From Defense to Disease: How the Immune System Fuels Epithelial–Mesenchymal Transition in Ovarian Cancer
Abstract
:1. Introduction
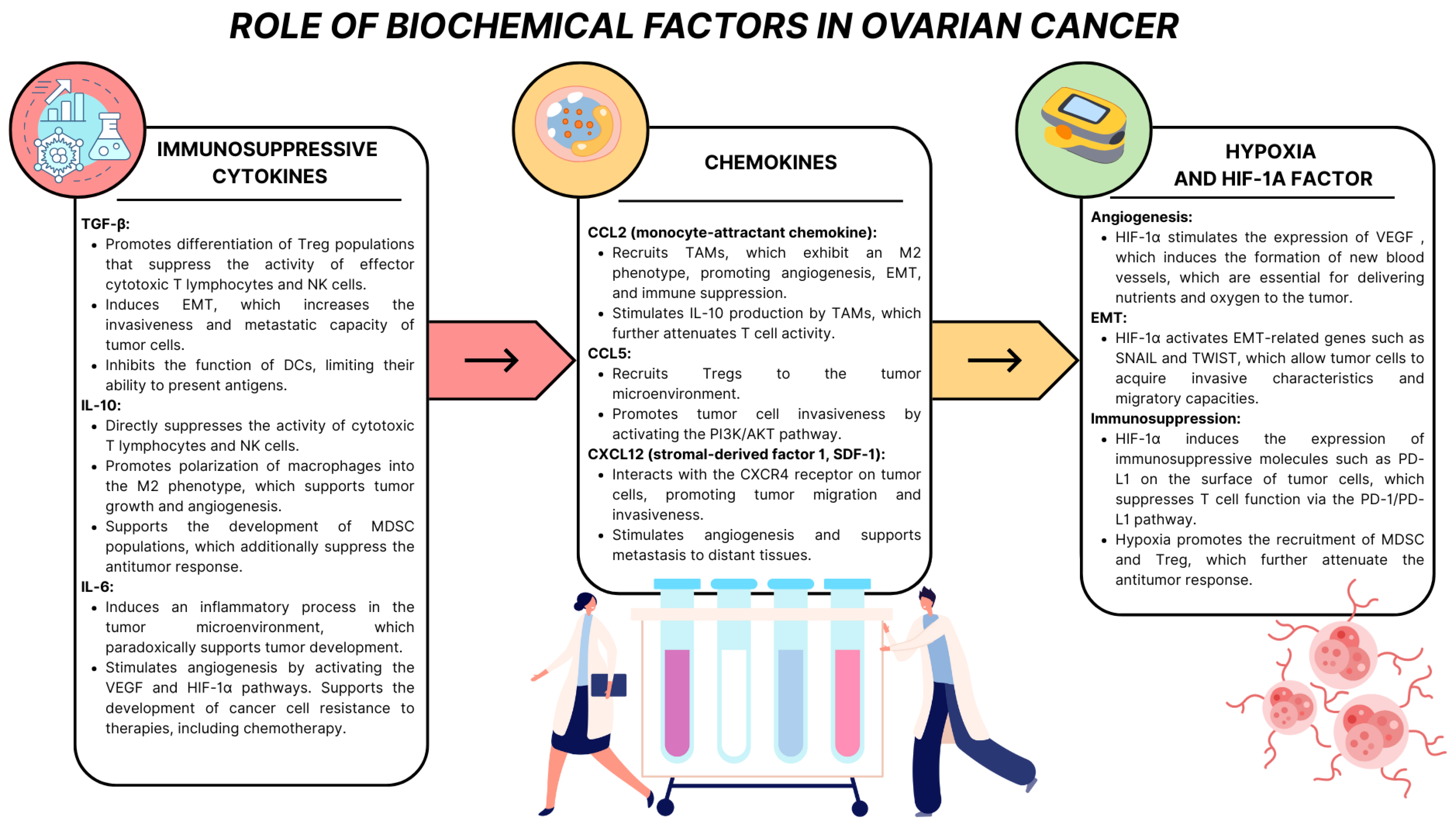
2. Ovarian Cancer Microenvironment: Complexity of Structure and Function
3. EMT in Ovarian Cancer
3.1. Types of EMT
3.2. Molecular Mechanisms of EMT
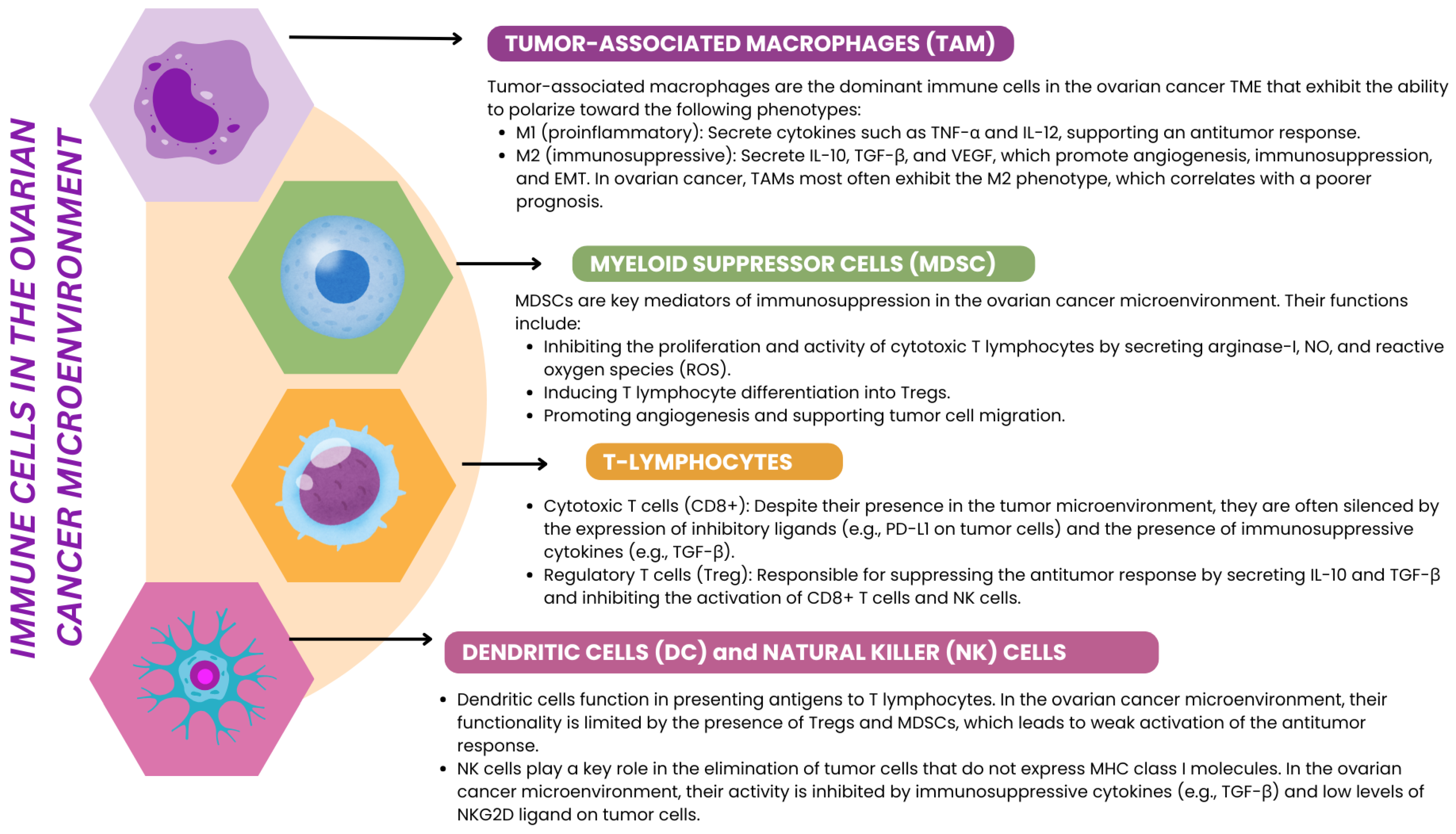
3.3. Immune Cells Involved in EMT in Ovarian Cancer
3.3.1. Macrophages
3.3.2. TILs in Ovarian Cancer
3.3.3. Th1, Th2, Th17, Th9, and Th22 Balance in Ovarian Cancer
| Cell Type | Subtype | Role in EMT | Key Cytokines/Factors | Mechanisms of Action | Prognostic Implication | References |
|---|---|---|---|---|---|---|
| Macrophages | M1 | Inhibit EMT | IL-12, TNF-α | Promote inflammation and cytotoxicity; oppose immunosuppressive signals; inhibit TGF-β signaling; enhance antigen presentation. | Higher M1/M2 ratio is linked to better clinical outcomes. | [85,86,87,88,89,90,91,92,93,94,95,96,97] |
| M2 (TAMs) | Promote EMT | TGF-β, IL-10, CCL18, IL-1β, | Activate Smad2/3, Wnt/β-catenin, EGFR, Hippo-YAP1/TAZ, and PI3K/AKT pathways; induce ZEB1, TWIST, and SLUG; increase MMP9 and CCL2; support angiogenesis and hypoxia. | Correlated with poor survival; CD163+Tim4+ TAMs drive EMT and metastasis. | ||
| T cells | CD8+ cytotoxic T cells | Inhibit EMT | IFN-γ | Suppress TGF-β/Smad pathway; maintain E-cadherin and cytokeratin expression; indirectly enhance M1 macrophage recruitment. | Associated with less invasive tumors and better survival. | [100,101,102,103] |
| T cells | Tregs (CD4+) | Promote EMT | IL-10, TGF-β | Suppress CD8+ T cell activity; induce EMT-supportive signaling; promote immunosuppression and tumor progression. | Linked to poor prognosis and tumor immune evasion. | [104,105,106] |
| CD39+ exhausted T cells | Promote EMT | - | Exhibit loss of effector function; express markers of tissue-resident memory T cells; fail to respond to tumor antigens, allowing EMT progression. | Indicate poor immunosurveillance and are associated with worse outcomes. | [110,111,112] | |
| Th cells | Th1 | Inhibit EMT | IFN-γ, IL-2 | Inhibit TGF-β/Smad and Wnt/β-catenin pathways; preserve epithelial markers (E-cadherin, cytokeratin); stimulate M1 and CD8+ activity. | Protective; Th1 deficiency correlates with advanced EMT and worse prognosis. | [115,116,117,118] |
| Th2 | Promote EMT | IL-4, IL-5, IL-13 | Activate the STAT6 pathway; upregulate vimentin and N-cadherin; promote M2 polarization; reinforce EMT signals. | Linked to aggressive tumors and therapy resistance. | ||
| Th17 | Dual (context-dependent) | IL-17 | Activate NF-κB, PI3K/AKT, and STAT3; increase mesenchymal markers (vimentin, N-cadherin); modulate angiogenesis and immunosuppression or enhance immune effectors. | Role varies with the TME context; it may promote or inhibit EMT depending on the cytokine milieu. | [119,120,121,122] | |
| Th9 | Inhibit EMT (potentially) | IL-9 | Stimulate CD8+ and M1 cells; suppress TGF-β/Smad signaling; may reduce EMT and tumor aggressiveness, though conflicting roles have been observed in other cancers. | Presence suggests favorable prognosis; therapeutic potential under investigation. | [123,124,125,126] | |
| Th22 | Promote EMT | IL-22 | Activate JAK/STAT3; induce EMT-related transcription factors (SNAIL, TWIST); support angiogenesis and cell migration; occasionally support tissue repair. | Strongly dependent on tumor microenvironment; mostly pro-EMT in ovarian cancer. | [127,128] |
3.4. Recent Evidence on the Role of EMT in the Tumor Biology of Ovarian Cancer
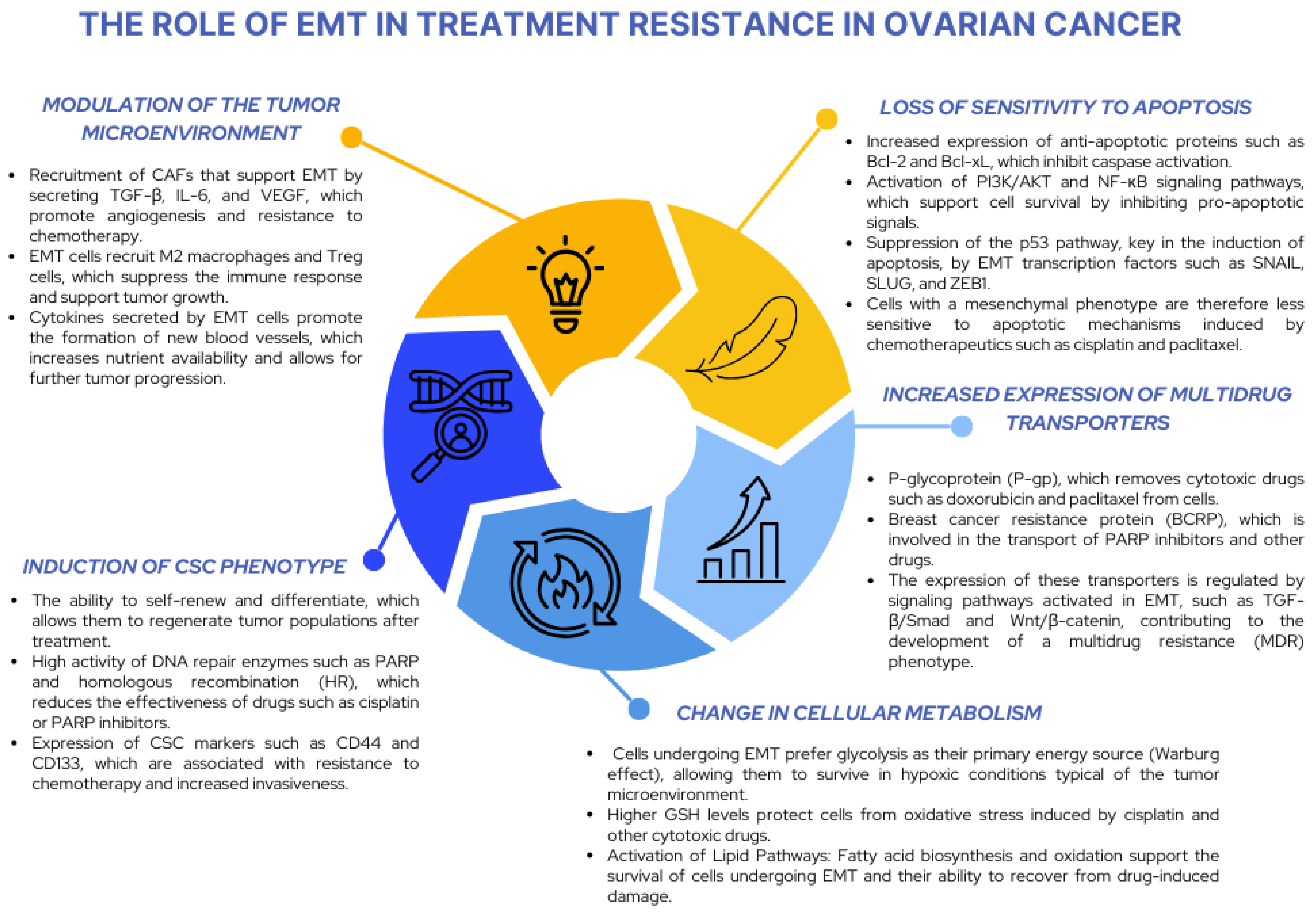
3.5. The Importance of EMT in Ovarian Cancer Chemotherapy
4. Further Research on EMT in the Development, Progression, and Treatment of Ovarian Cancer
4.1. EMT Transcription Factors and Apoptosis Resistance and Multidrug Resistance
4.2. The Relationship Between EMT and the Cancer Stem Cell (CSC) Phenotype and Resistance Mechanisms
4.3. EMT–MET Plasticity in Metastasis: Significance and Therapeutic Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ovarian Cancer Statistics. Available online: https://www.wcrf.org/preventing-cancer/cancer-statistics/ovarian-cancer-statistics/ (accessed on 23 December 2024).
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Women Health 2019, 11, 287–299. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, C.; Lin, Z.; Xiao, L.; Su, X.; Zheng, L.; Mu, Y.; Liao, M.; Ouyang, R.; Li, W.; et al. The Global Burden and Associated Factors of Ovarian Cancer in 1990–2019: Findings from the Global Burden of Disease Study 2019. BMC Public Health 2022, 22, 1455. [Google Scholar] [CrossRef] [PubMed]
- Global Ovarian Cancer Rates Rising|Figo. Available online: https://www.figo.org/news/global-ovarian-cancer-rates-rising (accessed on 23 December 2024).
- Nogueira-Rodrigues, A.; Giannecchini, G.V.; Secord, A.A. Real World Challenges and Disparities in the Systemic Treatment of Ovarian Cancer. Gynecol. Oncol. 2024, 185, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Gutic, B.; Bozanovic, T.; Mandic, A.; Dugalic, S.; Todorovic, J.; Dugalic, M.G.; Sengul, D.; Detanac, D.A.; Sengul, I.; Detanac, D.; et al. Preliminary Outcomes of Five-Year Survival for Ovarian Malignancies in Profiled Serbian Oncology Centre. Clinics 2023, 78, 100204. [Google Scholar] [CrossRef] [PubMed]
- Survival for Ovarian Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/ovarian-cancer/survival (accessed on 23 December 2024).
- Zamwar, U.M.; Anjankar, A.P. Aetiology, Epidemiology, Histopathology, Classification, Detailed Evaluation, and Treatment of Ovarian Cancer. Cureus 2022, 14, e30561. [Google Scholar] [CrossRef]
- Bukłaho, P.A.; Kiśluk, J.; Wasilewska, N.; Nikliński, J. Molecular Features as Promising Biomarkers in Ovarian Cancer. Adv. Clin. Exp. Med. 2023, 32, 1029–1040. [Google Scholar] [CrossRef]
- Hayashi, T.; Konishi, I. Molecular Histopathology for Establishing Diagnostic Method and Clinical Therapy for Ovarian Carcinoma. J. Clin. Med. Res. 2024, 15, 68–75. [Google Scholar] [CrossRef]
- Bradbury, M.; Borràs, E.; Pérez-Benavente, A.; Gil-Moreno, A.; Santamaria, A.; Sabidó, E. Proteomic Studies on the Management of High-Grade Serous Ovarian Cancer Patients: A Mini-Review. Cancers 2024, 13, 2067. [Google Scholar] [CrossRef]
- Charbonneau, B.; Goode, E.L.; Kalli, K.R.; Knutson, K.L.; DeRycke, M.S. The Immune System in the Pathogenesis of Ovarian Cancer. Crit. Rev. Immunol. 2013, 33, 137–164. [Google Scholar] [CrossRef]
- Macpherson, A.M.; Barry, S.C.; Ricciardelli, C.; Oehler, M.K. Epithelial Ovarian Cancer and the Immune System: Biology, Interactions, Challenges and Potential Advances for Immunotherapy. J. Clin. Med. 2020, 9, 2967. [Google Scholar] [CrossRef]
- Salas-Benito, D.; Vercher, E.; Conde, E.; Glez-Vaz, J.; Tamayo, I.; Hervas-Stubbs, S. Inflammation and Immunity in Ovarian Cancer. Eur. J. Cancer Suppl. 2020, 15, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Buckles, S. Harnessing the Immune System to Fight Ovarian Cancer. Available online: https://newsnetwork.mayoclinic.org/discussion/harnessing-the-immune-system-to-fight-ovarian-cancer/ (accessed on 23 December 2024).
- Rajtak, A.; Ostrowska-Leśko, M.; Żak, K.; Tarkowski, R.; Kotarski, J.; Okła, K. Integration of Local and Systemic Immunity in Ovarian Cancer: Implications for Immunotherapy. Front. Immunol. 2022, 13, 1018256. [Google Scholar] [CrossRef] [PubMed]
- Loret, N.; Denys, H.; Tummers, P.; Berx, G. The Role of Epithelial-to-Mesenchymal Plasticity in Ovarian Cancer Progression and Therapy Resistance. Cancers 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Chen, H.; Zhu, J.Y.; Wang, W.; Teng, Y.; Ding, H.-F.; Jing, Q.; Su, S.-B.; Huang, S. Epithelial-Mesenchymal Transition of Ovarian Cancer Cells Is Sustained by Rac1 through Simultaneous Activation of MEK1/2 and Src Signaling Pathways. Oncogene 2017, 36, 1546–1558. [Google Scholar] [CrossRef]
- Preston, C.C.; Goode, E.L.; Hartmann, L.C.; Kalli, K.R.; Knutson, K.L. Immunity and Immune Suppression in Human Ovarian Cancer. Immunotherapy 2011, 3, 539–556. [Google Scholar] [CrossRef]
- Immune Cells Within Ovarian Tumors Linked to Survival—NCI. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2017/ovarian-cancer-tils-survival (accessed on 23 December 2024).
- Launonen, I.-M.; Niemiec, I.; Hincapié-Otero, M.; Erkan, E.P.; Junquera, A.; Afenteva, D.; Falco, M.M.; Liang, Z.; Salko, M.; Chamchougia, F.; et al. Chemotherapy Induces Myeloid-Driven Spatially Confined T Cell Exhaustion in Ovarian Cancer. Cancer Cell 2024, 42, 2045–2063.e10. [Google Scholar] [CrossRef]
- Blanc-Durand, F.; Pautier, P.; Michels, J.; Leary, A. Targeting the Immune Microenvironment in Ovarian Cancer Therapy—Mission Impossible? ESMO Open 2024, 9, 102936. [Google Scholar] [CrossRef]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of Ovarian Cancer Antigen CA125/MUC16 to Mesothelin Mediates Cell Adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Gnjatic, S.; Aghajanian, C.; Konner, J.; Kim, S.H.; Sarasohn, D.; Soldan, K.; Tew, W.P.; Sarlis, N.J.; Zamarin, D.; et al. Phase I Study of a Multivalent WT1 Peptide Vaccine (Galinpepimut-S) in Combination with Nivolumab in Patients with WT1-Expressing Ovarian Cancer in Second or Third Remission. Cancers 2023, 15, 1458. [Google Scholar] [CrossRef]
- Kyi, C.; Doubrovina, E.; Zhou, Q.; Kravetz, S.; Iasonos, A.; Aghajanian, C.; Sabbatini, P.; Spriggs, D.; O’Reilly, R.J.; O’Cearbhaill, R.E. Phase I Dose Escalation Safety and Feasibility Study of Autologous WT1-Sensitized T Cells for the Treatment of Patients with Recurrent Ovarian Cancer. J. Immunother. Cancer 2021, 9, e002752. [Google Scholar] [CrossRef]
- Chow, S.; Berek, J.S.; Dorigo, O. Development of Therapeutic Vaccines for Ovarian Cancer. Vaccines 2020, 8, 657. [Google Scholar] [CrossRef]
- Hilliard, T.S. The Impact of Mesothelin in the Ovarian Cancer Tumor Microenvironment. Cancers 2018, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Wei, Y.; Zhao, Y.; Zhang, T.; Ma, X. The Role of Cancer-Associated Mesothelial Cells in the Progression and Therapy of Ovarian Cancer. Front. Immunol. 2022, 13, 1013506. [Google Scholar] [CrossRef]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galuzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef]
- Ghisoni, E.; Morotti, M.; Sarivalasis, A.; Grimm, A.J.; Kandalaft, L.; Laniti, D.D.; Coukos, G. Immunotherapy for Ovarian Cancer: Towards a Tailored Immunophenotype-Based Approach. Nat. Rev. Clin. Oncol. 2024, 21, 801–817. [Google Scholar] [CrossRef]
- Lasek, W. Cancer Immunoediting Hypothesis: History, Clinical Implications and Controversies. Cent. Eur. J. Immunol. 2022, 47, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Romanchik, D.; Albukhari, A.; Artibani, M.; Ahmed, A.A. Role of Immunotherapy in Ovarian Cancer: A Narrative Review. Gynecol. Pelvic Med. 2022, 5, 33. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Yang, J.; Zhao, X.; Wei, X. Tumor Microenvironment in Ovarian Cancer: Function and Therapeutic Strategy. Front. Cell Dev. Biol. 2020, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Garlisi, B.; Lauks, S.; Aitken, C.; Ogilvie, L.M.; Lockington, C.; Petrik, D.; Eichhorn, J.S.; Petrik, J. The Complex Tumor Microenvironment in Ovarian Cancer: Therapeutic Challenges and Opportunities. Curr. Oncol. 2024, 31, 3826–3844. [Google Scholar] [CrossRef]
- Blanc-Durand, F.; Clemence Wei Xian, L.; Tan, D.S.P. Targeting the Immune Microenvironment for Ovarian Cancer Therapy. Front. Immunol. 2023, 14, 1328651. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Ma, Y.; Zhang, Y. Recent Advances in Understanding the Immune Microenvironment in Ovarian Cancer. Front. Immunol. 2024, 15, 1412328. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Wang, Y.; Zhao, H.; Du, Y. The Role of Cancer-Associated Fibroblasts in Ovarian Cancer. Cancers 2022, 14, 2637. [Google Scholar] [CrossRef]
- Fang, Y.; Xiao, X.; Wang, J.; Dasari, S.; Pepin, D.; Nephew, K.P.; Zamarin, D.; Mitra, A.K. Cancer Associated Fibroblasts Serve as an Ovarian Cancer Stem Cell Niche through Noncanonical Wnt5a Signaling. npj Precis. Onc. 2024, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Axemaker, H.; Plesselova, S.; Calar, K.; Jorgensen, M.; Wollman, J.; de la Puente, P. Reprogramming of Normal Fibroblasts into Ovarian Cancer-Associated Fibroblasts via Non-Vesicular Paracrine Signaling Induces an Activated Fibroblast Phenotype. Biochim. Biophys. Acta BBA Mol. Cell Res. 2024, 1871, 119801. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Howell, V.M.; Colvin, E.K. The Extracellular Matrix in Epithelial Ovarian Cancer—A Piece of a Puzzle. Front. Oncol. 2015, 5, 245. [Google Scholar] [CrossRef] [PubMed]
- Puttock, E.H.; Tyler, E.J.; Manni, M.; Maniati, E.; Butterworth, C.; Burger Ramos, M.; Peerani, E.; Hirani, P.; Gauthier, V.; Liu, Y.; et al. Extracellular Matrix Educates an Immunoregulatory Tumor Macrophage Phenotype Found in Ovarian Cancer Metastasis. Nat. Commun. 2023, 14, 2514. [Google Scholar] [CrossRef]
- Brown, Y.; Hua, S.; Tanwar, P.S. Extracellular Matrix in High-Grade Serous Ovarian Cancer: Advances in Understanding of Carcinogenesis and Cancer Biology. Matrix Biol. 2023, 118, 16–46. [Google Scholar] [CrossRef]
- Yin, H.; Wang, J.; Li, H.; Yu, Y.; Wang, X.; Lu, L.; Lv, C.; Chang, B.; Jin, W.; Guo, W.; et al. Extracellular Matrix Protein-1 Secretory Isoform Promotes Ovarian Cancer through Increasing Alternative mRNA Splicing and Stemness. Nat. Commun. 2021, 12, 4230. [Google Scholar] [CrossRef]
- Gertych, A.; Walts, A.E.; Cheng, K.; Liu, M.; John, J.; Lester, J.; Karlan, B.Y.; Orsulic, S. Dynamic Changes in the Extracellular Matrix in Primary, Metastatic, and Recurrent Ovarian Cancers. Cells 2022, 11, 3769. [Google Scholar] [CrossRef]
- Nadiarnykh, O.; LaComb, R.B.; Brewer, M.A.; Campagnola, P.J. Alterations of the Extracellular Matrix in Ovarian Cancer Studied by Second Harmonic Generation Imaging Microscopy. BMC Cancer 2010, 10, 94. [Google Scholar] [CrossRef]
- Markowska, A.; Sawicki, W.; Zurawski, J.; Fechner, J.; Markowska, J. The Role of Selected Molecular Factors in Ovarian Cancer Metastasis. Ginekol. Pol. 2022, 93, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, W.; Wertel, I.; Marzec-Kotarska, B.; Ćwiklińska, A.; Kotarski, J. The Evaluation of Selected Clinical and Biochemical Parameters in Women with Ovarian Cancer in a 5-Year Observation Period. Menopause Rev. 2011, 10, 106–113. [Google Scholar]
- Nakayama, K.; Nakayama, N.; Katagiri, H.; Miyazaki, K. Mechanisms of Ovarian Cancer Metastasis: Biochemical Pathways. Int. J. Mol. Sci. 2012, 13, 11705–11717. [Google Scholar] [CrossRef]
- Fahmi, M.N.; Pradjatmo, H.; Astuti, I.; Nindrea, R.D. Cytokines as Prognostic Biomarkers of Epithelial Ovarian Cancer (EOC): A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2021, 22, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Cancer and Inflammation. Part 1. Pro-Inflammatory Cytokines. Available online: https://progressinhealthsciences.publisherspanel.com/article/01.3001.0012.1329/en (accessed on 23 December 2024).
- Browning, L.; Patel, M.R.; Horvath, E.B.; Tawara, K.; Jorcyk, C.L. IL-6 and Ovarian Cancer: Inflammatory Cytokines in Promotion of Metastasis. Cancer Manag. Res. 2018, 10, 6685–6693. [Google Scholar] [CrossRef]
- Luo, X.; Xu, J.; Yu, J.; Yi, P. Shaping Immune Responses in the Tumor Microenvironment of Ovarian Cancer. Front. Immunol. 2021, 12, 692360. [Google Scholar] [CrossRef]
- Yabuno, A.; Matsushita, H.; Hamano, T.; Tan, T.Z.; Shintani, D.; Fujieda, N.; Tan, D.S.P.; Huang, R.Y.-J.; Fujiwara, K.; Kakimi, K.; et al. Identification of Serum Cytokine Clusters Associated with Outcomes in Ovarian Clear Cell Carcinoma. Sci. Rep. 2020, 10, 18503. [Google Scholar] [CrossRef]
- Bose, S.; Saha, P.; Chatterjee, B.; Srivastava, A.K. Chemokines Driven Ovarian Cancer Progression, Metastasis and Chemoresistance: Potential Pharmacological Targets for Cancer Therapy. Semin. Cancer Biol. 2022, 86, 568–579. [Google Scholar] [CrossRef]
- Barbieri, F.; Bajetto, A.; Florio, T. Role of Chemokine Network in the Development and Progression of Ovarian Cancer: A Potential Novel Pharmacological Target. J. Oncol. 2010, 2010, 426956. [Google Scholar] [CrossRef]
- Popple, A.; Durrant, L.G.; Spendlove, I.; Rolland, P.; Scott, I.V.; Deen, S.; Ramage, J.M. The Chemokine, CXCL12, Is an Independent Predictor of Poor Survival in Ovarian Cancer. Br. J. Cancer 2012, 106, 1306–1313. [Google Scholar] [CrossRef]
- Huang, X.; Hao, J.; Tan, Y.Q.; Zhu, T.; Pandey, V.; Lobie, P.E. CXC Chemokine Signaling in Progression of Epithelial Ovarian Cancer: Theranostic Perspectives. Int. J. Mol. Sci. 2022, 23, 2642. [Google Scholar] [CrossRef] [PubMed]
- Klemba, A.; Bodnar, L.; Was, H.; Brodaczewska, K.K.; Wcislo, G.; Szczylik, C.A.; Kieda, C. Hypoxia-Mediated Decrease of Ovarian Cancer Cells Reaction to Treatment: Significance for Chemo- and Immunotherapies. Int. J. Mol. Sci. 2020, 21, 9492. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Cheng, X.; Li, L.; Tu, K. Hypoxia Promotes the Growth and Metastasis of Ovarian Cancer Cells by Suppressing Ferroptosis via Upregulating SLC2A12. Exp. Cell Res. 2023, 433, 113851. [Google Scholar] [CrossRef]
- Shih, H.-J.; Chang, H.-F.; Chen, C.-L.; Torng, P.-L. Differential Expression of Hypoxia-Inducible Factors Related to the Invasiveness of Epithelial Ovarian Cancer. Sci. Rep. 2021, 11, 22925. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Matuszewska, K.; Jamieson, C.; Petrik, J. Characterizing Endocrine Status, Tumor Hypoxia and Immunogenicity for Therapy Success in Epithelial Ovarian Cancer. Front. Endocrinol. 2021, 12, 772349. [Google Scholar] [CrossRef]
- Vergara, D.; Merlot, B.; Lucot, J.-P.; Collinet, P.; Vinatier, D.; Fournier, I.; Salzet, M. Epithelial–Mesenchymal Transition in Ovarian Cancer. Cancer Lett. 2010, 291, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, Z.; Wang, J.; Jiang, J.; Lin, B. An EMT-Based Gene Signature Enhances the Clinical Understanding and Prognostic Prediction of Patients with Ovarian Cancers. J. Ovarian Res. 2023, 16, 51. [Google Scholar] [CrossRef]
- Pasupulati, A.K.; Nishad, R.; Nakuluri, K.; Motrapu, M. Epithelial–mesenchymal Transition of Glomerular Podocytes: Implications in Proteinuria. MGM J. Med Sci. 2024, 4, 26–34. [Google Scholar] [CrossRef]
- Chirshev, E.; Hojo, N.; Bertucci, A.; Sanderman, L.; Nguyen, A.; Wang, H.; Suzuki, T.; Brito, E.; Martinez, S.R.; Castañón, C.; et al. Epithelial/Mesenchymal Heterogeneity of High-grade Serous Ovarian Carcinoma Samples Correlates with miRNA Let-7 Levels and Predicts Tumor Growth and Metastasis. Mol. Oncol. 2020, 14, 2796–2813. [Google Scholar] [CrossRef]
- Sohn, M.-H.; Kim, S.I.; Shin, J.-Y.; Kim, H.S.; Chung, H.H.; Kim, J.-W.; Lee, M.; Seo, J.-S. Classification of High-Grade Serous Ovarian Carcinoma by Epithelial-to-Mesenchymal Transition Signature and Homologous Recombination Repair Genes. Genes 2021, 12, 1103. [Google Scholar] [CrossRef]
- Jie, X.-X.; Zhang, M.; Du, M.; Cai, Q.-Q.; Cong, Q.; Xu, C.-J.; Zhang, X.-Y. Detection of Circulating Tumor Cells and Evaluation of Epithelial-Mesenchymal Transition Patterns of Circulating Tumor Cells in Ovarian Cancer. Transl. Cancer Res. 2022, 11, 2636–2646. [Google Scholar] [CrossRef]
- Xie, W.; Yu, J.; Yin, Y.; Zhang, X.; Zheng, X.; Wang, X. OCT4 Induces EMT and Promotes Ovarian Cancer Progression by Regulating the PI3K/AKT/mTOR Pathway. Front. Oncol. 2022, 12, 876257. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, K.; Li, S.; Tang, S.; Ren, Y.; Cen, Y.; Fei, W.; Zhang, B.; Shen, Y.; et al. Single-Cell RNA Sequencing Reveals the Tissue Architecture in Human High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2022, 28, 3590–3602. [Google Scholar] [CrossRef] [PubMed]
- Yakubovich, E.; Cook, D.P.; Rodriguez, G.M.; Vanderhyden, B.C. Mesenchymal Ovarian Cancer Cells Promote CD8+ T Cell Exhaustion through the LGALS3-LAG3 Axis. npj Syst. Biol. Appl. 2023, 9, 61. [Google Scholar] [CrossRef]
- Deng, Y.; Tan, Y.; Zhou, D.; Bai, Y.; Cao, T.; Zhong, C.; Huang, W.; Ou, Y.; Guo, L.; Liu, Q.; et al. Single-Cell RNA-Sequencing Atlas Reveals the Tumor Microenvironment of Metastatic High-Grade Serous Ovarian Carcinoma. Front. Immunol. 2022, 13, 923194. [Google Scholar] [CrossRef] [PubMed]
- Teeuwssen, M.; Fodde, R. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.L.; Hough, R.; Bernaudo, S.; Peng, C. Wnt/β-Catenin Signalling in Ovarian Cancer: Insights into Its Hyperactivation and Function in Tumorigenesis. J. Ovarian Res. 2019, 12, 122. [Google Scholar] [CrossRef]
- Lin, L.H.; Zamuco, R.D.; Shukla, P.S. Ovarian Clear Cell Carcinoma and Markers of Epithelial-Mesenchymal Transition (EMT): Immunohistochemical Characterization of Tumor Budding. Int. J. Gynecol. Pathol. 2023, 42, 602–612. [Google Scholar] [CrossRef]
- Kuroda, Y.; Chiyoda, T.; Kawaida, M.; Nakamura, K.; Aimono, E.; Yoshimura, T.; Takahashi, M.; Saotome, K.; Yoshihama, T.; Iwasa, N.; et al. ARID1A Mutation/ARID1A Loss Is Associated with a High Immunogenic Profile in Clear Cell Ovarian Cancer. Gynecol. Oncol. 2021, 162, 679–685. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, C.; Xu, Q.; An, Z.; Xu, S.; Xuan, G.; Lin, C.; Tang, C. ARID1A Restrains EMT and Stemness of Ovarian Cancer Cells through the Hippo Pathway. Int. J. Oncol. 2024, 65, 76. [Google Scholar] [CrossRef]
- Katagiri, A.; Nakayama, K.; Rahman, M.T.; Rahman, M.; Katagiri, H.; Nakayama, N.; Ishikawa, M.; Ishibashi, T.; Iida, K.; Kobayashi, H.; et al. Loss of ARID1A Expression Is Related to Shorter Progression-Free Survival and Chemoresistance in Ovarian Clear Cell Carcinoma. Mod. Pathol. 2012, 25, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Kyran, E.L.; Bedo, J.; Wakefield, M.J.; Ennis, D.P.; Mirza, H.B.; Vandenberg, C.J.; Lieschke, E.; Farrell, A.; Hadla, A.; et al. Epithelial-to-Mesenchymal Transition Supports Ovarian Carcinosarcoma Tumorigenesis and Confers Sensitivity to Microtubule Targeting with Eribulin. Cancer Res. 2022, 82, 4457–4473. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Haslehurst, A.M.; Koti, M.; Dharsee, M.; Nuin, P.; Evans, K.; Geraci, J.; Childs, T.; Chen, J.; Li, J.; Weberpals, J.; et al. EMT Transcription Factors Snail and Slug Directly Contribute to Cisplatin Resistance in Ovarian Cancer. BMC Cancer 2012, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Conant, A.; Curow, C.; Alexander, A.; Ioffe, Y.; Unternaehrer, J.J. Role of Epithelial-Mesenchymal Transition Factor SNAI1 and Its Targets in Ovarian Cancer Aggressiveness. J. Cancer Metastasis Treat. 2023, 9, 25. [Google Scholar] [CrossRef]
- Kielbik, M.; Przygodzka, P.; Szulc-Kielbik, I.; Klink, M. Snail Transcription Factors as Key Regulators of Chemoresistance, Stemness and Metastasis of Ovarian Cancer Cells. Biochim. Biophys. Acta BBA Rev. Cancer 2023, 1878, 189003. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Liu, S. RUNX1-Regulated Signaling Pathways in Ovarian Cancer. Biomedicines 2023, 11, 2357. [Google Scholar] [CrossRef]
- Perez-Fidalgo, J.A.; Ortega, B.; Simon, S.; Samartzis, E.P.; Boussios, S. NOTCH Signalling in Ovarian Cancer Angiogenesis. Ann. Transl. Med. 2020, 8, 1705. [Google Scholar] [CrossRef]
- Koutsaki, M.; Libra, M.; Spandidos, D.A.; Zaravinos, A. The miR-200 Family in Ovarian Cancer. Oncotarget 2017, 8, 66629–66640. [Google Scholar] [CrossRef]
- Choi, P.-W.; Ng, S.-W. The Functions of MicroRNA-200 Family in Ovarian Cancer: Beyond Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2017, 18, 1207. [Google Scholar] [CrossRef]
- Schweer, D.; McAtee, A.; Neupane, K.; Richards, C.; Ueland, F.; Kolesar, J. Tumor-Associated Macrophages and Ovarian Cancer: Implications for Therapy. Cancers 2022, 14, 2220. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-Y.; Xie, H.; Yuan, J.; Jiang, X.-Y.; Yong, J.-H.; Zeng, D.; Dou, Y.-Y.; Xiao, S.-S. M2-like Tumor-Associated Macrophages-Secreted EGF Promotes Epithelial Ovarian Cancer Metastasis via Activating EGFR-ERK Signaling and Suppressing lncRNA LIMT Expression. Cancer Biol. Ther. 2019, 20, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like Tumor-Associated Macrophages Is a Potential Therapeutic Approach to Overcome Antitumor Drug Resistance. npj Precis. Onc. 2024, 8, 31. [Google Scholar] [CrossRef]
- Brauneck, F.; Oliveira-Ferrer, L.; Muschhammer, J.; Sturmheit, T.; Ackermann, C.; Haag, F.; Schulze zur Wiesch, J.; Ding, Y.; Qi, M.; Hell, L.; et al. Immunosuppressive M2 TAMs Represent a Promising Target Population to Enhance Phagocytosis of Ovarian Cancer Cells in Vitro. Front. Immunol. 2023, 14, 1250258. [Google Scholar] [CrossRef] [PubMed]
- Asem, M.; Young, A.M.; Oyama, C.; Claure De La Zerda, A.; Liu, Y.; Yang, J.; Hilliard, T.S.; Johnson, J.; Harper, E.I.; Guldner, I.; et al. Host Wnt5a Potentiates Microenvironmental Regulation of Ovarian Cancer Metastasis. Cancer Res. 2020, 80, 1156–1170. [Google Scholar] [CrossRef]
- Abedini, A.; Sayed, C.; Carter, L.E.; Boerboom, D.; Vanderhyden, B.C. Non-Canonical WNT5a Regulates Epithelial-to-Mesenchymal Transition in the Mouse Ovarian Surface Epithelium. Sci. Rep. 2020, 10, 9695. [Google Scholar] [CrossRef]
- Dehghani-Ghobadi, Z.; Sheikh Hasani, S.; Arefian, E.; Hossein, G. Wnt5A and TGFβ1 Converges through YAP1 Activity and Integrin Alpha v Up-Regulation Promoting Epithelial to Mesenchymal Transition in Ovarian Cancer Cells and Mesothelial Cell Activation. Cells 2022, 11, 237. [Google Scholar] [CrossRef]
- Etzerodt, A.; Moulin, M.; Doktor, T.K.; Delfini, M.; Mossadegh-Keller, N.; Bajenoff, M.; Sieweke, M.H.; Moestrup, S.K.; Auphan-Anezin, N.; Lawrence, T. Tissue-Resident Macrophages in Omentum Promote Metastatic Spread of Ovarian Cancer. J. Exp. Med. 2020, 217, e20191869. [Google Scholar] [CrossRef]
- Zhang, M.; He, Y.; Sun, X.; Li, Q.; Wang, W.; Zhao, A.; Di, W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 2014, 7, 19. [Google Scholar] [CrossRef]
- Reinartz, S.; Schumann, T.; Finkernagel, F.; Wortmann, A.; Jansen, J.M.; Meissner, W.; Krause, M.; Schwörer, A.M.; Wagner, U.; Müller-Brüsselbach, S.; et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. Int. J. Cancer 2014, 134, 32–42. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Li, D.; Mao, Y.; Mo, F.; Du, W.; Ma, X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2017, 147, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Fanale, D.; Dimino, A.; Pedone, E.; Brando, C.; Corsini, L.R.; Filorizzo, C.; Fiorino, A.; Lisanti, M.C.; Magrin, L.; Randazzo, U.; et al. Prognostic and Predictive Role of Tumor-Infiltrating Lymphocytes (TILs) in Ovarian Cancer. Cancers 2022, 14, 4344. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Le Guellec, S.; Meignan, S.; Bécourt, S.; Pasquesoone, C.; El Hajj, H.; Martínez-Gómez, C.; Leblanc, É.; Narducci, F.; Ladoire, S. Tumor-Infiltrating Lymphocytes (TILs) in Epithelial Ovarian Cancer: Heterogeneity, Prognostic Impact, and Relationship with Immune Checkpoints. Cancers 2022, 14, 5332. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic Significance of Tumor-Infiltrating T Cells in Ovarian Cancer: A Meta-Analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Ovarian Tumor Tissue Analysis (OTTA) Consortium; Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Tołoczko, A.; Hein, A.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef]
- Karakaya, Y.A.; Atıgan, A.; Güler, Ö.T.; Demiray, A.G.; Bir, F. The Relation of CD3, CD4, CD8 and PD-1 Expression with Tumor Type and Prognosis in Epithelial Ovarian Cancers. Ginekol. Pol. 2021, 92, 344–351. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Zhang, W.; Fan, J.; Zhou, Y.; Li, W.; Yin, J.; Yang, X.; Guo, E.; Li, X.; et al. Spatial heterogeneity of infiltrating T cells in high-grade serous ovarian cancer revealed by multi-omics analysis. Cell Rep. Med. 2022, 3, 100856. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, Y.; Wan, T.; Deng, T.; Huang, H.; Liu, J. Significance of CD47 and Its Association With Tumor Immune Microenvironment Heterogeneity in Ovarian Cancer. Front. Immunol. 2021, 13, 768115. [Google Scholar] [CrossRef]
- Liu, C.; Wang, D.; Huang, X.; Song, Z.; Ye, L.; Zhou, G. The expression and clinical significance of cytokines Th1, Th2, and Th17 in ovarian cancer. Am. J. Med. Sci. 2024, 25, 346–353. [Google Scholar] [CrossRef]
- Ouyang, P.; Wang, L.; Wu, J.; Tian, Y.; Chen, C.; Li, D.; Yao, Z.; Chen, R.; Xiang, G.; Gong, J.; et al. Overcoming Cold Tumors: A Combination Strategy of Immune Checkpoint Inhibitors. Front. Immunol. 2024, 15, 1344272. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Coupland, S.E.; Aittokallio, T.; Figueiredo, C.R. Resistance to Immune Checkpoint Therapies by Tumour-Induced T-Cell Desertification and Exclusion: Key Mechanisms, Prognostication and New Therapeutic Opportunities. Br. J. Cancer 2023, 129, 1212–1224. [Google Scholar] [CrossRef]
- Zsiros, E.; Tanyi, J.; Balint, K.; Kandalaft, L.E. Immunotherapy for ovarian cancer. Curr. Opin. Oncol. 2024, 26, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Laumont, C.M.; Wouters, M.C.A.; Smazynski, J.; Gierc, N.S.; Chavez, E.A.; Chong, L.C.; Thornton, S.; Milne, K.; Webb, J.R.; Steidl, C.; et al. Single-Cell Profiles and Prognostic Impact of Tumor-Infiltrating Lymphocytes Coexpressing CD39, CD103, and PD-1 in Ovarian Cancer. Clin. Cancer Res. 2021, 27, 4089–4100. [Google Scholar] [CrossRef] [PubMed]
- Witt, M.; Oliveira-Ferrer, L.; Koch-Nolte, F.; Menzel, S.; Hell, L.; Sturmheit, T.; Seubert, E.; Weimer, P.; Ding, Y.; Qi, M.; et al. Expression of CD39 Is Associated with T Cell Exhaustion in Ovarian Cancer and Its Blockade Reverts T Cell Dysfunction. Oncoimmunology 2024, 13, 2346359. [Google Scholar] [CrossRef]
- Duhen, T.; Duhen, R.; Montler, R.; Moses, J.; Moudgil, T.; de Miranda, N.F.; Goodall, C.P.; Blair, T.C.; Fox, B.A.; McDermott, J.E.; et al. Co-Expression of CD39 and CD103 Identifies Tumor-Reactive CD8 T Cells in Human Solid Tumors. Nat. Commun. 2018, 9, 2724. [Google Scholar] [CrossRef]
- Ye, S.; Chen, W.; Zheng, Y.; Wu, Y.; Xiang, L.; Li, T.; Ping, B.; Zhang, X.; Yang, H. Peripheral lymphocyte populations in ovarian cancer patients and correlations with clinicopathological features. J. Ovarian Res. 2022, 15, 43. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Wu, X.; Zhang, T.; Zhu, Q.; Wang, X.; Wang, H.; Wang, K.; Lin, Y.; Wang, X. Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol. Res. 2018, 6, 1578–1592. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, X.; Feng, J.; Yan, R.; Xi, J. Machine Learning-Assisted Analysis of Epithelial Mesenchymal Transition Pathway for Prognostic Stratification and Immune Infiltration Assessment in Ovarian Cancer. Front. Endocrinol. 2023, 14, 1196094. [Google Scholar] [CrossRef]
- Govindaraj, C.; Scalzo-Inguanti, K.; Madondo, M.; Hallo, J.; Flanagan, K.; Quinn, M.; Plebanski, M. Impaired Th1 Immunity in Ovarian Cancer Patients Is Mediated by TNFR2 + Tregs within the Tumor Microenvironment. Clin. Immunol. 2013, 149, 97–110. [Google Scholar] [CrossRef]
- Su, H.; Jin, Y.; Tao, C.; Yang, H.; Yang, E.; Zhang, W.-G.; Feng, F. Th2 Cells Infiltrating High-Grade Serous Ovarian Cancer: A Feature That May Account for the Poor Prognosis. J. Gynecol. Oncol. 2023, 34, e48. [Google Scholar] [CrossRef]
- Kusuda, T.; Shigemasa, K.; Arihiro, K.; Fujii, T.; Nagai, N.; Ohama, K. Relative Expression Levels of Th1 and Th2 Cytokine mRNA Are Independent Prognostic Factors in Patients with Ovarian Cancer. Oncol. Rep. 2005, 13, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Bilska, M.; Pawłowska, A.; Zakrzewska, E.; Chudzik, A.; Suszczyk, D.; Gogacz, M.; Wertel, I. Th17 Cells and IL-17 As Novel Immune Targets in Ovarian Cancer Therapy. J. Oncol. 2020, 2020, 8797683. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, F.; Lieber, S.; Shinkevich, V.; Steitz, A.M.; Raifer, H.; Roth, K.; Finkernagel, F.; Worzfeld, T.; Burchert, A.; Keber, C.; et al. Reciprocal Crosstalk between Th17 and Mesothelial Cells Promotes Metastasis-associated Adhesion of Ovarian Cancer Cells. Clin. Transl. Med. 2024, 14, e1604. [Google Scholar] [CrossRef]
- Luo, Y.; Shreeder, B.; Jenkins, J.W.; Shi, H.; Lamichhane, P.; Zhou, K.; Bahr, D.A.; Kurian, S.; Jones, K.A.; Daum, J.I.; et al. Th17-Inducing Dendritic Cell Vaccines Stimulate Effective CD4 T Cell-Dependent Antitumor Immunity in Ovarian Cancer That Overcomes Resistance to Immune Checkpoint Blockade. J. Immunother. Cancer 2023, 11, e007661. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.; Woś, J.; Karczmarczyk, A.; Miotła, P.; Gogacz, M.; Skorupska, K.; Rechberger, T.; Tabarkiewicz, J.; Wolińska, E.; Skrzypczak, M. An Association of Circulating Tregs and Th17 Cells Producing IL-21 and IL-22 with the ROMA in Ovarian Cancer Patients. Cytokine 2020, 134, 155194. [Google Scholar] [CrossRef]
- Chen, T.; Guo, J.; Cai, Z.; Li, B.; Sun, L.; Shen, Y.; Wang, S.; Wang, Z.; Wang, Z.; Wang, Y.; et al. Th9 Cell Differentiation and Its Dual Effects in Tumor Development. Front. Immunol. 2020, 11, 1026. [Google Scholar] [CrossRef]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and Inflammation: Inseparable Actors of Cancer Progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Horta, C.A.; Yang, J. Regulation of Epithelial-Mesenchymal Transition by Tumor Microenvironmental Signals and Its Implication in Cancer Therapeutics. Semin. Cancer Biol. 2023, 88, 46–66. [Google Scholar] [CrossRef]
- Salazar, Y.; Zheng, X.; Brunn, D.; Raifer, H.; Picard, F.; Zhang, Y.; Winter, H.; Guenther, S.; Weigert, A.; Weigmann, B.; et al. Microenvironmental Th9 and Th17 lymphocytes induce metastatic spreading in lung cancer. J. Clin. Invest. 2020, 130, 3560–3575. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Xing, H.; Wang, L.; Zhang, G.; Yu, N.; Wang, J.; Guo, W.; Jiang, J. Elevated Th22 Cells and Related Cytokines in Patients with Epithelial Ovarian Cancer. Medicine 2017, 96, e8359. [Google Scholar] [CrossRef] [PubMed]
- Oncoscience|Chemokines and Cellular Plasticity of Ovarian Cancer Stem Cells. Available online: https://www.oncoscience.us/article/181/text/ (accessed on 23 December 2024).
- Davidson, B.; Trope, C.G.; Reich, R. Epithelial–Mesenchymal Transition in Ovarian Carcinoma. Front. Oncol. 2012, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Nagano, O.; Ishimoto, T.; Yae, T.; Suzuki, Y.; Shinoda, T.; Nakamura, S.; Niwa, S.; Ikeda, S.; Koga, H.; et al. Tumor necrosis factor-α regulates transforming growth factor-β-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J. Biol. Chem. 2010, 285, 4060–4073. [Google Scholar] [CrossRef]
- Alwosaibai, K.; Abedini, A.; Al-Hujaily, E.M.; Tang, Y.; Garson, K.; Collins, O.; Vanderhyden, B.C. PAX2 Maintains the Differentiation of Mouse Oviductal Epithelium and Inhibits the Transition to a Stem Cell-like State. Oncotarget 2017, 8, 76881–76897. [Google Scholar] [CrossRef]
- Zhou, J.; Du, Y.; Lu, Y.; Luan, B.; Xu, C.; Yu, Y.; Zhao, H. CD44 Expression Predicts Prognosis of Ovarian Cancer Patients Through Promoting Epithelial-Mesenchymal Transition (EMT) by Regulating Snail, ZEB1, and Caveolin-1. Front. Oncol. 2019, 9, 802. [Google Scholar] [CrossRef]
- Martincuks, A.; Li, P.-C.; Zhao, Q.; Zhang, C.; Li, Y.-J.; Yu, H.; Rodriguez-Rodriguez, L. CD44 in Ovarian Cancer Progression and Therapy Resistance—A Critical Role for STAT3. Front. Oncol. 2020, 10, 589601. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.C.; Hollemans, E.; Ezendam, N.; Feijen, H.; Boll, D.; Pijlman, B.; van der Putten, H.; Klinkhamer, P.; van Kuppevelt, T.H.; van der Wurff, A.A.M.; et al. MMP-14 and CD44 in Epithelial-to-Mesenchymal Transition (EMT) in Ovarian Cancer. J. Ovarian Res. 2016, 9, 53. [Google Scholar] [CrossRef]
- Lin, J.; Ding, D. The Prognostic Role of the Cancer Stem Cell Marker CD44 in Ovarian Cancer: A Meta-Analysis. Cancer Cell Int. 2017, 17, 8. [Google Scholar] [CrossRef]
- Lampropoulou, D.I.; Papadimitriou, M.; Papadimitriou, C.; Filippou, D.; Kourlaba, G.; Aravantinos, G.; Gazouli, M. The Role of EMT-Related lncRNAs in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 10079. [Google Scholar] [CrossRef]
- Strauss, R.; Li, Z.-Y.; Liu, Y.; Beyer, I.; Persson, J.; Sova, P.; Möller, T.; Pesonen, S.; Hemminki, A.; Hamerlik, P.; et al. Analysis of Epithelial and Mesenchymal Markers in Ovarian Cancer Reveals Phenotypic Heterogeneity and Plasticity. PLoS ONE 2011, 6, e16186. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Pre-Metastatic Niche: Formation, Characteristics and Therapeutic Implication|Signal Transduction and Targeted Therapy. Available online: https://www.nature.com/articles/s41392-024-01937-7 (accessed on 23 December 2024).
- Nan, X.; Wang, J.; Liu, H.N.; Wong, S.T.C.; Zhao, H. Epithelial-Mesenchymal Plasticity in Organotropism Metastasis and Tumor Immune Escape. J. Clin. Med. 2019, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Jinesh, G.G.; Brohl, A.S. Classical Epithelial-Mesenchymal Transition (EMT) and Alternative Cell Death Process-Driven Blebbishield Metastatic-Witch (BMW) Pathways to Cancer Metastasis. Signal Transduct. Target. Ther. 2022, 7, 296. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Frezza, C. Metabolic Reprogramming and Epithelial-to-Mesenchymal Transition in Cancer. FEBS J. 2017, 284, 3132–3144. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhou, X.; Ni, Y.; Zhao, X.; Liang, X. Metabolic Reprogramming of the Tumor Immune Microenvironment in Ovarian Cancer: A Novel Orientation for Immunotherapy. Front. Immunol. 2022, 13, 1030831. [Google Scholar] [CrossRef]
- Ramesh, V.; Brabletz, T.; Ceppi, P. Targeting EMT in Cancer with Repurposed Metabolic Inhibitors. Trends Cancer 2020, 6, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, L.; Chen, H.; Hao, J.; Ni, J.; Chang, L.; Duan, W.; Graham, P.; Li, Y. Targeting Epithelial-Mesenchymal Transition and Cancer Stem Cells for Chemoresistant Ovarian Cancer. Oncotarget 2016, 7, 55771–55788. [Google Scholar] [CrossRef]
- Chebouti, I.; Kasimir-Bauer, S.; Buderath, P.; Wimberger, P.; Hauch, S.; Kimmig, R.; Kuhlmann, J.D. EMT-like Circulating Tumor Cells in Ovarian Cancer Patients Are Enriched by Platinum-Based Chemotherapy. Oncotarget 2017, 8, 48820–48831. [Google Scholar] [CrossRef]
- Padilla, M.A.A.; Binju, M.; Wan, G.; Rahmanto, Y.S.; Kaur, P.; Yu, Y. Relationship between Ovarian Cancer Stem Cells, Epithelial Mesenchymal Transition and Tumour Recurrence. Cancer Drug Resist. 2019, 2, 1127–1135. [Google Scholar] [CrossRef]
- Zhang, H.; Steed, A.; Co, M.; Chen, X. Cancer Stem Cells, Epithelial-Mesenchymal Transition, ATP and Their Roles in Drug Resistance in Cancer. Cancer Drug Resist. 2021, 4, 684–709. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Kalantari, M.; Mohammadinejad, R.; Javaheri, T.; Sethi, G. Association of the Epithelial–Mesenchymal Transition (EMT) with Cisplatin Resistance. Int. J. Mol. Sci. 2020, 21, 4002. [Google Scholar] [CrossRef] [PubMed]
- Burger, G.A.; Nesenberend, D.N.; Lems, C.M.; Hille, S.C.; Beltman, J.B. Bidirectional Crosstalk between Epithelial-Mesenchymal Plasticity and IFNγ-Induced PD-L1 Expression Promotes Tumor Progression. R. Soc. Open Sci. 2022, 9, 220186. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.P.; Tong, P.; Diao, L.; Cardnell, R.J.; Gibbons, D.L.; William, W.N.; Skoulidis, F.; Parra, E.R.; Rodriguez-Canales, J.; Wistuba, I.I.; et al. A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin. Cancer Res. 2016, 22, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, J.; Ruan, Y.; Sun, L.; Xu, C.; Jiang, H. Sialyltransferase ST3GAL1 Promotes Cell Migration, Invasion, and TGF-Β1-Induced EMT and Confers Paclitaxel Resistance in Ovarian Cancer. Cell Death Dis. 2018, 9, 1102. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Q.; Chang, K.; Bao, L.; Yi, X. Integrated Analysis of Ferroptosis-Related Biomarker Signatures to Improve the Diagnosis and Prognosis Prediction of Ovarian Cancer. Front. Cell Dev. Biol. 2022, 9, 807862. [Google Scholar] [CrossRef]
- Su, R.; Jin, C.; Zhou, L.; Cao, Y.; Kuang, M.; Li, L.; Xiang, J. Construction of a ceRNA Network of Hub Genes Affecting Immune Infiltration in Ovarian Cancer Identified by WGCNA. BMC Cancer 2021, 21, 970. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Qiang, W.; Ge, W. Stanniocalcin-1 in Tumor Immunity: Acts via Macrophages. Front. Immunol. 2024, 15, 1510182. [Google Scholar] [CrossRef]
- Perales-Puchalt, A.; Wojtak, K.; Duperret, E.K.; Yang, X.; Slager, A.M.; Yan, J.; Muthumani, K.; Montaner, L.J.; Weiner, D.B. Engineered DNA Vaccination against Follicle-Stimulating Hormone Receptor Delays Ovarian Cancer Progression in Animal Models. Mol. Ther. 2019, 27, 314–325. [Google Scholar] [CrossRef]
- Hartl, C.A.; Bertschi, A.; Puerto, R.B.; Andresen, C.; Cheney, E.M.; Mittendorf, E.A.; Guerriero, J.L.; Goldberg, M.S. Combination Therapy Targeting Both Innate and Adaptive Immunity Improves Survival in a Pre-Clinical Model of Ovarian Cancer. J. Immunother. Cancer 2019, 7, 199. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, Q.; Liu, Y.; Li, X.; Brock, M.V.; Chen, M.; Dong, L.; Shi, L.; Wang, Y.; Guo, M.; et al. The Safety, Efficacy, and Treatment Outcomes of a Combination of Low-Dose Decitabine Treatment in Patients with Recurrent Ovarian Cancer. OncoImmunology 2017, 6, e1323619. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Waggoner, S.; Vidal, G.A.; Mita, M.; Moroney, J.W.; Holloway, R.; Van Le, L.; Sachdev, J.C.; Chapman-Davis, E.; Colon-Otero, G.; et al. Single-Arm Phases 1 and 2 Trial of Niraparib in Combination With Pembrolizumab in Patients With Recurrent Platinum-Resistant Ovarian Carcinoma. JAMA Oncol. 2019, 5, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Herold, C.; Gray, K.P.; Penson, R.T.; Horowitz, N.; Konstantinopoulos, P.A.; Castro, C.M.; Hill, S.J.; Curtis, J.; Luo, W.; et al. Assessment of Combined Nivolumab and Bevacizumab in Relapsed Ovarian Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 1731–1738. [Google Scholar] [CrossRef]
- Cai, J.; Gong, L.; Li, G.; Guo, J.; Yi, X.; Wang, Z. Exosomes in Ovarian Cancer Ascites Promote Epithelial–Mesenchymal Transition of Ovarian Cancer Cells by Delivery of miR-6780b-5p. Cell Death Dis. 2021, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, E.; Imbimbo, M.; Zimmermann, S.; Valabrega, G. Ovarian Cancer Immunotherapy: Turning up the Heat. Int. J. Mol. Sci. 2019, 20, 2927. [Google Scholar] [CrossRef]
- Peng, H.; He, X.; Wang, Q. Targeted Drug Delivery System for Ovarian Cancer Microenvironment: Improving the Effects of Immunotherapy. Front. Immunol. 2022, 13, 1035997. [Google Scholar] [CrossRef]
- Yang, C.; Xia, B.-R.; Zhang, Z.-C.; Zhang, Y.-J.; Lou, G.; Jin, W.-L. Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Front. Immunol. 2020, 11, 577869. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, J.; Li, Z.; Zhang, N.; Yin, H.; Liu, Z. The Role of Nuclear β-Catenin Accumulation in the Twist2-Induced Ovarian Cancer EMT. PLoS ONE 2013, 8, e78200. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Wu, J.; Lin, S.; Chen, Y.; Zheng, J. Identification and Clinical Validation of EMT-Associated Prognostic Features Based on Hepatocellular Carcinoma. Cancer Cell Int. 2021, 21, 621. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling Mechanisms of the Epithelial-Mesenchymal Transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Lei, Z.-N.; Teng, Q.-X.; Koya, J.; Liu, Y.; Chen, Z.; Zeng, L.; Chen, Z.-S.; Fang, S.; Wang, J.; Liu, Y.; et al. The Correlation between Cancer Stem Cells and Epithelial-Mesenchymal Transition: Molecular Mechanisms and Significance in Cancer Theragnosis. Front. Immunol. 2024, 15, 1417201. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and Drug Resistance: The Mechanistic Link and Clinical Implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Marie-Egyptienne, D.T.; Lohse, I.; Hill, R.P. Cancer Stem Cells, the Epithelial to Mesenchymal Transition (EMT) and Radioresistance: Potential Role of Hypoxia. Cancer Lett. 2013, 341, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Qin, S.; Zhang, Z.; Liu, Y.; Zhou, L.; Li, B.; Nice, E.C.; Zhang, Y.; Jing, J. Unraveling the Underlying Mechanisms of Cancer Stem Cells in Therapeutic Resistance for Optimizing Treatment Strategies. MedComm Oncol. 2025, 4, e70009. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-Mesenchymal Transitions in Tumour Progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Akhmetkaliyev, A.; Alibrahim, N.; Shafiee, D.; Tulchinsky, E. EMT/MET Plasticity in Cancer and Go-or-Grow Decisions in Quiescence: The Two Sides of the Same Coin? Mol. Cancer 2023, 22, 90. [Google Scholar] [CrossRef]

| Feature | EMT Type 1 | EMT Type 2 | EMT Type 3 |
|---|---|---|---|
| Primary Context | Embryogenesis and organ development | Tissue regeneration and wound healing | Tumor progression and metastasis |
| Physiological or Pathological Role | Physiological; tightly regulated | Both physiological and pathological, depending on context | Pathological; associated with malignancy |
| Triggering Factors | Developmental signals (e.g., Wnt, FGF, Notch) | Inflammatory cytokines, growth factors (e.g., TGF-β, IL-6), tissue injury | Oncogenic signals, hypoxia, TGF-β, tumor microenvironment components |
| Cellular Outcome | Formation of mesenchymal progenitor cells from epithelial precursors; essential for neural crest formation, heart development, etc. | Transient mesenchymal conversion to promote fibrosis resolution and tissue remodeling | Acquisition of mesenchymal traits by epithelial tumor cells, leading to increased motility, invasiveness, and therapy resistance |
| Molecular Features | Downregulation of E-cadherin, upregulation of N-cadherin, SNAI1, SNAI2 (developmentally regulated) | Similar molecular profile as type 3 but context-dependent and reversible under normal conditions | Persistent activation of EMT-TFs (SNAI1, SNAI2, ZEB1, ZEB2, TWIST1), stable repression of epithelial genes, activation of invasion/metastasis programs |
| Outcome in Cancer | Not associated with neoplastic transformation | May indirectly promote tumorigenesis in chronic inflammation via fibrosis and immunosuppression | Directly contributes to carcinogenesis, epithelial plasticity, intravasation, metastasis, and chemoresistance |
| Relevance to Ovarian Cancer | Not applicable | Chronic peritoneal inflammation may contribute to EMT induction and tumor-supportive stroma | Crucial in promoting dissemination of ovarian cancer cells within the peritoneal cavity, enhancing metastatic potential and resistance to platinum-based chemotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, M.; Mertowska, P.; Mertowski, S.; Roliński, J.; Krasińska-Płachta, A.; Urbanowicz, T.; Gogacz, M.; Grywalska, E. From Defense to Disease: How the Immune System Fuels Epithelial–Mesenchymal Transition in Ovarian Cancer. Int. J. Mol. Sci. 2025, 26, 4041. https://doi.org/10.3390/ijms26094041
Kos M, Mertowska P, Mertowski S, Roliński J, Krasińska-Płachta A, Urbanowicz T, Gogacz M, Grywalska E. From Defense to Disease: How the Immune System Fuels Epithelial–Mesenchymal Transition in Ovarian Cancer. International Journal of Molecular Sciences. 2025; 26(9):4041. https://doi.org/10.3390/ijms26094041
Chicago/Turabian StyleKos, Michał, Paulina Mertowska, Sebastian Mertowski, Jacek Roliński, Aleksandra Krasińska-Płachta, Tomasz Urbanowicz, Marek Gogacz, and Ewelina Grywalska. 2025. "From Defense to Disease: How the Immune System Fuels Epithelial–Mesenchymal Transition in Ovarian Cancer" International Journal of Molecular Sciences 26, no. 9: 4041. https://doi.org/10.3390/ijms26094041
APA StyleKos, M., Mertowska, P., Mertowski, S., Roliński, J., Krasińska-Płachta, A., Urbanowicz, T., Gogacz, M., & Grywalska, E. (2025). From Defense to Disease: How the Immune System Fuels Epithelial–Mesenchymal Transition in Ovarian Cancer. International Journal of Molecular Sciences, 26(9), 4041. https://doi.org/10.3390/ijms26094041







