Chitosan-Coated Silver Nanocomposites: Biosynthesis, Mechanical Properties, and Ag+ Release in Liquid and Biofilm Forms
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of AgChNPs
2.1.1. Visual Observation
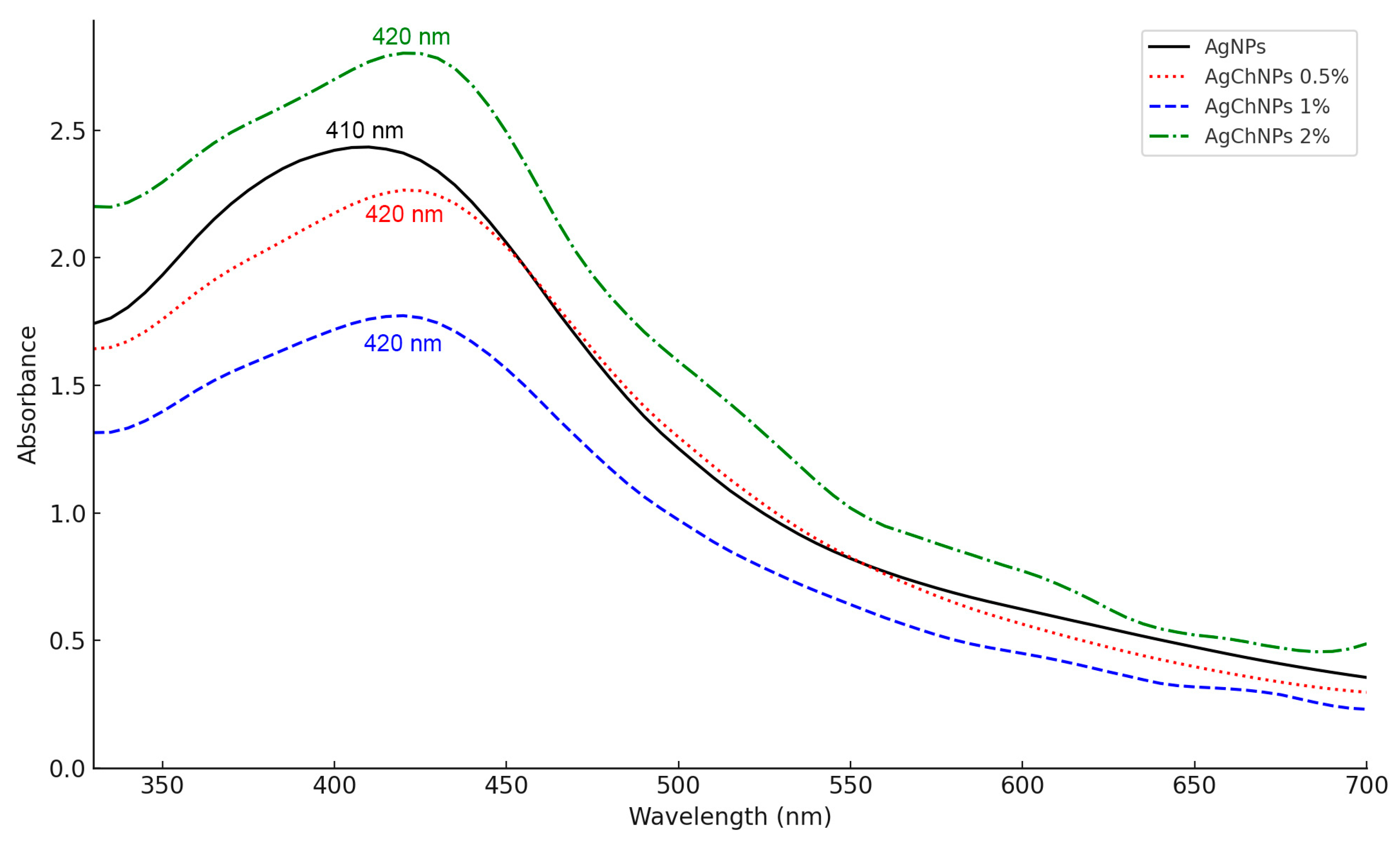
2.1.2. UV-Vis Spectroscopy of AgChNPs
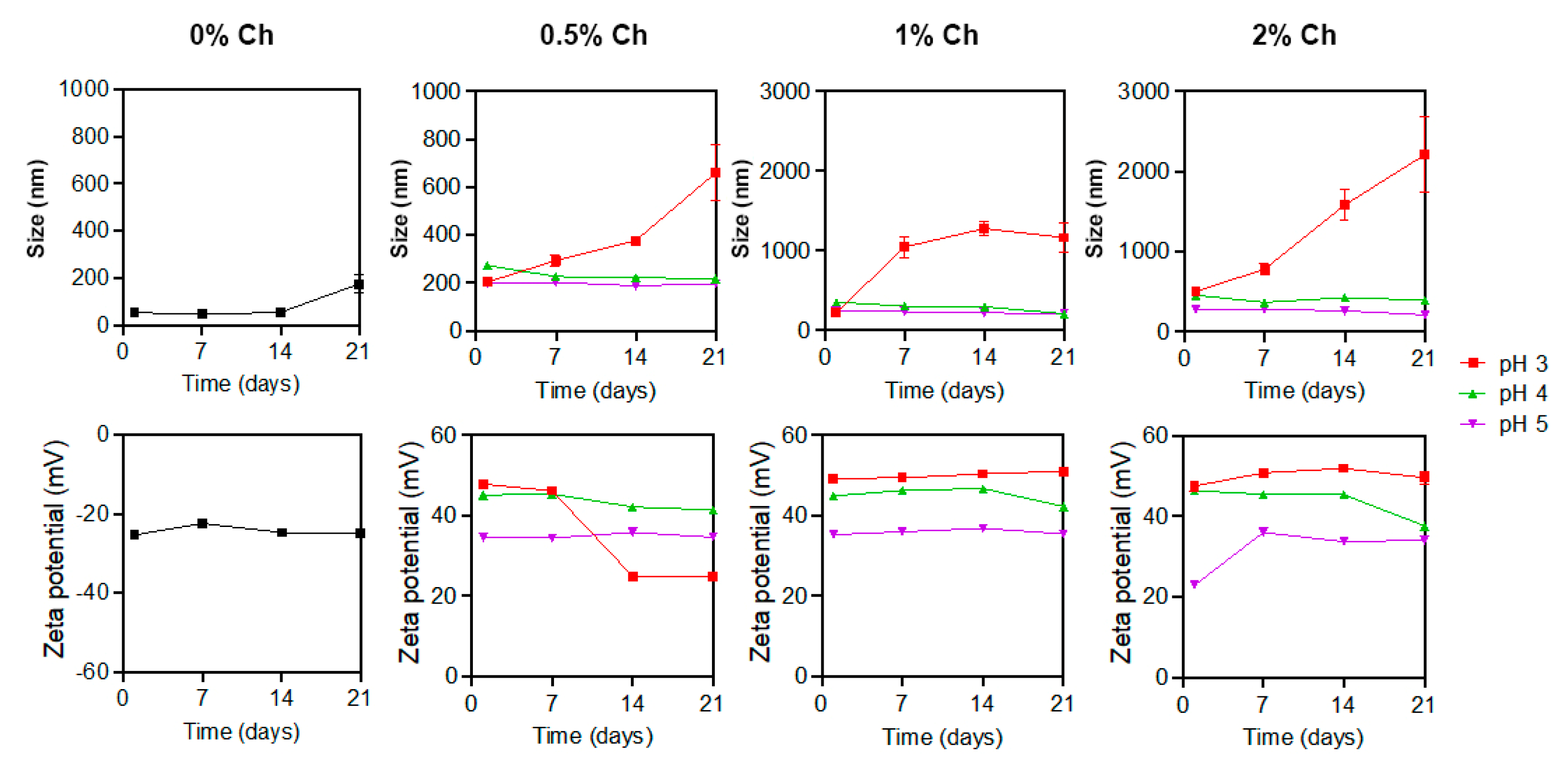
2.1.3. Dynamic Light Scattering (DLS) and Zeta Potential of AgChNPs
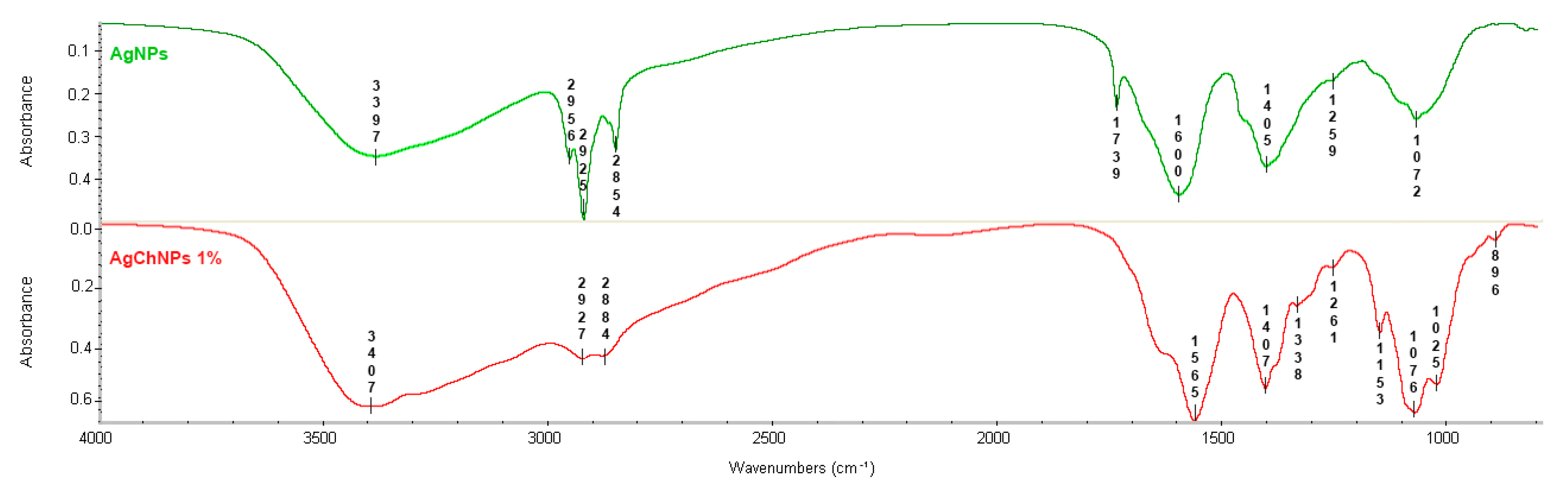
2.1.4. Fourier-Transform Infrared Spectroscopy (FTIR) of AgChNPs
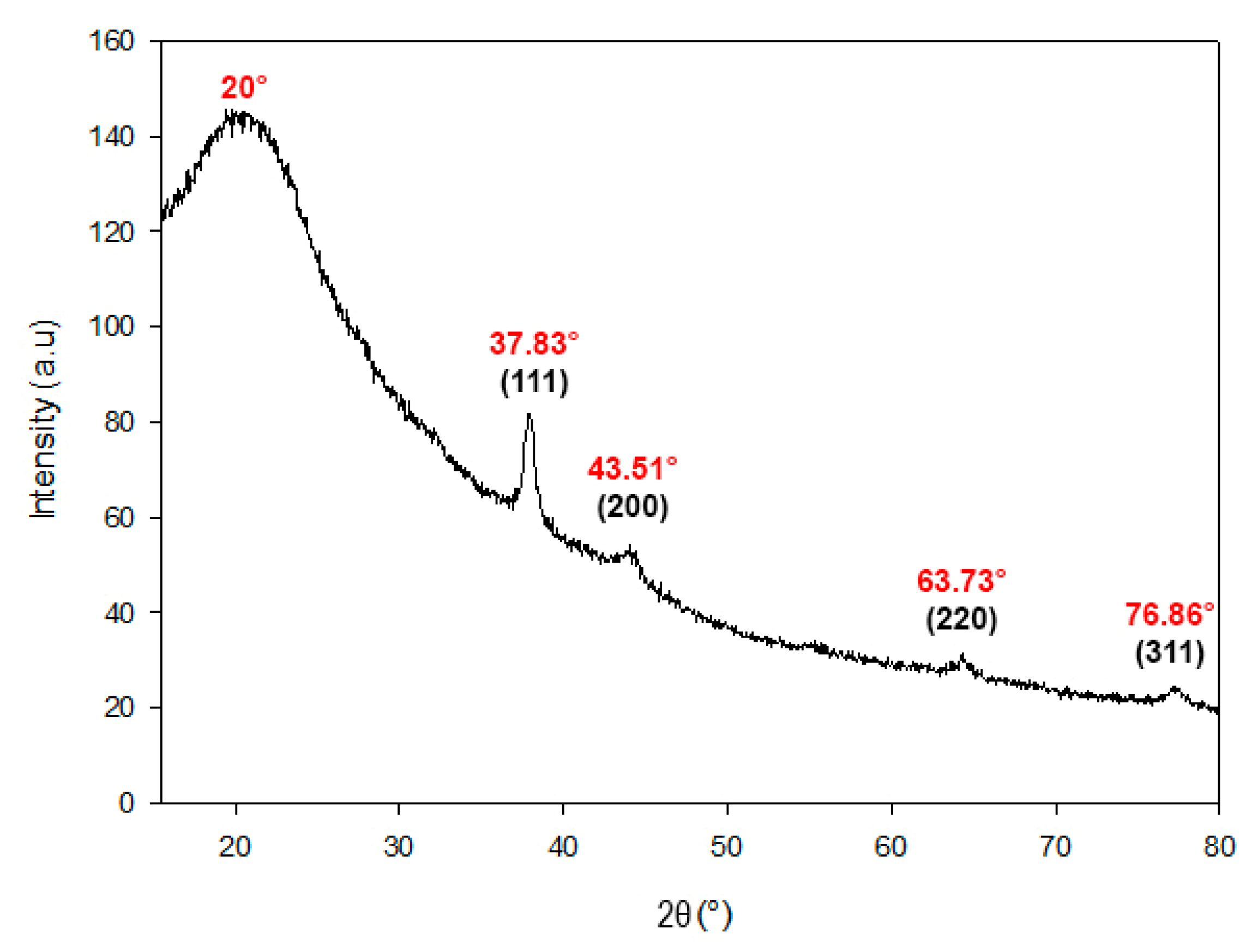
2.1.5. X-Ray Diffraction (XRD) Analysis of AgChNPs
2.1.6. Transmission Electron Microscopy (TEM-EDX) Analysis of AgChNPs
2.2. Biosynthesis of Ch-Coated Silver Nanoparticle Biofilm (bf-AgChNPs)
2.2.1. Physical, Structural, and Mechanical Properties Characterization Biofilms
2.2.2. Thermal Stability Analysis of AgChNP Biofilms
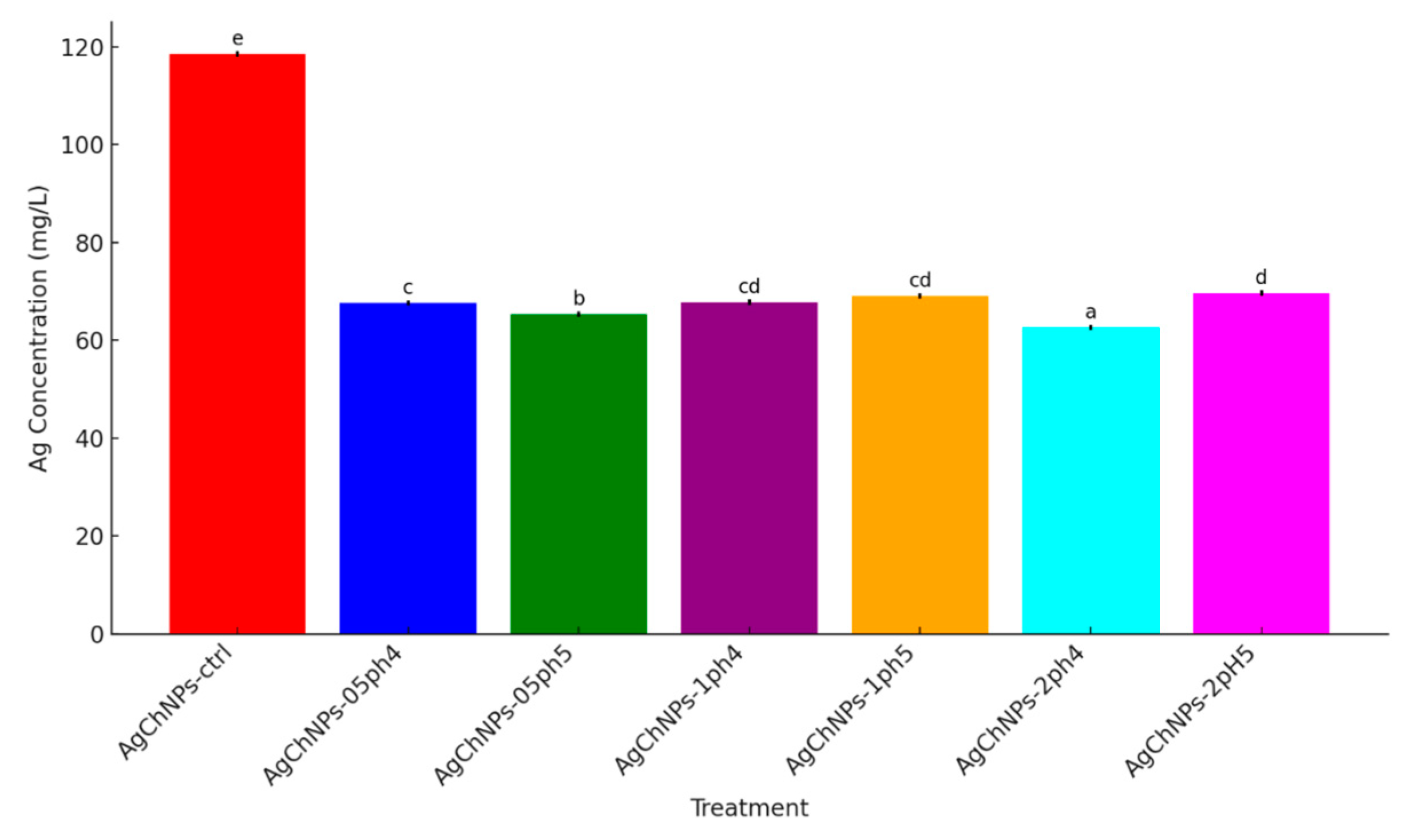
2.2.3. Release of Ag+ from AgChNP Nanocomposites
3. Materials and Methods
3.1. Plant Leaves and Chemical Obtention
3.2. Biosynthesis of AgNPs (AgNPs)
3.3. Biosynthesis of Ch-Coated AgNPs (AgChNPs)
3.4. Characterization of Ch-Coated AgNPs (AgChNPs)
3.4.1. Dynamic Light Scattering (DLS) and Zeta Potential
3.4.2. UV-Vis Spectroscopy
3.4.3. FTIR Spectroscopy
3.4.4. XRD Analysis
3.4.5. Transmission Electron Microscopy Coupled with Energy Dispersive X-Ray Spectroscopy (TEM-EDX)
3.5. Biosynthesis of Ch-Coated Silver Nanoparticle Biofilm
3.6. Physical and Structural Characterization of Ch-Coated Silver Nanoparticle Biofilm
3.6.1. Films Thickness
3.6.2. Mechanical Properties
3.7. Thermal Stability Analysis
3.8. Inductively Coupled Plasma Atomic of Biofilm (ICP-OES)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subramanyam, G.K.; Gaddam, S.A.; Kotakadi, V.S.; Venkata, R.K.C.; Kandasamy, S. Green fabrication of silver nanoparticles by leaf extract of Byttneria herbacea Roxb and their promising therapeutic applications and its interesting insightful observations in oral cancer. Artif. Cells Nanomed Biotechnol. 2023, 51, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, O.; Kalynovskyi, V.; Zelena, P.; Ivanchenko, S.; Bondarenko, I. Bactericidal activity of Ag nanoparticles biosynthesized from Capsicum annuum pericarps against phytopathogenic Clavibacter michiganensis. Sci. Nat. 2023, 110, 15. [Google Scholar] [CrossRef]
- Maharkan, A.; Gautam, R.; Lee, G.; Kim, D.; Lee, D.; Acharya, M.; Kim, H.; Heo, Y.; Kim, C. Assessment of skin sensitization potential of zinc oxide, aluminum oxide, manganese oxide, and copper oxide nanoparticles through the local lymph node assay: 5-bromo-deoxyuridine flow cytometry method. J. Toxicol. Environ. Health A Curr. Issues. 2025, 88, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Zhou, P.F.; Zhang, P.; Adeel, M.; Shakoor, N.; Li, Y.B.; Li, M.S.; Guo, M.L.; Zhao, W.C.; Lou, B.Z.; et al. Green synthesis of metal-based nanoparticles for sustainable agriculture. Environ. Pollut. 2022, 309, 119755. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.; Tandi, A.; Moharana, S.; Chakroborty, S.; Mohapatra, S.; Mondal, A.; Dey, S.; Chandra, P. Silver nanoparticle for biomedical applications: A review. Hybrid Adv. 2024, 6, 100184. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver nanoparticles.: Review of antiviral properties, mechanism of action and applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Mussin, K.; Giusiano, G. Biogenic silver nanoparticles as antifungal agents. Front. Chem. 2022, 10, 1023542. [Google Scholar] [CrossRef]
- Wilson, J.J.; Mahendran, S.; Sivakumar, T.; Ponmanickam, P.; Thangaraj, R. Mosquito larvicidal activity of silver nanoparticles synthesized using Azolla pinnata against Culex quinquefasciatus say (Diptera: Culicidae). S. Afr. J. 2023, 157, 380–386. [Google Scholar] [CrossRef]
- Qu, R.; Chen, M.; Liu, J.; Yu, H.; Liang, J. Blockage of ATPase-mediated energy supply inducing metabolic disturbances in algal cells under silver nanoparticles stress. J. Environ. Sci. 2023, 141, 141–150. [Google Scholar] [CrossRef]
- Manimegalai, T.; Raguvaran, K.; Kalpana, M.; Maheswaran, R. Green synthesis of silver nanoparticle using Leonotis nepetifolia and their toxicity against vector mosquitoes of Aedes aegypti and Culex quinquefasciatus and agricultural pests of Spodoptera litura and Helicoverpa armigera. Environ. Sci. Pollut. Res. 2020, 27, 43103–43116. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, M.; Samih, M.A.; Kalantari, S. Insecticidal effect of silica and silver nanoparticles on the cowpea seed beetle, Callosobruchus maculatus F. (Col.: Bruchidae). J. Entomol. Res. 2012, 4, 297–305. Available online: https://sid.ir/paper/201167/en (accessed on 20 January 2025).
- Kamaraj, C.; Rajakumar, G.; Rahuman, A.A. Feeding deterrent activity of synthesized silver nanoparticles using Manilkara zapota leaf extract against the house fly, Musca domestica (Diptera: Muscidae). Parasitol. Res. 2012, 111, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, M.; Samih, M.A.; Kalantari, S. Insecticide effect of silver and zinc nanoparticles against Aphis nerii Boye de fonscolombe (Hemiptera: Aphididae). Chil. J. Agric. Res. 2012, 72, 590–594. [Google Scholar] [CrossRef]
- Martínez-Cisterna, D.; Rubilar, O.; Tortela, G.; Chen, L.; Chacón-Fuentes, M.; Lizama, M.; Parra, P.; Bardehle, L. Silver nanoparticles as a Potent Nanopesticide: Toxic Effects and Action Mechanisms on Pest Insects of Agricultural Importance—A review. Molecules 2024, 29, 5520. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Huang, J.; Chen, C.; Wang, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Abdoon, F.; Hasan, H.; Salman, S.; Kareem, F.; Taha, R. Exploiting of green synthesized silver nanoparticles using Capparis spinosa L. fruit for spectrophotometric determination of diphenhydramine HCl in pure forms and commercial products. J. Exp. Nanosci. 2023, 18, 2161525. [Google Scholar] [CrossRef]
- Akyüz, G.; Kaymazlar, E.; Ay, H.; Inan, T.; Yildiz, M. Use of silver nanoparticles loaded locust bean gum coatings to extend the shelf-life of fruits. Biointerface Res. Appl. Chem. 2023, 13, 289. [Google Scholar] [CrossRef]
- Manosalva, N.; Tortella, G.; Diez, M.C.; Rubilar, O.; Schalchli, H.; Seabra, A.B. Green synthesis of silver nanoparticles: Effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef]
- Mishra, V.K.; Husen, A.; Rahman, Q. Plant-based fabrication of silver nanoparticles and their application. Nanomater. Plant Potential 2019, 5, 135–175. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, N.; Nanda, A.; Kaur, M.; Sharma, R.; Sohal, H.S. Recent advances in synthesis and bio-applications of natural stabilizers for metal nanoparticles. JMMM 2024, 34, 2145. [Google Scholar] [CrossRef]
- Hudek, M.; Johnston, K.; Kubiak-Ossowska, K.; Ferro, V.; Mulheran, P. Molecular dynamics study of chitosan adsorption at a silica surface. J. Phys. Chem. C. 2024, 128, 21531–21538. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Song, C.; Yang, C.; Guo, Q.; Yao, N. Low molecular weight chitosan-coated silver nanoparticles are effective for the treatment of MRSA-infected wounds. Int. J. Nanomed. 2017, 112, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Jannoo, K.; Teerapatsakul, C.; Punyanut, A.; Pasanphan, W. Electron beam assisted synthesis of silver nanoparticle in chitosan stabilizer: Preparation, stability and inhibition of building fungi studies. Radiat. Phys. Chem. 2015, 112, 177–188. [Google Scholar] [CrossRef]
- Shinde, S.; Folliero, V.; Chianese, A.; Zannella, C.; De Filippis, A.; Rosati, L.; Franci, G.; Galdiero, M.; Galdiero, M. Synthesis of chitosan-coated silver nanoparticle bioconjugates and their antimicrobial activity against multidrug-resistant bacteria. Appl. Sci. 2021, 11, 9340. [Google Scholar] [CrossRef]
- Poveda, J. Quitina: Compuesto natural y práctico. Mater. Investig. Joven. 2018, 5, 1–4. Available online: https://revistas.unlp.edu.ar/InvJov/article/view/4308 (accessed on 20 January 2025).
- González-López, D.; Bustos-Martínez, J.; Hamdam, A.; Schettino-Bermúdez, B.; Chamorro-Ramírez, F. Physical-mechanical and functional properties of Chitosan-based films incorporated with silver nanoparticles. Mundo Nano 2024, 17, 1e–18e. [Google Scholar] [CrossRef]
- Sivera, M.; Kvitek, L.; Soukupova, J.; Panacek, A.; Prucek, R.; Vecerova, R.; Zboril, R. Silver nanoparticles modified by gelatin with extraordinary pH stability and long-term antibacterial activity. PLoS ONE 2014, 9, e103675. [Google Scholar] [CrossRef]
- Farhadi, L.; Mohtashami, M.; Saeidi, J.; Azimi-Nezhad, M.; Taheri, G. Green synthesis of chitosan-coated silver nanoparticle, characterization, antimicrobial activities, and cytotoxicity analysis in cancerous and normal cell lines. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1637–1649. [Google Scholar] [CrossRef]
- Fan, M.; Si, J.; Xu, X.; Chen, L.; Chen, J.; Yang, C.; Zhu, J.W.; Wu, L.; Tian, J.; Chen, X. A versatile chitosan nanogel capable of generating AgNPs in-situ and long-acting slow-release of Ag+ for highly efficient antibacterial. Carbohydr. Polym. 2021, 257, 117636. [Google Scholar] [CrossRef]
- Samadi, A.; Azandeh, S.; Orazizadeh, M.; Bayati, V.; Rafienia, M.; Karami, M. Fabrication and characterization of glycerol/chitosan/polyvinyl alcohol-based transparent hydrogel films loaded with silver nanoparticles for antibacterial wound dressing applications. Adv. Biomed. Res. 2021, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Ediyilyam, S.; Lalitha, M.; George, B.; Shankar, S.S.; Waclawek, S.; Cernik, M.; Padil, V. Synthesis, Characterization and Physicochemical properties of Biogenic silver nanoparticle-encapsulated chitosan bionanocomposites. Polymers 2022, 14, 463. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Al Thabiani, A.; Subrata, T.; Chellasamy, P. Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance. Green. Process Synth. 2020, 9, 675–684. [Google Scholar] [CrossRef]
- Martínez-Cisterna, D.; Rubilar, O.; Chen, L.; Lizama, M.; Chacón-Fuentes, M.; Quiroz, A.; Parra, P.; Rebolledo, R.; Bardehle, L. Biosynthesized Chitosan-Coated Silver Nanoparticles: Insecticide Activity and Sublethal Effects Against Drosophila suzukii (Diptera: Drosophilidae). Biomolecules 2025, 15, 490. [Google Scholar] [CrossRef]
- Suresh, U.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Subramaniam, J. Suaeda maritima-based herbal coils and green nanoparticles as potential biopesticides against the dengue vector Aedes aegypti and the tobacco cutworm Spodoptera litura. Physiol. Mol. Plant Pathol. 2017, 101, 225–235. [Google Scholar] [CrossRef]
- Zainab, S.; Jadoon, M.; Sikandar, S.; Hussain, A.; Rehman, R. Thermomyces lanuginosus: A prospective thermophilic fungus for green synthesis and stabilization of BioAgNPs through glucoamylase. Materials 2023, 297, 127442. [Google Scholar] [CrossRef]
- Santos, A.F.; Reis, C.A.; Lima, M.S.; Silva, P.A. Effects of low concentrations of chitosan on biofilm formation and mechanical integrity in various pH conditions. Int. J. Biol. Macromol. 2019, 135, 495–503. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Tharani, M.; Rajeswari, V.; Sivakavinesan, M. Synthesis of greener silver nanoparticle-based chitosan nanocomposites and their potential antimicrobial activity against oral pathogens. Green Process. Synth. 2021, 10, 658–665. [Google Scholar] [CrossRef]
- Wulandari, I.; Pebriatin, B.; Valiana, V.; Indriati, N.; Pratiwi, R. Green synthesis of silver nanoparticles coated by water-soluble chitosan and its potency as non-alcoholic hand sanitizer formulation. Materials 2022, 15, 4641. [Google Scholar] [CrossRef]
- Hermosilla, E.; Diaz, M.; Vera, J.; Contreras, M.J.; Leal, K.; Salazar, R.; Barrientos, L.; Tortella, G.; Rubilar, O. Synthesis of antimicrobial Chitosan-silver nanoparticles mediated by reusable Chitosan fungal beads. Int. J. Mol. Sci. 2023, 24, 2318. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.; King, K.; Park, H. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Karuppaiah, A.; Babu, D.; Servaraj, D.; Ramalingam, G.; Muthusamy, A.; Saravanan, A. Building and behavior of a pH-stimuli responsive chitosan nanoparticles loaded with folic acid conjugated gemcitabine silver colloids in MDA-MB-453 metastatic breast cancer cell line and pharmacokinetics in rats. Eur. J. Pharm. Sci. 2021, 165, 105938. [Google Scholar] [CrossRef] [PubMed]
- Kanniah, P.; Chelliah, P.; Thangapandi, J.; Amalraj, A.; Gopi, S. Green synthesis of antibacterial and cytotoxic silver nanoparticles by Piper nigrum seed extract and development of antibacterial silver-based chitosan nanocomposite. Int. J. Biol. Macromol. 2021, 189, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Mbae, K.; Umesha, S. Physicochemical and antimicrobial properties of post-synthesis betanin and chitosan oligosaccharide functionalized silver nanoparticles. J. Nanopart. Res. 2020, 22, 346. [Google Scholar] [CrossRef]
- Barbosa, J.; Abdelsadig, M.; Conway, B.; Batchelor, H. Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their pKa. Int. J. Pharm. 2019, 561, 100024. [Google Scholar] [CrossRef]
- Chowdhury, M.; Hossain, N.; Mostofa, M.D.; Roy, M. Green synthesis and characterization of zirconium nanoparticle for dental implant applications. Heliyon 2023, 9, e12711. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Costa, D.; Rinaldi, R. Aloe vera silver nanoparticles addition in chitosan films: Improvement of physicochemical properties for eco-friendly food packaging material. J. Mater. Res. Technol. 2023, 24, 1015–1033. [Google Scholar] [CrossRef]
- Atrooz, O.; Al-Nadaf, A.; Uysal, H.; El-Gendy, M. Biosynthesis of silver nanoparticles using Coriandrum sativum L. extract and evaluation of their antibacterial, anti-inflammatory and antinociceptive activities. S. Afr. J. Bot. 2023, 158, 219–227. [Google Scholar] [CrossRef]
- Pavunraj, M.; Baskar, K.; Duraipandiyan, V.; Al-Dhabi, N.A.; Ignacimuthu, S. Toxicity of Ag nanoparticles synthesized using stearic acid from Catharanthus roseus leaf extract against Earias vittela and mosquito vectors (Culex quinquefasciatus and Aedes aegypti). J. Clust. Sci. 2017, 28, 2477–2492. [Google Scholar] [CrossRef]
- Muthukumaran, U.; Govindarajan, M.; Rajeswary, M.; Hoti, S.L. Synthesis and characterization of silver nanoparticles using Gmelina asiatica leaf extract against filariasis, dengue, and malaria vector mosquitoes. Parasitol. Res. 2015, 114, 1817–1827. [Google Scholar] [CrossRef]
- Ghramh, H.; Al-Ghamdi, A.; Mahyoub, J.; Mohammed, M.G.; Al-Qahtani, A.; El-Gendy, I.R. Chrysanthemum extract and extract-prepared silver nanoparticles as biocides to control Aedes aegypti (L.), the vector of dengue fever. J. Asia Pac. Entomol. 2018, 21, 205–210. [Google Scholar] [CrossRef]
- Akif, H.K.; Olutas, E.; Avcioglu, F.; Kaya, H.; Sahin, B. Quercetin- and caffeic acid-functionalized chitosan-capped colloidal silver nanoparticles: One-pot synthesis, characterization, and anticancer and antibacterial activities. Beilstein J. Nanotechnol. 2023, 14, 362–376. [Google Scholar] [CrossRef]
- Costa, M.; Carreiro, E.; Filho, C.; Sousa, J. Chitosan salts as stabilizing agents for the synthesis of silver nanoparticles (AgNPs). ChemistrySelect 2023, 8, e202203413. [Google Scholar] [CrossRef]
- Malakhovska, T.; Pogodin, A.; Filep, M.; Barannik, A.; Kravets, A. Optical characteristics of silver-based nanocomposites fabricated by an environmentally friendly method. Semicond. Phys. Quantum. Electron. 2023, 26, 076–083. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2020, 38, 43–74. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jimenez, A.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Chitosan coated—Biogenic silver nanoparticles from wheat residues as green antifungal and nanoprimig in wheat seeds. Int. J. Biol. Macromol. 2023, 225, 964–973. [Google Scholar] [CrossRef]
- Shehabeldine, A.; Salem, S.; Ali, O.; Abd-Elsalam, K.; Elkady, F.; Hashem, A. Multifunctional Silver Nanoparticles Based on Chitosan: Antibacterial, Antibiofilm, Antifungal, Antioxidant, and Wound-Healing Activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef]
- Dara, P.K.; Mahadevan, R.; Digita, P.A.; Visnuvinayagam, S.; Kumar, L.; Suseela, M.; Ravishankar, C.N.; Anandan, R. Synthesis and biochemical characterization of silver nanoparticles grafted chitosan (Chi-Ag-NPs): In vitro studies on antioxidant and antibacterial applications. SN Appl. Sci. 2020, 2, 665. [Google Scholar] [CrossRef]
- Abdellatif, A.; Abdelfattah, A.; Aldalaan, S.; Tawfeek, H. Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells. Nanotechnol. Rev. 2023, 12, 20220546. [Google Scholar] [CrossRef]
- Reiad, N. Green synthesis of antibacterial chitosan films loaded with silver nanoparticles. Chin. J. Polym. Sci. 2013, 31, 984–993. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczynski, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepknowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs. 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Kausar, H.; Afzal, A.; Ahmad, F.; Hussain, A. Antifungal activity of Juglans regia-mediated silver nanoparticles (AgNPs) against Aspergillus ochraceus-induced toxicity in in vitro and in vivo settings. J. Funct. Biomater. 2023, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Naveen; Dhatwalia, J.; Thakur, S.; Radhakrishnan, A.; Chauhan, A.; Chandan, G.; Hyune Choi, B.; Neetika; Nidhi. Antioxidant, antimicrobial, and cytotoxic potential of Euphorbia royleana extract-mediated silver and copper oxide nanoparticles. Chem Pap. 2023, 77, 4643–4657. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 9, 381. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Azimi, F.; Mahmoudi, F.; Mahmoudi, F.; Amini, M.M. Synthesis of silver nanoparticles by Galega officinalis and its hypoglycemic effects in type 1 diabetic rats. Nanomed J. 2021, 8, 255–263. [Google Scholar] [CrossRef]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804. [Google Scholar] [CrossRef]
- Affes, S.; Maalej, H.; Aranaz, I.; Nasri, M.; Acosta, N. Controlled size green synthesis of bioactive silver nanoparticles assisted by chitosan and its derivatives and their application in biofilm preparation. Carbohydr. Polym. 2020, 236, 116063. [Google Scholar] [CrossRef]
- Cheng, Z.; Tang, S.; Feng, J.; Wu, Y. Biosynthesis and antibacterial activity of silver nanoparticles using Flos sophorae Immaturus extract. Heliyon 2022, 8, e10010. [Google Scholar] [CrossRef]
- Wang, W.; Ma, P.; Zhao, Q.; Liu, M.; Zheng, Y. Beneficial properties of the biosynthesized silver/chitosan nanoparticles mediated by Mentha piperita in rats with heart failure following myocardial infarction. Inorg. Chem. Commun. 2022, 141, 109581. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.H.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Pava, L. Preparation and characterization of plasticized chitosan film: A potential biomaterial in medical field. Rev. Reto. 2019, 7, 11–24. [Google Scholar] [CrossRef]
- Shi, B.; Hao, Z.; Du, Y.; Jia, M.; Xie, S. Mechanical and barrier properties of chitosan-based composite film as food packaging: A review. Bioresources 2024, 19, 4001–4014. [Google Scholar] [CrossRef]
- Rohaeti, E.; Laksono, E.; Rakhmawati, A. Mechanical properties and antibacterial activity of cellulose composite based coconut water with addition glycerol, chitosan, and silver nanoparticle. Orient. J. Chem. 2018, 34, 3. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Advances in Functional biopolymer-based nanocomposites for active food packaging applications. Polymers 2021, 13, 4198. [Google Scholar] [CrossRef]
- Molleman, B.; Hiemstra, T. Surface structure of silver nanoparticles as a model for understanding the oxidative dissolution of silver ions. Langmuir 2015, 31, 13361–13372. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Mahmoud, G.A. pH-responsive chitosan/acrylamide/gold/nanocomposite supported with silver nanoparticles for controlled release of anticancer drug. Sci. Rep. 2023, 13, 7818. [Google Scholar] [CrossRef]
- Abd El-Hady, M.; Saeed, S.E. Antibacterial properties and pH-sensitive swelling of in situ formed silver-curcumin nanocomposite based chitosan hydrogel. Polymers 2020, 12, 2451. [Google Scholar] [CrossRef]
- Yan, K.; Xu, F.; Wei, W.; Yang, C.; Wang, D.; Shi, X. Electrochemical synthesis of chitosan/silver nanoparticles multilayer hydrogel coating with pH-dependent controlled release capability and antibacterial property. Colloids Surf. B Biointerfaces 2021, 202, 111711. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
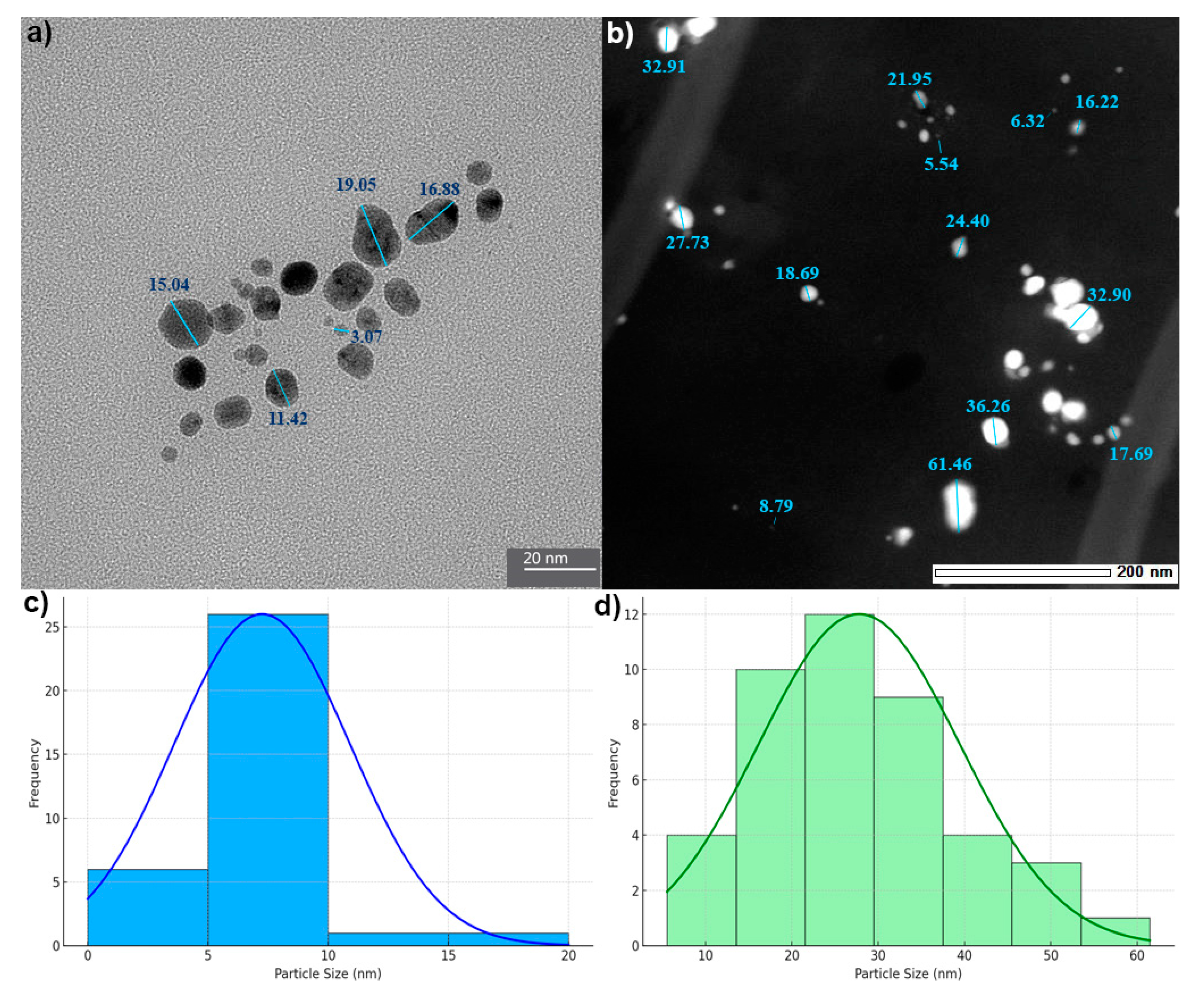



| Ch Concentration (%) | pH | PDI | |||
|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | ||
| 0.5 | 3 | 0.53 ± 0.14 | 0.39 ± 0.04 | 0.27 ± 0.08 | 0.68 ± 0.10 |
| 4 | 0.31 ± 0.02 | 0.43 ± 0.09 | 0.36 ± 0.05 | 0.45 ± 0.12 | |
| 5 | 0.42 ± 0.10 | 0.44 ± 0.11 | 0.43 ± 0.12 | 0.44 ± 0.11 | |
| 1 | 3 | 0.48 ± 0.12 | 0.79 ± 0.10 | 0.69 ± 0.04 | 0.67 ± 0.15 |
| 4 | 0.41 ± 0.05 | 0.39 ± 0.06 | 0.39 ± 0.06 | 0.62 ± 0.16 | |
| 5 | 0.38 ± 0.07 | 0.37 ± 0.06 | 0.46 ± 0.12 | 0.44 ± 0.12 | |
| 2 | 3 | 0.46 ± 0.07 | 0.80 ± 0.07 | 0.84 ± 0.10 | 0.75 ± 0.11 |
| 4 | 0.48 ± 0.09 | 0.54 ± 0.12 | 0.40 ± 0.05 | 0.41 ± 0.07 | |
| 5 | 0.52 ± 0.12 | 0.50 ± 0.12 | 0.49 ± 0.12 | 0.55 ± 0.15 | |
| 0 | 12 | 0.66 ± 0.15 | 0.68 ± 0.14 | 0.71 ± 0.13 | 0.52 ± 0.01 |
| Biofilm Sample | Biofilm Characteristics | |||||
|---|---|---|---|---|---|---|
| Glycerol (%) | Ch (%) | pH | Assigned Label | Thickness (mm) | Load at Tensile Strength (N) | Extension at Break (mm) |
| 5 | 0.5 | 4 | bf-glychph-5-05-4 | nbf | nbf | nbf |
| 0.5 | 5 | bf-glychph-5-05-5 | nbf | nbf | nbf | |
| 1.0 | 4 | bf-glychph-5-1-4 | 0.23 ± 0.04 a | 0.80 ± 0.23 a | 6.29 ± 1.54 a | |
| 1.0 | 5 | bf-glychph-5-1-5 | 0.28 ± 0.05 ab | 1.41 ± 0.01 ab | 16.05 ± 1.51 ab | |
| 2.0 | 4 | bf-glychph-5-2-4 | 0.23 ± 0.02 a | 2.94 ± 0.50 cd | 24.99 ± 3.62 b | |
| 2.0 | 5 | bf-glychph-5-2-5 | 0.25 ± 0.05 ab | 3.48 ± 0.06 d | 15.68 ± 1.86 ab | |
| 10 | 0.5 | 4 | bf-glychph-10-05-4 | nbf | nbf | nbf |
| 0.5 | 5 | bf-glychph-10-05-5 | nbf | nbf | nbf | |
| 1.0 | 4 | bf-glychph-10-1-4 | 0.25 ± 0.03 ab | 0.71 ± 0.23 a | 6.80 ± 1.32 a | |
| 1.0 | 5 | bf-glychph-10-1-5 | 0.23 ± 0.04 a | 0.97 ± 0.12 ab | 10.59 ± 2.31 a | |
| 2.0 | 4 | bf-glychph-10-2-4 | 0.50 ± 0.07 ab | 2.16 ± 0.10 bc | 11.82 ± 2.16 a | |
| 2.0 | 5 | bf-glychph-10-2-5 | 0.28 ± 0.01 ab | 2.75 ± 0.38 cd | 12.65 ± 2.67 a | |
| 15 | 0.5 | 4 | bf-glychph-15-05-4 | nbf | nbf | nbf |
| 0.5 | 4 | bf-glychph-15-05-5 | nbf | nbf | nbf | |
| 1.0 | 4 | bf-glychph-15-1-4 | nbf | nbf | nbf | |
| 1.0 | 5 | bf-glychph-15-1-5 | nbf | nbf | nbf | |
| 2.0 | 4 | bf-glychph-15-2-4 | 0.52 ± 0.12 a | 1.09 ± 0.15 ab | 12.73 ± 2.79 a | |
| 2.0 | 5 | bf-glychph-15-2-5 | 0.39 ± 0.05 ab | 1.32 ± 0.25 ab | 8.33 ± 097 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cisterna, D.; Chen, L.; Bardehle, L.; Hermosilla, E.; Tortella, G.; Chacón-Fuentes, M.; Rubilar, O. Chitosan-Coated Silver Nanocomposites: Biosynthesis, Mechanical Properties, and Ag+ Release in Liquid and Biofilm Forms. Int. J. Mol. Sci. 2025, 26, 4130. https://doi.org/10.3390/ijms26094130
Martínez-Cisterna D, Chen L, Bardehle L, Hermosilla E, Tortella G, Chacón-Fuentes M, Rubilar O. Chitosan-Coated Silver Nanocomposites: Biosynthesis, Mechanical Properties, and Ag+ Release in Liquid and Biofilm Forms. International Journal of Molecular Sciences. 2025; 26(9):4130. https://doi.org/10.3390/ijms26094130
Chicago/Turabian StyleMartínez-Cisterna, Daniel, Lingyun Chen, Leonardo Bardehle, Edward Hermosilla, Gonzalo Tortella, Manuel Chacón-Fuentes, and Olga Rubilar. 2025. "Chitosan-Coated Silver Nanocomposites: Biosynthesis, Mechanical Properties, and Ag+ Release in Liquid and Biofilm Forms" International Journal of Molecular Sciences 26, no. 9: 4130. https://doi.org/10.3390/ijms26094130
APA StyleMartínez-Cisterna, D., Chen, L., Bardehle, L., Hermosilla, E., Tortella, G., Chacón-Fuentes, M., & Rubilar, O. (2025). Chitosan-Coated Silver Nanocomposites: Biosynthesis, Mechanical Properties, and Ag+ Release in Liquid and Biofilm Forms. International Journal of Molecular Sciences, 26(9), 4130. https://doi.org/10.3390/ijms26094130








