.2. Chemotherapy in Healthy Animals: Diverse Pathways to Neurotoxicity
3.4.3. Impact of Ovariectomy on Chemotherapy-Induced Neurotoxicity
3.4.4. BDNF Reduction: A Core but Not Universal Mechanism in Chemotherapy-Induced Neurotoxicity
3.5. Effects of the Interventions
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, S. Breast Cancer: Presentation, Investigation and Management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 17 June 2024).
- Shah, C.; Tendulkar, R.; Smile, T.; Nanavati, A.; Manyam, B.; Balagamwala, E.; Pham, Y.; Takiar, R.; Wobb, J.; Khan, A.; et al. Adjuvant Radiotherapy in Early-Stage Breast Cancer: Evidence-Based Options. Ann. Surg. Oncol. 2016, 23, 3880–3890. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Zhu, L.; Su, F.; Song, E.; Jacobs, L.K. Comparative Effectiveness Study of Breast-Conserving Surgery and Mastectomy in the General Population: A NCDB Analysis. Oncotarget 2015, 6, 40127–40140. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Silva, D.D.F.R.; Alessi, J.V.M.; Mano, M.S. Neoadjuvant Endocrine Therapy in Breast Cancer: Current Role and Future Perspectives. Ecancermedicalscience 2016, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- Kümmel, S.; Holtschmidt, J.; Loibl, S. Surgical Treatment of Primary Breast Cancer in the Neoadjuvant Setting. Br. J. Surg. 2014, 101, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Iwamoto, M.; Kimura, K.; Matsunami, N.; Morishima, H.; Yoshidome, K.; Nomura, T.; Morimoto, T.; Yamamoto, D.; Tsubota, Y.; et al. Phase II Study of Neoadjuvant Anthracycline-Based Regimens Combined with Nanoparticle Albumin-Bound Paclitaxel and Trastuzumab for Human Epidermal Growth Factor Receptor 2-Positive Operable Breast Cancer. Clin. Breast Cancer 2015, 15, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zheng, L.-W.; Ding, Y.; Chen, Y.-F.; Cai, Y.-W.; Wang, L.-P.; Huang, L.; Liu, C.-C.; Shao, Z.-M.; Yu, K.-D. Breast Cancer: Pathogenesis and Treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- American Cancer Society. Breast Cancer Facts and Figures 2024–2025; American Cancer Society: Atlanta, GA, USA, 2024. [Google Scholar]
- Martín, M.; Lluch, A.; Seguí, M.A.; Ruiz, A.; Ramos, M.; Adrover, E.; Rodríguez-Lescure, Á.; Grosse, R.; Calvo, L.; Fernandez-Chacón, C.; et al. Toxicity and Health-Related Quality of Life in Breast Cancer Patients Receiving Adjuvant Docetaxel, Doxorubicin, Cyclophosphamide (TAC) or 5-Fluorouracil, Doxorubicin and Cyclophosphamide (FAC): Impact of Adding Primary Prophylactic Granulocyte-Colony Stimulating Factor to the TAC Regimen. Ann. Oncol. 2006, 17, 1205–1212. [Google Scholar] [CrossRef]
- Durán-Gómez, N.; López-Jurado, C.F.; Nadal-Delgado, M.; Montanero-Fernández, J.; Palomo-López, P.; Cáceres, M.C. Prevalence of Psychoneurological Symptoms and Symptom Clusters in Women with Breast Cancer Undergoing Treatment: Influence on Quality of Life. Semin. Oncol. Nurs. 2023, 39, 151451. [Google Scholar] [CrossRef]
- Hsu, H.-T.; Lin, K.-C.; Wu, L.-M.; Juan, C.-H.; Hou, M.-F.; Hwang, S.-L.; Liu, Y.; Dodd, M.J. Symptom Cluster Trajectories During Chemotherapy in Breast Cancer Outpatients. J. Pain. Symptom Manag. 2017, 53, 1017–1025. [Google Scholar] [CrossRef]
- Yap, N.Y.; Toh, Y.L.; Tan, C.J.; Acharya, M.M.; Chan, A. Relationship between Cytokines and Brain-Derived Neurotrophic Factor (BDNF) in Trajectories of Cancer-Related Cognitive Impairment. Cytokine 2021, 144, 155556. [Google Scholar] [CrossRef]
- Oliva, G.; Giustiniani, A.; Danesin, L.; Burgio, F.; Arcara, G.; Conte, P. Cognitive Impairment Following Breast Cancer Treatments: An Umbrella Review. Oncologist 2024, 29, e848–e863. [Google Scholar] [CrossRef]
- Menning, S.; de Ruiter, M.B.; Veltman, D.J.; Koppelmans, V.; Kirschbaum, C.; Boogerd, W.; Reneman, L.; Schagen, S.B. Multimodal MRI and Cognitive Function in Patients with Breast Cancer Prior to Adjuvant Treatment—The Role of Fatigue. Neuroimage Clin. 2015, 7, 547–554. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Ng, T.; Shwe, M.; Ho, H.K.; Foo, K.M.; Cham, M.T.; Lee, J.A.; Fan, G.; Tan, Y.P.; Yong, W.S.; et al. Association of Proinflammatory Cytokines and Chemotherapy-Associated Cognitive Impairment in Breast Cancer Patients: A Multi-Centered, Prospective, Cohort Study. Ann. Oncol. 2015, 26, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Wieneke, M.H.; Dienst, E.R. Neuropsychological Assessment of Cognitive Functioning Following Chemotherapy for Breast Cancer. Psychooncology 1995, 4, 61–66. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Cappola, A.R.; Stricker, C.T.; Sweeney, C.; Norman, S.A. The Intersection of Cancer and Aging: Establishing the Need for Breast Cancer Rehabilitation. Cancer Epidemiol. Biomark. Prev. 2007, 16, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ranadive, N.; Kinra, M.; Nampoothiri, M.; Arora, D.; Mudgal, J. An Overview on Chemotherapy-Induced Cognitive Impairment and Potential Role of Antidepressants. Curr. Neuropharmacol. 2020, 18, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, A.; Ilnytskyy, Y.; Rodriguez-Juarez, R.; Katz, A.; Sidransky, D.; Kolb, B.; Kovalchuk, O. Growth of Triple Negative and Progesterone Positive Breast Cancer Causes Oxidative Stress and Down-Regulates Neuroprotective Transcription Factor NPAS4 and NPAS4-Regulated Genes in Hippocampal Tissues of TumorGraft Mice—An Aging Connection. Front. Genet. 2018, 9, 58. [Google Scholar] [CrossRef]
- Hutchinson, A.D.; Hosking, J.R.; Kichenadasse, G.; Mattiske, J.K.; Wilson, C. Objective and Subjective Cognitive Impairment Following Chemotherapy for Cancer: A Systematic Review. Cancer Treat. Rev. 2012, 38, 926–934. [Google Scholar] [CrossRef]
- Ganz, P.A.; Kwan, L.; Castellon, S.A.; Oppenheim, A.; Bower, J.E.; Silverman, D.H.S.; Cole, S.W.; Irwin, M.R.; Ancoli-Israel, S.; Belin, T.R. Cognitive Complaints After Breast Cancer Treatments: Examining the Relationship with Neuropsychological Test Performance. JNCI J. Natl. Cancer Inst. 2013, 105, 791–801. [Google Scholar] [CrossRef]
- Schagen, S.B.; Muller, M.J.; Boogerd, W.; Mellenbergh, G.J.; van Dam, F.S.A.M. Change in Cognitive Function After Chemotherapy: A Prospective Longitudinal Study in Breast Cancer Patients. JNCI J. Natl. Cancer Inst. 2006, 98, 1742–1745. [Google Scholar] [CrossRef]
- Winocur, G.; Henkelman, M.; Wojtowicz, J.M.; Zhang, H.; Binns, M.A.; Tannock, I.F. The Effects of Chemotherapy on Cognitive Function in a Mouse Model: A Prospective Study. Clin. Cancer Res. 2012, 18, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Seigers, R.; Fardell, J.E. Neurobiological Basis of Chemotherapy-Induced Cognitive Impairment: A Review of Rodent Research. Neurosci. Biobehav. Rev. 2011, 35, 729–741. [Google Scholar] [CrossRef]
- Briones, T.L.; Woods, J. Chemotherapy-Induced Cognitive Impairment Is Associated with Decreases in Cell Proliferation and Histone Modifications. BMC Neurosci. 2011, 12, 124. [Google Scholar] [CrossRef]
- Schroyen, G.; Vissers, J.; Smeets, A.; Gillebert, C.R.; Lemiere, J.; Sunaert, S.; Deprez, S.; Sleurs, C. Blood and Neuroimaging Biomarkers of Cognitive Sequelae in Breast Cancer Patients throughout Chemotherapy: A Systematic Review. Transl. Oncol. 2022, 16, 101297. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Bi, Z.; Jing, Y.; Yin, X.; Zhang, X.; Yao, S.; Zhao, J.; Cheng, H. Changes in Cytokine Levels in Breast Cancer Patients with CRCI before or after CALM Intervention. Am. J. Cancer Res. 2021, 11, 5415. [Google Scholar] [PubMed]
- Jehn, C.F.; Becker, B.; Flath, B.; Nogai, H.; Vuong, L.; Schmid, P.; Lüftner, D. Neurocognitive Function, Brain-Derived Neurotrophic Factor (BDNF) and IL-6 Levels in Cancer Patients with Depression. J. Neuroimmunol. 2015, 287, 88–92. [Google Scholar] [CrossRef]
- Yap, N.Y.; Tan, N.Y.T.; Tan, C.J.; Loh, K.W.-J.; Ng, R.C.H.; Ho, H.K.; Chan, A. Associations of Plasma Brain-Derived Neurotrophic Factor (BDNF) and Val66Met Polymorphism (Rs6265) with Long-Term Cancer-Related Cognitive Impairment in Survivors of Breast Cancer. Breast Cancer Res. Treat. 2020, 183, 683–696. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Barbosa, I.G.; Diniz, B.S.; Kummer, A. Circulating Levels of Brain-Derived Neurotrophic Factor: Correlation with Mood, Cognition and Motor Function. Biomark. Med. 2010, 4, 871–887. [Google Scholar] [CrossRef]
- Panja, D.; Bramham, C.R. BDNF Mechanisms in Late LTP Formation: A Synthesis and Breakdown. Neuropharmacology 2014, 76, 664–676. [Google Scholar] [CrossRef]
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 Cells): Multifunctional Cells with Lineage Plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Heo, S.; McLaren, M.; Pence, B.D.; Martin, S.A.; Vieira, V.J.; Woods, J.A.; et al. Brain-Derived Neurotrophic Factor Is Associated with Age-Related Decline in Hippocampal Volume. J. Neurosci. 2010, 30, 5368–5375. [Google Scholar] [CrossRef]
- Bone, R.C.; Grodzin, C.J.; Balk, R.A. Sepsis: A New Hypothesis for Pathogenesis of the Disease Process. Chest 1997, 112, 235–243. [Google Scholar] [CrossRef]
- Liles, W.C.; Van Voorhis, W.C. Review: Nomenclature and Biologic Significance of Cytokines Involved in Inflammation and the Host Immune Response. J. Infect. Dis. 1995, 172, 1573–1580. [Google Scholar] [CrossRef]
- Lucey, D.R.; Clerici, M.; Shearer, G.M. Type 1 and Type 2 Cytokine Dysregulation in Human Infectious, Neoplastic, and Inflammatory Diseases. Clin. Microbiol. Rev. 1996, 9, 532–562. [Google Scholar] [CrossRef] [PubMed]
- Gliwińska, A.; Czubilińska-Łada, J.; Więckiewicz, G.; Świętochowska, E.; Badeński, A.; Dworak, M.; Szczepańska, M. The Role of Brain-Derived Neurotrophic Factor (BDNF) in Diagnosis and Treatment of Epilepsy, Depression, Schizophrenia, Anorexia Nervosa and Alzheimer’s Disease as Highly Drug-Resistant Diseases: A Narrative Review. Brain Sci. 2023, 13, 163. [Google Scholar] [CrossRef]
- Leung, R.; Proitsi, P.; Simmons, A.; Lunnon, K.; Güntert, A.; Kronenberg, D.; Pritchard, M.; Tsolaki, M.; Mecocci, P.; Kloszewska, I.; et al. Inflammatory Proteins in Plasma Are Associated with Severity of Alzheimer’s Disease. PLoS ONE 2013, 8, e64971. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.N.; Al-Hussainy, A.F.; Sanghvi, G.; Ballal, S.; Singh, A.; Sabarivani, A.; Mishra, S.; Rizaev, J.; Taher, S.G.; Alwan, M.; et al. BDNF Biosensors for Neurodegenerative Disease. Clin. Chim. Acta 2025, 576, 120412. [Google Scholar] [CrossRef]
- Baune, B.T.; Ponath, G.; Rothermundt, M.; Riess, O.; Funke, H.; Berger, K. Association between Genetic Variants of IL-1β, IL-6 and TNF-α Cytokines and Cognitive Performance in the Elderly General Population of the MEMO-Study. Psychoneuroendocrinology 2008, 33, 68–76. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.-M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.E.; Cohen, R.; Chen, H.; Kelly, D.L.; McCain, N.L.; Starkweather, A.; Ahn, H.; Sturgill, J.; Jackson-Cook, C.K. Relationship of Systemic Cytokine Concentrations to Cognitive Function over Two Years in Women with Early Stage Breast Cancer. J. Neuroimmunol. 2016, 301, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.; Janelsins, M.; Koovakkattu, D.; Palesh, O.; Mustian, K.; Morrow, G.; Dhabhar, F.S. Reduced Hippocampal Volume and Verbal Memory Performance Associated with Interleukin-6 and Tumor Necrosis Factor-Alpha Levels in Chemotherapy-Treated Breast Cancer Survivors. Brain Behav. Immun. 2013, 30, S109–S116. [Google Scholar] [CrossRef] [PubMed]
- Lomeli, N.; Lepe, J.; Gupta, K.; Bota, D.A. Cognitive Complications of Cancer and Cancer-Related Treatments–Novel Paradigms. Neurosci. Lett. 2021, 749, 135720. [Google Scholar] [CrossRef]
- Joshi, G.; Aluise, C.D.; Cole, M.P.; Sultana, R.; Pierce, W.M.; Vore, M.; St Clair, D.K.; Butterfield, D.A. Alterations in Brain Antioxidant Enzymes and Redox Proteomic Identification of Oxidized Brain Proteins Induced by the Anti-Cancer Drug Adriamycin: Implications for Oxidative Stress-Mediated Chemobrain. Neuroscience 2010, 166, 796–807. [Google Scholar] [CrossRef]
- Jaiswara, P.K.; Shukla, S.K. Chemotherapy-Mediated Neuronal Aberration. Pharmaceuticals 2023, 16, 1165. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating Guidance for Reporting Systematic Reviews: Development of the PRISMA 2020 Statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Walker, A.K.; Chang, A.; Ziegler, A.I.; Dhillon, H.M.; Vardy, J.L.; Sloan, E.K. Low Dose Aspirin Blocks Breast Cancer-Induced Cognitive Impairment in Mice. PLoS ONE 2018, 13, e0208593. [Google Scholar] [CrossRef] [PubMed]
- Pyter, L.M.; Cochrane, S.F.; Ouwenga, R.L.; Patel, P.N.; Pineros, V.; Prendergast, B.J. Mammary Tumors Induce Select Cognitive Impairments. Brain Behav. Immun. 2010, 24, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Netherby-Winslow, C.; Thompson, B.; Lotta, L.; Gallagher, M.; Van Haute, P.; Yang, R.; Hott, D.; Hasan, H.; Bachmann, K.; Bautista, J.; et al. Effects of Mammary Cancer and Chemotherapy on Neuroimmunological Markers and Memory Function in a Preclinical Mouse Model. Brain Behav. Immun. Health 2023, 34, 100699. [Google Scholar] [CrossRef]
- John, J.; Kinra, M.; Ranadive, N.; Keni, R.; Nayak, P.G.; Jagdale, R.N.; Ahmed, S.M.; Raghavendra, K.V.; Mudgal, J.; Nandakumar, K. Neuroprotective Effect of Mulmina Mango against Chemotherapy-Induced Cognitive Decline in Mouse Model of Mammary Carcinoma. Sci. Rep. 2022, 12, 3072. [Google Scholar] [CrossRef]
- Lyu, W.; Ouyang, M.; Ma, X.; Han, T.; Pi, D.; Qiu, S. Kai-Xin-San Attenuates Doxorubicin-Induced Cognitive Impairment by Reducing Inflammation, Oxidative Stress, and Neural Degeneration in 4T1 Breast Cancer Mice. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Wu, Y.; Che, J.; Dong, J.; Zhang, X.; Deng, Y.; Chen, W.; Zhang, J. CCR5 Antagonist Maraviroc Alleviates Doxorubicin-Induced Neuroinflammation and Neurobehavioral Deficiency by Regulating NF-ΚB/NLRP3 Signaling in a Breast Cancer Mouse Model. Neuropharmacology 2024, 254, 109981. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Q.; Li, J.; Lai, B.; Pei, X.; Chen, N. Effect of Fangxia-Dihuang Decoction on Doxorubicin-Induced Cognitive Impairment in Breast Cancer Animal Model. Front. Oncol. 2025, 15, 1515498. [Google Scholar] [CrossRef]
- Usmani, M.T.; Krattli, R.P.; El-Khatib, S.M.; Le, A.C.D.; Smith, S.M.; Baulch, J.E.; Ng, D.Q.; Acharya, M.M.; Chan, A. BDNF Augmentation Using Riluzole Reverses Doxorubicin-Induced Decline in Cognitive Function and Neurogenesis. Neurotherapeutics 2023, 20, 838–852. [Google Scholar] [CrossRef]
- Salas-Ramirez, K.Y.; Bagnall, C.; Frias, L.; Abdali, S.A.; Ahles, T.A.; Hubbard, K. Doxorubicin and Cyclophosphamide Induce Cognitive Dysfunction and Activate the ERK and AKT Signaling Pathways. Behav. Brain Res. 2015, 292, 133–141. [Google Scholar] [CrossRef]
- Flanigan, T.J.; Anderson, J.E.; Elayan, I.; Allen, A.R.; Ferguson, S.A. Effects of Cyclophosphamide and/or Doxorubicin in a Murine Model of Postchemotherapy Cognitive Impairment. Toxicol. Sci. 2018, 162, 462–474. [Google Scholar] [CrossRef]
- Allen, B.D.; Apodaca, L.A.; Syage, A.R.; Markarian, M.; Baddour, A.A.D.; Minasyan, H.; Alikhani, L.; Lu, C.; West, B.L.; Giedzinski, E.; et al. Attenuation of Neuroinflammation Reverses Adriamycin-Induced Cognitive Impairments. Acta Neuropathol. Commun. 2019, 7, 186. [Google Scholar] [CrossRef]
- Alsaud, M.M.; Alhowail, A.H.; Aldubayan, M.A.; Almami, I.S. The Ameliorative Effect of Pioglitazone against Neuroinflammation Caused by Doxorubicin in Rats. Molecules 2023, 28, 4775. [Google Scholar] [CrossRef]
- Alotayk, L.I.; Aldubayan, M.A.; Alenezi, S.K.; Anwar, M.J.; Alhowail, A.H. Comparative Evaluation of Doxorubicin, Cyclophosphamide, 5-Fluorouracil, and Cisplatin on Cognitive Dysfunction in Rats: Delineating the Role of Inflammation of Hippocampal Neurons and Hypothyroidism. Biomed. Pharmacother. 2023, 165, 115245. [Google Scholar] [CrossRef] [PubMed]
- Mega, O.O.; Faith, F.Y.; Ejiro, O.P.; Uchechukwu, J.G.; Temitope, O.G.; Oghenetega, O.B.; Victor, E.; Edesiri, T.P.; Rume, R.A.; Rotu, R.A.; et al. Diosmin Alleviates Doxorubicin-Induced Chemobrain in Rats via Inhibition of Oxido-Inflammation, Apoptosis and Modulation of Autophagy. Brain Disord. 2024, 13, 100111. [Google Scholar] [CrossRef]
- Altarifi, A.A.; Sawali, K.; Alzoubi, K.H.; Saleh, T.; Abu Al-Rub, M.; Khabour, O. Effect of Vitamin E on Doxorubicin and Paclitaxel-Induced Memory Impairments in Male Rats. Cancer Chemother. Pharmacol. 2024, 93, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.E.; Trujillo, M.; McElroy, T.; Groves, T.; Alexander, T.; Kiffer, F.; Allen, A.R. Early Effects of Cyclophosphamide, Methotrexate, and 5-Fluorouracil on Neuronal Morphology and Hippocampal-Dependent Behavior in a Murine Model. Toxicol. Sci. 2020, 173, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, S.; Kim, J.; Kim, J.-C.; Kim, S.-H.; Son, Y.; Shin, T.; Youn, B.; Kim, J.-S.; Wang, H.; et al. Chronic Treatment with Combined Chemotherapeutic Agents Affects Hippocampal Micromorphometry and Function in Mice, Independently of Neuroinflammation. Exp. Neurobiol. 2018, 27, 419–436. [Google Scholar] [CrossRef]
- Belcher, E.K.; Culakova, E.; Gilmore, N.J.; Hardy, S.J.; Kleckner, A.S.; Kleckner, I.R.; Lei, L.; Heckler, C.; Sohn, M.B.; Thompson, B.D.; et al. Inflammation, Attention, and Processing Speed in Patients with Breast Cancer Before and After Chemotherapy. JNCI J. Natl. Cancer Inst. 2022, 114, 712–721. [Google Scholar] [CrossRef]
- Zhao, J.; Zuo, H.; Ding, K.; Zhang, X.; Bi, Z.; Cheng, H. Changes in Plasma IL-1β, TNF-α and IL-4 Levels Are Involved in Chemotherapy-Related Cognitive Impairment in early-Stage Breast Cancer Patients. Am. J. Transl. Res. 2020, 12, 3046. [Google Scholar]
- Culig, Z. Cytokine Disbalance in Common Human Cancers. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2011, 1813, 308–314. [Google Scholar] [CrossRef]
- Sheikhpour, E.; Noorbakhsh, P.; Foroughi, E.; Farahnak, S.; Nasiri, R.; Neamatzadeh, H. A Survey on the Role of Interleukin-10 in Breast Cancer: A Narrative. Rep. Biochem. Mol. Biol. 2018, 7, 30–37. [Google Scholar]
- RALLIS, K.S.; CORRIGAN, A.E.; DADAH, H.; GEORGE, A.M.; KESHWARA, S.M.; SIDERIS, M.; SZABADOS, B. Cytokine-Based Cancer Immunotherapy: Challenges and Opportunities for IL-10. Anticancer Res. 2021, 41, 3247–3252. [Google Scholar] [CrossRef]
- Gomes, C.; Ferreira, R.; George, J.; Sanches, R.; Rodrigues, D.I.; Gonçalves, N.; Cunha, R.A. Activation of Microglial Cells Triggers a Release of Brain-Derived Neurotrophic Factor (BDNF) Inducing Their Proliferation in an Adenosine A2A Receptor-Dependent Manner: A2A Receptor Blockade Prevents BDNF Release and Proliferation of Microglia. J. Neuroinflamm. 2013, 10, 780. [Google Scholar] [CrossRef]
- Malekan, M.; Nezamabadi, S.S.; Samami, E.; Mohebalizadeh, M.; Saghazadeh, A.; Rezaei, N. BDNF and Its Signaling in Cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 2621–2636. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Mokhtari-Zaer, A.; Rezaee, M.; Afzaljavan, F.; Rivandi, M.; Hassanian, S.M.; Ferns, G.A.; Pasdar, A.; Avan, A. Therapeutic Potentials of BDNF/TrkB in Breast Cancer; Current Status and Perspectives. J. Cell Biochem. 2017, 118, 2502–2515. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.T.; Willson, M.L.; Goel, S.; Beith, J.; Goodwin, A. Ovarian Suppression for Adjuvant Treatment of Hormone Receptor-Positive Early Breast Cancer. Cochrane Database Syst. Rev. 2020, 3, CD013538. [Google Scholar] [CrossRef]
- Dees, E.; Davidson, N.E. Ovarian Ablation as Adjuvant Therapy for Breast Cancer. Semin. Oncol. 2001, 28, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Charoenphandhu, N.; Nuntapornsak, A.; Wongdee, K.; Krishnamra, N.; Charoenphandhu, J. Upregulated MRNA Levels of SERT, NET, MAOB, and BDNF in Various Brain Regions of Ovariectomized Rats Exposed to Chronic Aversive Stimuli. Mol. Cell. Biochem. 2012, 375, 49–58. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J.E.; DeLeo, J.A.; Hickey, W.F.; Ahles, T.A.; Saykin, A.J.; Bucci, D.J. Cancer Chemotherapy Impairs Contextual but Not Cue-Specific Fear Memory. Behav. Brain Res. 2007, 181, 168–172. [Google Scholar] [CrossRef]
- Grusdat, N.P.; Stäuber, A.; Tolkmitt, M.; Schnabel, J.; Schubotz, B.; Wright, P.R.; Schulz, H. Routine Cancer Treatments and Their Impact on Physical Function, Symptoms of Cancer-Related Fatigue, Anxiety, and Depression. Support. Care Cancer 2022, 30, 3733–3744. [Google Scholar] [CrossRef]
- Chalmers, O.; Waddell, A.; Choudhury, A.; Sale, C.; Harwood, A.E. Elucidating the Mechanisms of Lifestyle Interventions in Mitigating Radiotherapy Adverse Effects: A Scoping Review. BMJ Oncol. 2025, 4, e000615. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis Induced in Mice with Dextran Sulfate Sodium (DSS) Is Mediated by the NLRP3 Inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Apte, R.N. IL-1 in Colon Inflammation, Colon Carcinogenesis and Invasiveness of Colon Cancer. Cancer Microenviron. 2015, 8, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Han, G.-C.; Wang, R.-X.; Xiao, H.; Hou, C.-M.; Guo, R.-F.; Dou, Y.; Shen, B.-F.; Li, Y.; et al. Neutrophil Infiltration Favors Colitis-Associated Tumorigenesis by Activating the Interleukin-1 (IL-1)/IL-6 Axis. Mucosal Immunol. 2014, 7, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell 2009, 15, 103–113, Erratum in Cancer Cell 2009, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, X.; Shi, P.; Yang, G.; Zhou, Z.; Li, W.; Mao, X.; Jiang, D.; Chen, C. TNF-α Increases Breast Cancer Stem-like Cells through up-Regulating TAZ Expression via the Non-Canonical NF-ΚB Pathway. Sci. Rep. 2020, 10, 1804. [Google Scholar] [CrossRef]
- Schilder, C.M.T.; Seynaeve, C.; Linn, S.C.; Boogerd, W.; Beex, L.V.A.M.; Gundy, C.M.; Nortier, J.W.R.; van de Velde, C.J.H.; van Dam, F.S.A.M.; Schagen, S.B. Self-reported Cognitive Functioning in Postmenopausal Breast Cancer Patients before and during Endocrine Treatment: Findings from the Neuropsychological TEAM Side-study. Psychooncology 2012, 21, 479–487. [Google Scholar] [CrossRef]
- Falleti, M.G.; Sanfilippo, A.; Maruff, P.; Weih, L.; Phillips, K.-A. The Nature and Severity of Cognitive Impairment Associated with Adjuvant Chemotherapy in Women with Breast Cancer: A Meta-Analysis of the Current Literature. Brain Cogn. 2005, 59, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Mousset, A.; Lecorgne, E.; Bourget, I.; Lopez, P.; Jenovai, K.; Cherfils-Vicini, J.; Dominici, C.; Rios, G.; Girard-Riboulleau, C.; Liu, B.; et al. Neutrophil Extracellular Traps Formed during Chemotherapy Confer Treatment Resistance via TGF-β Activation. Cancer Cell 2023, 41, 757–775.e10. [Google Scholar] [CrossRef] [PubMed]
- Pun, P.B.L.; Lu, J.; Moochhala, S. Involvement of ROS in BBB Dysfunction. Free Radic. Res. 2009, 43, 348–364. [Google Scholar] [CrossRef]
- Wardill, H.R.; Mander, K.A.; Van Sebille, Y.Z.A.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Sonis, S.T. Cytokine-mediated Blood Brain Barrier Disruption as a Conduit for Cancer/Chemotherapy-associated Neurotoxicity and Cognitive Dysfunction. Int. J. Cancer 2016, 139, 2635–2645. [Google Scholar] [CrossRef]
- Pratama, F.M.; Ghaib, H.; Ali, I. Correlation between Serum Interleukin-6 Levels and Clinical Response to Anthracycline-Based Neoadjuvant Chemotherapy Regimen in Locally Advanced Breast Cancer Patients. Int. J. Res. Rev. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Hu, F.; Song, D.; Yan, Y.; Huang, C.; Shen, C.; Lan, J.; Chen, Y.; Liu, A.; Wu, Q.; Sun, L.; et al. IL-6 Regulates Autophagy and Chemotherapy Resistance by Promoting BECN1 Phosphorylation. Nat. Commun. 2021, 12, 3651. [Google Scholar] [CrossRef]
- Antoon, J.W.; Lai, R.; Struckhoff, A.P.; Nitschke, A.M.; Elliott, S.; Martin, E.C.; Rhodes, L.V.; Yoon, N.S.; Salvo, V.A.; Shan, B.; et al. Altered Death Receptor Signaling Promotes Epithelial-to-Mesenchymal Transition and Acquired Chemoresistance. Sci. Rep. 2012, 2, 539. [Google Scholar] [CrossRef]
- Qodir, N.; Pramudhito, D.; Hafy, Z.; Iman, M.B.; Syafira, F.; Afladhanti, P.M.; Daenasa, R.S.; Indra, B. Tumor Necrosis Factor-Alpha and Its Association with Breast Cancer: A Systematic Review. World J. Oncol. 2025, 16, 143–151. [Google Scholar] [CrossRef]
- Tyagi, K.; Masoom, M.; Majid, H.; Garg, A.; Bhurani, D.; Agarwal, N.B.; Khan, M.A. Role of Cytokines in Chemotherapy-Related Cognitive Impairment of Breast Cancer Patients: A Systematic Review. Curr. Rev. Clin. Exp. Pharmacol. 2023, 18, 110–119. [Google Scholar] [CrossRef]
- Totpal, K.; Aggarwal, B.B. Interleukin 4 Potentiates the Antiproliferative Effects of Tumor Necrosis Factor on Various Tumor Cell Lines. Cancer Res. 1991, 51, 4266–4270. [Google Scholar] [PubMed]
- Nagai, S.; Toi, M. Interleukin-4 and Breast Cancer. Breast Cancer 2000, 7, 181–186. [Google Scholar] [CrossRef]
- Chang, C.-M.; Lam, H.Y.P.; Hsu, H.-J.; Jiang, S.-J. Interleukin-10: A Double-Edged Sword in Breast Cancer. Tzu Chi Med. J. 2021, 33, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.K.; Cho, K.-J.; Ishido, S.; Roche, P.A. Interleukin 10 (IL-10)-Mediated Immunosuppression. J. Biol. Chem. 2015, 290, 27158–27167. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, H.K.; Bansal, V.K.; Nepal, B.; Srivastava, S.; Dinda, A.K.; Misra, M.C. Is Interleukin 10 (IL10) Expression in Breast Cancer a Marker of Poor Prognosis? Indian. J. Surg. Oncol. 2016, 7, 320–325. [Google Scholar] [CrossRef]
- Schroyen, G.; Blommaert, J.; van Weehaeghe, D.; Sleurs, C.; Vandenbulcke, M.; Dedoncker, N.; Hatse, S.; Goris, A.; Koole, M.; Smeets, A.; et al. Neuroinflammation and Its Association with Cognition, Neuronal Markers and Peripheral Inflammation after Chemotherapy for Breast Cancer. Cancers 2021, 13, 4198. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Smeele, P.; d’Almeida, S.M.; Meiller, C.; Chéné, A.-L.; Liddell, C.; Cellerin, L.; Montagne, F.; Deshayes, S.; Benziane, S.; Copin, M.-C.; et al. Brain-Derived Neurotrophic Factor, a New Soluble Biomarker for Malignant Pleural Mesothelioma Involved in Angiogenesis. Mol. Cancer 2018, 17, 148. [Google Scholar] [CrossRef]
- Kamińska, K.; Cudnoch-Jędrzejewska, A. A Review on the Neurotoxic Effects of Doxorubicin. Neurotox. Res. 2023, 41, 383–397. [Google Scholar] [CrossRef]
- Chughtai, S.; Doyle, D.; Tata, S.; Ram, D.; Oymagil, I. Chemotherapy-Induced Cognitive Impairment: Mechanisms, Emerging Biomarkers, and Therapeutic Interventions. Biochem. Biophys. Res. Commun. 2025, 779, 152456. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Pei, C.; Chen, J.; Lv, Q.; Zhang, F.; Cheng, Z. Efficacy of Acupuncture Therapy for Chemotherapy-Related Cognitive Impairment in Breast Cancer Patients. Med. Sci. Monit. 2018, 24, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Milbury, K.; Chaoul, A.; Biegler, K.; Wangyal, T.; Spelman, A.; Meyers, C.A.; Arun, B.; Palmer, J.L.; Taylor, J.; Cohen, L. Tibetan Sound Meditation for Cognitive Dysfunction: Results of a Randomized Controlled Pilot Trial. Psychooncology 2013, 22, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Lower, E.E.; Fleishman, S.; Cooper, A.; Zeldis, J.; Faleck, H.; Yu, Z.; Manning, D. Efficacy of Dexmethylphenidate for the Treatment of Fatigue After Cancer Chemotherapy: A Randomized Clinical Trial. J. Pain. Symptom Manag. 2009, 38, 650–662. [Google Scholar] [CrossRef]
- Lawrence, J.A.; Griffin, L.; Balcueva, E.P.; Groteluschen, D.L.; Samuel, T.A.; Lesser, G.J.; Naughton, M.J.; Case, L.D.; Shaw, E.G.; Rapp, S.R. A Study of Donepezil in Female Breast Cancer Survivors with Self-Reported Cognitive Dysfunction 1 to 5 Years Following Adjuvant Chemotherapy. J. Cancer Surviv. 2016, 10, 176–184. [Google Scholar] [CrossRef]
- Kohli, S.; Fisher, S.G.; Tra, Y.; Adams, M.J.; Mapstone, M.E.; Wesnes, K.A.; Roscoe, J.A.; Morrow, G.R. The Effect of Modafinil on Cognitive Function in Breast Cancer Survivors. Cancer 2009, 115, 2605–2616. [Google Scholar] [CrossRef]
- Ferguson, R.J.; McDonald, B.C.; Rocque, M.A.; Furstenberg, C.T.; Horrigan, S.; Ahles, T.A.; Saykin, A.J. Development of CBT for Chemotherapy-related Cognitive Change: Results of a Waitlist Control Trial. Psychooncology 2012, 21, 176–186. [Google Scholar] [CrossRef]
- Kesler, S.; Hadi Hosseini, S.M.; Heckler, C.; Janelsins, M.; Palesh, O.; Mustian, K.; Morrow, G. Cognitive Training for Improving Executive Function in Chemotherapy-Treated Breast Cancer Survivors. Clin. Breast Cancer 2013, 13, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Kam, J.W.Y.; Neil-Sztramko, S.E.; Liu Ambrose, T.; Handy, T.C.; Lim, H.J.; Hayden, S.; Hsu, L.; Kirkham, A.A.; Gotay, C.C.; et al. Effect of Aerobic Exercise on Cancer-associated Cognitive Impairment: A Proof-of-concept RCT. Psychooncology 2018, 27, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Welzel, G.; Steinvorth, S.; Wenz, F. Cognitive Effects of Chemotherapy and/or Cranial Irradiation in Adults. Strahlenther. Onkol. 2005, 181, 141–156. [Google Scholar] [CrossRef]
- Trudeau, J.; Ng, D.Q.; Sayer, M.; Tan, C.J.; Ke, Y.; Chan, R.J.; Chan, A. Brain-Derived Neurotrophic Factor and Cytokines as Predictors of Cognitive Impairment in Adolescent and Young Adult Cancer Patients Receiving Chemotherapy: A Longitudinal Study. BMC Cancer 2025, 25, 1045. [Google Scholar] [CrossRef]
- Kharsodiya, K.; Das, K.P.; Dhingra, H.K. Predictive Value of IL-6, IL-1β, TNF-α, and Vaginal PH in Diagnosing Vaginal Microbial Infections: A Host-Inflammatory Axis Perspective. J. Microbiol. Methods 2025, 237, 107212. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Liu, B.; Xiang, J. Combined Detection of Serum Brain-Derived Neurotrophic Factor and Interleukin-6 for Evaluating Therapeutic Efficacy in Major Depressive Disorder. Anal. Chem. 2025, 97, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
| Strain/Model | Cancer Treatment (Dosage and Duration) | Intervention (Dosage and Duration) | Cognitive Function: Paradigm | Biomarker (Biological Sample) | Results | References | |
|---|---|---|---|---|---|---|---|
| Female BALB/c and C57BL/6J mice/Orthotopic implantation of mammary adenocarcinoma tumor cells (4T1.2 or EO771, respectively). | Aspirin (25 mg/kg/day for 2 weeks) | Episodic memory: NOR. Spatial memory: NPR | IL-1α, IL-6, MIP-1α, G-CSF, TNF-α and CXCL1 (Plasma) | Tumor-bearing mice exhibited increased plasma levels of IL-1α, IL-6, MIP-1α, G-CSF and decreased levels of TNF-α and CXCL1. These changes coincided with the development of NOR and NPR memory deficits between days 4 and 7. Low-dose aspirin (oral) completely prevented tumor-induced memory impairments without affecting tumor burden or locomotion. | [53] | 10.1371/journal.pone.0208593 | |
| Wistar female rats/N-nitroso-N-methylurea (NMU)-induced mammary tumor model | Episodic memory: NOR. Spatial learning and working memory: MWM. Reference and working Memory: RAM | BDNF and IL-1β (Hippocampus) | Rats with tumors showed increased hippocampal gene expression of IL-1β, with no significant changes in BDNF levels observed. Cognitively, they showed impaired spatial reference memory (RAM) and were unable to recognize new objects (NOR), while working memory and performance in the MWM were unaffected. | [54] | 10.1016/j.bbi.2010.02.004 | ||
| Female C57BL/6 mice/Orthotopic implantation of mammary adenocarcinoma tumor cells. | DOX (10 mg/kg) + CYP (200 mg/kg) (once a week for 4 weeks) | Spatial learning and memory: Delayed spatial alternation | TNF-α, IL-1β, IL-2, MCP-1, IL-10, IL-6, IL-17, IL-4 (Serum) | The presence of tumor was associated with elevated serum concentrations of TNF-α, IL-1β, IL-2, MCP-1, and IL-10, which correlated with impairments in delayed spatial alternation memory. Treatment with chemotherapy (DOX + CYP) further amplified inflammatory response and worsened spatial memory deficits. | [55] | 10.1016/j.bbih.2023.100699 | |
| Female BALB/c mice/Orthotopic implantation of TNBC tumor cells (4T1) | CMF: CYP (50 mg/kg) + MTX (5 mg/kg) + 5-FU (50 mg/kg) (once a week for 3 weeks) | Mulmina MN (40 mL/kg and 80 mL/kg) and Donepezil DPL: (2 mg/kg). Daily oral treatment of MN and DPL began one week before CMF. | Spatial learning and memory: MWM | BDNF and TNF-α, IL-1β, IL-6, MIP-1α (Brain) | In tumor-bearing mice, hippocampal levels of IL-1β, IL-6, TNF-α and BDNF were elevated. The presence of tumor did not cause significant cognitive impairment. Administration of CMF was associated with a further increase in cytokine levels and induction of cognitive deficits. Co-treatment with both MN and DPL significantly reduced IL-1β, IL-6, and BDNF levels, and improved cognitive function compared to CMF alone. | [56] | 10.1038/s41598-022-06862-9 |
| Female BALB/c mice/Orthotopic implantation of TNBC tumor cells (4T1). | DOX (5 mg/kg once a week for 3 weeks) | Kai-Xin-San KXS: (1.5 g/kg for 21 consecutive days). | Spatial learning and working memory: MWM | IL-1β, IL-6, TNF-α, IL-12p70, IL-4, and IL-10 (Hippocampus and Serum) | The presence of tumor induced a mild elevation in serum levels of IL-6 and IL-12p70 but did not cause cognitive impairment. DOX treatment significantly worsened spatial memory, amplified hippocampal levels of IL-6, IL-12p70, TNF-α, IL-1β, and suppressed the anti-inflammatory activity of IL-4 and IL-10. Intervention with KXS reversed cognitive deficits, reduced pro-inflammatory cytokine levels, and restored the balance of IL-4 and IL-10. | [57] | 10.1155/2021/5521739 |
| Female C57BL/6J mice/Orthotopic implantation of E0771 TNBC tumor cells. | DOX (5 mg/kg once a week for 4 weeks) | Maraviroc MVC: (10 mg/kg, five times a week for 4 weeks) | Spatial learning and working memory: MWM | BDNF and IL-1β, TNF-α, IL-6, CCL3, CCL4 (Hippocampus) | Treatment with DOX resulted in increased mRNA expression and protein levels of the chemokines CCL3 and CCL4, as well as increased protein levels of IL-1β and TNF-α in the hippocampus. DOX treatment also decreased BDNF levels and induced spatial memory impairment. Administration of MVC attenuated the DOX-induced neuroinflammation and systemic inflammation, restored hippocampal BDNF levels, and improved cognitive performance. | [58] | 10.1016/j.neuropharm.2024.109981 |
| Female BALB/c mice/Orthotopic implantation of TNBC tumor cells (4T1) | DOX (5 mg/kg once a week for 3 weeks) | Fangxia-Dihuang Decoction FXDH (0.28 g/mL, 0.56 g/mL, 0.78 g/mL daily for 3 weeks) | Episodic memory: NOR. Working memory: Y-Maze | TNF-α, IL-12p70, IL-4, IL-10, IL-6 (Serum and hippocampus) | Tumor-bearing mice showed elevated serum levels of IL-6 and mild, non-significant, cognitive impairments. DOX treatment increased the levels of IL-6, IL-12p70, TNF-α while reducing the levels of IL-10 and IL-4; it also impaired working and recognition memory. Treatment with FXDH resulted in reduced levels of pro-inflammatory cytokines, increased levels of anti-inflammatory cytokines, and improved cognitive performance. | [59] | 10.3389/fonc.2025.1515498 |
| Female C57BL/6J wild-type mice/Chemotherapy applied in healthy mice. | DOX (2 mg/kg once a week for 4 weeks) | Riluzole RZ: (13 mg/kg for 30 days). | Spatial memory: NPR. Fear extinction memory: FE | BDNF (Hippocampus) | DOX treatment resulted in ~45% reduction in hippocampal BDNF levels and impaired spatial and recognition memory. Administration of RZ restored BDNF levels and reversed cognitive deficits. | [60] | 10.1007/s13311-022-01339-z |
| Female Sprague-Dawley rats/Chemotherapy in healthy and ovariectomized rats. | DOX (4 mg/kg) + CYP (40 mg/kg) (once a week for 3 weeks) | Working memory: Y-Maze. Episodic and spatial Memory: NOR | BDNF (Hippocampus) | Treatment with DOX + CYP did not alter hippocampal BDNF levels, while ovariectomized animals showed higher BDNF levels than intact controls regardless of chemotherapy. Chemotherapy was associated with significant cognitive deficits (spatial and working memory). Ovariectomy did not affect the severity of these cognitive impairments. | [61] | 10.1016/j.bbr.2015.06.028 | |
| Female C57BL/6J mice/Chemotherapy to ovariectomized mice. | DOX (2 mg/kg) and/or CYP (50 mg/kg) (once a week for 4 weeks) | Learning and spatial memory: MWM. Spatial Memory: NPR. Episodic Memory: NOR | IL-1α, IL-1β, IL-2 to IL-6, IL-10, IL-12p70, IL-17, MCP-1, IFN-γ, TNF-α, MIP-1α, RANTES (Hippocampus) | Levels of IL-1β, IL-6, and IL-12 in DOX + CYP-treated mice were significantly decreased compared with saline treated mice. Density of stubby spines, but not mushroom or thin spines, in the dentate gyrus was significantly decreased in the DOX, CYP, and DOX + CYP groups. These treatments were not associated with long-term cognitive impairment. | [62] | 10.1093/toxsci/kfx267 | |
| Male wild-type C57BL/6 mice/Chemotherapy applied in healthy mice. | DOX (2 mg/kg once a week for 4 weeks) | 1. Inhibition of CSF1R (PLX5622) 2. iMG-EV | Episodic memory: NOR. Spatial memory: NPR. Fear memory consolidation: FC | Cytokine gene expression: IL-1α, IL-1β, IL-3, IL-4, IL-5, IL-12, GM-CSF, RANTES, MIP-1α and CCL5 (Hippocampus) | DOX treatment resulted in significantly increased levels of IL-1β, IL-3, IL-5, IL-12, and GM-CSF, and impaired memory. Co-treatment with PLX5622 was associated with restored cognitive performance, normalization of IL-1α, IL-1β, IL-3, IL-4, IL-5, and GM-CSF levels, and increased RANTES and MIP-1α levels. Treatment with iMG-EVs also led to a complete restoration of cognitive performance and attenuated microglial activation. | [63] | 10.1186/s40478-019-0838-8 |
| Wistar female rats/Chemotherapy applied in healthy rats. | DOX (5 mg/kg twice a week for 14 days) | Pioglitazone PIO: (2 mg/kg, twice per week for 14 days) | Episodic memory: NOR. Working memory: Y-Maze | IL-6, IL-1β, and TNF-α (Brain) | DOX treatment increased levels of IL-1β, TNF-α, and IL-6 and impaired cognitive performance in both Y-Maze and NOR. Co-treatment with PIO significantly reduced TNF-α and IL-6 levels and normalized Y-Maze performance (non-significant improvement in NOR). | [64] | 10.3390/molecules28124775 |
| Wistar female rats/Chemotherapy applied in healthy rats. | DOX (25 mg/kg), CYP (200 mg/kg), 5-FU (100 mg/kg), CIS (8 mg/kg) (single dose) | Episodic memory: NOR. Working memory: Y-Maze | TNF-α, IL-1β, IL-6 (Hippocampus) | DOX treatment increased TNF-α, IL-1β and IL-6 levels, and was associated with impairments in spatial learning and memory, recognition of new objects, and spontaneous alternation. CYP treatment also elevated TNF-α and IL-1β levels, but to a lesser extent, with no significant changes in IL-6, and induced mild cognitive deficits in spatial and working memory. 5-FU treatment increased TNF-α and IL-6 levels, but not IL-1β levels, impaired spatial learning and recognition of new objects. Treatment with CIS appeared to impair short-term memory, with no effects in TNF-α, IL-1β, and IL-6. | [65] | 10.1016/j.biopha.2023.115245 | |
| Male Wistar rats/Chemotherapy applied in healthy rats. | DOX (3 mg/kg once weekly for 56 days) | Diosmin DIOS: (40 mg/kg daily for 56 days) | Episodic memory: NOR. Working memory: Y-Maze | IL-6, IL-1β, TNF-α, MMP-9 and COX-2 (Brain) | Treatment with DOX elevated the levels of IL-6, IL-1β, TNF-α, MMP-9 and COX-2, and impaired episodic memory. Co-treatment with DIOS largely reversed the cytokine elevations and fully restored cognitive performance in the NOR task and Y-maze. | [66] | 10.1016/j.dscb.2023.100111 |
| Female Sprague-Dawley rats/Chemotherapy applied in healthy rats. | DOX (2 mg/kg/week) or PAC (2 mg/kg, every other day) (4 weeks) | Vitamin E α-tocoferol: (100 mg/kg daily for 4 weeks) | Learning and memory: Radial arm water maze (RAWM) | BDNF (Hippocampus) | Treatment with DOX and PAC decreased BDNF levels and resulted in short-term memory impairment. Co-administration of vitamin E restored BDNF levels and improved cognitive outcomes. | [67] | 10.1007/s00280-023-04602-y |
| Male C57BL/6J mice/Chemotherapy applied in healthy mice. | CMF: CYP (100 mg/kg) + MTX (10 mg/kg) + 5-FU (100 mg/kg) (once a week for 4 weeks) | Episodic memory: NOR. Working memory: Y-Maze | BDNF and IL-1α, IL-1β, IL-3, IL-10, TNF-α (Hippocampus) | CMF treatment resulted in decreased BDNF levels, increased levels of pro-inflammatory cytokines (IL-1α, IL-1β, IL-3, IL-10, and TNF-α), and deficits in spatial working memory and recognition memory. | [68] | 10.1093/toxsci/kfz213 | |
| Female C57BL/6 mice/Chemotherapy applied in healthy mice. | DOX (2 mg/kg) + CYP (50 mg/kg) (once per week for 4 weeks). | Working memory: Y-Maze. Episodic memory: NOR | BDNF and TNF-α, IL-1β, IL-6 (Hippocampus) | Treatment with DOX + CYP did not increase pro-inflammatory cytokine levels, but it significantly reduced BDNF protein levels. Treated mice showed impaired recognition memory (NOR task) compared to controls. | [69] | 10.5607/en.2018.27.5.419 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fierro-Salgado, Y.T.; Reiriz, M.; Beltrán-Velasco, A.I.; Calleja-Conde, J.; Hernández-Oñativia, X.; Uceda, S.; Echeverry-Alzate, V. Cytokines and Brain-Derived Neurotrophic Factor as Biomarkers of Cognitive Impairment Related to Breast Cancer and Its Treatments: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 10074. https://doi.org/10.3390/ijms262010074
Fierro-Salgado YT, Reiriz M, Beltrán-Velasco AI, Calleja-Conde J, Hernández-Oñativia X, Uceda S, Echeverry-Alzate V. Cytokines and Brain-Derived Neurotrophic Factor as Biomarkers of Cognitive Impairment Related to Breast Cancer and Its Treatments: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(20):10074. https://doi.org/10.3390/ijms262010074
Chicago/Turabian StyleFierro-Salgado, Yenny Trinidad, Manuel Reiriz, Ana Isabel Beltrán-Velasco, Javier Calleja-Conde, Xabier Hernández-Oñativia, Sara Uceda, and Víctor Echeverry-Alzate. 2025. "Cytokines and Brain-Derived Neurotrophic Factor as Biomarkers of Cognitive Impairment Related to Breast Cancer and Its Treatments: A Systematic Review" International Journal of Molecular Sciences 26, no. 20: 10074. https://doi.org/10.3390/ijms262010074
APA StyleFierro-Salgado, Y. T., Reiriz, M., Beltrán-Velasco, A. I., Calleja-Conde, J., Hernández-Oñativia, X., Uceda, S., & Echeverry-Alzate, V. (2025). Cytokines and Brain-Derived Neurotrophic Factor as Biomarkers of Cognitive Impairment Related to Breast Cancer and Its Treatments: A Systematic Review. International Journal of Molecular Sciences, 26(20), 10074. https://doi.org/10.3390/ijms262010074








