Effect of Arginine Vasopressin on Human Neutrophil Function Under Physiological and Sepsis-Associated Conditions
Abstract
1. Introduction
2. Results
2.1. Flow Cytometry
2.1.1. The Percentage of Propidium Jodide (pj)-Stained Cells
2.1.2. Antigen Expression
2.1.3. Reactive Oxygen Species Production
2.2. Live Cell Imaging
2.2.1. Quantification of ROS Production and NETosis
| Experimental Group | TmaxROS | SD | ET50MPO | SD | ET50NETosis | SD |
|---|---|---|---|---|---|---|
| control | 107.22 | 65.51 | 165.44 | 47.56 | 250.23 | 49.71 |
| ClB 1.24 | 119.80 | 25.40 | 219.14 | 14.86 | 214.26 | 39.80 |
| ClB 12,400 | 93.66 | 38.74 | 225.06 | 25.00 | 227.21 | 16.75 |
| Total ClB | 100.55 | 36.88 | 223.61 | 32.23 | 230.80 | 127.40 |
| AVP/CLB 1.24 | 129.37 | 43.90 | 276.77 | 60.03 | 260.86 | 32.75 |
| AVP/CLB 12.4 | 36.03 | 40.66 | 139.70 | 1.24 | 209.84 | 78.54 |
| AVP/CLB 124 | 109.55 | 48.71 | 161.62 | 43.15 | 229.43 | 75.72 |
| AVP/CLB 1240 | 135.29 | 61.22 | 165.90 | 23.38 | 323.51 | 132.08 |
| AVP/CLB 12,400 | 61.16 | 29.46 | 185.95 | 45.83 | 317.59 | 140.30 |
| AVP/CLB Max | 94.39 | 10.03 | 171.62 | 24.68 | 218.27 | 17.37 |
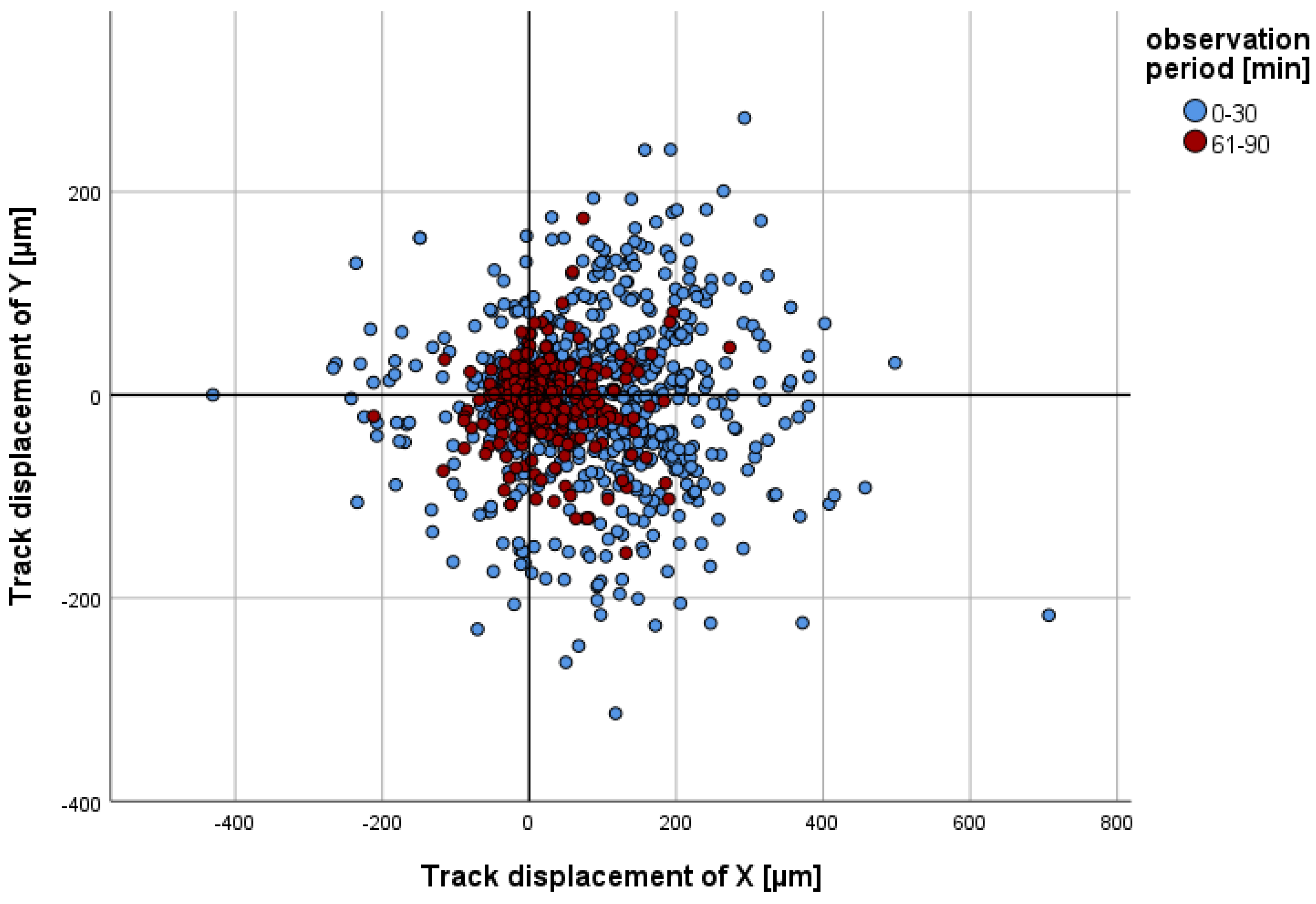
2.2.2. Migration
- (a)
- Track displacement of Y
- (b)
- Track length
- i.
- ClB compared to the control, total observation timeA comparison of the track lengths over the entire observation period revealed a significant difference (p < 0.001 in each case) between the experimental groups with ClB offset PMNs and the PMNs in the control group (Table 2).
- ii.
- ClB compared to the control, individual periodsThe results demonstrate a statistically significant increase (p < 0.001) in the track length for each concentration of ClB, with the greatest change occurring during the first two half-hour intervals (cf. Figure 3).
- iii.
- AVP/ClB compared to the control, total observation timeA comparison of the track length over the entire observation period revealed a significant difference (p < 0.001) between all of the AVP/ClB test groups, with the exception of those spiked with 12.4 pg/mL AVP/ClB, and the PMNs of the control group (Table 2).
- iv.
- AVP/ClB compared to the control, individual periodsFurthermore, the track length was markedly increased by 1.24 pg/mL AVP/ClB during the initial three half-hour periods (cf. Figure 3). As the AVP/ClB concentration increased, the track length exhibited a progressive decline, resulting in some PMNs spiked with higher AVP/ClB concentrations having significantly shorter track lengths than those of the control (cf. Table 2). The PMNs that were spiked with a concentration of 12.4 pg/mL AVP/ClB exhibited a significantly (p < 0.01) reduced track length compared to that in the control group during the initial 30 min. During the observation period of 31 to 60 min, the track lengths of the experimental group were found to be similar to those of the control group. During the observation periods of 61–90 min and 91–120 min, the track lengths were found to be significantly (p < 0.01) higher.
- v.
- AVP/ClB compared to ClB, total observation timeA direct comparison of the AVP/ClB group with the corresponding ClB group revealed no significant difference in the track length between the PMNs of the AVP/ClB 1.24 group and the PMNs of the corresponding ClB experimental group (ClB 1.24 group) over the entire observation period. The PMNs of the AVP/ClB 12,400 group exhibited a markedly reduced track length in comparison to that in the PMNs of the corresponding ClB group (ClB 12,400) throughout the observation period, with a statistically significant difference (p < 0.001).
- vi.
- AVP/ClB compared to ClB, individual periodsA direct comparison of the AVP/ClB group with the corresponding ClB group revealed no significant difference in the track length between the PMNs of the AVP/ClB 1.24 group and the PMNs of the corresponding ClB experimental group (ClB 1.24) at any time point. The PMNs of the AVP/ClB 12,400 group exhibited a significantly (p < 0.001) reduced track length in comparison to the PMNs of the corresponding ClB group (ClB 12,400) throughout the entire observation period.
- (c)
- Straightness
- i.
- ClB compared to control, total observation timeThe PMNs of all ClB test groups exhibited a statistically significant difference (p < 0.001 in each case) in straightness compared to that in the control group over the entire observation period.
- ii.
- ClB compared to the control, individual periodsThe PMNs of the 1.24 ClB and 12,400 ClB group exhibited a markedly higher degree of straightness (p < 0.001 in each case) compared to that in the control group during the initial three half-hour intervals.
- iii.
- AVP/ClB compared to the control, total observation timeThe PMNs of all of the AVP/ClB test groups exhibited a statistically significant difference (p < 0.001 in each case) in straightness compared to that in the control group over the entire observation period.
- iv.
- AVP/ClB compared to the control, individual periodsIn the first three half hours, the PMNs incubated with 12.4 pg/mL AVP/ClB or higher exhibited a significantly reduced straightness (cf. Figure 4) in comparison to that in the PMNs in the control group. The incubation of the PMNs with 1.24 pg/mL AVP/ClB resulted in a significantly higher straightness at all time intervals (p < 0.01 in each case) compared to that in the PMNs in the control group. The straightness of the cells demonstrated a decline with an increase in the AVP/ClB concentration. The greatest reduction in straightness was observed in the PMNs incubated with the highest AVP/ClB concentration (AVP/ClB Max).
- v.
- AVP/ClB compared to ClB, total observation timeThe PMNs of the AVP/ClB 12,400 group exhibited a markedly diminished straightness throughout the observation period, as evidenced by a p-value of less than 0.01 in each instance, in comparison to that in the PMNs of the corresponding ClB group (ClB 12,400 group).
- vi.
- AVP/ClB compared to ClB, individual periodsA direct comparison demonstrated that the PMNs incubated with 1.24 pg/mL AVP/ClB exhibited markedly elevated straightness in the second (p < 0.05) and third half-hour (p < 0.01) periods in comparison to the PMNs incubated with the corresponding ClB concentration (ClB = 1.24). The cells spiked with 12,400 pg/mL AVP/ClB demonstrated this significantly (p < 0.01 in each case) in the first three half-hour periods when compared to the PMNs incubated with the corresponding ClB concentration (ClB = 12,400).
- (d)
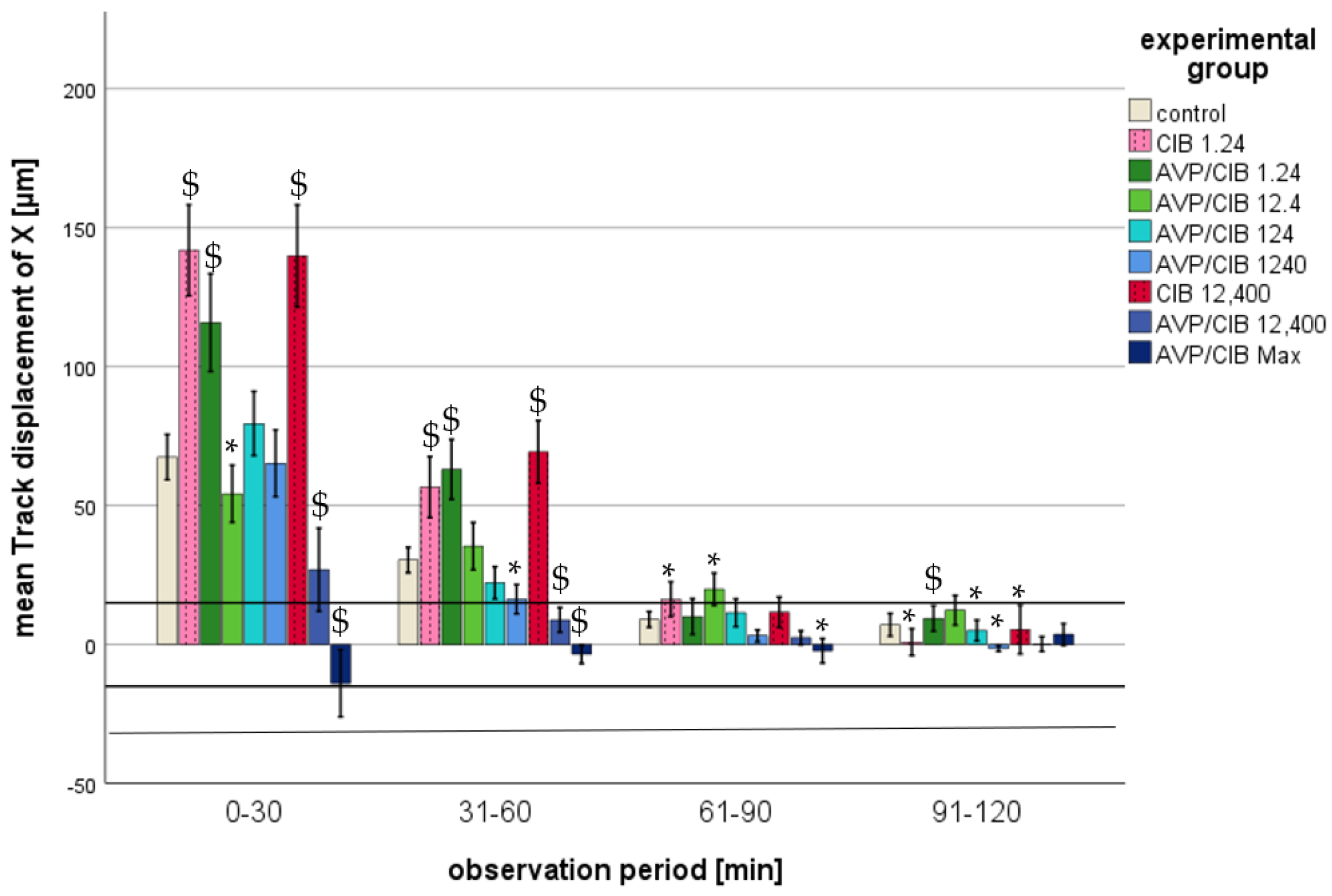
- Track displacement of X
- i.
- ClB compared to the control, total observation timeThroughout the observation period, all of the PMNs spiked with ClB showed a significantly higher track displacement of X than that of the PMNs in the control group (cf. Table 2).
- ii.
- ClB compared to the control, individual periodsAll of the PMNs that were exposed to pure ClB exhibited a positive track displacement of X at all time points, indicating a trajectory towards the reservoir with the chemoattractant. A disparity of greater than one PMN cell diameter from the control group was observed during the initial two half-hour intervals. As the time progressed, the values of the track displacement of X parameter converged. From the fourth half hour (from 91 min after the commencement of microscopy), the track displacement of X parameter of the ClB test groups no longer exhibited a statistically significant difference from that of the control group, nor did it demonstrate a difference of less than one PMN diameter. In the initial half hour, all of the PMNs spiked with ClB exhibited a markedly elevated track displacement of X in comparison to that in the control group (p < 0.01) (cf. Figure 5).
- iii.
- AVP/ClB compared to the control, total observation timeThe PMNs spiked with AVP/ClB showed a significantly different track displacement of X than that of the PMNs in the control group at the lowest and the three highest concentrations used throughout the observation period (cf. Table 2). The PMNs incubated with 12.4 or 124 pg/mL AVP/ClB demonstrated no statistically significant difference in the track displacement of X parameter when compared to that in the control group over the entire observation period.
- iv.
- AVP/ClB compared to the control, individual periodsWith the exception of the PMNs belonging to the highest-AVP-concentration group, all of the PMNs that had been spiked with AVP/ClB demonstrated a positive track displacement of X at all time points, indicating a movement towards the reservoir with the chemoattractant. The PMNs incubated with the highest AVP concentration exhibited a negative track displacement, corresponding to a distance of less than one PMN cell diameter. A difference of greater than one PMN cell diameter from the control group was observed during the initial two half-hour periods. As the time progressed, the values of the track displacement of X parameter for all groups converged. From the fourth half hour (91 min after the commencement of microscopy), the track displacement of X parameter of the AVP/ClB experimental groups no longer exhibited a statistically significant difference from that of the control group, nor did it show a difference of less than one PMN diameter.In the initial 30 min, the PMNs treated with 1.24 pg/mL AVP/ClB exhibited a markedly elevated track displacement of X in comparison to that of the control group (p < 0.01 each). Higher AVP/ClB concentrations demonstrated a similar or significantly reduced track displacement of X in comparison to that in the control group (cf. Figure 5).
- v.
- AVP/ClB compared to ClB, total observation timeThe PMNs of the AVP/ClB 1.24 group demonstrated no statistically significant difference when compared to the PMNs of the corresponding ClB group (ClB 1.24) over the entire observation period.
- vi.
- AVP/ClB compared to ClB, individual periodsIn the initial 30-minute period, the PMNs of the AVP/ClB 1.24 group exhibited a markedly reduced track displacement of X in comparison to that in the corresponding ClB group (ClB 1.24). Subsequently, no notable discrepancy was observed. In the initial four half-hour intervals, the PMNs of the AVP/ClB 12,400 group exhibited a markedly diminished track displacement of X in comparison to that in the corresponding ClB group (ClB 12,400) (p < 0.01 in the first two half-hour intervals, p < 0.05 in the third and fourth half-hour intervals).
| Experimental Group | Track Length | Straightness | Track Displacement of X |
|---|---|---|---|
| ClB 1.24 | <0.001 | <0.001 | <0.001 |
| ClB 12,400 | <0.001 | <0.001 | <0.001 |
| AVP/ClB 1.24 | <0.001 | <0.001 | <0.001 |
| AVP/ClB 12.4 | No significance | <0.001 | No significance |
| AVP/ClB 124 | <0.001 | <0.001 | No significance |
| AVP/ClB 1240 | <0.001 | <0.001 | <0.05 |
| AVP/ClB 12,400 | <0.001 | <0.001 | <0.001 |
| AVP/ClB Max | <0.001 | <0.001 | <0.001 |
3. Discussion
3.1. The Proportion of Dead Cells, Antigen Production, ROS Production, and NETosis in Relation to AVP/ClBClB and ClB
3.2. Chemokinesis and Chemotaxis in Relation to AVP/ClB and ClB
| Chemotaxis | Chemokinesis |
|---|---|
| track displacement of X, track displacement of Y, straightness | track displacement of Y, track length, straightness |
| Clinical Significance of the Analysed AVP-Concentration | Chemokinesis | Chemotaxis | Experimental Group |
| physiological AVP-plasma concentration | increased | not decreased | AVP/ClB 1.24 |
| AVP-plasma concentration during Sepsis | dependent on time | AVP/ClB 12.4 | |
| AVP-plasma concentration during continuous AVP-infusion | no effect | no effect | AVP/ClB 124 |
| Supraclinical AVP- concentration | decreased | decreased | AVP/ClB 1240 |
| Supraclinical AVP- concentration | decreased | decreased | AVP/ClB 12,400 |
| Supraclinical AVP- concentration | decreased | decreased | AVP/ClB Max |
| Chemokinesis | Chemotaxis | Experimental group | |
| Chlorobutanol concentration | increased | increased | ClB 1.24 |
| increased | increased | ClB 12,400 | |
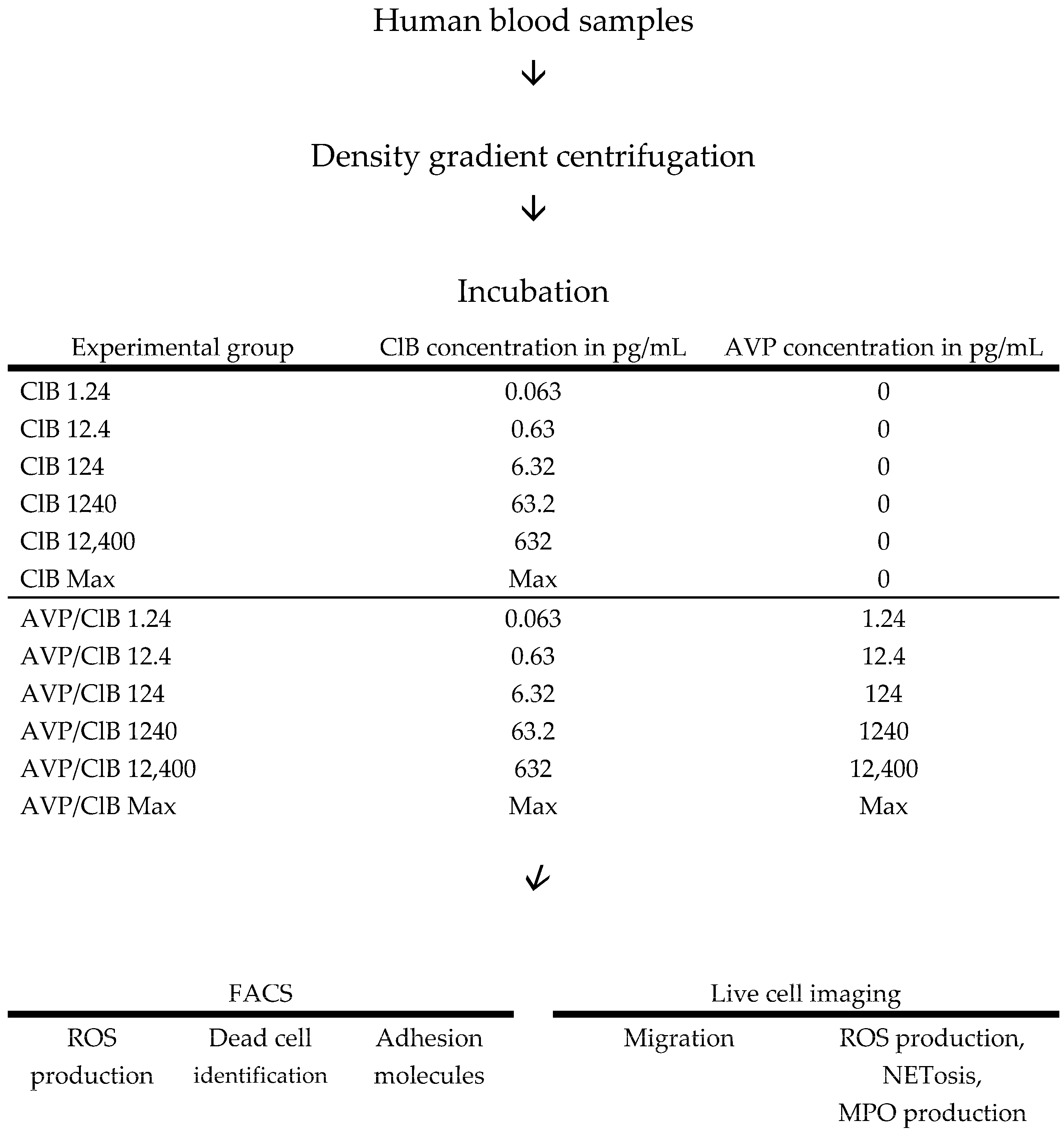
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Experimental Group | TmaxROS | ET50NETosis | ET50MPO |
|---|---|---|---|
| control | 15 | 16 | 18 |
| ClB 1.24 | 3 | 3 | 3 |
| ClB 12,400 | 2 | 2 | 2 |
| All analysed ClB concentrations | 5 | 5 | 5 |
| AVP/ClB 1.24 | 3 | 2 | 2 |
| AVP/ClB 12.4 | 5 | 4 | 2 |
| AVP/ClB 124 | 6 | 7 | 7 |
| AVP/ClB 1240 | 6 | 6 | 6 |
| AVP/ClB 12,400 | 4 | 4 | 6 |
| AVP/ClB Max | 3 | 3 | 3 |
| All analysed AVP/ClB concentrations | 27 | 26 | 26 |
| Experimental Group | 0–30 min | 31–60 min | 61–90 min | 91–120 min | 120–150 min | 150–180 min |
|---|---|---|---|---|---|---|
| control | 786 | 738 | 725 | 464 | 322 | 76 |
| ClB 1.24 | 197 | 197 | 171 | 124 | 122 | - |
| ClB 12,400 | 149 | 143 | 126 | 39 | - | - |
| AVP/ClB 1.24 | 200 | 200 | 186 | 128 | 140 | - |
| AVP/ClB 12.4 | 300 | 297 | 294 | 199 | 71 | 32 |
| AVP/ClB 124 | 261 | 326 | 288 | 251 | 115 | 36 |
| AVP/ClB 1240 | 214 | 253 | 255 | 226 | 136 | - |
| AVP/ClB 12,400 | 222 | 258 | 175 | 134 | 51 | - |
| AVP/ClB Max | 124 | 119 | 80 | 45 | 42 | - |
References
- Zhou, Y.-Y.; Sun, B.-W. Recent advances in neutrophil chemotaxis abnormalities during sepsis. Chin. J. Traumatol. 2022, 25, 317–324. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Sharshar, T.; Blanchard, A.; Paillard, M.; Raphael, J.C.; Gajdos, P.; Annane, D. Circulating vasopressin levels in septic shock. Crit. Care Med. 2003, 31, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.; Lang, F.; Schmidt, R.F. Physiologie des Menschen; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Ukor, I.-F.; Walley, K.R. Vasopressin in Vasodilatory Shock. Crit. Care Clin. 2019, 35, 247–261. [Google Scholar] [CrossRef]
- Wickramasinghe, S.N.; Valdimarsson, H.; Williams, B. Interaction between a synthetic analogue of vasopressin and human peripheral blood lymphocytes. J. Clin. Lab. Immunol. 1982, 7, 67–70. [Google Scholar] [PubMed]
- Torres, B.A.; Johnson, H.M. Arginine vasopressin (AVP) replacement of helper cell requirement in IFN-gamma production. Evidence for a novel AVP receptor on mouse lymphocytes. J. Immunol. 1988, 140, 2179–2183. [Google Scholar] [CrossRef]
- Johnson, H.M.; Torres, B.A. Regulation of lymphokine production by arginine vasopressin and oxytocin: Modulation of lymphocyte function by neurohypophyseal hormones. J. Immunol. 1985, 135 (Suppl. S2), 773s–775s. [Google Scholar] [CrossRef]
- Andrianov, I.G.; Dobkin, A.N.; Kiselev, O.I.; Okulov, V.B.; Semiglazov, V.F. Immunomoduliatsiia aktivnosti estestvennykh killerov u bol’nykh s opukholiami molochnoĭ zhelezy s pomoshch’iu vazopressina i interleĭkina-2 in vitro. Vopr. Onkol. 1989, 35, 1186–1191. [Google Scholar]
- Khegai, I.I.; Gulyaeva, M.A.; Popova, N.A.; Zakharova, L.A.; Ivanova, L.N. Immune system in vasopressin-deficient rats during ontogeny. Bull. Exp. Biol. Med. 2003, 136, 448–450. [Google Scholar] [CrossRef]
- Block, L.H.; Locher, R.; Tenschert, W.; Siegenthaler, W.; Hofmann, T.; Mettler, E.; Vetter, W. 125I-8-L-arginine vasopressin binding to human mononuclear phagocytes. J. Clin. Investig. 1981, 68, 374–381. [Google Scholar] [CrossRef]
- Bell, J.; Adler, M.W.; Greenstein, J.I.; Liu-Chen, L.Y. Identification and characterization of 125Iarginine vasopressin binding sites on human peripheral blood mononuclear cells. Life Sci. 1993, 52, 95–105. [Google Scholar] [CrossRef]
- Elands, J.; Resink, A.; de Kloet, E.R. Neurohypophyseal hormone receptors in the rat thymus, spleen, and lymphocytes. Endocrinology 1990, 126, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Ruff, M.; Schiffmann, E.; Terranova, V.; Pert, C.B. Neuropeptides are chemoattractants for human tumor cells and monocytes: A possible mechanism for metastasis. Clin. Immunol. Immunopathol. 1985, 37, 387–396. [Google Scholar] [CrossRef]
- Rostron, A.J.; Avlonitis, V.S.; Cork, D.M.; Grenade, D.S.; Kirby, J.A.; Dark, J.H. Hemodynamic resuscitation with arginine vasopressin reduces lung injury after brain death in the transplant donor. Transplantation 2008, 85, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, F.J.; Watzinger, K.; Stichlberger, M.; Joannidis, M.; Kaehler, C.; Lederer, W. Effects of Arginine Vasopressin on Migration and Respiratory Burst Activity in Human Leukocytes. Open Med. 2018, 13, 122–129. [Google Scholar] [CrossRef]
- Doherty, C.C.; LaBelle, P.; Collins, J.F.; Brautbar, N.; Massry, S.G. Effect of parathyroid hormone on random migration of human polymorphonuclear leukocytes. Am. J. Nephrol. 1988, 8, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, C.; Dünser, M.W.; Wenzel, V.; Jochberger, S.; Mayr, V.; Schmittinger, C.A.; Lorenz, I.; Schmid, S.; Westphal, M.; Grander, W.; et al. Comparing two different arginine vasopressin doses in advanced vasodilatory shock: A randomized, controlled, open-label trial. Intensive Care Med. 2010, 36, 57–65. [Google Scholar] [CrossRef]
- Bankir, L.; Bichet, D.G.; Morgenthaler, N.G. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef]
- Leng, G.; Sabatier, N. Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. J. Neuroendocrinol. 2016, 28, 1–13. [Google Scholar] [CrossRef]
- Meck, J.V.; Waters, W.W.; Ziegler, M.G.; Deblock, H.F.; Mills, P.J.; Robertson, D.; Huang, P.L. Mechanisms of postspaceflight orthostatic hypotension: Low alpha1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1486–H1495. [Google Scholar] [CrossRef]
- Breum Jakobsen, N.F.; Laugesen, E.; Rolighed, L.; Nissen, P.H.; Poulsen, P.L.; Pedersen, E.B.; Mosekilde, L.; Rejnmark, L. The cardiovascular system in familial hypocalciuric hypercalcemia: A cross-sectional study on physiological effects of inactivating variants in the calcium-sensing receptor gene. Eur. J. Endocrinol. 2016, 175, 299–309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miwa, K. Down-regulation of renin-aldosterone and antidiuretic hormone systems in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J. Cardiol. 2017, 69, 684–688. [Google Scholar] [CrossRef]
- Kagerbauer, S.M.; Martin, J.; Schuster, T.; Blobner, M.; Kochs, E.F.; Landgraf, R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocrinol. 2013, 25, 668–673. [Google Scholar] [CrossRef]
- Jochberger, S.; Dünser, M.W. Influences of hydrocortisone therapy on arginine vasopressin plasma levels in septic shock. Wien. Klin. Wochenschr. 2011, 123, 245–247. [Google Scholar] [CrossRef]
- Parker, K.J.; Kenna, H.A.; Zeitzer, J.M.; Keller, J.; Blasey, C.M.; Amico, J.A.; Schatzberg, A.F. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010, 178, 359–362. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Factor, P. Role of vasopressin in the management of septic shock. Intensive Care Med. 2004, 30, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Gressner, A.M.; Arndt, T. (Eds.) Lexikon der Medizinischen Laboratoriumsdiagnostik; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Siegel, A.J.; Verbalis, J.G.; Clement, S.; Mendelson, J.H.; Mello, N.K.; Adner, M.; Shirey, T.; Glowacki, J.; Lee-Lewandrowski, E.; Lewandrowski, K.B. Hyponatremia in marathon runners due to inappropriate arginine vasopressin secretion. Am. J. Med. 2007, 120, e11–e17. [Google Scholar] [CrossRef]
- Sollanek, K.J.; Staab, J.S.; Kenefick, R.W.; Cheuvront, S.N. Biological variation of arginine vasopressin. Eur. J. Appl. Physiol. 2020, 120, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, A.J.; Wen, S.W.; Hall, P.; Hickey, M.J.; Wong, C.H.Y. Activation of the sympathetic nervous system modulates neutrophil function. J. Leukoc. Biol. 2018, 103, 295–309. [Google Scholar] [CrossRef]
- Wenisch, C.; Parschalk, B.; Weiss, A.; Zedwitz-Liebenstein, K.; Hahsler, B.; Wenisch, H.; Georgopoulos, A.; Graninger, W. High-dose catecholamine treatment decreases polymorphonuclear leukocyte phagocytic capacity and reactive oxygen production. Clin. Diagn. Lab. Immunol. 1996, 3, 423–428. [Google Scholar] [CrossRef]
- Davis, J.M.; Albert, J.D.; Tracy, K.J.; Calvano, S.E.; Lowry, S.F.; Shires, G.T.; Yurt, R.W. Increased neutrophil mobilization and decreased chemotaxis during cortisol and epinephrine infusions. J. Trauma Acute Care Surg. 1991, 31, 725–731, discussion 731–732. [Google Scholar] [CrossRef]
- Trabold, B.; Gruber, M.; Fröhlich, D. Synthetic inotropes inhibit the expression of adhesion molecules and augment the expression of L-selectin in polymorphonuclear neutrophils. Resuscitation 2007, 74, 352–356. [Google Scholar] [CrossRef]
- Deitch, E.A.; Bridges, R.M. Stress hormones modulate neutrophil and lymphocyte activity in vitro. J. Trauma 1987, 27, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Habara, Y.; Kanno, T. Dual effects of chlorobutanol on secretory response and intracellular Ca2+ dynamics in isolated pancreatic acini of the rat. Br. J. Pharmacol. 1993, 109, 685–692. [Google Scholar] [CrossRef]
- Chen, S.L.; Yang, W.C.; Huang, T.P.; Wann, S.A.; Teng, C.M. Chlorobutanol, a preservative of desmopressin, inhibits human platelet aggregation and release in vitro. Thromb. Haemost. 1990, 64, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Leino, L.; Hurttia, H.; Peltonen, E. Diacylglycerol in peripheral blood neutrophils from patients with localized juvenile periodontitis. J. Periodontal Res. 1994, 29, 334–338. [Google Scholar] [CrossRef]
- Paragh, G.; Kovács, É.; Seres, I.; Keresztes, T.; Balogh, Z.; Szabó, J.; Teichmann, F.; Fóris, G. Altered signal pathway in granulocytes from patients with hypercholesterolemia. J. Lipid Res. 1999, 40, 1728–1733. [Google Scholar] [CrossRef]
- Evans, G.A.; Farrar, W.L. Phorbol Esters. In Encyclopedia of Immunology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1940–1942. [Google Scholar]
- Savitha, G.; Salimath, B.P. Cross-talk between protein kinase C and protein kinase A down-regulates the respiratory burst in polymorphonuclear leukocytes. Cell Signal 1993, 5, 107–117. [Google Scholar] [CrossRef]
- Kuwano, Y.; Tominaga, K.; Kawahara, T.; Sasaki, H.; Takeo, K.; Nishida, K.; Masuda, K.; Kawai, T.; Teshima-Kondo, S.; Rokutan, K. Tumor necrosis factor alpha activates transcription of the NADPH oxidase organizer 1 (NOXO1) gene and upregulates superoxide production in colon epithelial cells. Free Radic. Biol. Med. 2008, 45, 1642–1652. [Google Scholar] [CrossRef]
- Fischer, M. Effects of chlorobutanol on primary and secondary endings of isolated cat muscle spindles. Brain Res. 2000, 854, 106–121. [Google Scholar] [CrossRef]
- Sim, A.T.; White, M.D.; Denborough, M.A. The effects of chlorbutol on skeletal muscle sarcoplasmic reticulum function in porcine malignant hyperpyrexia. Int. J. Biochem. 1987, 19, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Pai, D.; Gruber, M.; Pfaehler, S.-M.; Bredthauer, A.; Lehle, K.; Trabold, B. Polymorphonuclear Cell Chemotaxis and Suicidal NETosis: Simultaneous Observation Using fMLP, PMA, H7, and Live Cell Imaging. J. Immunol. Res. 2020, 2020, 1415947. [Google Scholar] [CrossRef] [PubMed]
- Bredthauer, A.; Geiger, A.; Gruber, M.; Pfaehler, S.M.; Petermichl, W.; Bitzinger, D.; Metterlein, T.; Seyfried, T. Propofol Ameliorates Exaggerated Human Neutrophil Activation in a LPS Sepsis Model. J. Inflamm. Res. 2021, 14, 3849–3862. [Google Scholar] [CrossRef]
- Doblinger, N.; Bredthauer, A.; Mohrez, M.; Hähnel, V.; Graf, B.; Gruber, M.; Ahrens, N. Impact of hydroxyethyl starch and modified fluid gelatin on granulocyte phenotype and function. Transfusion 2019, 59, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.L. Stimulated neutrophil locomotion: Chemokinesis and chemotaxis. Arch. Pathol. Lab. Med. 1977, 101, 509–513. [Google Scholar]
- Xu, J.; Cai, H.; Zheng, X. Timing of vasopressin initiation and mortality in patients with septic shock: Analysis of the MIMIC-III and MIMIC-IV databases. BMC Infect. Dis. 2023, 23, 199. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haile, S.-M.; Gruber, M.; Bollwein, G.; Trabold, B. Effect of Arginine Vasopressin on Human Neutrophil Function Under Physiological and Sepsis-Associated Conditions. Int. J. Mol. Sci. 2025, 26, 2512. https://doi.org/10.3390/ijms26062512
Haile S-M, Gruber M, Bollwein G, Trabold B. Effect of Arginine Vasopressin on Human Neutrophil Function Under Physiological and Sepsis-Associated Conditions. International Journal of Molecular Sciences. 2025; 26(6):2512. https://doi.org/10.3390/ijms26062512
Chicago/Turabian StyleHaile, Sophie-Marie, Michael Gruber, Gabriele Bollwein, and Benedikt Trabold. 2025. "Effect of Arginine Vasopressin on Human Neutrophil Function Under Physiological and Sepsis-Associated Conditions" International Journal of Molecular Sciences 26, no. 6: 2512. https://doi.org/10.3390/ijms26062512
APA StyleHaile, S.-M., Gruber, M., Bollwein, G., & Trabold, B. (2025). Effect of Arginine Vasopressin on Human Neutrophil Function Under Physiological and Sepsis-Associated Conditions. International Journal of Molecular Sciences, 26(6), 2512. https://doi.org/10.3390/ijms26062512







