N-Acetylcysteine in the Treatment of Acute Lung Injury: Perspectives and Limitations
Abstract
1. Introduction
2. ALI and ARDS
2.1. Definitions of ALI and ARDS
2.2. Epidemiology and Mortality of ARDS
2.3. Causes and Risk Factors of ARDS
2.4. Major Pathomechanisms of ARDS
2.5. ARDS Due to Severe Viral Diseases and COVID-19
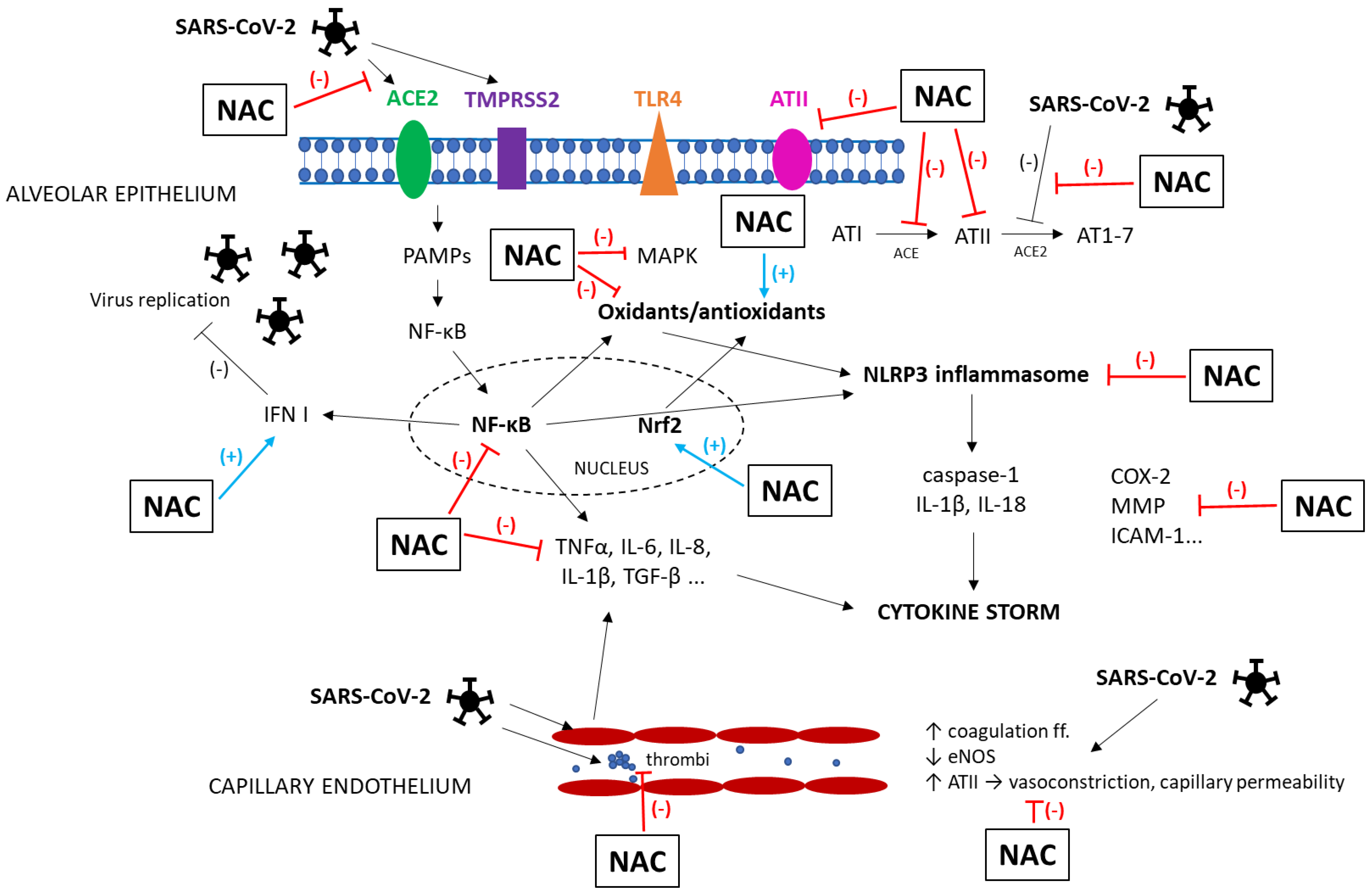
Pathophysiology of COVID-19
2.6. Standard Treatment of ARDS
3. Pharmacological Effects of NAC Potentially Beneficial in ARDS
4. NAC in Animal Models of ALI
4.1. Characteristics of Animal Models of Direct and Indirect ALI
4.2. NAC in Animal Models of Direct ALI
4.2.1. NAC in a Model of Bacterial Pneumonia-Induced ALI
4.2.2. NAC in Models of i.t. LPS-Induced ALI
4.2.3. NAC in a Model of Ventilator-Induced ALI
4.2.4. NAC in Models of Phosgene-Induced ALI
4.2.5. NAC in a Model of Meconium-Induced ALI
4.2.6. NAC in Models of Lung Contusion-Induced ALI
4.2.7. NAC in Models of Lung Ischemia/Reperfusion-Induced ALI
4.3. NAC in Animal Models of Indirect ALI
4.3.1. NAC in Models of i.p. or i.v. LPS-Induced ALI
4.3.2. NAC in Models of CLP-Sepsis-Induced ALI
4.3.3. NAC in Models of Hemorrhagic Shock-Induced ALI
4.3.4. NAC in a Model of Renal Ischemia/Reperfusion-Induced ALI
4.3.5. NAC in Models of OA-Induced ALI
4.3.6. NAC in a Model of Acute Pancreatitis-Induced ALI
5. NAC in Patients with ARDS
6. NAC in Patients with COVID-19
7. Limitations and Future Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzyme |
| AKI | acute kidney injury |
| ALI | acute lung injury |
| ARDS | acute respiratory distress syndrome |
| BALF | bronchoalveolar lavage fluid |
| CLP | ligation and perforation of the cecum |
| COVID-19 | Coronavirus disease 2019 |
| COX-2 | cyclooxygenase-2 |
| CRP | C-reactive protein |
| eNOS | Endothelial nitric oxide synthase |
| FiO2 | fraction of inspired oxygen |
| G6PD | glucose-6-phosphate dehydrogenase |
| GSH | glutathione |
| i.m. | intramuscular |
| i.p. | intraperitoneal |
| i.t. | intratracheal |
| i.v. | intravenous |
| ICAM-1 | intercellular adhesion molecule-1 |
| ICU | intensive care unit |
| IFN | interferon |
| IL | interleukin |
| iNOS | Inducible nitric oxide synthase |
| LIS | lung injury score |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MCP | monocyte chemoattractant protein |
| MDA | malondialdehyde |
| MMP | matrix metalloproteinases |
| MPO | myeloperoxidase |
| NAC | N-acetylcysteine |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NETs | neutrophil extracellular traps |
| NF-κB | nuclear factor-kappa B |
| NLRP3 | NLR family pyrin domain containing 3 |
| NO | nitric oxide |
| Nrf2 | nuclear factor erythroid-derived 2-like 2 |
| NT | nitrotyrosine |
| OA | oleic acid |
| OTZ | L-2-oxothiazolidine-4-carboxylate |
| p.o. | (per)oral |
| PAF | platelet-activating factor |
| PAI-1 | plasminogen activator inhibitor-1 |
| PAMPs | pathogen-associated molecular patterns |
| PaO2 | arterial partial pressure of oxygen |
| PEEP | positive end-expiratory pressure |
| RAGE | receptor for advanced glycation end products |
| RNA | Ribonucleic acid |
| ROS | reactive oxygen species |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus-2 |
| SOD | superoxide dismutase |
| SP | surfactant protein |
| TGF-β | transforming growth factor-beta |
| TLR | Toll-like receptor |
| TMPRSS | transmembrane protease serine |
| TNFα | tumor necrosis factor-alpha |
| VAP | ventilator-associated pneumonia |
| VEGF | vascular endothelial growth factor |
| VILI | ventilator-induced lung injury |
| vWF | von Willebrand factor |
References
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress in adults. Lancet 1967, 2, 319–323. [Google Scholar] [CrossRef]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149, 818–824. [Google Scholar] [CrossRef] [PubMed]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. J. Am. Med. Assoc. 2012, 307, 2526–2533. [Google Scholar]
- Mokrá, D. Acute lung injury—From pathophysiology to treatment. Physiol. Res. 2020, 69, S353–S366. [Google Scholar] [CrossRef]
- Seeley, E.J. Updates in the management of acute lung injury: A focus on the overlap between AKI and ARDS. Adv. Chronic Kidney Dis. 2013, 20, 14–20. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. J. Am. Med. Assoc. 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, C.L.; Zhuo, H.; Delucchi, K.; Deiss, T.; Liu, T.; Jauregui, A.; Ke, S.; Vessel, K.; Lippi, M.; Seeley, E.; et al. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 2020, 46, 1222–1231. [Google Scholar] [CrossRef]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Herridge, M.S.; Moss, M.; Hough, C.L.; Hopkins, R.O.; Rice, T.W.; Bienvenu, O.J.; Azoulay, E. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016, 42, 725–738. [Google Scholar] [CrossRef]
- Bein, T.; Weber-Carstens, S.; Apfelbacher, C. Long-term outcome after the acute respiratory distress syndrome: Different from general critical illness? Curr. Opin. Crit. Care 2018, 24, 35–40. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Park, M.; Turrini, C.; Tunstall, T.; Thwaites, R.; Mauri, T.; Ragazzi, R.; Ruggeri, P.; Hansel, T.T.; Caramori, G.; et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J. Inflamm. 2019, 16, 1. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Invest. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef]
- Cardinal-Fernández, P.; Bajwa, E.K.; Dominguez-Calvo, A.; Menéndez, J.M.; Papazian, L.; Thompson, B.T. The Presence of Diffuse Alveolar Damage on Open Lung Biopsy Is Associated with Mortality in Patients with Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Chest 2016, 149, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L.; Barkauskas, C.E.; Chapman, H.A.; Epstein, J.A.; Jain, R.; Hsia, C.C.; Niklason, L.; Calle, E.; Le, A.; Randell, S.H.; et al. Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell. Stem Cell 2014, 15, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.; Janz, D.R.; Bernard, G.R.; May, A.K.; Kangelaris, K.N.; Matthay, M.A.; Ware, L.B. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015, 147, 1539–1548. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- Famous, K.R.; Delucchi, K.; Ware, L.B.; Kangelaris, K.N.; Liu, K.D.; Thompson, B.T.; Calfee, C.S.; ARDS Network. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am. J. Respir. Crit. Care Med. 2017, 195, 331–338. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F.; et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Roche, L.; Mesta, F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox biology of respiratory viral infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef]
- Fraternale, A.; Zara, C.; De Angelis, M.; Nencioni, L.; Palamara, A.T.; Retini, M.; Di Mambro, T.; Magnani, M.; Crinelli, R. Intracellular Redox-Modulated Pathways as Targets for Effective Approaches in the Treatment of Viral Infection. Int. J. Mol. Sci. 2021, 22, 3603. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- du Preez, H.N.; Aldous, C.; Hayden, M.R.; Kruger, H.G.; Lin, J. Pathogenesis of COVID-19 described through the lens of an undersulfated and degraded epithelial and endothelial glycocalyx. FASEB J. 2022, 36, e22052. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Czajkowsky, D.M. SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor. Rev. 2022, 63, 44–57. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar]
- Karki, R.; Kanneganti, T.D. Innate immunity, cytokine storm, and inflammatory cell death in COVID-19. J. Transl. Med. 2022, 20, 542. [Google Scholar] [CrossRef]
- Itri, R.; Junqueira, H.C.; Mertins, O.; Baptista, M.S. Membrane changes under oxidative stress: The impact of oxidized lipids. Biophys. Rev. 2014, 6, 47–61. [Google Scholar] [CrossRef]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Di, B.; Xu, L.L. The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor. Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef]
- Norooznezhad, A.H.; Mansouri, K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19). Microvasc. Res 2021, 137, 104188. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Simani, L. Central nervous system manifestations of COVID-19: A systematic review. J. Neurol. Sci. 2020, 413, 116832. [Google Scholar] [CrossRef]
- Akanchise, T.; Angelova, A. Potential of Nano-Antioxidants and Nanomedicine for Recovery from Neurological Disorders Linked to Long COVID Syndrome. Antioxidants 2023, 12, 393. [Google Scholar] [CrossRef]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; D’Andrea, L.; Vitale, G.; Pesenti, A.; Gattinoni, L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1994, 149, 8–13. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Manthous, C.A.; Hall, J.B.; Olson, D.; Singh, M.; Chatila, W.; Pohlman, A.; Kushner, R.; Schmidt, G.A.; Wood, L.D. Effect of cooling on oxygen consumption in febrile critically ill patients. Am. J. Respir. Crit. Care Med. 1995, 151, 10–14. [Google Scholar] [CrossRef]
- Kress, J.P.; O’Connor, M.F.; Pohlman, A.S.; Olson, D.; Lavoie, A.; Toledano, A.; Hall, J.B. Sedation of critically ill patients during mechanical ventilation. A comparison of propofol and midazolam. Am. J. Respir. Crit. Care Med. 1996, 153, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Kaufman, D. The effects of neuromuscular paralysis on systemic and splanchnic oxygen utilization in mechanically ventilated patients. Chest 1996, 109, 1038–1042. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; de Boisblanc, B.; Connors, A.F., Jr.; Hite, R.D.; Harabin, A.L. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef]
- Lewis, S.R.; Pritchard, M.W.; Thomas, C.M.; Smith, A.F. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst. Rev. 2019, 7, CD004477. [Google Scholar] [CrossRef]
- Hussain, M.; Khurram Syed, S.; Fatima, M.; Shaukat, S.; Saadullah, M.; Alqahtani, A.M.; Alqahtani, T.; Bin Emran, T.; Alamri, A.H.; Barkat, M.Q.; et al. Acute Respiratory Distress Syndrome and COVID-19: A Literature Review. J. Inflamm. Res. 2021, 14, 7225–7242. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Tusman, G.; Berra, L.; Rodríguez-Suárez, P.; Suárez-Sipmann, F. Unsuccessful and Successful Clinical Trials in Acute Respiratory Distress Syndrome: Addressing Physiology-Based Gaps. Front. Physiol. 2021, 12, 774025. [Google Scholar] [CrossRef]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Urbanic Purkart, T.; Waltensdorfer, A.; Münch, A.; Warnkross, H.; et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. J. Am. Med. Assoc. 2014, 312, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Ginde, A.A.; Brower, R.G.; Caterino, J.M.; Finck, L.; Banner-Goodspeed, V.M.; Grissom, C.K.; Hayden, D.; Hough, C.L.; Hyzy, R.C.; et al. Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients. N. Engl. J. Med. 2019, 381, 2529–2540. [Google Scholar] [PubMed]
- Fowler, A.A., 3rd; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. J. Am. Med. Assoc. 2019, 322, 1261–1270. [Google Scholar]
- Labbani-Motlagh, Z.; Amini, S.; Aliannejad, R.; Sadeghi, A.; Shafiee, G.; Heshmat, R.; Jafary, M.; Talaschian, M.; Akhtari, M.; Jamshidi, A.; et al. High-dose Intravenous Vitamin C in Early Stages of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Double-blind, Randomized, Controlled Clinical Trial. J. Res. Pharm. Pract. 2022, 11, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Mokry, J.; Barosova, R.; Hanusrichterova, J. Advances in the Use of N-Acetylcysteine in Chronic Respiratory Diseases. Antioxidants 2023, 12, 1713. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Zhitkovich, A. N-Acetylcysteine: Antioxidant, Aldehyde Scavenger, and More. Chem. Res. Toxicol. 2019, 32, 1318–1319. [Google Scholar] [CrossRef]
- Izquierdo-Alonso, J.L.; Pérez-Rial, S.; Rivera, C.G.; Peces-Barba, G. N-acetylcysteine for prevention and treatment of COVID-19: Current state of evidence and future directions. J. Infect. Public. Health 2022, 15, 1477–1483. [Google Scholar] [CrossRef]
- Ji, L.; Liu, R.; Zhang, X.D.; Chen, H.L.; Bai, H.; Wang, X.; Zhao, H.L.; Liang, X.; Hai, C.X. N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal. Toxicol. 2010, 22, 535–542. [Google Scholar] [CrossRef]
- Oter, S.; Jin, S.; Cucullo, L.; Dorman, H.J. Oxidants and antioxidants: Friends or foes? Oxid. Antioxid. Med. Sci. 2012, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Martínez-Expósito, F.; Montero, P.; Roger, I.; Bayarri, M.A.; Ribera, P.; Oishi-Konari, M.N.; Alba-García, J.R.; Zapater, E.; Cortijo, J. N-acetylcysteine Reduces Inflammasome Activation Induced by SARS-CoV-2 Proteins In Vitro. Int. J. Mol. Sci. 2022, 23, 14518. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gon, Y.; Matsumoto, K.; Takeshita, I.; Horie, T. N-acetylcysteine attenuates TNF-alpha-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells. Br. J. Pharmacol. 2001, 132, 270–276. [Google Scholar] [CrossRef]
- Chen, J.; Reheman, A.; Gushiken, F.C.; Nolasco, L.; Fu, X.; Moake, J.L.; Ni, H.; López, J.A. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J. Clin. Invest. 2011, 121, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Lizarrondo, S.; Gakuba, C.; Herbig, B.A.; Repessé, Y.; Ali, C.; Denis, C.V.; Lenting, P.J.; Touzé, E.; Diamond, S.L.; Vivien, D.; et al. Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi. Circulation 2017, 136, 646–660. [Google Scholar] [CrossRef]
- Morris, G.; Bortolasci, C.C.; Puri, B.K.; Olive, L.; Marx, W.; O’Neil, A.; Athan, E.; Carvalho, A.; Maes, M.; Walder, K.; et al. Preventing the development of severe COVID-19 by modifying immunothrombosis. Life Sci. 2021, 264, 118617. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Rogliani, P.; Salvi, S.S.; Ora, J.; Matera, M.G. Use of Thiols in the Treatment of COVID-19: Current Evidence. Lung 2021, 199, 335–343. [Google Scholar] [CrossRef]
- Araki, S.; Dobashi, K.; Kubo, K.; Yamamoto, Y.; Asayama, K.; Shirahata, A. N-acetylcysteine attenuates TNF-α induced changes in secretion of interleukin-6, plasminogen activator inhibitor-1 and adiponectin from 3T3-L1 adipocytes. Life Sci. 2006, 79, 2405–2412. [Google Scholar] [CrossRef]
- Zuo, Y.; Warnock, M.; Harbaugh, A.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Knight, J.S.; Kanthi, Y.; Lawrence, D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021, 11, 1580. [Google Scholar] [CrossRef]
- Ullian, M.E.; Gelasco, A.K.; Fitzgibbon, W.R.; Beck, C.N.; Morinelli, T.A. N-acetylcysteine decreases angiotensin II receptor binding in vascular smooth muscle cells. J. Am. Soc. Nephrol. 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Capettini, L.S.; Montecucco, F.; Mach, F.; Stergiopulos, N.; Santos, R.A.; da Silva, R.F. Role of renin-angiotensin system in inflammation, immunity and aging. Curr. Pharm. Des. 2012, 18, 963–970. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; DiNicolantonio, J.J. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog. Cardiovasc. Dis. 2020, 63, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Bhattacharyya, S. Impact of thiol-disulfide balance on the binding of Covid-19 spike protein with Angiotensin Converting Enzyme 2 receptor. ACS. Omega 2020, 5, 16292–16298. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
- Mokra, D.; Mikolka, P.; Kosutova, P.; Calkovska, A. Experimental models of acute lung injury: Their advantages and limitations. Acta Med. Martin. 2020, 20, 90–102. [Google Scholar] [CrossRef]
- Blondonnet, R.; Constantin, J.M.; Sapin, V.; Jabaudon, M. A Pathophysiologic Approach to Biomarkers in Acute Respiratory Distress Syndrome. Dis. Markers 2016, 2016, 3501373. [Google Scholar] [CrossRef]
- Xu, J.F.; Qu, J.M.; Li, H.P. N-Acetylcysteine modulates acute lung injury induced by Pseudomonas aeruginosa in rats. Clin. Exp. Pharmacol. Physiol. 2011, 38, 345–351. [Google Scholar] [CrossRef]
- Leal, A.P.F.; Nieto Marín, V.; Cabistany, V.V.; Morales, J.; Buccini, D.F.; Franco, O.L. Applicability of mouse models for induction of severe acute lung injury. Pulm. Pharmacol. Ther. 2024, 86, 102316. [Google Scholar] [CrossRef]
- Demiralay, R.; Gürsan, N.; Ozbilim, G.; Erdogan, G.; Demirci, E. Comparison of the effects of erdosteine and N-acetylcysteine on apoptosis regulation in endotoxin-induced acute lung injury. J. Appl. Toxicol. 2006, 26, 301–308. [Google Scholar] [CrossRef]
- Lima Trajano, E.T.; Sternberg, C.; Caetano, M.; Santos Silva, M.A.; Porto, L.C.; Santos, J.C.; Ribeiro, M.L.; Magalhães, C.B.; Zin, W.A.; Benjamim, C.F.; et al. Endotoxin-induced acute lung injury is dependent upon oxidative response. Inhal. Toxicol. 2011, 23, 918–926. [Google Scholar] [CrossRef]
- Chen, H.; Ma, N.; Song, X.; Wei, G.; Zhang, H.; Liu, J.; Shen, X.; Zhuge, X.; Chang, G. Protective Effects of N-Acetylcysteine on Lipopolysaccharide-Induced Respiratory Inflammation and Oxidative Stress. Antioxidants 2022, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Kolomaznik, M.; Mikolka, P.; Hanusrichterova, J.; Kosutova, P.; Matasova, K., Jr.; Mokra, D.; Calkovska, A. N-Acetylcysteine in Mechanically Ventilated Rats with Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome: The Effect of Intravenous Dose on Oxidative Damage and Inflammation. Biomedicines 2021, 9, 1885. [Google Scholar] [CrossRef]
- Mokra, D.; Calkovska, A. Experimental models of acute lung injury in the newborns. Physiol. Res. 2017, 66, S187–S201. [Google Scholar] [CrossRef]
- Chen, C.; Guan, X.; Quinn, D.A.; Ouyang, B. N-Acetylcysteine Inhibits Ventilation-Induced Collagen Accumulation in the Rat Lung. Tohoku J. Exp. Med. 2015, 236, 255–261. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, L.; Shen, J. Phosgene-Induced acute lung injury: Approaches for mechanism-based treatment strategies. Front. Immunol. 2022, 13, 917395. [Google Scholar] [CrossRef] [PubMed]
- Rendell, R.; Fairhall, S.; Graham, S.; Rutter, S.; Auton, P.; Smith, A.; Perrott, R.; Jugg, B. Assessment of N-acetylcysteine as a therapy for phosgene-induced acute lung injury. Toxicol. Lett. 2018, 290, 145–152. [Google Scholar] [CrossRef]
- Mokra, D.; Drgova, A.; Petras, M.; Mokry, J.; Antosova, M.; Calkovska, A. N-acetylcysteine alleviates the meconium-induced acute lung injury. Adv. Exp. Med. Biol. 2015, 832, 59–67. [Google Scholar] [PubMed]
- Mokra, D.; Drgova, A.; Mokry, J.; Antosova, M.; Durdik, P.; Calkovska, A. N-acetylcysteine effectively diminished meconium-induced oxidative stress in adult rabbits. J. Physiol. Pharmacol. 2015, 66, 101–110. [Google Scholar]
- Mokra, D.; Tonhajzerova, I.; Pistekova, H.; Visnovcova, Z.; Drgova, A.; Mokry, J.; Calkovska, A. Cardiovascular effects of N-acetylcysteine in meconium-induced acute lung injury. Adv. Exp. Med. Biol. 2015, 832, 35–43. [Google Scholar]
- Požgain, Z.; Kristek, D.; Lovrić, I.; Kondža, G.; Jelavić, M.; Kocur, J.; Danilović, M. Pulmonary contusions after blunt chest trauma: Clinical significance and evaluation of patient management. Eur. J. Trauma. Emerg. Surg. 2018, 44, 773–777. [Google Scholar] [CrossRef]
- Türüt, H.; Ciralik, H.; Kilinc, M.; Ozbag, D.; Imrek, S.S. Effects of early administration of dexamethasone, N-acetylcysteine and aprotinin on inflammatory and oxidant-antioxidant status after lung contusion in rats. Injury 2009, 40, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Chavko, M.; Adeeb, S.; Ahlers, S.T.; McCarron, R.M. Attenuation of pulmonary inflammation after exposure to blast overpressure by N-acetylcysteine amide. Shock 2009, 32, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Inci, I.; Zhai, W.; Arni, S.; Hillinger, S.; Vogt, P.; Weder, W. N-acetylcysteine attenuates lung ischemia-reperfusion injury after lung transplantation. Ann. Thorac. Surg. 2007, 84, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Forgiarini, L.F.; Forgiarini, L.A., Jr.; da Rosa, D.P.; Silva, M.B.; Mariano, R.; Paludo Ade, O.; Andrade, C.F. N-acetylcysteine administration confers lung protection in different phases of lung ischaemia-reperfusion injury. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 894–899. [Google Scholar] [CrossRef]
- Kao, S.J.; Wang, D.; Lin, H.I.; Chen, H.I. N-acetylcysteine abrogates acute lung injury induced by endotoxin. Clin. Exp. Pharmacol. Physiol. 2006, 33, 33–40. [Google Scholar] [CrossRef]
- Baboolal, H.A.; Ichinose, F.; Ullrich, R.; Kawai, N.; Bloch, K.D.; Zapol, W.M. Reactive oxygen species scavengers attenuate endotoxin-induced impairment of hypoxic pulmonary vasoconstriction in mice. Anesthesiology 2002, 97, 1227–1233. [Google Scholar] [CrossRef]
- Demiralay, R.; Gürsan, N.; Erdem, H. Regulation of sepsis-induced apoptosis of pulmonary cells by posttreatment of erdosteine and N-aceylcysteine. Toxicology 2006, 228, 151–161. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, H.S.; Seo, K.H.; Na, J.O.; Kim, Y.H.; Uh, S.T.; Park, C.S.; Oh, M.H.; Lee, S.H.; Kim, Y.T. The effect of post-treatment N-acetylcysteine in LPS-induced acute lung injury of rats. Tuberc. Respir. Dis. 2012, 73, 22–31. [Google Scholar] [CrossRef]
- Campos, R.; Shimizu, M.H.; Volpini, R.A.; de Bragança, A.C.; Andrade, L.; Lopes, F.D.; Olivo, C.; Canale, D.; Seguro, A.C. N-acetylcysteine prevents pulmonary edema and acute kidney injury in rats with sepsis submitted to mechanical ventilation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L640–L650. [Google Scholar] [CrossRef]
- Le, J.W.; Sun, M.; Zhu, J.H.; Fan, H. Protective effect of N-acetylcysteine on acute lung injury in septic rats by inhibiting inflammation, oxidation, and apoptosis. Iran. J. Basic. Med. Sci. 2022, 25, 859–864. [Google Scholar]
- Ozdulger, A.; Cinel, I.; Koksel, O.; Cinel, L.; Avlan, D.; Unlu, A.; Okcu, H.; Dikmengil, M.; Oral, U. The protective effect of N-acetylcysteine on apoptotic lung injury in cecal ligation and puncture-induced sepsis model. Shock 2003, 19, 366–372. [Google Scholar] [CrossRef]
- Gürer, A.; Ozdoğan, M.; Gökakin, A.K.; Gömceli, I.; Gülbahar, O.; Arikök, A.T.; Kulaçoğlu, H.; Aydin, R. Tissue oxidative stress level and remote organ injury in two-hit trauma model of sequential burn injury and peritoneal sepsis are attenuated with N-acetylcysteine treatment in rats. Ulus. Travma Acil Cerrahi Derg. 2009, 15, 1–6. [Google Scholar] [PubMed]
- Pfeifer, R.; Andruszkow, J.H.; Busch, D.; Hoepken, M.; Barkatali, B.M.; Horst, K.; Pape, H.C.; Hildebrand, F. Development of a standardized trauma-related lung injury model. J. Surg. Res. 2015, 196, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Alkan, A.; Eroğlu, F.; Eroğlu, E.; Ergin, C.; Cerçi, C.; Alsancak, G. Protective effects of N-acetylcysteine and erdosteine on hemorrhagic shock-induced acute lung injury. Eur. J. Emerg. Med. 2006, 13, 281–285. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, Y.H.; Kim, K.; Lee, J.H.; Rim, K.P.; Kwon, W.Y.; Suh, G.J.; Rhee, J.E. Effect of N-acetylcysteine (NAC) on acute lung injury and acute kidney injury in hemorrhagic shock. Resuscitation 2013, 84, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Saad, K.R.; Saad, P.F.; Dantas Filho, L.; Brito, J.M.; Koike, M.K.; Zanoni, F.L.; Dolhnikoff, M.; Montero, E.F. Pulmonary impact of N-acetylcysteine in a controlled hemorrhagic shock model in rats. J. Surg. Res. 2013, 182, 108–115. [Google Scholar] [CrossRef]
- Azarkish, F.; Nematbakhsh, M.; Fazilati, M.; Talebi, A.; Pilehvarian, A.A.; Pezeshki, Z.; Moeini, M.; Mansouri, A.; Safari, T. N-acetylcysteine Prevents Kidney and Lung Disturbances in Renal Ischemia/Reperfusion Injury in Rat. Int. J. Prev. Med. 2013, 4, 1139–1146. [Google Scholar]
- Koksel, O.; Cinel, I.; Tamer, L.; Cinel, L.; Ozdulger, A.; Kanik, A.; Ercan, B.; Oral, U. N-acetylcysteine inhibits peroxynitrite-mediated damage in oleic acid-induced lung injury. Pulm. Pharmacol. Ther. 2004, 17, 263–270. [Google Scholar] [CrossRef]
- Kumar, S.; Bhagat, P.; Pandey, S.; Pandey, R. The Role of Antioxidant Agent (N-Acetylcysteine) in Oleic Acid-Induced Acute Lung Injury in a Rat Model. Cureus 2022, 14, e29478. [Google Scholar] [CrossRef]
- Bhatia, M.; Wong, F.L.; Cao, Y.; Lau, H.Y.; Huang, J.; Puneet, P.; Chevali, L. Pathophysiology of acute pancreatitis. Pancreatology 2005, 5, 132–144. [Google Scholar] [CrossRef]
- Yubero, S.; Ramudo, L.; Manso, M.A.; Collía, F.; De Dios, I. Evaluation of N-acetylcysteine treatment in acute pancreatitis-induced lung injury. Inflamm. Res. 2012, 61, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Suter, P.M.; Domenighetti, G.; Schaller, M.D.; Laverrière, M.C.; Ritz, R.; Perret, C. N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest 1994, 105, 190–194. [Google Scholar] [CrossRef]
- Bernard, G.R.; Wheeler, A.P.; Arons, M.M.; Morris, P.E.; Paz, H.L.; Russell, J.A.; Wright, P.E. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest 1997, 112, 164–172. [Google Scholar] [CrossRef]
- Domenighetti, G.; Suter, P.M.; Schaller, M.D.; Ritz, R.; Perret, C. Treatment with N-acetylcysteine during acute respiratory distress syndrome: A randomized, double-blind, placebo-controlled clinical study. J. Crit. Care 1997, 12, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Soltan-Sharifi, M.S.; Mojtahedzadeh, M.; Najafi, A.; Reza Khajavi, M.; Reza Rouini, M.; Moradi, M.; Mohammadirad, A.; Abdollahi, M. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: Evidence for underlying toxicological mechanisms. Hum. Exp. Toxicol. 2007, 26, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ju, Y.; Ma, Y.; Wang, T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: A randomized controlled trial. Medicine 2018, 97, e13087. [Google Scholar] [CrossRef] [PubMed]
- Sharafkhah, M.; Abdolrazaghnejad, A.; Zarinfar, N.; Mohammadbeigi, A.; Massoudifar, A.; Abaszadeh, S. Safety and efficacy of N-acetyl-cysteine for prophylaxis of ventilator-associated pneumonia: A randomized, double blind, placebo-controlled clinical trial. Med. Gas. Res. 2018, 8, 19–23. [Google Scholar]
- Ghorbi, M.; Rashidi, M.; Olapour, A.; Javaherforooshzadeh, F.; Akhondzadeh, R. Effect of N-Acetylcysteine on the treatment of acute respiratory distress syndrome in mechanically ventilated patients admitted to the intensive care unit. Med. J. Islam. Repub. Iran. 2021, 35, 87. [Google Scholar] [CrossRef]
- Mohanty, R.R.; Padhy, B.M.; Das, S.; Meher, B.R. Therapeutic potential of N-acetyl cysteine (NAC) in preventing cytokine storm in COVID-19: Review of current evidence. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2802–2807. [Google Scholar]
- Wong, K.K.; Lee, S.W.H.; Kua, K.P. N-Acetylcysteine as Adjuvant Therapy for COVID-19—A Perspective on the Current State of the Evidence. J. Inflamm. Res. 2021, 14, 2993–3013. [Google Scholar] [CrossRef]
- Di Marco, F.; Foti, G.; Corsico, A.G. Where are we with the use of N-acetylcysteine as a preventive and adjuvant treatment for COVID-19? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 715–721. [Google Scholar]
- Liu, T.H.; Wu, J.Y.; Huang, P.Y.; Tsai, Y.W.; Hsu, W.H.; Chuang, M.H.; Tang, H.J.; Lai, C.C. Clinical efficacy of N-acetylcysteine for COVID-19: A systematic review and meta-analysis of randomized controlled trials. Heliyon 2024, 10, e25179. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Perl, A.; Smith, D.; Lewis, T.; Kon, Z.; Goldenberg, R.; Yarta, K.; Staniloae, C.; Williams, M. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin. Immunol. 2020, 219, 108544. [Google Scholar] [CrossRef]
- de Alencar, J.C.G.; Moreira, C.L.; Müller, A.D.; Chaves, C.E.; Fukuhara, M.A.; da Silva, E.A.; Miyamoto, M.F.S.; Pinto, V.B.; Bueno, C.G.; Lazar Neto, F.; et al. Double-blind, Randomized, Placebo-controlled Trial with N-acetylcysteine for Treatment of Severe Acute Respiratory Syndrome Caused by Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 72, e736–e741. [Google Scholar] [CrossRef]
- Taher, A.; Lashgari, M.; Sedighi, L.; Rahimi-Bashar, F.; Poorolajal, J.; Mehrpooya, M. A pilot study on intravenous N-Acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome. Pharmacol. Rep. 2021, 73, 1650–1659. [Google Scholar] [CrossRef]
- Faverio, P.; Rebora, P.; Rossi, E.; Del Giudice, S.; Montanelli, F.; Garzillo, L.; Busnelli, S.; Luppi, F.; Valsecchi, M.G.; Pesci, A. Impact of N-acetyl-l-cysteine on SARS-CoV-2 pneumonia and its sequelae: Results from a large cohort study. ERJ. Open Res. 2021, 8, 00542–2021. [Google Scholar] [CrossRef] [PubMed]
- Avdeev, S.N.; Gaynitdinova, V.V.; Merzhoeva, Z.M.; Berikkhanov, Z.G. N-acetylcysteine for the treatment of COVID-19 among hospitalized patients. J. Infect. 2022, 84, 94–118. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Aretha, D.; Komninos, D.; Dimitropoulou, D.; Lagadinou, M.; Leonidou, L.; Oikonomou, I.; Mouzaki, A.; Marangos, M. N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: A two-center retrospective cohort study. Infect. Dis. 2021, 53, 847–854. [Google Scholar] [CrossRef]
- Izquierdo, J.L.; Soriano, J.B.; González, Y.; Lumbreras, S.; Ancochea, J.; Echeverry, C.; Rodríguez, J.M. Use of N-Acetylcysteine at high doses as an oral treatment for patients hospitalized with COVID-19. Sci. Prog. 2022, 105, 368504221074574. [Google Scholar] [CrossRef]
- Chavarría, A.P.; Vázquez, R.R.V.; Cherit, J.G.D.; Bello, H.H.; Suastegui, H.C.; Moreno-Castañeda, L.; Alanís Estrada, G.; Hernández, F.; González-Marcos, O.; Saucedo-Orozco, H.; et al. Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19. Comput. Struct. Biotechnol. J. 2021, 19, 1379–1390. [Google Scholar] [CrossRef]
- Atefi, N.; Goodarzi, A.; Riahi, T.; Khodabandehloo, N.; Talebi Taher, M.; Najar Nobari, N.; Seirafianpour, F.; Mahdi, Z.; Baghestani, A.; Valizadeh, R. Evaluation of the efficacy and safety of oral N-acetylcysteine in patients with COVID-19 receiving the routine antiviral and hydroxychloroquine protocol: A randomized controlled clinical trial. Immun. Inflamm. Dis. 2023, 11, e1083. [Google Scholar] [CrossRef]
- Panahi, Y.; Ghanei, M.; Rahimi, M.; Samim, A.; Vahedian-Azimi, A.; Atkin, S.L.; Sahebkar, A. Evaluation the efficacy and safety of N-acetylcysteine inhalation spray in controlling the symptoms of patients with COVID-19: An open-label randomized controlled clinical trial. J. Med. Virol. 2023, 95, e28393. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Puyo, C.A. N-acetylcysteine to combat COVID-19: An evidence review. Ther. Clin. Risk Manag. 2020, 16, 1047–1055. [Google Scholar] [CrossRef]
- Lai, K.Y.; Au, S.Y.; Sin, K.C.; Yung, S.K.; Leung, A.K.H. High-dose N-acetylcysteine in an immunocompromised patient with COVID-19: A case report. Hong. Kong Med. J. 2024, 30, 69–71. [Google Scholar]
- Liu, Y.; Wang, M.; Luo, G.; Qian, X.; Wu, C.; Zhang, Y.; Chen, B.; Leung, E.L.; Tang, Y. Experience of N-acetylcysteine airway management in the successful treatment of one case of critical condition with COVID-19: A case report. Medicine 2020, 99, e22577. [Google Scholar] [CrossRef] [PubMed]
- Bellone, S.; Siegel, E.R.; Santin, A.D. N-acetylcysteine (NAC) supplementation improves dyspnea and may normalize von Willebrand plasma levels in gynecologic patients with Post-Acute-COVID-Sequela (PASC)/Long COVID. Gynecol. Oncol. Rep. 2025, 57, 101682. [Google Scholar] [CrossRef]
- Carothers, C.; Birrer, K.; Vo, M. Acetylcysteine for the Treatment of Suspected Remdesivir-Associated Acute Liver Failure in COVID-19: A Case Series. Pharmacotherapy 2020, 40, 1166–1171. [Google Scholar] [CrossRef]
- Lu, X.; Ma, Y.; He, J.; Li, Y.; Zhu, H.; Yu, X. N-acetylcysteine for adults with acute respiratory distress syndrome: A meta-analysis of randomized controlled trials. Hong. Kong J. Emerg. Med. 2019, 26, 288–298. [Google Scholar] [CrossRef]
- Qiao, Q.; Liu, X.; Yang, T.; Cui, K.; Kong, L.; Yang, C.; Zhang, Z. Nanomedicine for acute respiratory distress syndrome: The latest application, targeting strategy, and rational design. Acta Pharm. Sin. B 2021, 11, 3060–3091. [Google Scholar] [CrossRef]
- Moss, D.M.; Curley, P.; Kinvig, H.; Hoskins, C.; Owen, A. The Biological Challenges and Pharmacological Opportunities of Orally Administered Nanomedicine Delivery. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 223–236. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Xiang, D.; Ju, L.; Shen, D.; Wang, X.; Wang, Y. Friend or Foe? The Roles of Antioxidants in Acute Lung Injury. Antioxidants 2021, 10, 1956. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Inorganics and hormesis. Crit. Rev. Toxicol. 2003, 33, 215–304. [Google Scholar] [CrossRef] [PubMed]
- Holdiness, M.R. Clinical pharmacokinetics of N-acetylcysteine. Clin. Pharmacokinet. 1991, 20, 123–134. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Lana, A.V.S.D.; Rodrigues, Q.S.; Santos, G.S.; Navani, R.; Navani, A.; da Fonseca, L.F.; Azzini, G.O.M.; Setti, T.; Mosaner, T.; et al. Nebulization of glutathione and N-Acetylcysteine as an adjuvant therapy for COVID-19 onset. Adv. Redox Res. 2021, 3, 100015. [Google Scholar] [CrossRef]
- Delić, N.; Matetic, A.; Domjanović, J.; Kljaković-Gašpić, T.; Šarić, L.; Ilić, D.; Došenović, S.; Domazet, J.; Kovač, R.; Runjić, F.; et al. Effects of Different Inhalation Therapy on Ventilator-Associated Pneumonia in Ventilated COVID-19 Patients: A Randomized Controlled Trial. Microorganisms 2022, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Murgia, X.; de Souza Carvalho, C.; Lehr, C.M. Overcoming the pulmonary barrier: New insights to improve the efficiency of inhaled therapeutics. Eur. J. Nanomed. 2014, 6, 157e69. [Google Scholar] [CrossRef]
- Ritter, C.; Andrades, M.E.; Reinke, A.; Menna-Barreto, S.; Moreira, J.C.; Dal-Pizzol, F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit. Care Med. 2004, 32, 342–349. [Google Scholar] [CrossRef]
- Ritter, C.; da Cunha, A.A.; Echer, I.C.; Andrades, M.; Reinke, A.; Lucchiari, N.; Rocha, J.; Streck, E.L.; Menna-Barreto, S.; Moreira, J.C.; et al. Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit. Care Med. 2006, 34, 471–477. [Google Scholar] [CrossRef]
- Kolomaznik, M.; Hanusrichterova, J.; Mikolka, P.; Kosutova, P.; Vatecha, M.; Zila, I.; Mokra, D.; Calkovska, A. Efficiency of exogenous surfactant combined with intravenous N-acetylcysteine in two-hit rodent model of ARDS. Respir. Physiol. Neurobiol. 2023, 316, 104138. [Google Scholar] [CrossRef]
- Kopincová, J.; Mokrá, D.; Mikolka, P.; Kolomazník, M.; Čalkovská, A. N-acetylcysteine advancement of surfactant therapy in experimental meconium aspiration syndrome: Possible mechanisms. Physiol. Res. 2014, 63, S629–S642. [Google Scholar] [CrossRef]
- Mikolka, P.; Kopincova, J.; Mikusiakova, L.T.; Kosutova, P.; Calkovska, A.; Mokra, D. Antiinflammatory Effect of N-Acetylcysteine Combined with Exogenous Surfactant in Meconium-Induced Lung Injury. Adv. Exp. Med. Biol. 2016, 934, 63–75. [Google Scholar]
- Kopincova, J.; Kolomaznik, M.; Mikolka, P.; Kosutova, P.; Topercerova, J.; Matasova, K., Jr.; Calkovska, A.; Mokra, D. Recombinant Human Superoxide Dismutase and N-Acetylcysteine Addition to Exogenous Surfactant in the Treatment of Meconium Aspiration Syndrome. Molecules 2019, 24, 905. [Google Scholar] [CrossRef] [PubMed]
- Wigenstam, E.; Koch, B.; Bucht, A.; Jonasson, S. N-acetyl cysteine improves the effects of corticosteroids in a mouse model of chlorine-induced acute lung injury. Toxicology 2015, 328, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Martini, N.; Singla, P.; Arbuckle, E.; Goyal, G.; Liu, Q.; Santos-Zabala, M.L.; Zainah, H. SARS-CoV-2-Induced Autoimmune Hepatitis. Cureus 2023, 15, e38932. [Google Scholar] [CrossRef] [PubMed]
- Leme, A.S.; Lichtenstein, A.; Arantes-Costa, F.M.; Landucci, E.C.; Martins, M.A. Acute lung injury in experimental pancreatitis in rats: Pulmonary protective effects of crotapotin and N-acetylcysteine. Shock 2002, 18, 428–433. [Google Scholar] [CrossRef]
- Guo, D.W.; Wang, C.Y.; Shih, H.C. N-acetylcysteine and atorvastatin alleviates lung injury due to ischemia-reperfusion injury in rats. J. Chin. Med. Assoc. 2019, 82, 909–914. [Google Scholar] [CrossRef]
- Song, Q.; Lin, L.; Chen, L.; Cheng, L.; Zhong, W. Co-administration of N-acetylcysteine and dexmedetomidine plays a synergistic effect on protection of LPS-induced acute lung injury via correcting Th1/Th2/Th17 cytokines imbalance. Clin. Exp. Pharmacol. Physiol. 2020, 47, 294–301. [Google Scholar] [CrossRef]
- Warren, B.; Royall, N.; Smith, H.; Bhullar, I.S. Novel Treatment of Acute Respiratory Distress Syndrome after Chlorine Gas Inhalation Injury. Am. Surg. 2016, 82, e219–e220. [Google Scholar] [CrossRef]
- Dube, K.M.; Ditch, K.L.; Hills, L. Use of Nebulized Heparin, Nebulized N-Acetylcysteine, and Nebulized Epoprostenol in a Patient with Smoke Inhalational Injury and Acute Respiratory Distress Syndrome. J. Pharm. Pract. 2017, 30, 663–667. [Google Scholar] [CrossRef]
- Shaikh, N.; Chanda, A.H.; Rahman, M.A.; Nainthramveetil, M.M.; Kumar, A.; Mathias, R.M.; Nashwan, A.J. Inhalational injury and use of heparin & N-acetylcysteine nebulization: A case report. Respir. Med. Case Rep. 2022, 37, 101640. [Google Scholar]
- Kim, S.; Kim, S.Y.; Rho, S.J.; Kim, S.H.; Song, S.H.; Kim, C.H.; Lee, H.; Kim, S.K. Biocompatible N-acetyl-nanoconstruct alleviates lipopolysaccharide-induced acute lung injury in vivo. Sci. Rep. 2021, 11, 22662. [Google Scholar] [CrossRef] [PubMed]
- Mitsopoulos, P.; Omri, A.; Alipour, M.; Vermeulen, N.; Smith, M.G.; Suntres, Z.E. Effectiveness of liposomal-N-acetylcysteine against LPS-induced lung injuries in rodents. Int. J. Pharm. 2008, 363, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Mitsopoulos, P.; Suntres, Z.E. Protective Effects of Liposomal N-Acetylcysteine against Paraquat-Induced Cytotoxicity and Gene Expression. J. Toxicol. 2011, 2011, 808967. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, H.; Zheng, D.; Sun, Y.; An, L.; Li, G.; Zhao, Z. Integrating network pharmacology and pharmacological evaluation to reveal the therapeutic effects and potential mechanism of S-allylmercapto-N-acetylcysteine on acute respiratory distress syndrome. Int. Immunopharmacol. 2023, 121, 110516. [Google Scholar] [CrossRef]
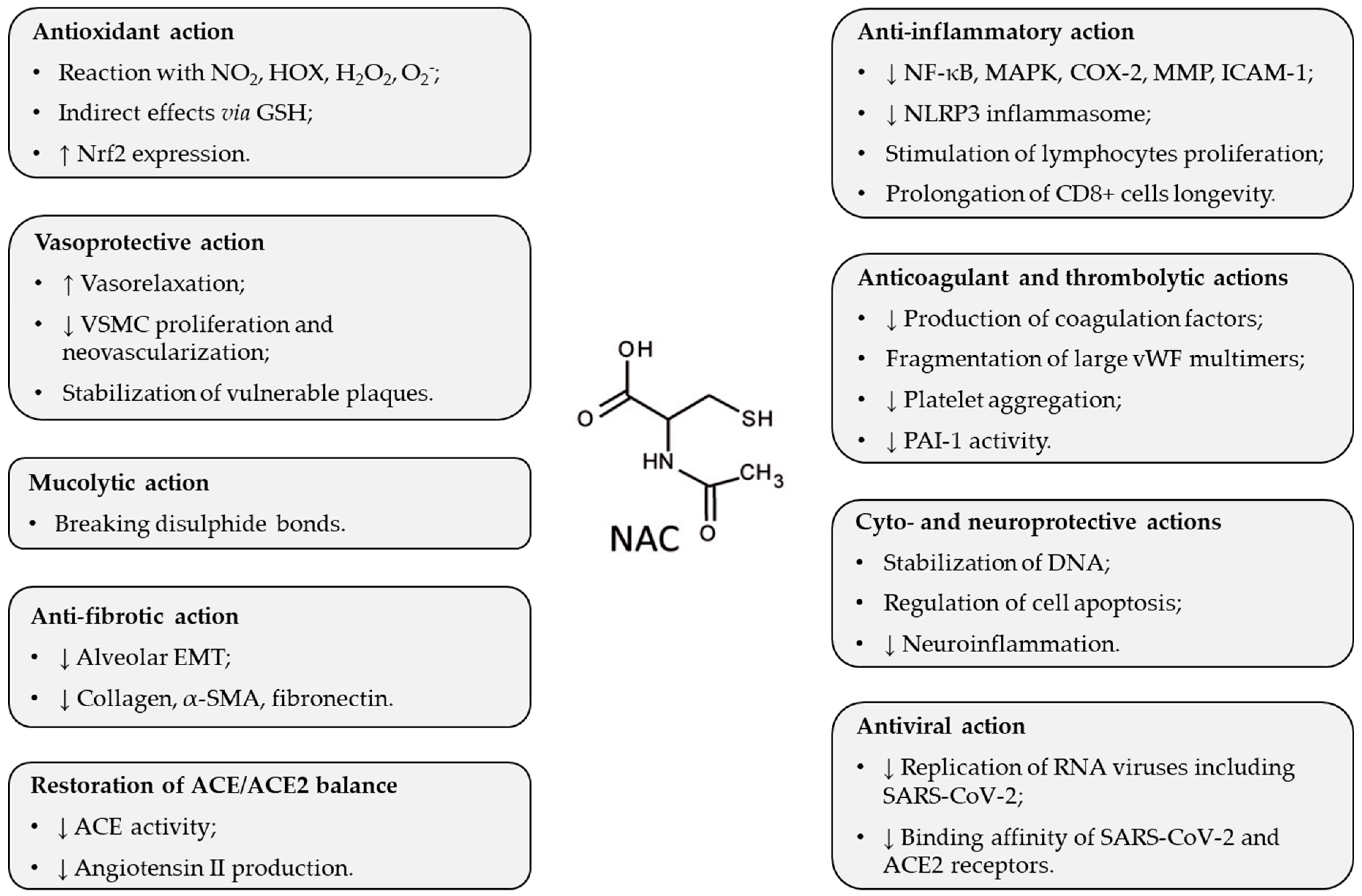
| Triggering Factor | Species | NAC Dose/Way of Delivery | Major Findings | Ref. |
|---|---|---|---|---|
| I.t. LPS | Wistar rats | NAC (10 mg/kg or 20 mg/kg i.v.) after elicitation of respiratory insufficiency | ↑ ventilatory parameters and oxygenation, ↓ lung edema, ↓ oxidative stress, ↓ inflammation | [82] |
| Phosgene inhalation | SD rats | NAC (50, 100, or 200 mg/kg i.p.) after exposure to phosgene | ↓ lung edema, ↓ markers of oxidative stress, ↑ Nrf2 | [60] |
| Phosgene inhalation | Juvenile pigs | NAC (cca 350 mg) given by nebulization 0.5, 2, 4, 6, 8, 10, and 12 h post-exposure | No improvement in survival, gas exchange, shunt fraction, lung edema, or histological score | [86] |
| Meconium instillation | Rabbits | NAC (10 mg/kg i.v.) 30 min after the induction of respiratory insufficiency | ↑ oxygenation, ↓ right-to-left pulmonary shunts, ↓ lung edema, ↓ oxidative stress, ↓ inflammation, and ↓ AW hyperreactivity | [87,88] |
| Lung contusion | SD rats | NAC (100 mg/kg i.p.) 30, 60 min, and 24 h after the blast damage | ↓ neutrophil infiltration of lungs and ↓blast-induced inflammatory response | [92] |
| Lung ischemia/reperfusion | Wistar rats | NAC (50 mg/kg i.v.) treatment before or after the insult | ↓ nitrotyrosine, cleaved caspase-3, NF-κB, TNFα, and IL-1β levels | [94] |
| Triggering Factor | Species | NAC Dose/Way of Delivery | Major Findings | Ref. |
|---|---|---|---|---|
| I.p. LPS | SV129/B6F1 mice | NAC (500 mg/kg i.p. or 100 mg/kg i.t. by aerosol) given 1 h and 22 h after i.p. LPS challenge | Early delivery of i.p. or i.t. NAC ↓ hypoxic pulmonary vasoconstriction | [96] |
| I.p. LPS | Wistar rats | NAC (150 mg/kg p.o.) following i.p. LPS | ↓ apoptosis of epithelial lung cells and ↓ TNFα, VEGF, and MPO | [97] |
| I.v. LPS | SD rats | NAC (20 mg/kg i.p.) given 3, 6, and 12 h after LPS i.v. injection | ↓ lipid peroxidation in the lung, ↓ MPO activity, ↓ NF-kB, and ↓ extent of lung injury | [98] |
| CLP sepsis | Wistar rats | NAC (150 mg/kg/day i.m.) initiated 6 h after operations, for 1 week | ↓ MPO activity, ↓ MDA in lung, improved histopathology, and ↓ apoptosis | [101] |
| Two-hit (burn + CLP) insult | Wistar rats | NAC (150 mg/kg/day i.p.), 72 h after CLP | ↓ MDA in liver and ileum, ↑ lung GSH, and ↓ lung injury score | [102] |
| Hemorrhagic shock | SD rats | NAC (150 mg/kg/h i.v.) initiated 15 min after the insult | ↓ MDA, ↓ nitrite/nitrate, ↓ IL-6, ↓ NF-κB p65 DNA activity, and improved histopathology | [105] |
| Hemorrhagic shock | Wistar rats | Addition of NAC (150 mg/kg) in resuscitation Ringer’s lactate solution | ↓ cell counts in BALF, ↓ MDA, and ↓ inflammatory infiltration | [106] |
| Subtype of ARDS | No. of Patients | NAC Dose/Way of Delivery | Major Findings/Outcomes | Ref. |
|---|---|---|---|---|
| Mild-to-moderate ARDS | NAC n = 32, placebo n = 29 | NAC (40 mg/kg/d i.v.) or placebo in controls, given for 3 days | ↑ oxygenation, regression of lung injury score, ↓ need for ventilation, and no adverse effects | [112] |
| ARDS requiring mechanical ventilation | NAC n = 14, OTZ n = 17, placebo n = 15 | NAC (70 mg/kg) or procystein (OTZ, 63 mg/kg), or placebo given by i.v. infusion every 8 h for 10 days | Repletion of GSH in red blood cells, ↑ cardiac index, and ↓ number of days with ARDS | [113] |
| ARDS requiring mechanical ventilation | NAC n = 22, placebo n = 20 | NAC (190 mg/kg/d) or placebo, continuous i.v. infusion, for the first 3 days | ↓ lung injury score, no improvement in oxygenation, and no reduction in the need for ventilation | [114] |
| ARDS requiring mechanical ventilation | NAC n = 17, NAC-nontreated n = 10 | NAC (150 mg/kg i.v. on the first day followed by 50 mg/kg/day for 3 days) and controls obtained the standard therapy | ↑ extracellular total antioxidant power, ↑ total thiols, ↑ GSH, and improved outcome | [115] |
| Community-acquired pneumonia | NAC n = 37, NAC-nontreated n = 24 | NAC (600 mg tablets, a dose of 1200 mg/d p.o., for 10 days) + conventional therapy and controls treated by conventional therapy | ↓ plasma MDA and TNFα, ↑ total antioxidant capacity, no effect on SOD, and no improvement in CT | [116] |
| Ventilator-associated pneumonia | NAC n = 30, NAC-nontreated n = 30 | NAC (600 mg) given twice daily via nasogastric tube in addition to routine care | ↓ development of clinically confirmed pneumonia, shorter stay in ICU, and more patients with complete recovery | [117] |
| ARDS requiring mechanical ventilation | NAC n = 30, NAC-nontreated n = 30 | NAC (150 mg/kg on the day 1 of admission, then 50 mg/kg up to day 4 of admission) and control group given routine care without NAC | Improved level of consciousness, oxygenation, and PEEP within 3–4 days of intervention | [118] |
| No. of Patients | NAC Dose/Way of Delivery | Major Findings/Outcomes | Ref. |
|---|---|---|---|
| n = 10 | One G6PD-deficient patient: 30,000 mg i.v. NAC in 2 days, one patient: 20,000 mg i.v. NAC in 2 days, and additional eight patients: 600 mg i.v. NAC every 12 h | ↓ liver enzymes, CRP, and ferritin in G6PD-deficient patient; improved clinical status; and ↓ inflammatory markers (CRP and ferritin) | [123] |
| n = 135 | NAC in a single dose of 21 g (300 mg/kg) for 20 h | No significant improvement in mortality, duration of mechanical ventilation, or need for ICU admission | [124] |
| n = 47 | NAC (40 mg/kg/day i.v.) for 3 consecutive days in addition to standard treatment | Slight improvement in the patients’ condition, but no differences in mortality, need for ventilation, or hospital stay | [125] |
| n = 585 | NAC for at least 5 days, initially a dose of 300 mg i.v. three times a day, then reduced to 600 mg p.o. twice a day after the patient’s condition stabilized | Shorter hospitalization period, but no differences in mortality or ICU admission | [126] |
| n = 24 | NAC i.v. at a daily dose of 1200–1800 mg | Improvements in oxygenation, CRP, and NEWS2 score, but no differences in mortality, need for ventilation or ICU admission | [127] |
| n = 42 | NAC (600 mg p.o. twice a day) to a standard treatment for 14 days | ↓ disease progression, ↑ oxygenation, ↓ blood leukocytes, ↓ CRP, D-dimers, LDH, and ↓ mortality and need for ventilation | [128] |
| n = 2071 | NAC (600 mg p.o. every 8 h) added to standard therapy | ↓ mortality, but no differences in need for ventilation, ICU admission, or duration of hospitalization | [129] |
| n = 125 | NAC inhaler spray (one puff per 12 h, for 7 days) + routine treatment | ↓ mortality, ↓ leukocyte count, ↓ CRP, and ↓ AST, but no differences in ICU admission or duration of hospitalization | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokra, D.; Porvaznik, I.; Mokry, J. N-Acetylcysteine in the Treatment of Acute Lung Injury: Perspectives and Limitations. Int. J. Mol. Sci. 2025, 26, 2657. https://doi.org/10.3390/ijms26062657
Mokra D, Porvaznik I, Mokry J. N-Acetylcysteine in the Treatment of Acute Lung Injury: Perspectives and Limitations. International Journal of Molecular Sciences. 2025; 26(6):2657. https://doi.org/10.3390/ijms26062657
Chicago/Turabian StyleMokra, Daniela, Igor Porvaznik, and Juraj Mokry. 2025. "N-Acetylcysteine in the Treatment of Acute Lung Injury: Perspectives and Limitations" International Journal of Molecular Sciences 26, no. 6: 2657. https://doi.org/10.3390/ijms26062657
APA StyleMokra, D., Porvaznik, I., & Mokry, J. (2025). N-Acetylcysteine in the Treatment of Acute Lung Injury: Perspectives and Limitations. International Journal of Molecular Sciences, 26(6), 2657. https://doi.org/10.3390/ijms26062657






