Carbon Monoxide and Prokaryotic Energy Metabolism
Abstract
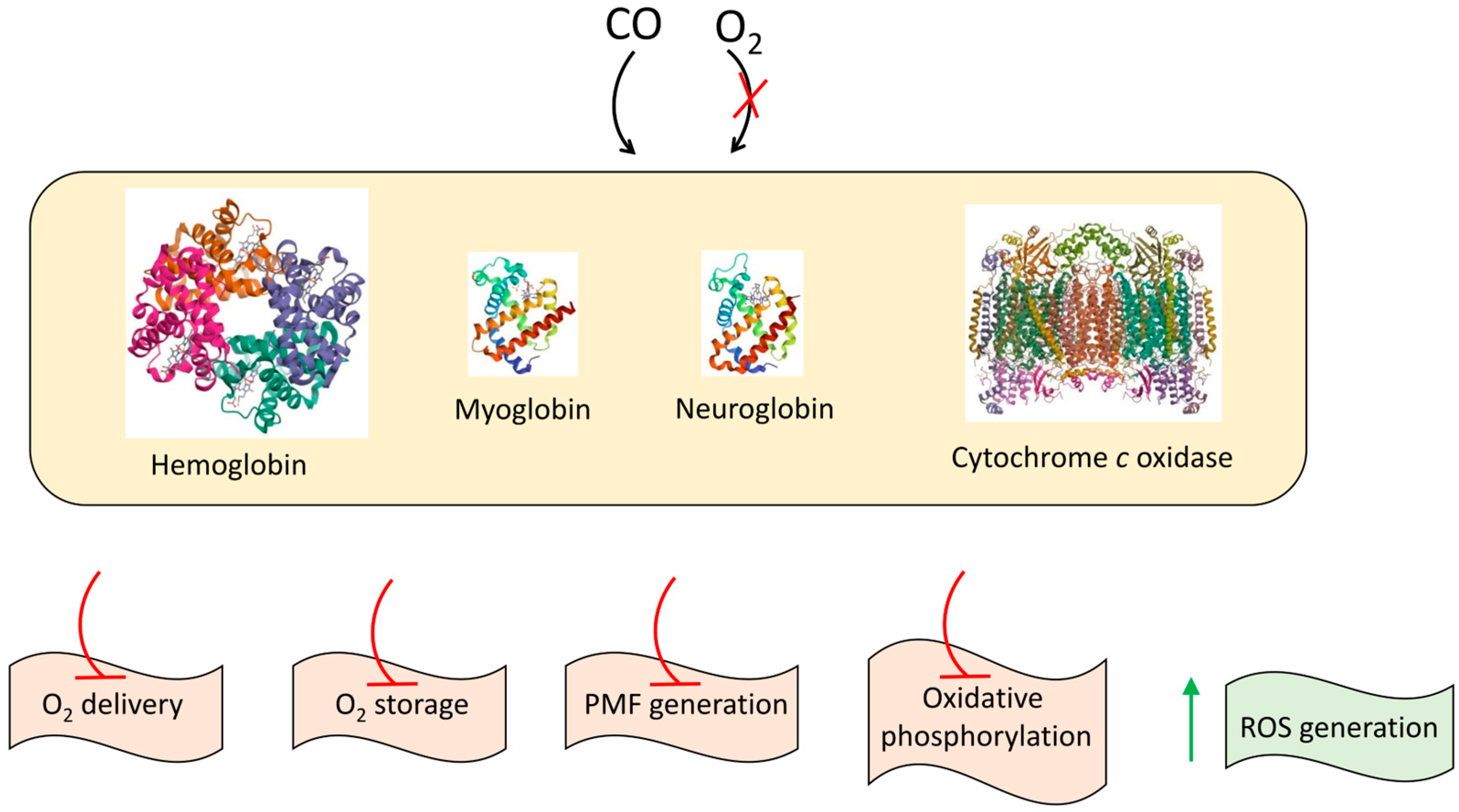
1. Introduction
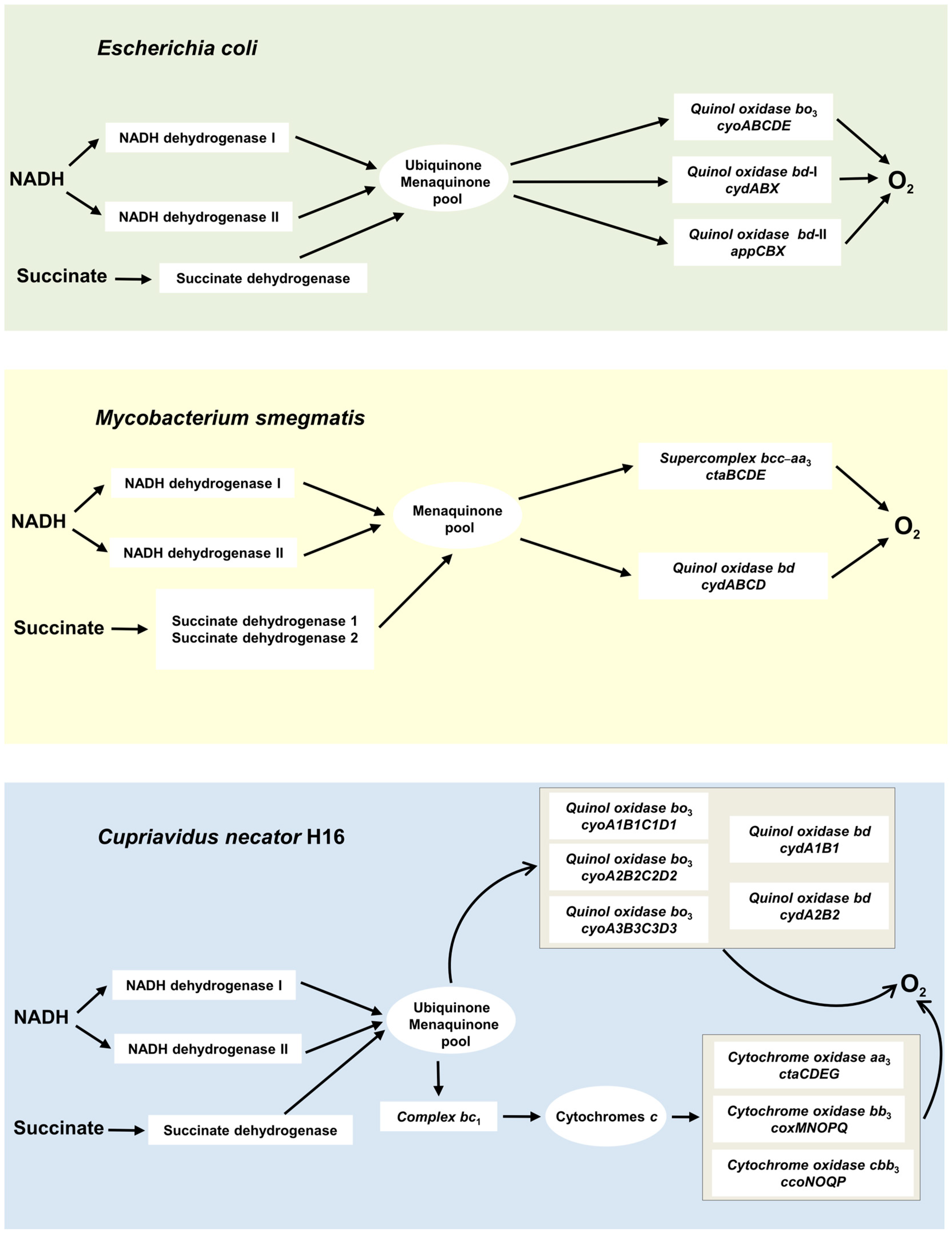
2. Two Superfamilies of Terminal Oxidases
3. Effect of CO on Bacterial Growth and Aerobic Respiration
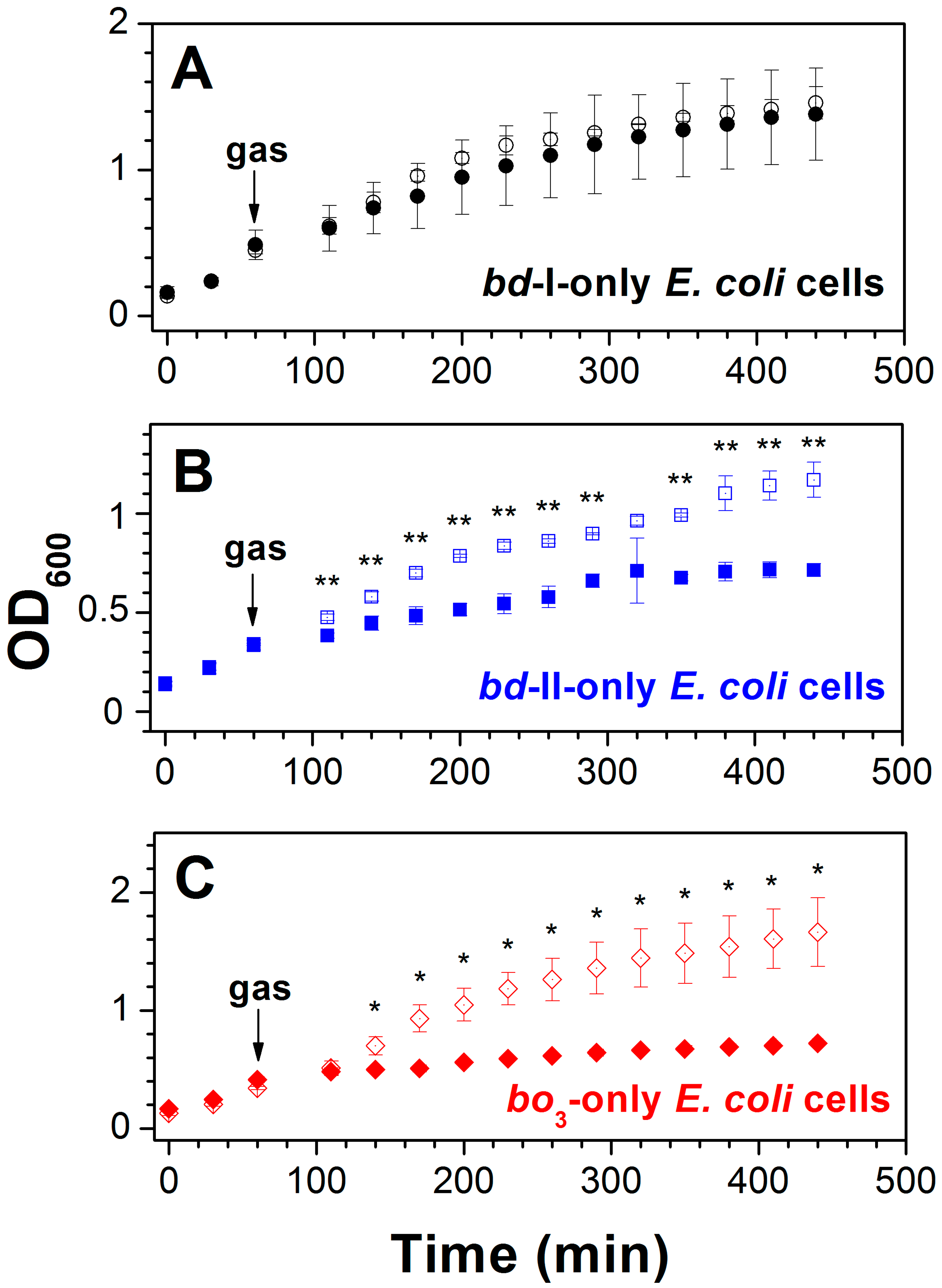
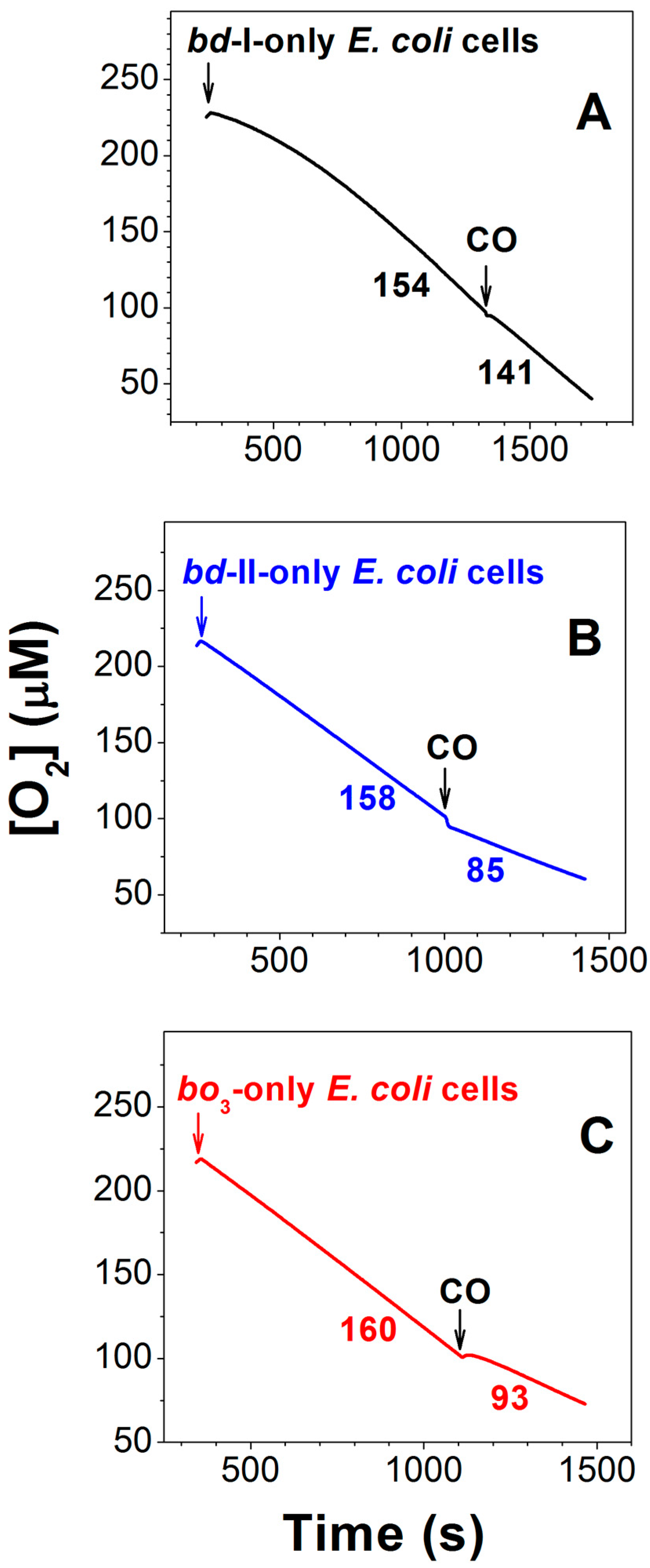
3.1. Effect of CO on E. coli Cell Growth and Aerobic Respiration
3.2. Effect of CO on M. smegmatis Cell Growth and Aerobic Respiration
3.3. Two bd-Type Terminal Oxidases of C. necator H16 Are Differently Sensitive to CO
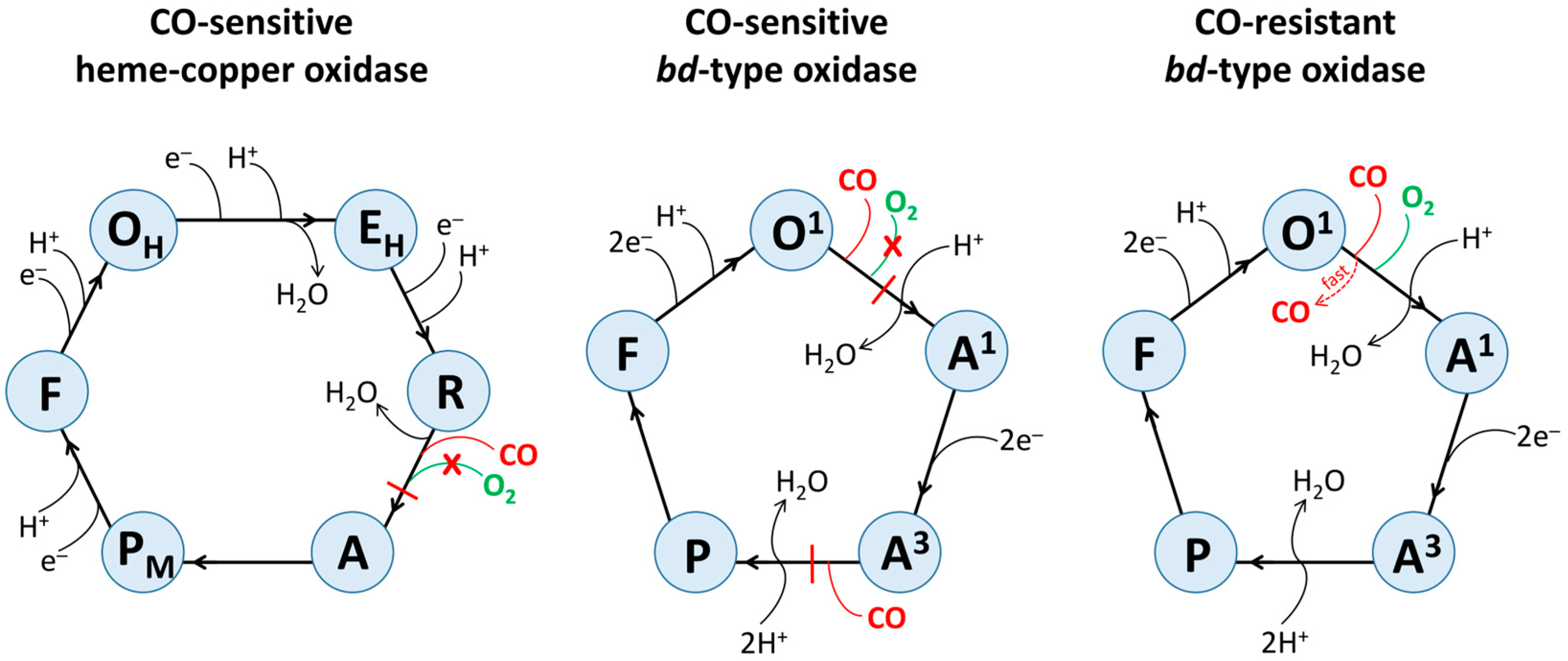
4. Possible Mechanisms Underlying Inhibitory Effects of CO on Different Terminal Oxidases
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BNC | binuclear center |
| CO | carbon monoxide |
| CODH | CO dehydrogenase |
| CORM | carbon monoxide-releasing molecule |
| ECH | energy-converting hydrogenase |
| IC50 | half-maximal inhibitory concentration |
| Ki | inhibition constant |
| OD | optical density |
| PMF | proton motive force |
References
- Delvau, N.; Elens, L.; Penaloza, A.; Liistro, G.; Thys, F.; Roy, P.M.; Gianello, P.; Hantson, P. Carboxyhemoglobin half-life toxicokinetic profiles during and after normobaric oxygen therapy: On a swine model. Toxicol. Rep. 2024, 12, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Myers, I.; Angelova, P.R. Carbon Monoxide: A Pleiotropic Redox Regulator of Life and Death. Antioxidants 2024, 13, 1121. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, I.; Zatsepin, N.A.; Hikita, M.; Conrad, C.E.; Nelson, G.; Coe, J.D.; Basu, S.; Grant, T.D.; Seaberg, M.H.; Sierra, R.G.; et al. Crystal structure of CO-bound cytochrome c oxidase determined by serial femtosecond X-ray crystallography at room temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 8011–8016. [Google Scholar] [CrossRef]
- Petersen, L.C. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim. Biophys. Acta 1977, 460, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.G.; Nicholls, P.; Wilson, M.T.; Cooper, C.E. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2006, 103, 708–713. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y. Mechanisms and therapeutic targets of carbon monoxide poisoning: A focus on reactive oxygen species. Chem. Biol. Interact. 2024, 403, 111223. [Google Scholar] [CrossRef]
- Katsnelson, A. The Good Side of Carbon Monoxide. ACS Cent. Sci. 2019, 5, 1632–1635. [Google Scholar] [CrossRef]
- Wareham, L.K.; Southam, H.M.; Poole, R.K. Do nitric oxide, carbon monoxide and hydrogen sulfide really qualify as ‘gasotransmitters’ in bacteria? Biochem. Soc. Trans. 2018, 46, 1107–1118. [Google Scholar] [CrossRef]
- Yuan, Z.; De La Cruz, L.K.; Yang, X.; Wang, B. Carbon monoxide signaling: Examining its engagement with various molecular targets in the context of binding affinity, concentration, and biologic response. Pharmacol. Rev. 2022, 74, 823–873. [Google Scholar] [CrossRef]
- Makino, R.; Obata, Y.; Tsubaki, M.; Iizuka, T.; Hamajima, Y.; Kato-Yamada, Y.; Mashima, K.; Shiro, Y. Mechanistic Insights into the Activation of Soluble Guanylate Cyclase by Carbon Monoxide: A Multistep Mechanism Proposed for the BAY 41-2272 Induced Formation of 5-Coordinate CO-Heme. Biochemistry 2018, 57, 1620–1631. [Google Scholar] [CrossRef]
- Kabe, Y.; Yamamoto, T.; Kajimura, M.; Sugiura, Y.; Koike, I.; Ohmura, M.; Nakamura, T.; Tokumoto, Y.; Tsugawa, H.; Handa, H.; et al. Cystathionine beta-synthase and PGRMC1 as CO sensors. Free Radic. Biol. Med. 2016, 99, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Sato, E.; Sato, A.; Sagami, I.; Shimizu, T.; Kitagawa, T. CO-dependent activity-controlling mechanism of heme-containing CO-sensor protein, neuronal PAS domain protein 2. J. Biol. Chem. 2005, 280, 21358–21368. [Google Scholar] [CrossRef]
- Kapetanaki, S.M.; Burton, M.J.; Basran, J.; Uragami, C.; Moody, P.C.E.; Mitcheson, J.S.; Schmid, R.; Davies, N.W.; Dorlet, P.; Vos, M.H.; et al. A mechanism for CO regulation of ion channels. Nat. Commun. 2018, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Schelvis, J.P.M.; Chen, Z.; Messina, M.A.; Catalano, J. Effect of CO binding to P450 BM3 F393 mutants on electron density distribution in the heme cofactor. J. Inorg. Biochem. 2024, 259, 112660. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Losada, P.A. Direct Inhibitory Effects of Carbon Monoxide on Six Venoms Containing Fibrinogenolytic Metalloproteinases. Basic Clin. Pharmacol. Toxicol. 2017, 120, 207–212. [Google Scholar] [CrossRef]
- Bae, H.; Kim, T.; Lim, I. Carbon monoxide activates large-conductance calcium-activated potassium channels of human cardiac fibroblasts through various mechanisms. Korean J. Physiol. Pharmacol. 2021, 25, 227–237. [Google Scholar] [CrossRef]
- Klemz, R.; Reischl, S.; Wallach, T.; Witte, N.; Jurchott, K.; Klemz, S.; Lang, V.; Lorenzen, S.; Knauer, M.; Heidenreich, S.; et al. Reciprocal regulation of carbon monoxide metabolism and the circadian clock. Nat. Struct. Mol. Biol. 2017, 24, 15–22. [Google Scholar] [CrossRef]
- Peng, Y.J.; Zhang, X.; Gridina, A.; Chupikova, I.; McCormick, D.L.; Thomas, R.J.; Scammell, T.E.; Kim, G.; Vasavda, C.; Nanduri, J.; et al. Complementary roles of gasotransmitters CO and H2S in sleep apnea. Proc. Natl. Acad. Sci. USA 2017, 114, 1413–1418. [Google Scholar] [CrossRef]
- Rahman, F.U.; Park, D.R.; Joe, Y.; Jang, K.Y.; Chung, H.T.; Kim, U.H. Critical Roles of Carbon Monoxide and Nitric Oxide in Ca2+ Signaling for Insulin Secretion in Pancreatic Islets. Antioxid. Redox Signal. 2019, 30, 560–576. [Google Scholar] [CrossRef]
- Hopper, C.P.; De La Cruz, L.K.; Lyles, K.V.; Wareham, L.K.; Gilbert, J.A.; Eichenbaum, Z.; Magierowski, M.; Poole, R.K.; Wollborn, J.; Wang, B. Role of carbon monoxide in host-gut microbiome communication. Chem. Rev. 2020, 120, 13273–13311. [Google Scholar] [CrossRef]
- Wang, M.; Liao, W. Carbon Monoxide as a Signaling Molecule in Plants. Front. Plant Sci. 2016, 7, 572. [Google Scholar] [CrossRef]
- Otterbein, L.E.; May, A.; Chin, B.Y. Carbon monoxide increases macrophage bacterial clearance through Toll-like receptor (TLR)4 expression. Cell. Mol. Biol. 2005, 51, 433–440. [Google Scholar] [PubMed]
- Wegiel, B.; Larsen, R.; Gallo, D.; Chin, B.Y.; Harris, C.; Mannam, P.; Kaczmarek, E.; Lee, P.J.; Zuckerbraun, B.S.; Flavell, R.; et al. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J. Clin. Investig. 2014, 124, 4926–4940. [Google Scholar] [CrossRef]
- Chung, S.W.; Liu, X.; Macias, A.A.; Baron, R.M.; Perrella, M.A. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Investig. 2008, 118, 239–247. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.J.; Coronata, A.A.; Fredenburgh, L.E.; Chung, S.W.; Perrella, M.A.; Nakahira, K.; Ryter, S.W.; Choi, A.M. Carbon monoxide confers protection in sepsis by enhancing beclin 1-dependent autophagy and phagocytosis. Antioxid. Redox Signal. 2014, 20, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.; Ghanta, S.; Li, Q.; Lamattina, A.M.; Murzin, E.; Lederer, J.A.; El-Chemaly, S.; Chung, S.W.; Liu, X.; Perrella, M.A. Carbon monoxide-induced autophagy enhances human mesenchymal stromal cell function via paracrine actions in murine polymicrobial sepsis. Mol. Ther. 2024, 32, 2232–2247. [Google Scholar] [CrossRef]
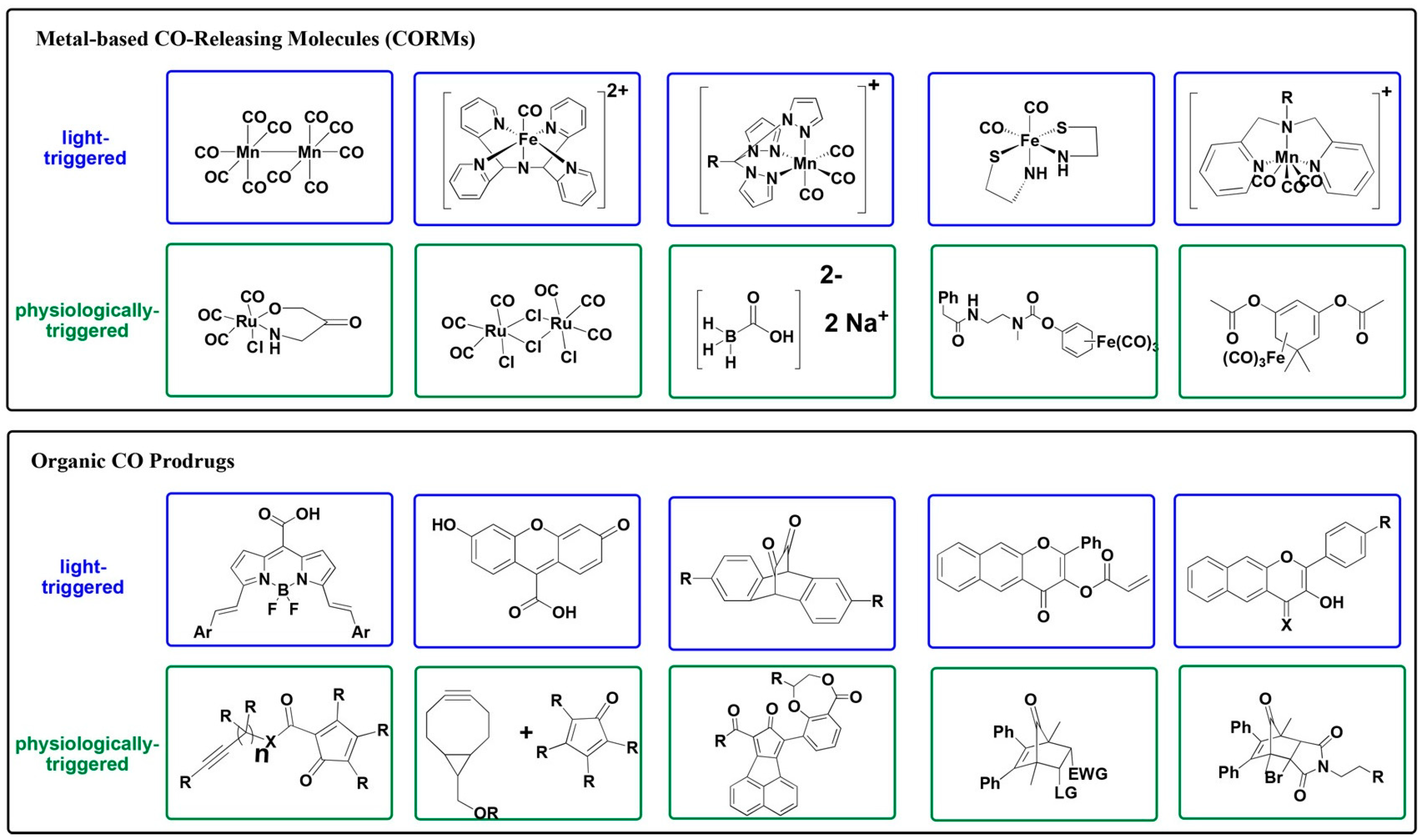
- Ismailova, A.; Kuter, D.; Bohle, D.S.; Butler, I.S. An Overview of the Potential Therapeutic Applications of CO-Releasing Molecules. Bioinorg. Chem. Appl. 2018, 2018, 8547364. [Google Scholar] [CrossRef]
- Davidge, K.S.; Sanguinetti, G.; Yee, C.H.; Cox, A.G.; McLeod, C.W.; Monk, C.E.; Mann, B.E.; Motterlini, R.; Poole, R.K. Carbon monoxide-releasing antibacterial molecules target respiration and global transcriptional regulators. J. Biol. Chem. 2009, 284, 4516–4524. [Google Scholar] [CrossRef]
- Tavares, A.F.; Parente, M.R.; Justino, M.C.; Oleastro, M.; Nobre, L.S.; Saraiva, L.M. The bactericidal activity of carbon monoxide-releasing molecules against Helicobacter pylori. PLoS ONE 2013, 8, e83157. [Google Scholar] [CrossRef]
- Wareham, L.K.; McLean, S.; Begg, R.; Rana, N.; Ali, S.; Kendall, J.J.; Sanguinetti, G.; Mann, B.E.; Poole, R.K. The broad-spectrum antimicrobial potential of [Mn(CO)4(S2CNMe(CH2CO2H))], a water-soluble CO-releasing molecule (CORM-401): Intracellular accumulation, transcriptomic and statistical analyses, and membrane polarization. Antioxid. Redox Signal. 2018, 28, 1286–1308. [Google Scholar] [CrossRef]
- Mansour, A.M.; Khaled, R.M.; Ferraro, G.; Shehab, O.R.; Merlino, A. Metal-based carbon monoxide releasing molecules with promising cytotoxic properties. Dalton Trans. 2024, 53, 9612–9656. [Google Scholar] [CrossRef]
- Hanson, M.G.; Ambre, R.; Joshi, R.; Amidon, J.D.; Snow, J.B.; Lawless, V.C.; Worrell, B.T. Visible Light Triggerable CO Releasing Micelles. J. Am. Chem. Soc. 2024, 146, 35029–35034. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.G.; Liu, W.; Ke, C.; Zhao, Y.Q.; Xu, X. A “turn-on” red cyclometalated iridium (III) complex for long-term tracking the diffusion of CORM-2 in cells and zebrafish. Anal. Chim. Acta 2024, 1288, 342153. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, J.; He, R.; Chen, K.; Zhang, J.; Liu, X.; Hu, J.; Lu, Y. Red light-induced localized release of carbon monoxide for alleviating postoperative cognitive dysfunction. Biomaterials 2025, 312, 122744. [Google Scholar] [CrossRef]
- Choi, Y.K.; Maki, T.; Liang, A.C.; Hayakawa, K.; Koh, S.H.; Kim, Y.M.; Whalen, M.J.; Seo, J.H.; Lok, J.; Gelman, I.H.; et al. A-kinase anchor protein 12 promotes oligodendrogenesis and cognitive recovery in carbon monoxide therapy for traumatic brain injury. J. Cereb. Blood Flow Metab. 2025, 271678X251314371. [Google Scholar] [CrossRef]
- Southam, H.M.; Smith, T.W.; Lyon, R.L.; Liao, C.; Trevitt, C.R.; Middlemiss, L.A.; Cox, F.L.; Chapman, J.A.; El-Khamisy, S.F.; Hippler, M.; et al. A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3. Redox Biol. 2018, 18, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Yang, W.; Zhang, Y.; Chen, Y.; Ding, T.; Chen, Y.; Wang, F.; Rosenholm, J.; Li, Y.; Zhang, H.; et al. In situ generating CO gas for destroying bacterial biofilms. Nano Today 2024, 56, 102296. [Google Scholar] [CrossRef]
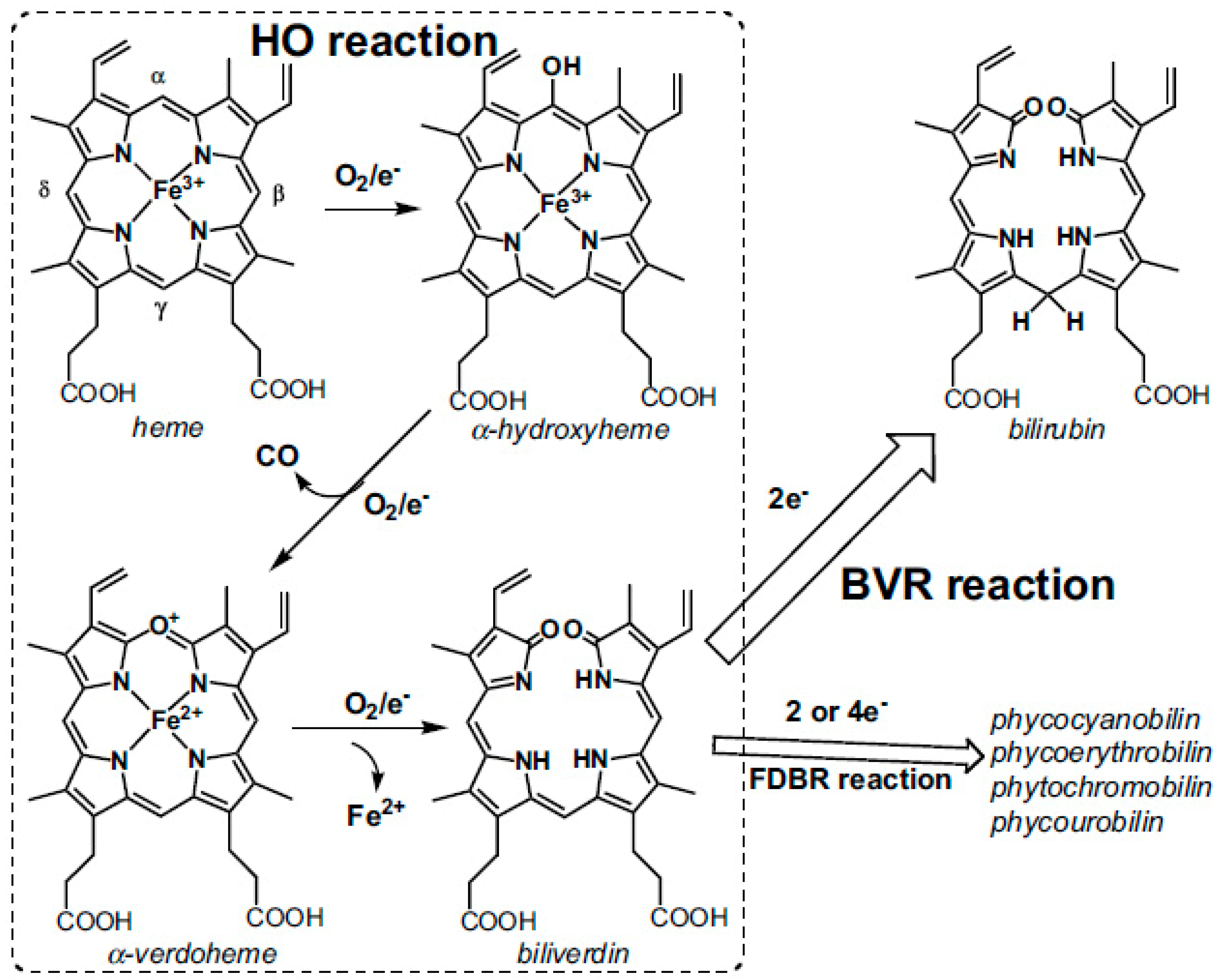
- Sugishima, M.; Wada, K.; Fukuyama, K. Recent Advances in the Understanding of the Reaction Chemistries of the Heme Catabolizing Enzymes HO and BVR Based on High Resolution Protein Structures. Curr. Med. Chem. 2020, 27, 3499–3518. [Google Scholar] [CrossRef]
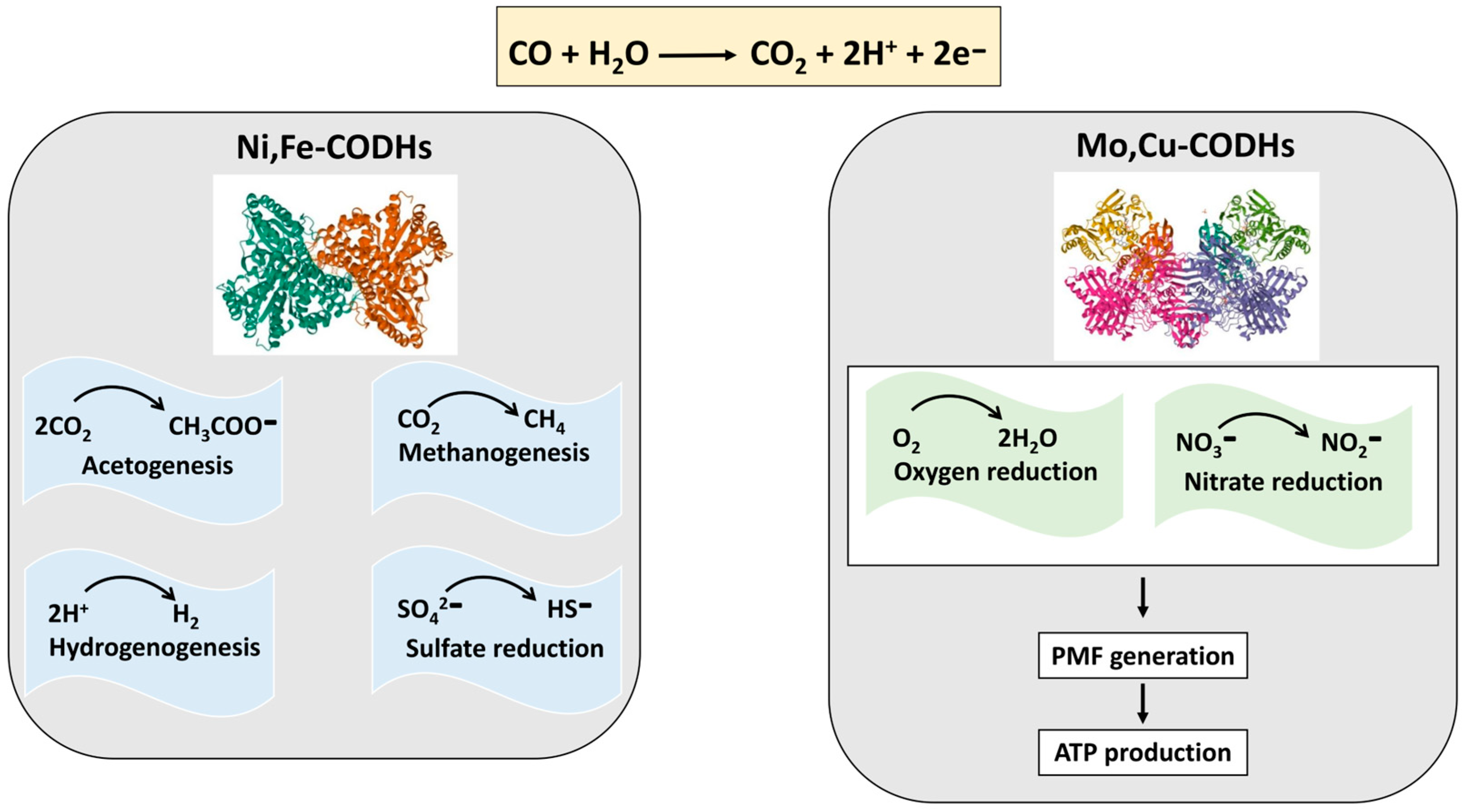
- Dent, M.R.; Weaver, B.R.; Roberts, M.G.; Burstyn, J.N. Carbon monoxide-sensing transcription factors: Regulators of microbial carbon monoxide oxidation pathway gene expression. J. Bacteriol. 2023, 205, e0033222. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Inoue, M.; Omae, K.; Yoshida, T.; Sako, Y. Anaerobic and hydrogenogenic carbon monoxide-oxidizing prokaryotes: Versatile microbial conversion of a toxic gas into an available energy. Adv. Appl. Microbiol. 2020, 110, 99–148. [Google Scholar] [CrossRef]
- Bahrle, R.; Bohnke, S.; Englhard, J.; Bachmann, J.; Perner, M. Current status of carbon monoxide dehydrogenases (CODH) and their potential for electrochemical applications. Bioresour. Bioprocess. 2023, 10, 84. [Google Scholar] [CrossRef]
- Nicholls, P.; Chanady, G.A. Interactions of cytochrome aa3 with oxygen and carbon monoxide. The role of the 607 nm complex. Biochim. Biophys. Acta 1981, 634, 256–265. [Google Scholar] [CrossRef]
- Siletsky, S.; Kaulen, A.D.; Konstantinov, A.A. Resolution of electrogenic steps couples to conversion of cytochrome c oxidase from the peroxy to the ferryl-oxo state. Biochemistry 1999, 38, 4853–4861. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.A.; Egawa, T.; Shinzawa-Itoh, K.; Yoshikawa, S.; Guallar, V.; Yeh, S.R.; Rousseau, D.L.; Gerfen, G.J. Two tyrosyl radicals stabilize high oxidation states in cytochrome c oxidase for efficient energy conservation and proton translocation. J. Am. Chem. Soc. 2012, 134, 4753–4761. [Google Scholar] [CrossRef]
- Tomkova, A.; Cizmar, E.; Jancura, D.; Fabian, M. High stability of the radical at the catalytic center of cytochrome c oxidase. Arch. Biochem. Biophys. 2025, 764, 110271. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yamashita, E.; Inoue, N.; Yao, M.; Fei, M.J.; Libeu, C.P.; Mizushima, T.; et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science 1998, 280, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.P.; Youn, H.; Kerby, R.L. CO-sensing mechanisms. Microbiol. Mol. Biol. Rev. 2004, 68, 453–473. [Google Scholar] [CrossRef]
- Mendes, S.S.; Miranda, V.; Saraiva, L.M. Hydrogen sulfide and carbon monoxide tolerance in bacteria. Antioxidants 2021, 10, 729. [Google Scholar] [CrossRef]
- Vos, M.H.; Salman, M.; Liebl, U. Early processes in heme-based CO-sensing proteins. Front. Mol. Biosci. 2022, 9, 1046412. [Google Scholar] [CrossRef]
- Sousa, F.L.; Alves, R.J.; Ribeiro, M.A.; Pereira-Leal, J.B.; Teixeira, M.; Pereira, M.M. The superfamily of heme-copper oxygen reductases: Types and evolutionary considerations. Biochim. Biophys. Acta 2012, 1817, 629–637. [Google Scholar] [CrossRef]
- Murali, R.; Gennis, R.B.; Hemp, J. Evolution of the cytochrome bd oxygen reductase superfamily and the function of CydAA’ in Archaea. ISME J. 2021, 15, 3534–3548. [Google Scholar] [CrossRef]
- Murali, R.; Hemp, J.; Gennis, R.B. Evolution of quinol oxidation within the heme-copper oxidoreductase superfamily. Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148907. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, T.T.; Kayastha, K.; Waterham, C.Y.J.; Brunle, S.; Jeuken, L.J.C. Menaquinone-specific turnover by M. tuberculosis cytochrome bd is redox regulated by the Q-loop disulfide bond. J. Biol. Chem. 2025, 301, 108094. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Montalvo, M.A.; Sorescu, J.M.; Baltes, G.; Juarez, O.; Tuz, K. The respiratory chain of Klebsiella aerogenes in urine-like conditions: Critical roles of NDH-2 and bd-terminal oxidases. Front. Microbiol. 2024, 15, 1479714. [Google Scholar] [CrossRef]
- Blomberg, M.R.A.; Adelroth, P. Reduction of molecular oxygen in flavodiiron proteins—Catalytic mechanism and comparison to heme-copper oxidases. J. Inorg. Biochem. 2024, 255, 112534. [Google Scholar] [CrossRef] [PubMed]
- Janczak, M.; Vilhjalmsdottir, J.; Adelroth, P. Proton transfer in cytochrome bd-I from E. coli involves Asp-105 in CydB. Biochim. Biophys. Acta Bioenerg. 2024, 1865, 149489. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, G.; Zhang, W.; Yu, S.; Fei, J.; An, T.; Yi, J.; Li, F.; Huang, T.; Yang, J.; et al. Mitochondrial-cytochrome c oxidase II promotes glutaminolysis to sustain tumor cell survival upon glucose deprivation. Nat. Commun. 2025, 16, 212. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, X.; Chang, H.; Yap, J.; Sun, W.; Gao, H. Inhibitory effects of nitrite and sulfite/peroxymonosulfate on bacteria are mediated respectively through respiration and intracellular GSH homeostasis. Microbiol. Res. 2025, 290, 127962. [Google Scholar] [CrossRef]
- Smirnova, I.; Wu, F.; Brzezinski, P. Stimulation of cytochrome c oxidase activity by detergents. Biochim. Biophys. Acta Bioenerg. 2025, 1866, 149509. [Google Scholar] [CrossRef]
- Chobert, S.C.; Roger-Margueritat, M.; Flandrin, L.; Berraies, S.; Lefevre, C.T.; Pelosi, L.; Junier, I.; Varoquaux, N.; Pierrel, F.; Abby, S.S. Dynamic quinone repertoire accompanied the diversification of energy metabolism in Pseudomonadota. Isme J. 2025, 19, wrae253. [Google Scholar] [CrossRef]
- Srivastav, S.; Biswas, A.; Anand, A. Interplay of niche and respiratory network in shaping bacterial colonization. J. Biol. Chem. 2025, 301, 108052. [Google Scholar] [CrossRef] [PubMed]
- Inskeep, W.P.; Jay, Z.J.; McKay, L.J.; Dlakic, M. Respiratory processes of early-evolved hyperthermophiles in sulfidic and low-oxygen geothermal microbial communities. Nat. Commun. 2025, 16, 277. [Google Scholar] [CrossRef]
- Vogt, S.; Ramzan, R.; Grossman, L.I.; Singh, K.K.; Ferguson-Miller, S.; Yoshikawa, S.; Lee, I.; Huttemann, M. Mitochondrial respiration is controlled by allostery, subunit composition and phosphorylation sites of cytochrome c oxidase: A trailblazer’s tale—Bernhard Kadenbach. Mitochondrion 2021, 60, 228–233. [Google Scholar] [CrossRef]
- Capitanio, G.; Papa, F.; Papa, S. The allosteric protein interactions in the proton-motive function of mammalian redox enzymes of the respiratory chain. Biochimie 2021, 189, 1–12. [Google Scholar] [CrossRef]
- Wikstrom, M.; Gennis, R.B.; Rich, P.R. Structures of the intermediates in the catalytic cycle of mitochondrial cytochrome c oxidase. Biochim. Biophys. Acta. Bioenerg. 2023, 1864, 148933. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Tsukihara, T.; Yoshikawa, S. Recent progress in experimental studies on the catalytic mechanism of cytochrome c oxidase. Front. Chem. 2023, 11, 1108190. [Google Scholar] [CrossRef]
- Rousseau, D.L.; Ishigami, I.; Yeh, S.R. Structural and functional mechanisms of cytochrome c oxidase. J. Inorg. Biochem. 2025, 262, 112730. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.K.; Cook, G.M. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv. Microb. Physiol. 2000, 43, 165–224. [Google Scholar] [CrossRef]
- Borisov, V.B.; Gennis, R.B.; Hemp, J.; Verkhovsky, M.I. The cytochrome bd respiratory oxygen reductases. Biochim. Biophys. Acta 2011, 1807, 1398–1413. [Google Scholar] [CrossRef]
- Kaila, V.R.I.; Wikstrom, M. Architecture of bacterial respiratory chains. Nat. Rev. Microbiol. 2021, 19, 319–330. [Google Scholar] [CrossRef]
- Li, J.; Han, L.; Vallese, F.; Ding, Z.; Choi, S.K.; Hong, S.; Luo, Y.; Liu, B.; Chan, C.K.; Tajkhorshid, E.; et al. Cryo-EM structures of Escherichia coli cytochrome bo3 reveal bound phospholipids and ubiquinone-8 in a dynamic substrate binding site. Proc. Natl. Acad. Sci. USA 2021, 118, e2106750118. [Google Scholar] [CrossRef]
- Siletsky, S.A.; Soulimane, T.; Belevich, I.; Gennis, R.B.; Wikstrom, M. Specific inhibition of proton pumping by the T315V mutation in the K channel of cytochrome ba3 from Thermus thermophilus. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148450. [Google Scholar] [CrossRef]
- Hederstedt, L. Diversity of cytochrome c oxidase assembly proteins in bacteria. Microorganisms 2022, 10, 926. [Google Scholar] [CrossRef]
- Makarchuk, I.; Gerasimova, T.; Kagi, J.; Wohlwend, D.; Melin, F.; Friedrich, T.; Hellwig, P. Mutating the environment of heme b595 of E. coli cytochrome bd-I oxidase shifts its redox potential by 200 mV without inactivating the enzyme. Bioelectrochemistry 2023, 151, 108379. [Google Scholar] [CrossRef] [PubMed]
- Makarchuk, I.; Kagi, J.; Gerasimova, T.; Wohlwend, D.; Friedrich, T.; Melin, F.; Hellwig, P. pH-dependent kinetics of NO release from E. coli bd-I and bd-II oxidase reveals involvement of Asp/Glu58(B). Biochim. Biophys. Acta Bioenerg. 2023, 1864, 148952. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Hakke, S.; Mohren, R.; Sijbers, L.; Peters, P.J.; Ravelli, R.B.G. Cryo-EM structure of cytochrome bo(3) quinol oxidase assembled in peptidiscs reveals an “open” conformation for potential ubiquinone-8 release. Biochim. Biophys. Acta Bioenerg. 2024, 1865, 149045. [Google Scholar] [CrossRef]
- Deutschmann, S.; Tauber, S.T.; Rimle, L.; Biner, O.; Schori, M.; Stanic, A.M.; von Ballmoos, C. Modulating Liposome Surface Charge for Maximized ATP Regeneration in Synthetic Nanovesicles. ACS Synth. Biol. 2024, 13, 4061–4073. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.V.; Shirsath, A.M.; Anand, A. Dioxygen reductase heterogeneity is crucial for robust aerobic growth physiology of Escherichia coli. iScience 2024, 27, 111498. [Google Scholar] [CrossRef]
- Rauch, J.; Kurscheidt, K.; Shen, K.W.; Andrei, A.; Daum, N.; Ozturk, Y.; Melin, F.; Layer, G.; Hellwig, P.; Daldal, F.; et al. The small membrane protein CcoS is involved in cofactor insertion into the cbb(3)-type cytochrome c oxidase. Biochim. Biophys. Acta Bioenerg. 2025, 1866, 149524. [Google Scholar] [CrossRef]
- Siddeeque, R.; Heger, L.; Kagi, J.; Friedrich, T.; Melin, F.; Hellwig, P. Interplay of acidic residues in the proton channel of E. coli cytochrome bd-I oxidase to promote oxygen reduction and NO release. Biochim. Biophys. Acta Bioenerg. 2025, 1866, 149537. [Google Scholar] [CrossRef]
- Paszti, S.; Biner, O.; Liu, Y.; Bolli, K.; Jeggli, S.D.; Pessi, G.; Eberl, L. Insights into the diverse roles of the terminal oxidases in Burkholderia cenocepacia H111. Sci. Rep. 2025, 15, 2390. [Google Scholar] [CrossRef] [PubMed]
- Batista, B.B.; de Lima, V.M.; Will, W.R.; Fang, F.C.; da Silva Neto, J.F. A cytochrome bd repressed by a MarR family regulator confers resistance to metals, nitric oxide, sulfide, and cyanide in Chromobacterium violaceum. Appl. Environ. Microbiol. 2025, 91, e02360-24. [Google Scholar] [CrossRef] [PubMed]
- Siletsky, S.A. Investigation of the Mechanism of Membrane Potential Generation by Heme-Copper Respiratory Oxidases in a Real Time Mode. Biochemistry 2023, 88, 1513–1527. [Google Scholar] [CrossRef]
- Pereira, M.M.; Teixeira, M. Proton pathways, ligand binding and dynamics of the catalytic site in haem-copper oxygen reductases: A comparison between the three families. Biochim. Biophys. Acta 2004, 1655, 340–346. [Google Scholar] [CrossRef]
- Letts, J.A.; Fiedorczuk, K.; Sazanov, L.A. The architecture of respiratory supercomplexes. Nature 2016, 537, 644–648. [Google Scholar] [CrossRef]
- Melo, A.M.; Teixeira, M. Supramolecular organization of bacterial aerobic respiratory chains: From cells and back. Biochim. Biophys. Acta 2016, 1857, 190–197. [Google Scholar] [CrossRef]
- Moe, A.; Dimogkioka, A.R.; Rapaport, D.; Ojemyr, L.N.; Brzezinski, P. Structure and function of the S. pombe III-IV-cyt c supercomplex. Proc. Natl. Acad. Sci. USA 2023, 120, e2307697120. [Google Scholar] [CrossRef] [PubMed]
- Di Trani, J.M.; Gheorghita, A.A.; Turner, M.; Brzezinski, P.; Adelroth, P.; Vahidi, S.; Howell, P.L.; Rubinstein, J.L. Structure of the bc1-cbb3 respiratory supercomplex from Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2023, 120, e2307093120. [Google Scholar] [CrossRef] [PubMed]
- Lobez, A.P.; Wu, F.; Di Trani, J.M.; Rubinstein, J.L.; Oliveberg, M.; Brzezinski, P.; Moe, A. Electron transfer in the respiratory chain at low salinity. Nat. Commun. 2024, 15, 8241. [Google Scholar] [CrossRef]
- Riepl, D.; Gamiz-Hernandez, A.P.; Kovalova, T.; Krol, S.M.; Mader, S.L.; Sjostrand, D.; Hogbom, M.; Brzezinski, P.; Kaila, V.R.I. Long-range charge transfer mechanism of the III(2)IV(2) mycobacterial supercomplex. Nat. Commun. 2024, 15, 5276. [Google Scholar] [CrossRef]
- Safarian, S.; Rajendran, C.; Muller, H.; Preu, J.; Langer, J.D.; Ovchinnikov, S.; Hirose, T.; Kusumoto, T.; Sakamoto, J.; Michel, H. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science 2016, 352, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Thesseling, A.; Rasmussen, T.; Burschel, S.; Wohlwend, D.; Kagi, J.; Muller, R.; Bottcher, B.; Friedrich, T. Homologous bd oxidases share the same architecture but differ in mechanism. Nat. Commun. 2019, 10, 5138. [Google Scholar] [CrossRef] [PubMed]
- Safarian, S.; Hahn, A.; Mills, D.J.; Radloff, M.; Eisinger, M.L.; Nikolaev, A.; Meier-Credo, J.; Melin, F.; Miyoshi, H.; Gennis, R.B.; et al. Active site rearrangement and structural divergence in prokaryotic respiratory oxidases. Science 2019, 366, 100–104. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Tang, Y.; Zhou, X.; Lai, Y.; Zhou, S.; Zhang, Y.; Yang, X.; Liu, F.; Guddat, L.W.; et al. Cryo-EM structure of mycobacterial cytochrome bd reveals two oxygen access channels. Nat. Commun. 2021, 12, 4621. [Google Scholar] [CrossRef]
- Safarian, S.; Opel-Reading, H.K.; Wu, D.; Mehdipour, A.R.; Hards, K.; Harold, L.K.; Radloff, M.; Stewart, I.; Welsch, S.; Hummer, G.; et al. The cryo-EM structure of the bd oxidase from M. tuberculosis reveals a unique structural framework and enables rational drug design to combat TB. Nat. Commun. 2021, 12, 5236. [Google Scholar] [CrossRef]
- Grauel, A.; Kagi, J.; Rasmussen, T.; Makarchuk, I.; Oppermann, S.; Moumbock, A.F.A.; Wohlwend, D.; Muller, R.; Melin, F.; Gunther, S.; et al. Structure of Escherichia coli cytochrome bd-II type oxidase with bound aurachin D. Nat. Commun. 2021, 12, 6498. [Google Scholar] [CrossRef]
- Grund, T.N.; Radloff, M.; Wu, D.; Goojani, H.G.; Witte, L.F.; Josting, W.; Buschmann, S.; Muller, H.; Elamri, I.; Welsch, S.; et al. Mechanistic and structural diversity between cytochrome bd isoforms of Escherichia coli. Proc. Natl. Acad. Sci. USA 2021, 118, e2114013118. [Google Scholar] [CrossRef]
- Friedrich, T.; Wohlwend, D.; Borisov, V.B. Recent advances in structural studies of cytochrome bd and its potential application as a drug target. Int. J. Mol. Sci. 2022, 23, 3166. [Google Scholar] [CrossRef] [PubMed]
- Grund, T.N.; Kabashima, Y.; Kusumoto, T.; Wu, D.; Welsch, S.; Sakamoto, J.; Michel, H.; Safarian, S. The cryoEM structure of cytochrome bd from C. glutamicum provides novel insights into structural properties of actinobacterial terminal oxidases. Front. Chem. 2023, 10, 1085463. [Google Scholar] [CrossRef]
- Nastasi, M.R.; Caruso, L.; Giordano, F.; Mellini, M.; Rampioni, G.; Giuffre, A.; Forte, E. Cyanide insensitive oxidase confers hydrogen sulfide and nitric oxide tolerance to Pseudomonas aeruginosa aerobic respiration. Antioxidants 2024, 13, 383. [Google Scholar] [CrossRef]
- Borisov, V.B.; Murali, R.; Verkhovskaya, M.L.; Bloch, D.A.; Han, H.; Gennis, R.B.; Verkhovsky, M.I. Aerobic respiratory chain of Escherichia coli is not allowed to work in fully uncoupled mode. Proc. Natl. Acad. Sci. USA 2011, 108, 17320–17324. [Google Scholar] [CrossRef]
- Borisov, V.B. Generation of membrane potential by cytochrome bd. Biochemistry 2023, 88, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- D’mello, R.; Hill, S.; Poole, R.K. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two-oxygen-binding haems: Implicaitons for regulation of activity in vivo by oxygen inihibition. Microbiology 1996, 142, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Belevich, I.; Borisov, V.B.; Konstantinov, A.A.; Verkhovsky, M.I. Oxygenated complex of cytochrome bd from Escherichia coli: Stability and photolability. FEBS Lett. 2005, 579, 4567–4570. [Google Scholar] [CrossRef]
- Arutyunyan, A.M.; Sakamoto, J.; Inadome, M.; Kabashima, Y.; Borisov, V.B. Optical and magneto-optical activity of cytochrome bd from Geobacillus thermodenitrificans. Biochim. Biophys. Acta 2012, 1817, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Borisov, V.B.; Vicente, J.B.; Giuffre, A. Cytochrome bd and gaseous ligands in bacterial physiology. Adv. Microb. Physiol. 2017, 71, 171–234. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Paiardini, A.; Hoogewijs, D.; Forte, E.; Giuffre, A.; Poole, R.K. Bacterial oxidases of the cytochrome bd family: Redox enzymes of unique structure, function and utility as drug targets. Antioxid. Redox Signal. 2021, 34, 1280–1318. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Terminal oxidase cytochrome bd protects bacteria against hydrogen sulfide toxicity. Biochemistry 2021, 86, 22–32. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Nastasi, M.R.; Forte, E. ROS defense systems and terminal oxidases in bacteria. Antioxidants 2021, 10, 839. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Impact of hydrogen sulfide on mitochondrial and bacterial bioenergetics. Int. J. Mol. Sci. 2021, 22, 12688. [Google Scholar] [CrossRef]
- Seregina, T.A.; Lobanov, K.V.; Shakulov, R.S.; Mironov, A.S. Inactivation of terminal oxidase bd-I leads to supersensitivity of E. coli to quinolone and beta-lactam antibiotics. Mol. Biol. 2022, 56, 619–627. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Bioenergetics and reactive nitrogen species in bacteria. Int. J. Mol. Sci. 2022, 23, 7321. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Giardina, G.; Pistoia, G.; Forte, E. Cytochrome bd-type oxidases and environmental stressors in microbial physiology. Adv. Microb. Physiol. 2025, in press. [Google Scholar] [CrossRef]
- Lee, B.S.; Hards, K.; Engelhart, C.A.; Hasenoehrl, E.J.; Kalia, N.P.; Mackenzie, J.S.; Sviriaeva, E.; Chong, S.M.S.; Manimekalai, M.S.S.; Koh, V.H.; et al. Dual inhibition of the terminal oxidases eradicates antibiotic-tolerant Mycobacterium tuberculosis. EMBO Mol. Med. 2021, 13, e13207. [Google Scholar] [CrossRef] [PubMed]
- Radloff, M.; Elamri, I.; Grund, T.N.; Witte, L.F.; Hohmann, K.F.; Nakagaki, S.; Goojani, H.G.; Nasiri, H.; Hideto, M.; Bald, D.; et al. Short-chain aurachin D derivatives are selective inhibitors of E. coli cytochrome bd-I and bd-II oxidases. Sci. Rep. 2021, 11, 23852. [Google Scholar] [CrossRef]
- Makarchuk, I.; Nikolaev, A.; Thesseling, A.; Dejon, L.; Lamberty, D.; Stief, L.; Speicher, A.; Friedrich, T.; Hellwig, P.; Nasiri, H.R.; et al. Identification and optimization of quinolone-based inhibitors against cytochrome bd oxidase using an electrochemical assay. Electrochim. Acta 2021, 381, 138293. [Google Scholar] [CrossRef]
- Harikishore, A.; Chong, S.S.M.; Ragunathan, P.; Bates, R.W.; Gruber, G. Targeting the menaquinol binding loop of mycobacterial cytochrome bd oxidase. Mol. Divers. 2021, 25, 517–524. [Google Scholar] [CrossRef]
- Hopfner, S.M.; Lee, B.S.; Kalia, N.P.; Miller, M.J.; Pethe, K.; Moraski, G.C. Structure guided generation of thieno[3,2-d]pyrimidin-4-amine Mycobacterium tuberculosis bd oxidase inhibitors. RSC Med. Chem. 2021, 12, 73–77. [Google Scholar] [CrossRef]
- Anand, P.; Akhter, Y. A review on enzyme complexes of electron transport chain from Mycobacterium tuberculosis as promising drug targets. Int. J. Biol. Macromol. 2022, 212, 474–494. [Google Scholar] [CrossRef]
- Hards, K.; Cheung, C.Y.; Waller, N.; Adolph, C.; Keighley, L.; Tee, Z.S.; Harold, L.K.; Menorca, A.; Bujaroski, R.S.; Buckley, B.J.; et al. An amiloride derivative is active against the F1Fo-ATP synthase and cytochrome bd oxidase of Mycobacterium tuberculosis. Commun. Biol. 2022, 5, 166. [Google Scholar] [CrossRef]
- Harikishore, A.; Mathiyazakan, V.; Pethe, K.; Gruber, G. Novel targets and inhibitors of the Mycobacterium tuberculosis cytochrome bd oxidase to foster anti-tuberculosis drug discovery. Expert Opin. Drug Discov. 2023, 18, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Capela, R.; Felix, R.; Clariano, M.; Nunes, D.; Perry, M.J.; Lopes, F. Target Identification in Anti-Tuberculosis Drug Discovery. Int. J. Mol. Sci. 2023, 24, 10482. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, L.N.; Ardrey, A.; Hafiz, T.A.; Dyer, L.A.; Warman, A.J.; Mosallam, N.; Nixon, G.L.; Fisher, N.E.; Hong, W.D.; Leung, S.C.; et al. Identification of 2-aryl-quinolone inhibitors of cytochrome bd and chemical validation of combination strategies for respiratory inhibitors against Mycobacterium tuberculosis. ACS Infect. Dis. 2023, 9, 221–238. [Google Scholar] [CrossRef]
- Kagi, J.; Sloan, W.; Schimpf, J.; Nasiri, H.R.; Lashley, D.; Friedrich, T. Exploring ND-011992, a quinazoline-type inhibitor targeting quinone reductases and quinol oxidases. Sci. Rep. 2023, 13, 12226. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, M.; Wang, W.; Cheung, C.Y.; Wu, Y.; Yu, H.; Hu, X.; Cook, G.M.; Gong, H.; Lu, X. Discovery of 1-hydroxy-2-methylquinolin-4(1H)-one derivatives as new cytochrome bd oxidase inhibitors for tuberculosis therapy. Eur. J. Med. Chem. 2023, 245, 114896. [Google Scholar] [CrossRef]
- Henry, S.A.; Webster, C.M.; Shaw, L.N.; Torres, N.J.; Jobson, M.E.; Totzke, B.C.; Jackson, J.K.; McGreig, J.E.; Wass, M.N.; Robinson, G.K.; et al. Steroid drugs inhibit bacterial respiratory oxidases and are lethal toward methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 2024, 230, e149–e158. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Das, S.; Indurthi, H.K.; Kumar, R.; Roy, A.; Kalia, N.P.; Sharma, D.K. Cytochrome bd oxidase: An emerging anti-tubercular drug target. RSC Med. Chem. 2024, 15, 769–787. [Google Scholar] [CrossRef]
- Seitz, C.; Ahn, S.H.; Wei, H.; Kyte, M.; Cook, G.M.; Krause, K.L.; McCammon, J.A. Targeting Tuberculosis: Novel Scaffolds for Inhibiting Cytochrome bd Oxidase. J. Chem. Inf. Model. 2024, 64, 5232–5241. [Google Scholar] [CrossRef]
- Nastasi, M.R.; Borisov, V.B.; Forte, E. The terminal oxidase cytochrome bd-I confers carbon monoxide resistance to Escherichia coli cells. J. Inorg. Biochem. 2023, 247, 112341. [Google Scholar] [CrossRef]
- Nastasi, M.R.; Borisov, V.B.; Forte, E. Membrane-bound redox enzyme cytochrome bd-I promotes carbon monoxide-resistant Escherichia coli growth and respiration. Int. J. Mol. Sci. 2024, 25, 1277. [Google Scholar] [CrossRef]
- Alexeeva, S.; Hellingwerf, K.; Teixeira de Mattos, M.J. Quantitative assessment of oxygen availability: Perceived aerobiosis and its effect on flux distribution in the respiratory chain of Escherichia coli. J. Bacteriol. 2002, 184, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, M.D.; Ter Beek, A.; Graham, A.I.; Trotter, E.W.; Asif, H.M.; Sanguinetti, G.; de Mattos, J.T.; Poole, R.K.; Green, J. Transcript profiling and inference of Escherichia coli K-12 ArcA activity across the range of physiologically relevant oxygen concentrations. J. Biol. Chem. 2011, 286, 10147–10154. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Borisov, V.B.; Falabella, M.; Colaco, H.G.; Tinajero-Trejo, M.; Poole, R.K.; Vicente, J.B.; Sarti, P.; Giuffre, A. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Sci. Rep. 2016, 6, 23788. [Google Scholar] [CrossRef]
- Wareham, L.K.; Begg, R.; Jesse, H.E.; Van Beilen, J.W.; Ali, S.; Svistunenko, D.; McLean, S.; Hellingwerf, K.J.; Sanguinetti, G.; Poole, R.K. Carbon monoxide gas is not inert, but global, in its consequences for bacterial gene expression, iron acquisition, and antibiotic resistance. Antioxid. Redox Signal. 2016, 24, 1013–1028. [Google Scholar] [CrossRef]
- Forte, E.; Borisov, V.B.; Siletsky, S.A.; Petrosino, M.; Giuffre, A. In the respiratory chain of Escherichia coli cytochromes bd-I and bd-II are more sensitive to carbon monoxide inhibition than cytochrome bo3. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 148088. [Google Scholar] [CrossRef]
- Cook, G.M.; Hards, K.; Vilcheze, C.; Hartman, T.; Berney, M. Energetics of respiration and oxidative phosphorylation in mycobacteria. Microbiol. Spectr. 2014, 2, MGM2-0015-2013. [Google Scholar] [CrossRef]
- Liang, Y.; Plourde, A.; Bueler, S.A.; Liu, J.; Brzezinski, P.; Vahidi, S.; Rubinstein, J.L. Structure of mycobacterial respiratory complex I. Proc. Natl. Acad. Sci. USA 2023, 120, e2214949120. [Google Scholar] [CrossRef]
- Kalia, N.P.; Singh, S.; Hards, K.; Cheung, C.Y.; Sviriaeva, E.; Banaei-Esfahani, A.; Aebersold, R.; Berney, M.; Cook, G.M.; Pethe, K.M. Tuberculosis relies on trace oxygen to maintain energy homeostasis and survive in hypoxic environments. Cell Rep. 2023, 42, 112444. [Google Scholar] [CrossRef]
- Cordero, P.R.F.; Bayly, K.; Man Leung, P.; Huang, C.; Islam, Z.F.; Schittenhelm, R.B.; King, G.M.; Greening, C. Atmospheric carbon monoxide oxidation is a widespread mechanism supporting microbial survival. ISME J. 2019, 13, 2868–2881. [Google Scholar] [CrossRef]
- Bayly, K.; Cordero, P.R.F.; Kropp, A.; Huang, C.; Schittenhelm, R.B.; Grinter, R.; Greening, C. Mycobacteria tolerate carbon monoxide by remodeling their respiratory chain. mSystems 2021, 6, e01292-01220. [Google Scholar] [CrossRef]
- Cramm, R. Genomic view of energy metabolism in Ralstonia eutropha H16. J. Mol. Microbiol. Biotechnol. 2009, 16, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.; Crang, N.; Gynna, A.H.; Kabova, D.; Frielingsdorf, S.; Lenz, O.; Charpentier, E.; Hudson, E.P. The energy metabolism of Cupriavidus necator in different trophic conditions. Appl. Environ. Microbiol. 2024, 90, e0074824. [Google Scholar] [CrossRef] [PubMed]
- Windhorst, C.; Gescher, J. Efficient biochemical production of acetoin from carbon dioxide using Cupriavidus necator H16. Biotechnol. Biofuels 2019, 12, 163. [Google Scholar] [CrossRef]
- Wickham-Smith, C.; Malys, N.; Winzer, K. Improving carbon monoxide tolerance of Cupriavidus necator H16 through adaptive laboratory evolution. Front. Bioeng. Biotechnol. 2023, 11, 1178536. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.L.; Berka, V.; Martin, E.; Olson, J.S. A “sliding scale rule” for selectivity among NO, CO, and O2 by heme protein sensors. Biochemistry 2012, 51, 172–186. [Google Scholar] [CrossRef]
- Bartlett, G.J.; Newberry, R.W.; VanVeller, B.; Raines, R.T.; Woolfson, D.N. Interplay of hydrogen bonds and n→π* interactions in proteins. J. Am. Chem. Soc. 2013, 135, 18682–18688. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E.; Sarti, P.; Brunori, M.; Konstantinov, A.A.; Giuffre, A. Redox control of fast ligand dissociation from Escherichia coli cytochrome bd. Biochem. Biophys. Res. Commun. 2007, 355, 97–102. [Google Scholar] [CrossRef]
- Gibson, Q.; Greenwood, C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem. J. 1963, 86, 541–554. [Google Scholar] [CrossRef]
- Borisov, V.; Arutyunyan, A.M.; Osborne, J.P.; Gennis, R.B.; Konstantinov, A.A. Magnetic circular dichroism used to examine the interaction of Escherichia coli cytochrome bd with ligands. Biochemistry 1999, 38, 740–750. [Google Scholar] [CrossRef]
- Borisov, V.B.; Arutyunyan, A.M. The fully reduced terminal oxidase bd-I isolated from Escherichia coli binds cyanide. J. Inorg. Biochem. 2024, 259, 112653. [Google Scholar] [CrossRef]
- Belevich, I.; Borisov, V.B.; Verkhovsky, M.I. Discovery of the true peroxy intermediate in the catalytic cycle of terminal oxidases by real-time measurement. J. Biol. Chem. 2007, 282, 28514–28519. [Google Scholar] [CrossRef] [PubMed]
- Paulus, A.; Rossius, S.G.; Dijk, M.; de Vries, S. Oxoferryl-porphyrin radical catalytic intermediate in cytochrome bd oxidases protects cells from formation of reactive oxygen species. J. Biol. Chem. 2012, 287, 8830–8838. [Google Scholar] [CrossRef] [PubMed]
- Belevich, I.; Borisov, V.B.; Zhang, J.; Yang, K.; Konstantinov, A.A.; Gennis, R.B.; Verkhovsky, M.I. Time-resolved electrometric and optical studies on cytochrome bd suggest a mechanism of electron-proton coupling in the di-heme active site. Proc. Natl. Acad. Sci. USA 2005, 102, 3657–3662. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisov, V.B.; Forte, E. Carbon Monoxide and Prokaryotic Energy Metabolism. Int. J. Mol. Sci. 2025, 26, 2809. https://doi.org/10.3390/ijms26062809
Borisov VB, Forte E. Carbon Monoxide and Prokaryotic Energy Metabolism. International Journal of Molecular Sciences. 2025; 26(6):2809. https://doi.org/10.3390/ijms26062809
Chicago/Turabian StyleBorisov, Vitaliy B., and Elena Forte. 2025. "Carbon Monoxide and Prokaryotic Energy Metabolism" International Journal of Molecular Sciences 26, no. 6: 2809. https://doi.org/10.3390/ijms26062809
APA StyleBorisov, V. B., & Forte, E. (2025). Carbon Monoxide and Prokaryotic Energy Metabolism. International Journal of Molecular Sciences, 26(6), 2809. https://doi.org/10.3390/ijms26062809






