Sex Differences in a Novel Mouse Model of Spinocerebellar Ataxia Type 1 (SCA1)
Abstract
:1. Introduction
2. Results
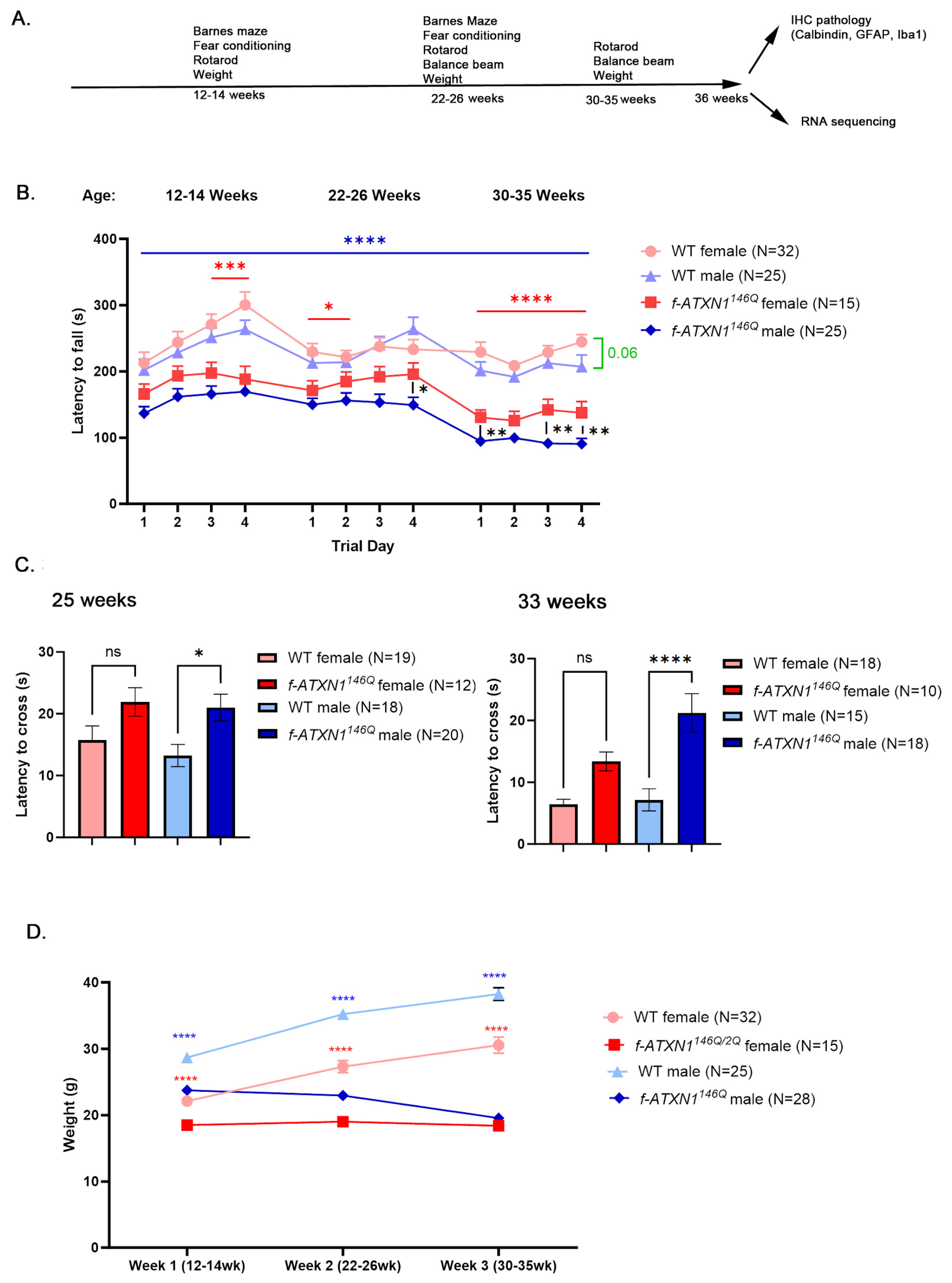
2.1. Male SCA1 Mice Perform Worse on the Rotarod and Show Weight Loss
2.2. Sex Differences in Terms of Failure to Gain Weight and Weight Loss in f-ATXN1146Q Mice
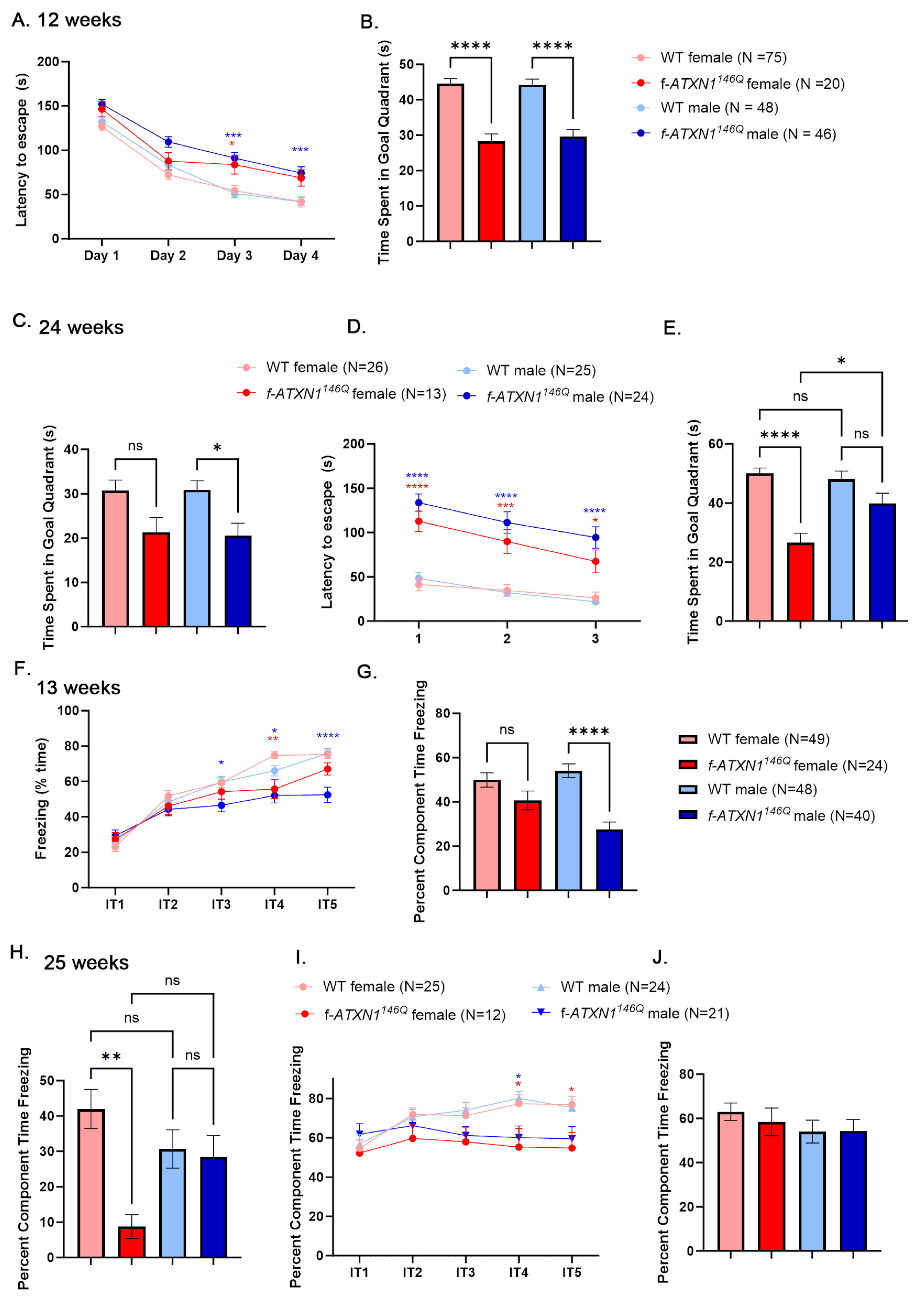
2.3. Sex Differences in Cognitive Deficits in f-ATXN1146Q Mice
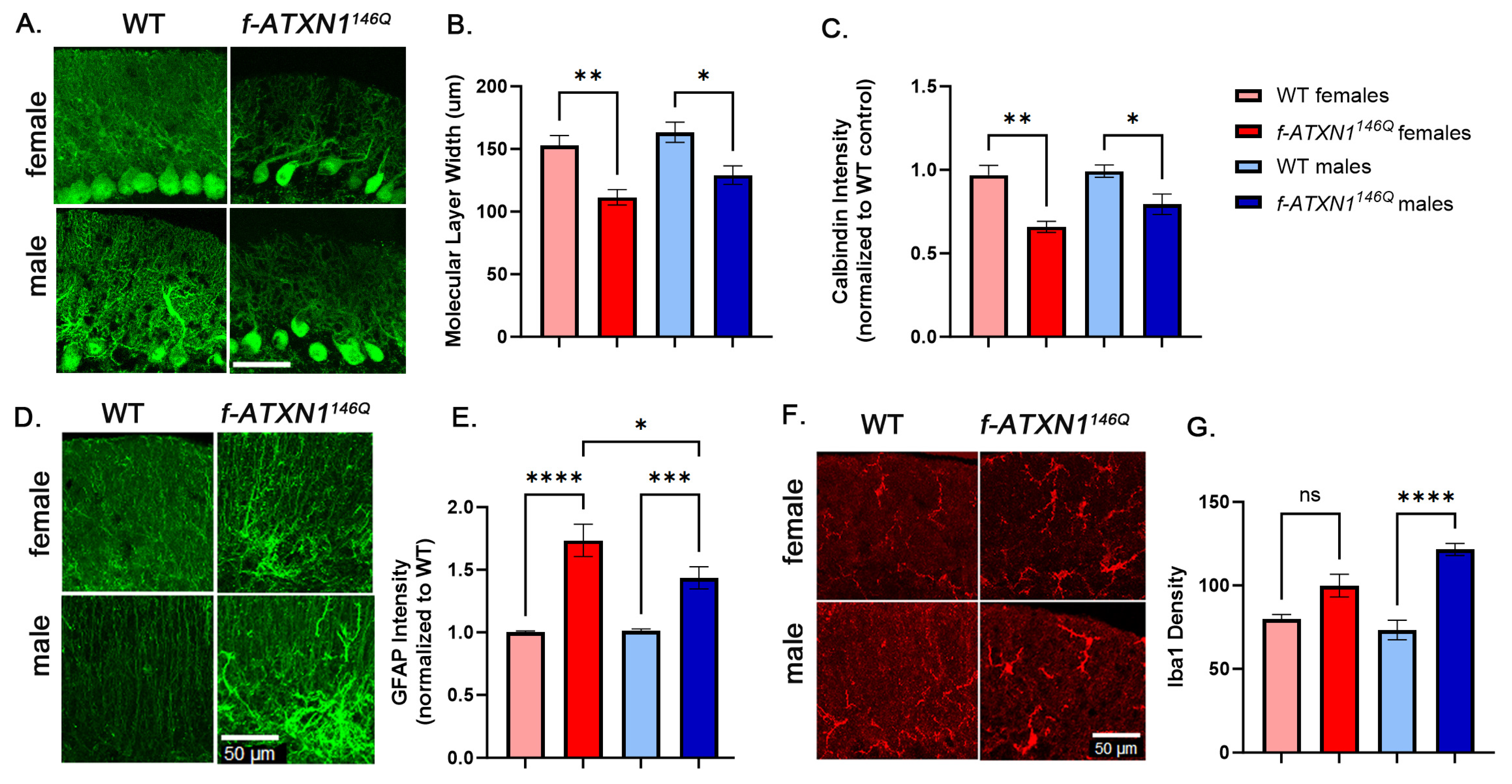
2.4. Sex Differences in Cerebellar Pathology
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Genotyping
4.3. Behavioral Testing
4.4. Motor Testing
4.5. Cognitive Testing
4.6. RNAseq
4.7. Immunofluorescence
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCA1 | Spinocerebellar ataxia type 1 |
| ATXN1 | Ataxin-1s |
| GFAP | Glial Fibrillary Acidic Protein |
| Iba1 | Ionized calcium-binding adaptor protein 1 |
References
- Lopez-Lee, C.; Kodama, L.; Gan, L. Sex Differences in Neurodegeneration: The Role of the Immune System in Humans. Biol. Psychiatry 2022, 91, 72–80. [Google Scholar] [CrossRef]
- Arabia, G.; De Martino, A.; Moro, E. Sex and gender differences in movement disorders: Parkinson’s disease, essential tremor, dystonia and chorea. in Int. Rev. Neurobiol. 2022, 164, 101–128. [Google Scholar]
- Bourque, M.; Dluzen, D.E.; Di Paolo, T. Neuroprotective actions of sex steroids in Parkinson’s disease. Front. Neuroendocrinol. 2009, 30, 142–157. [Google Scholar] [CrossRef]
- Zielonka, D.; Stawinska-Witoszynska, B. Gender Differences in Non-sex Linked Disorders: Insights From Huntington’s Disease. Front. Neurol. 2020, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, H.; Bauer, P.; Giunti, P.; Labrum, R.; Sweeney, M.; Charles, P.; Dürr, A.; Marelli, C.; Globas, C.; Linnemann, C.; et al. The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: A 2-year follow-up study. Neurology 2011, 77, 1035–1041. [Google Scholar] [CrossRef]
- Jacobi, H.; du Montcel, S.T.; Bauer, P.; Giunti, P.; Cook, A.; Labrum, R.; Parkinson, M.H.; Durr, A.; Brice, A.; Charles, P.; et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: A longitudinal cohort study. Lancet Neurol. 2015, 14, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.; Rosa, J.-G.; Rainwater, O.; Duvick, L.; Bennyworth, M.; Lai, R.-Y.; Sca, C.; Kuo, S.-H.; Cvetanovic, M. Cerebellar contribution to the cognitive alterations in SCA1: Evidence from mouse models. Hum. Mol. Genet. 2020, 29, 117–131. [Google Scholar] [CrossRef]
- Orr, H.; Chung, M.-Y.; Banfi, S.; Kwiatkowski, T.J.; Servadio, A.; Beaudet, A.L.; McCall, A.E.; Duvick, L.A.; Ranum, L.P.W.; Zoghbi, H. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat. Genet. 1993, 4, 221–226. [Google Scholar] [CrossRef]
- Orr, H.T.; Zoghbi, H.Y. Trinucleotide Repeat Disorders. Annu. Rev. Neurosci. 2007, 30, 575–621. [Google Scholar] [CrossRef]
- Paulson, H.L.; Shakkottai, V.G.; Clark, H.B.; Orr, H.T. Polyglutamine spinocerebellar ataxias—From genes to potential treatments. Nat. Rev. Neurosci. 2017, 18, 613–626. [Google Scholar] [CrossRef]
- Zoghbi, H.Y.; Orr, H.T. Pathogenic Mechanisms of a Polyglutamine-mediated Neurodegenerative Disease, Spinocerebellar Ataxia Type 1. J. Biol. Chem. 2009, 284, 7425–7429. [Google Scholar] [CrossRef] [PubMed]
- Cendelin, J.; Cvetanovic, M.; Gandelman, M.; Hirai, H.; Orr, H.T.; Pulst, S.M.; Strupp, M.; Tichanek, F.; Tuma, J.; Manto, M. Consensus Paper: Strengths and Weaknesses of Animal Models of Spinocerebellar Ataxias and Their Clinical Implications. Cerebellum 2022, 21, 452–481. [Google Scholar] [CrossRef] [PubMed]
- Duvick, L.; Southern, W.M.; Benzow, K.A.; Burch, Z.N.; Handler, H.P.; Mitchell, J.S.; Kuivinen, H.; Gadiparthi, U.; Yang, P.; Soles, A.; et al. Mapping SCA1 regional vulnerabilities reveals neural and skeletal muscle contributions to disease. JCI Insight 2024, 9, e176057. [Google Scholar] [CrossRef] [PubMed]
- Handler, H.P.; Duvick, L.; Mitchell, J.S.; Cvetanovic, M.; Reighard, M.; Soles, A.; Mather, K.B.; Rainwater, O.; Serres, S.; Nichols-Meade, T.; et al. Decreasing mutant ATXN1 nuclear localization improves a spectrum of SCA1-like phenotypes and brain region transcriptomic profiles. Neuron 2023, 111, 493–507.e6. [Google Scholar] [CrossRef]
- Illouz, T.; Madar, R.; Clague, C.; Griffioen, K.J.; Louzoun, Y.; Okun, E. Unbiased classification of spatial strategies in the Barnes maze. Bioinformatics 2016, 32, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Siswanto, S.; Brunt, E.R.; den Dunnen, W.; Korf, H.W.; Rüb, U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012, 124, 1–21. [Google Scholar] [CrossRef]
- Dell’Orco, J.M.; Wasserman, A.H.; Chopra, R.; Ingram, M.A.; Hu, Y.S.; Singh, V.; Wulff, H.; Opal, P.; Orr, H.T.; Shakkottai, V.G. Neuronal Atrophy Early in Degenerative Ataxia Is a Compensatory Mechanism to Regulate Membrane Excitability. J. Neurosci. 2015, 35, 11292–11307. [Google Scholar] [CrossRef]
- Gennarino, V.A.; Singh, R.K.; White, J.J.; De Maio, A.; Han, K.; Kim, J.-Y.; Jafar-Nejad, P.; di Ronza, A.; Kang, H.; Sayegh, L.S.; et al. Pumilio1 Haploinsufficiency Leads to SCA1-like Neurodegeneration by Increasing Wild-Type Ataxin1 Levels. Cell 2015, 160, 1087–1098. [Google Scholar] [CrossRef]
- Cvetanovic, M.; Ingram, M.; Orr, H.; Opal, P. Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1. Neuroscience 2015, 289, 289–299. [Google Scholar] [CrossRef]
- Kim, J.H.; Lukowicz, A.; Qu, W.; Johnson, A.; Cvetanovic, M. Astroglia contribute to the pathogenesis of spinocerebellar ataxia Type 1 (SCA1) in a biphasic, stage-of-disease specific manner. Glia 2018, 66, 1972–1987. [Google Scholar] [CrossRef]
- Qu, W.; Johnson, A.; Kim, J.H.; Lukowicz, A.; Svedberg, D.; Cvetanovic, M. Inhibition of colony-stimulating factor 1 receptor early in disease ameliorates motor deficits in SCA1 mice. J. Neuroinflamm. 2017, 14, 107. [Google Scholar] [CrossRef]
- Tejwani, L.; Ravindra, N.G.; Lee, C.; Cheng, Y.; Nguyen, B.; Luttik, K.; Ni, L.; Zhang, S.; Morrison, L.M.; Gionco, J.; et al. Longitudinal single-cell transcriptional dynamics throughout neurodegeneration in SCA1. Neuron 2024, 112, 362–383.e15. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, S.J.B.; De Laat, W. CTCF: The protein, the binding partners, the binding sites and their chromatin loops. Phil. Trans. R. Soc. B 2013, 368, 20120369. [Google Scholar] [CrossRef] [PubMed]
- McConnell, B.B.; Yang, V.W. Mammalian Krüppel-Like Factors in Health and Diseases. Physiol. Rev. 2010, 90, 1337–1381. [Google Scholar] [CrossRef]
- Mariottini, C.; Munari, L.; Gunzel, E.; Seco, J.M.; Tzavaras, N.; Hansen, J.; Stern, S.A.; Gao, V.; Aleyasin, H.; Sharma, A.; et al. Wilm’s tumor 1 promotes memory flexibility. Nat. Commun. 2019, 10, 3756. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Tanabe, M.; Kawazoe, Y.; Inazawa, J.; Morimoto, R.I.; Nagata, K. HSF4, a New Member of the Human Heat Shock Factor Family Which Lacks Properties of a Transcriptional Activator. Mol. Cell. Biol. 1997, 17, 469–481. [Google Scholar] [CrossRef]
- López-Sánchez, N.; Garrido-García, A.; Ramón-Landreau, M.; Cano-Daganzo, V.; Frade, J.M. E2F4-Based Gene Therapy Mitigates the Phenotype of the Alzheimer’s Disease Mouse Model 5xFAD. Neurotherapeutics 2021, 18, 2484–2503. [Google Scholar] [CrossRef]
- Hoch-Kraft, P.; White, R.; Tenzer, S.; Krämer-Albers, E.-M.; Trotter, J.; Gonsior, C. Dual role of the RNA helicase DDX5 in post-transcriptional regulation of myelin basic protein in oligodendrocytes. J. Cell Sci. 2018, 131, jcs204750. [Google Scholar] [CrossRef]
- Li, F.; Ling, X.; Chakraborty, S.; Fountzilas, C.; Wang, J.; Jamroze, A.; Liu, X.; Kalinski, P.; Tang, D.G. Role of the DEAD-box RNA helicase DDX5 (p68) in cancer DNA repair, immune suppression, cancer metabolic control, virus infection promotion, and human microbiome (microbiota) negative influence. J. Exp. Clin. Cancer Res. 2023, 42, 213. [Google Scholar] [CrossRef]
- Duan, R.; Shi, Y.; Yu, L.; Zhang, G.; Li, J.; Lin, Y.; Guo, J.; Wang, J.; Shen, L.; Jiang, H.; et al. UBA5 Mutations Cause a New Form of Autosomal Recessive Cerebellar Ataxia. PLoS ONE 2016, 11, e0149039. [Google Scholar] [CrossRef]
- Gehman, L.T.; Meera, P.; Stoilov, P.; Shiue, L.; O’Brien, J.E.; Meisler, M.H.; Ares, M.; Otis, T.S.; Black, D.L. The splicing regulator Rbfox2 is required for both cerebellar development and mature motor function. Genes Dev. 2012, 26, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.; Jacobi, H.; Cook, A.; Labrum, R.; Durr, A.; Brice, A.; Charles, P.; Marelli, C.; Mariotti, C.; Nanetti, L.; et al. Survival in patients with spinocerebellar ataxia types 1, 2, 3, and 6 (EUROSCA): A longitudinal cohort study. Lancet Neurol. 2018, 17, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.P.; Marsden, C.D. Menstrual-related fluctuations in Parkinson’s disease. Mov. Disord. 1986, 1, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Diaz Brinton, R. Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology 2012, 153, 3571–3578. [Google Scholar] [CrossRef]
- Hamel, K.; Moncada, E.L.; Sheeler, C.; Rosa, J.-G.; Gilliat, S.; Zhang, Y.; Cvetanovic, M. Cerebellar Heterogeneity and Selective vulnerability in Spinocerebellar Ataxia Type 1 (SCA1). Neurobiol. Dis. 2024, 197, 106530. [Google Scholar] [CrossRef]
- Rosa, J.-G.; Hamel, K.; Sheeler, C.; Borgenheimer, E.; Gilliat, S.; Soles, A.; Ghannoum, F.J.; Sbrocco, K.; Handler, H.P.; Rainwater, O.; et al. Spatial and Temporal Diversity of Astrocyte Phenotypes in Spinocerebellar Ataxia Type 1 Mice. Cells 2022, 11, 3323. [Google Scholar] [CrossRef]
- Rosa, J.-G.; Hamel, K.; Soles, A.; Sheeler, C.; Borgenheimer, E.; Gilliat, S.; Sbrocco, K.; Ghanoum, F.; Handler, H.P.; Forster, C.; et al. BDNF is altered in a brain-region specific manner and rescues deficits in Spinocerebellar Ataxia Type 1. Neurobiol. Dis. 2023, 178, 106023. [Google Scholar] [CrossRef]
- Geller, S.E.; Koch, A.; Pellettieri, B.; Carnes, M. Inclusion, Analysis, and Reporting of Sex and Race/Ethnicity in Clinical Trials: Have We Made Progress? J. Women’s Health 2011, 20, 315–320. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selimovic, A.; Sbrocco, K.; Talukdar, G.; McCall, A.; Gilliat, S.; Zhang, Y.; Cvetanovic, M. Sex Differences in a Novel Mouse Model of Spinocerebellar Ataxia Type 1 (SCA1). Int. J. Mol. Sci. 2025, 26, 2623. https://doi.org/10.3390/ijms26062623
Selimovic A, Sbrocco K, Talukdar G, McCall A, Gilliat S, Zhang Y, Cvetanovic M. Sex Differences in a Novel Mouse Model of Spinocerebellar Ataxia Type 1 (SCA1). International Journal of Molecular Sciences. 2025; 26(6):2623. https://doi.org/10.3390/ijms26062623
Chicago/Turabian StyleSelimovic, Adem, Kaelin Sbrocco, Gourango Talukdar, Adri McCall, Stephen Gilliat, Ying Zhang, and Marija Cvetanovic. 2025. "Sex Differences in a Novel Mouse Model of Spinocerebellar Ataxia Type 1 (SCA1)" International Journal of Molecular Sciences 26, no. 6: 2623. https://doi.org/10.3390/ijms26062623
APA StyleSelimovic, A., Sbrocco, K., Talukdar, G., McCall, A., Gilliat, S., Zhang, Y., & Cvetanovic, M. (2025). Sex Differences in a Novel Mouse Model of Spinocerebellar Ataxia Type 1 (SCA1). International Journal of Molecular Sciences, 26(6), 2623. https://doi.org/10.3390/ijms26062623






