Implications of Chitinase 3-like 1 Protein in the Pathogenesis of Multiple Sclerosis in Autopsied Brains and a Murine Model
Abstract
1. Introduction
2. Results
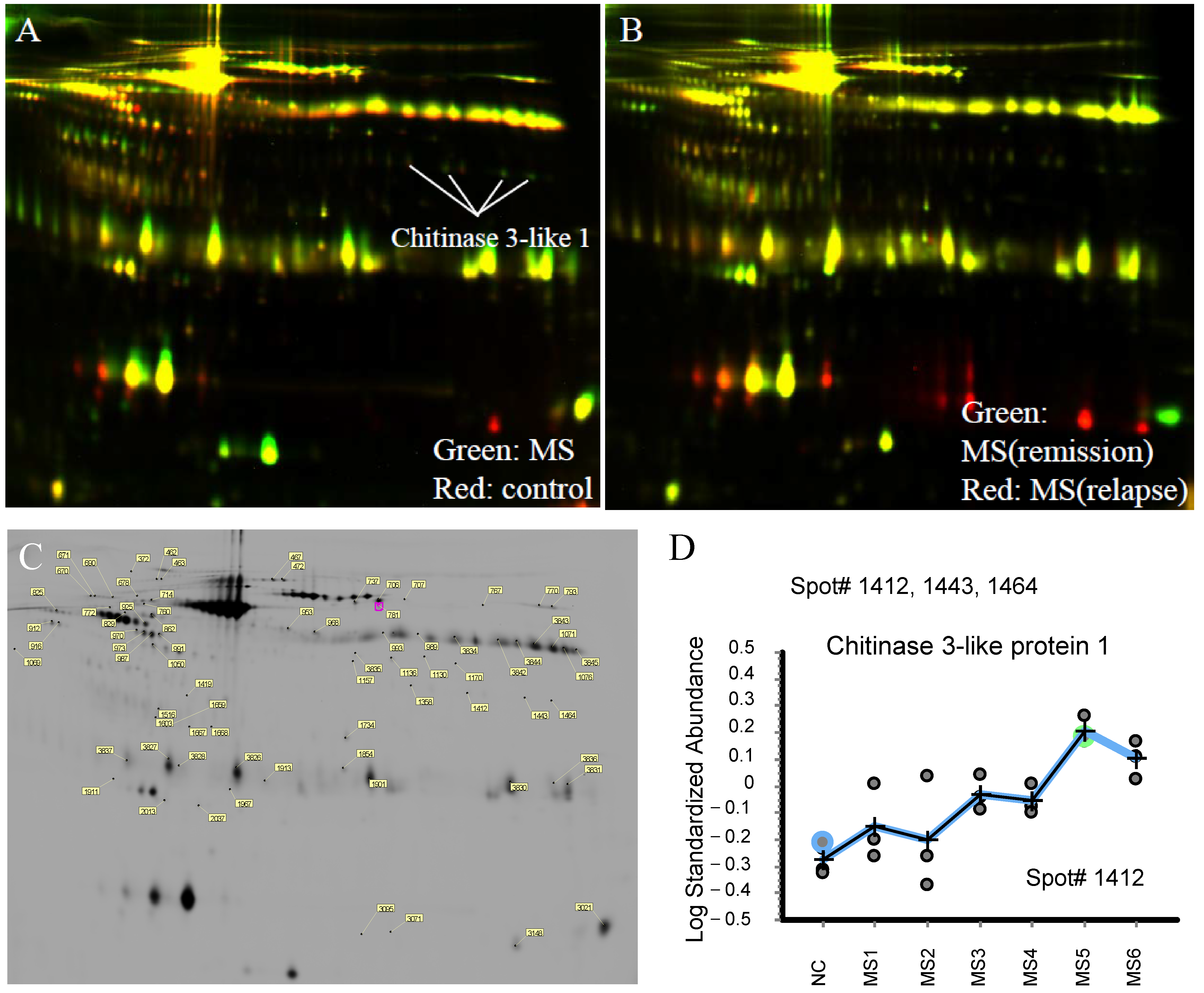
2.1. Proteomic Analysis in CSF of RRMS Patients
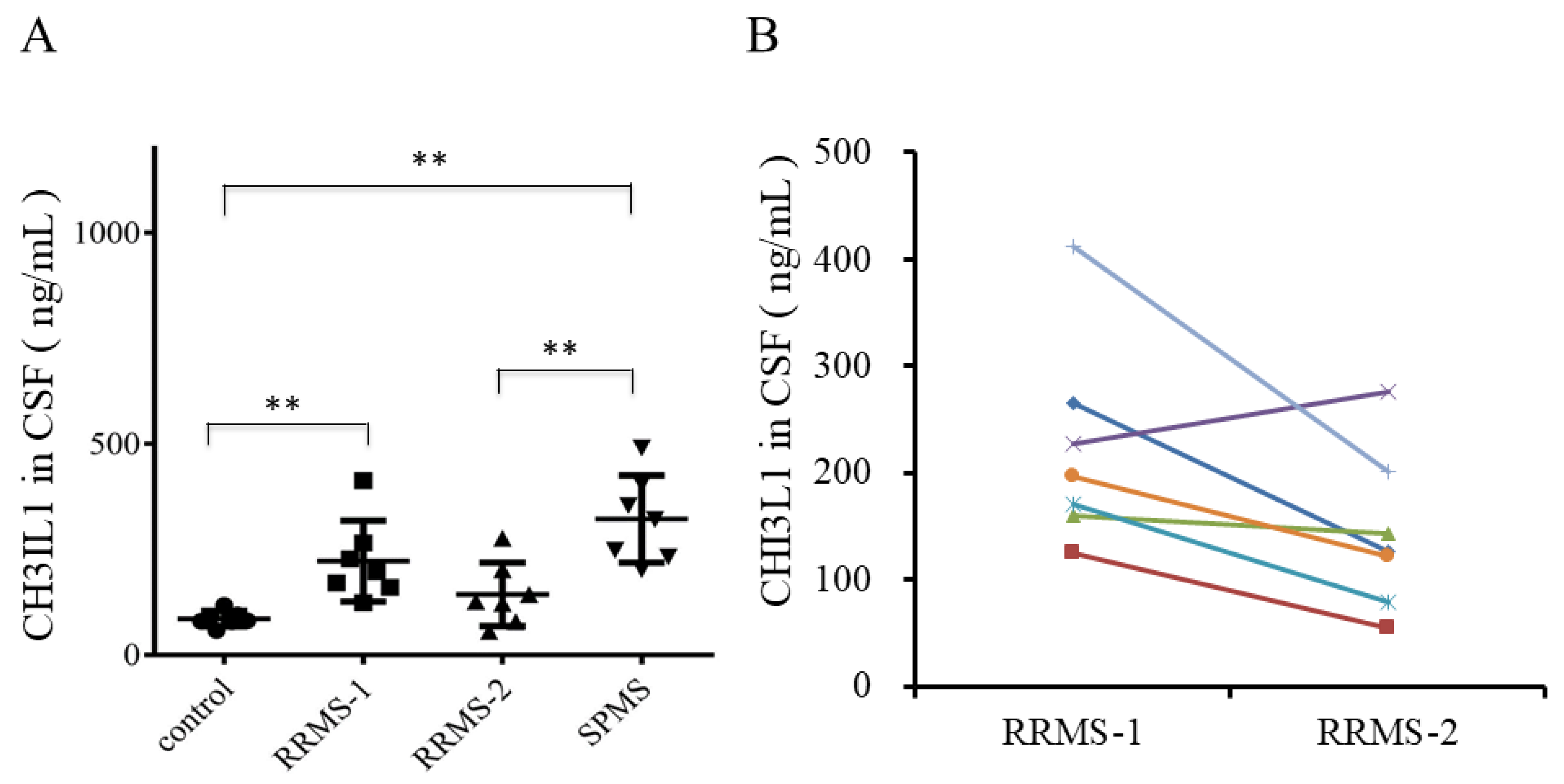
2.2. Upregulation of CHI3L1 in the CSF of Recurrent RRMS Patients by ELISA
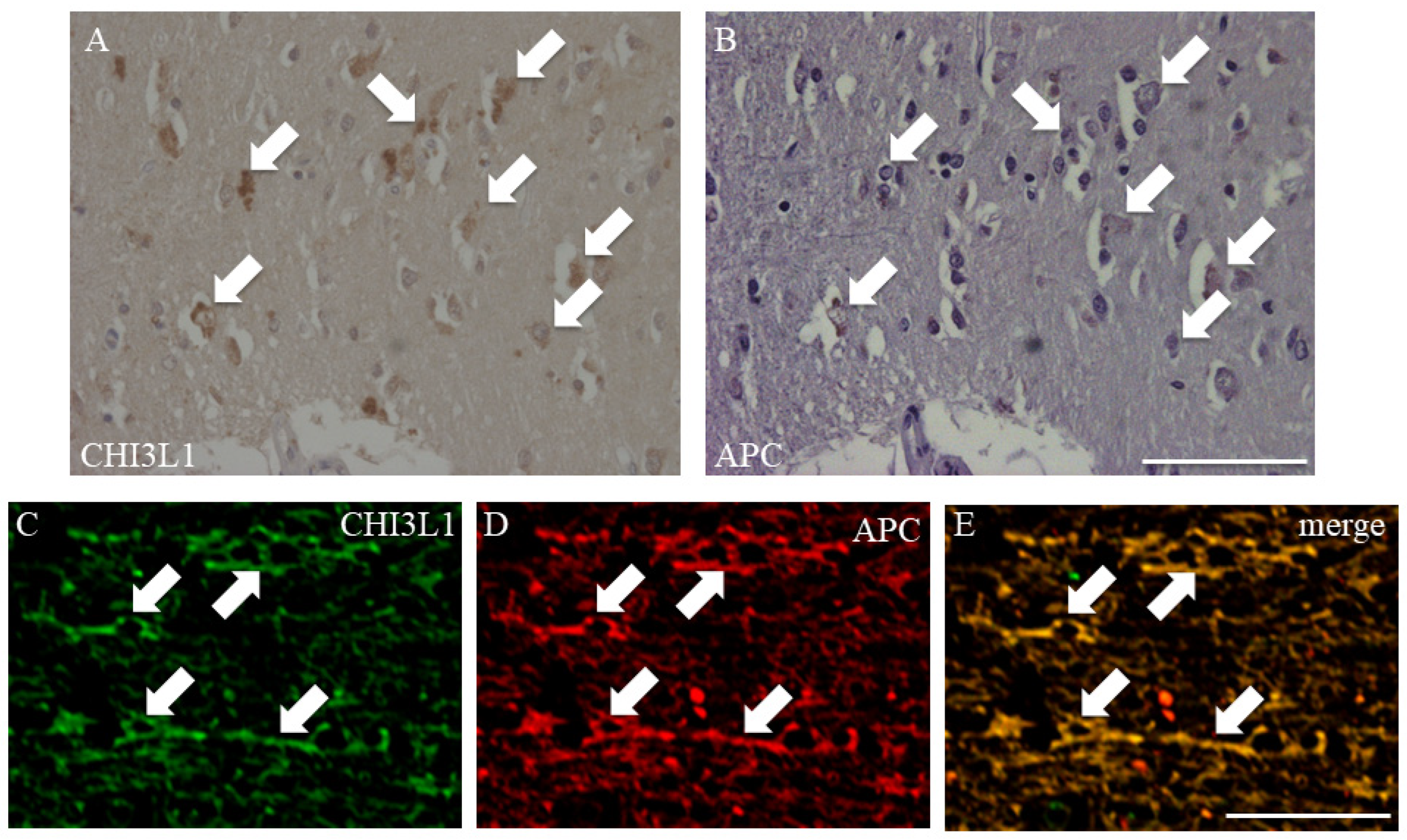
2.3. CHI3L1 Expression in Oligodendrocytes in MS Autopsied Brain Tissue
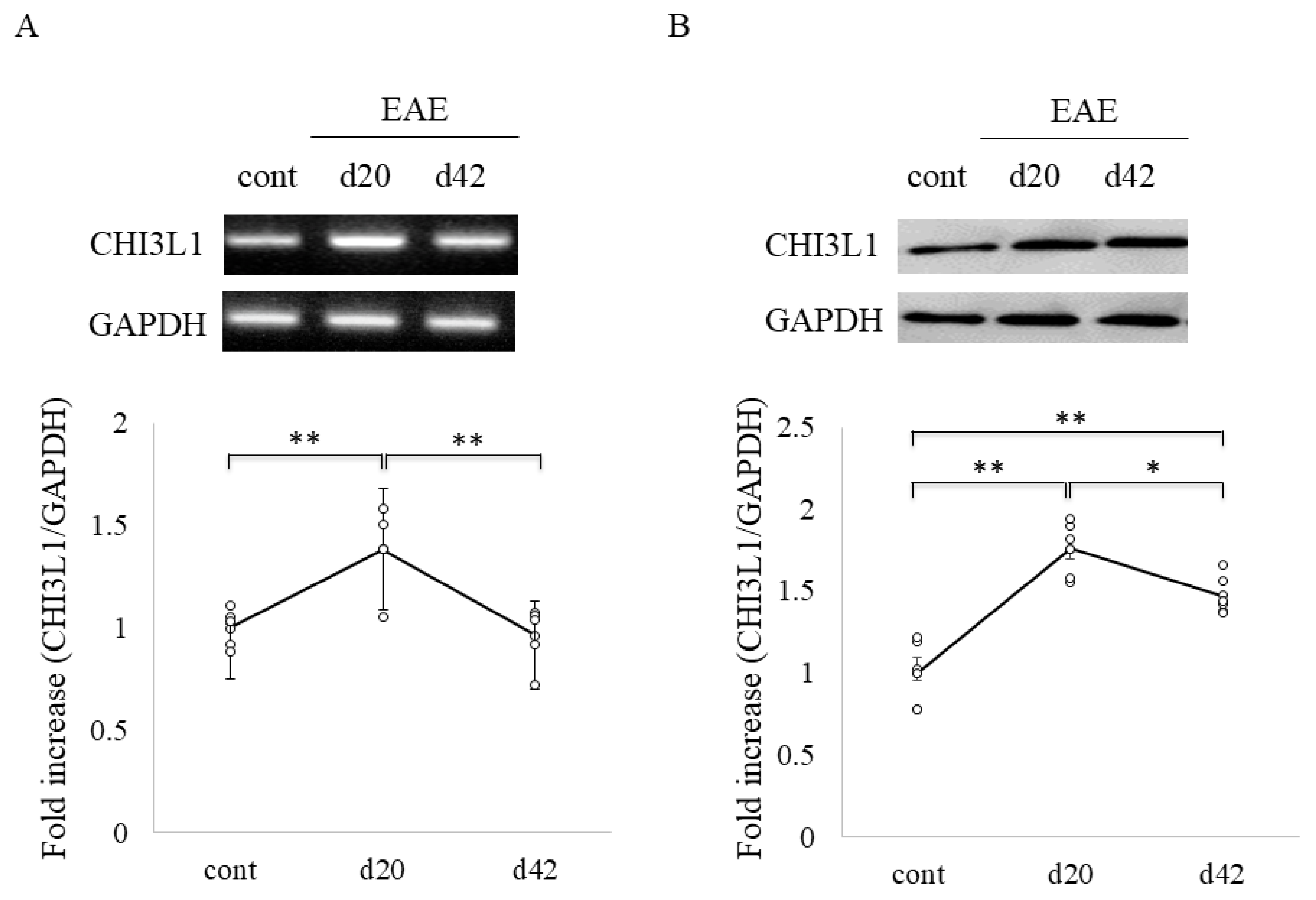
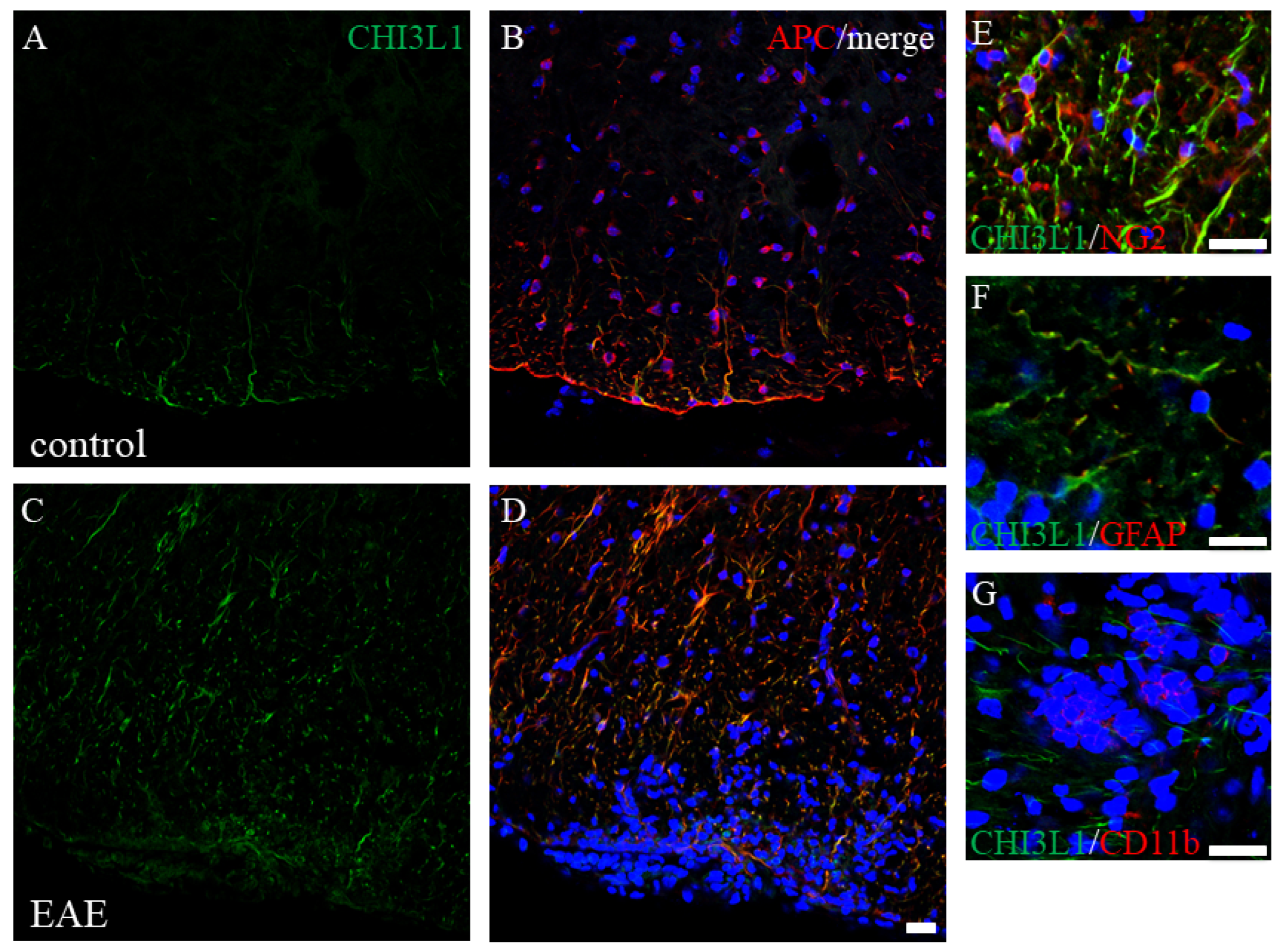
2.4. Elevated CHI3L1 Expression in Oligodendrocytes and Macrophages/Microglia in the EAE Spinal Cord
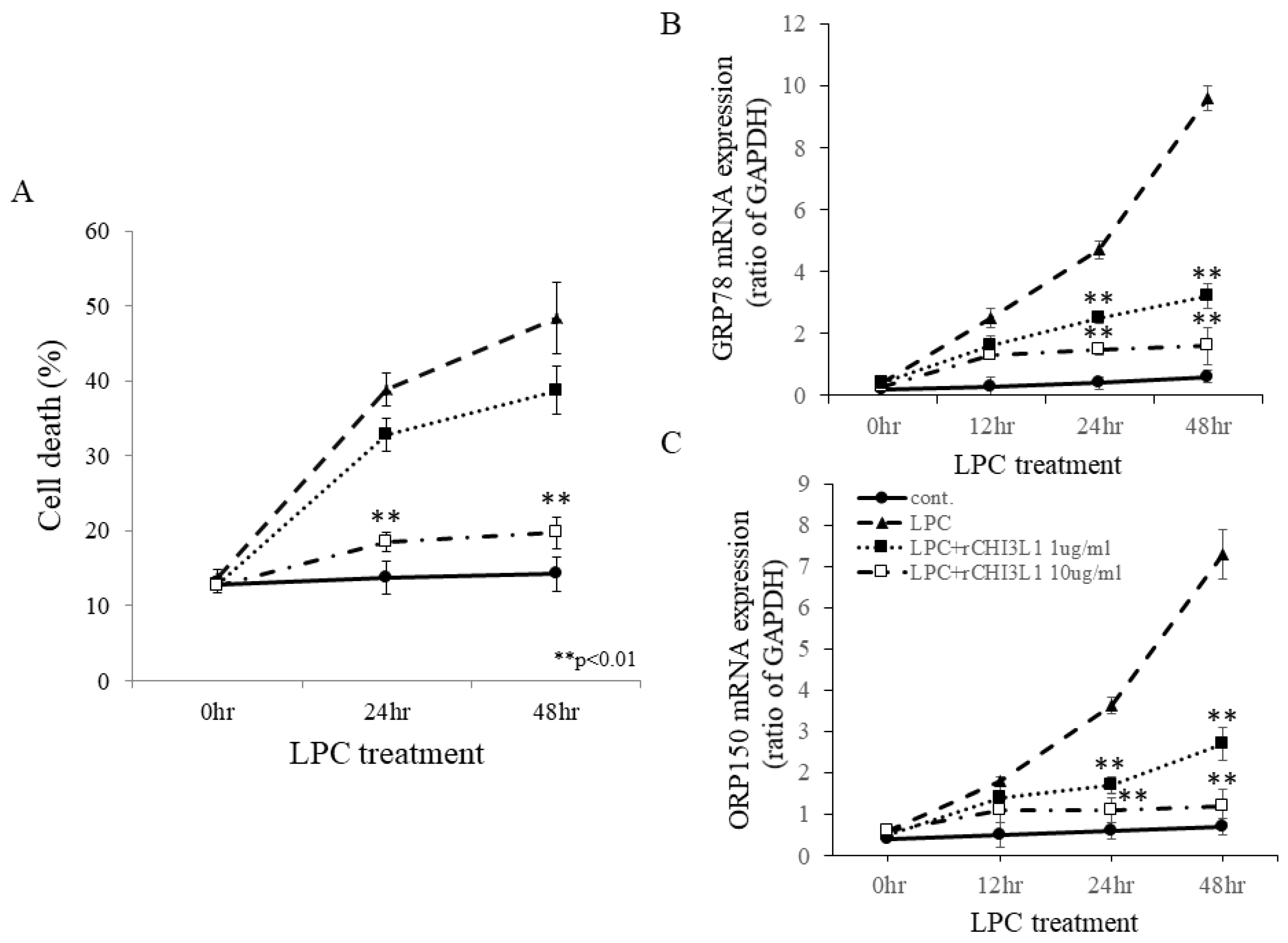
2.5. Protective Effects of rCHI3L1 Against LPC-Induced Cell Death in FBD-102b Cells
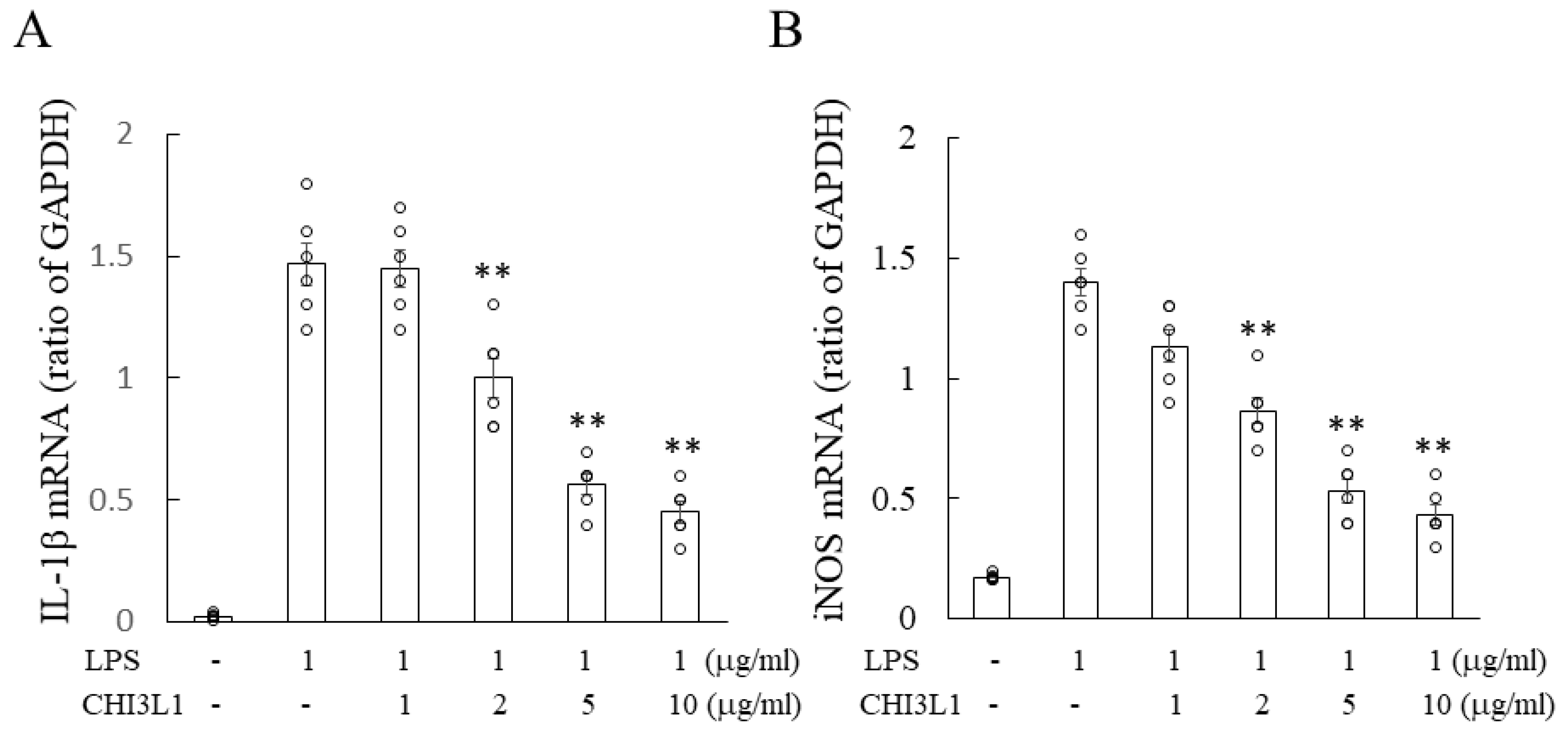
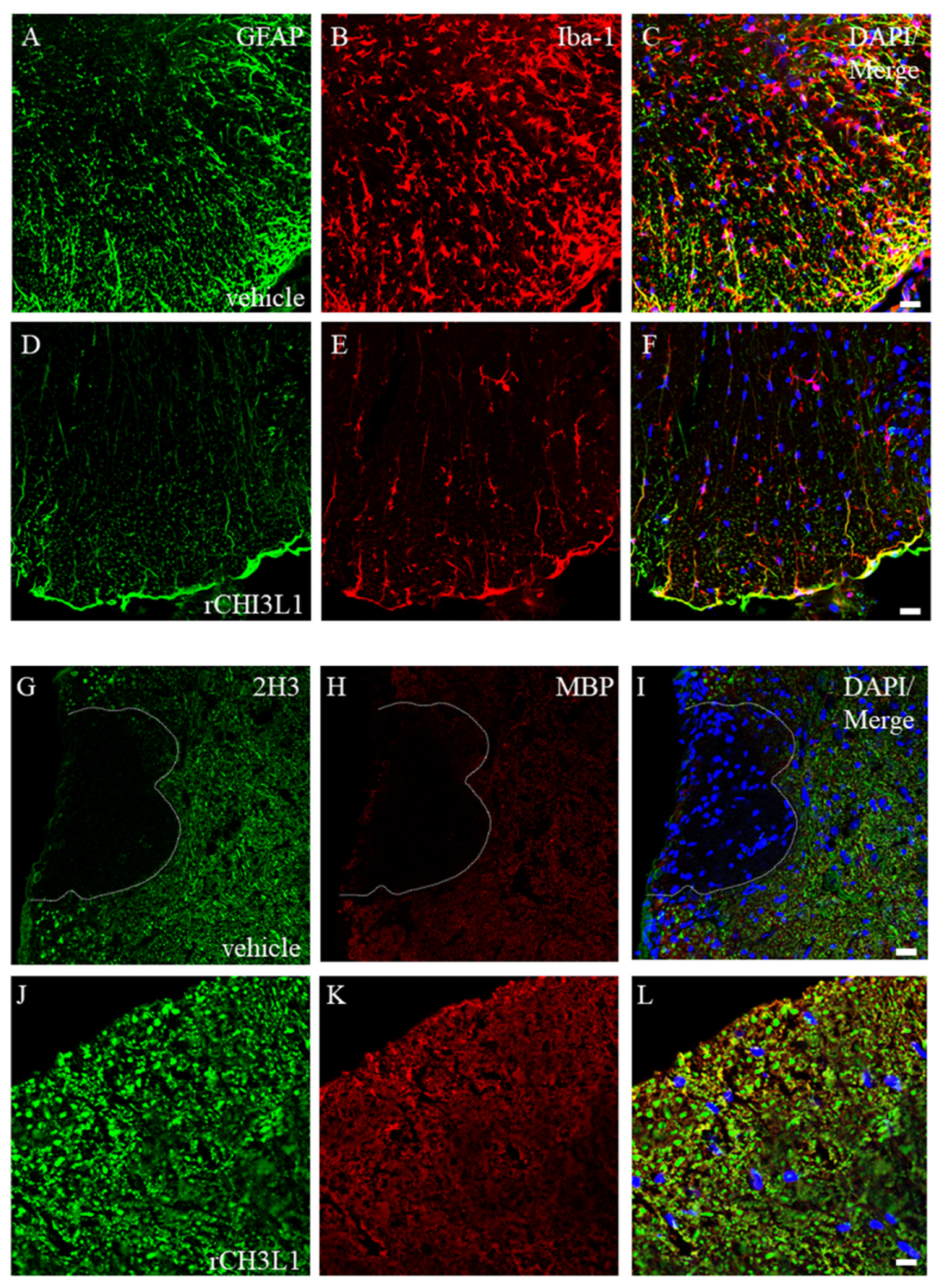
2.6. Attenuation of Microglial Activation by rCHI3L1
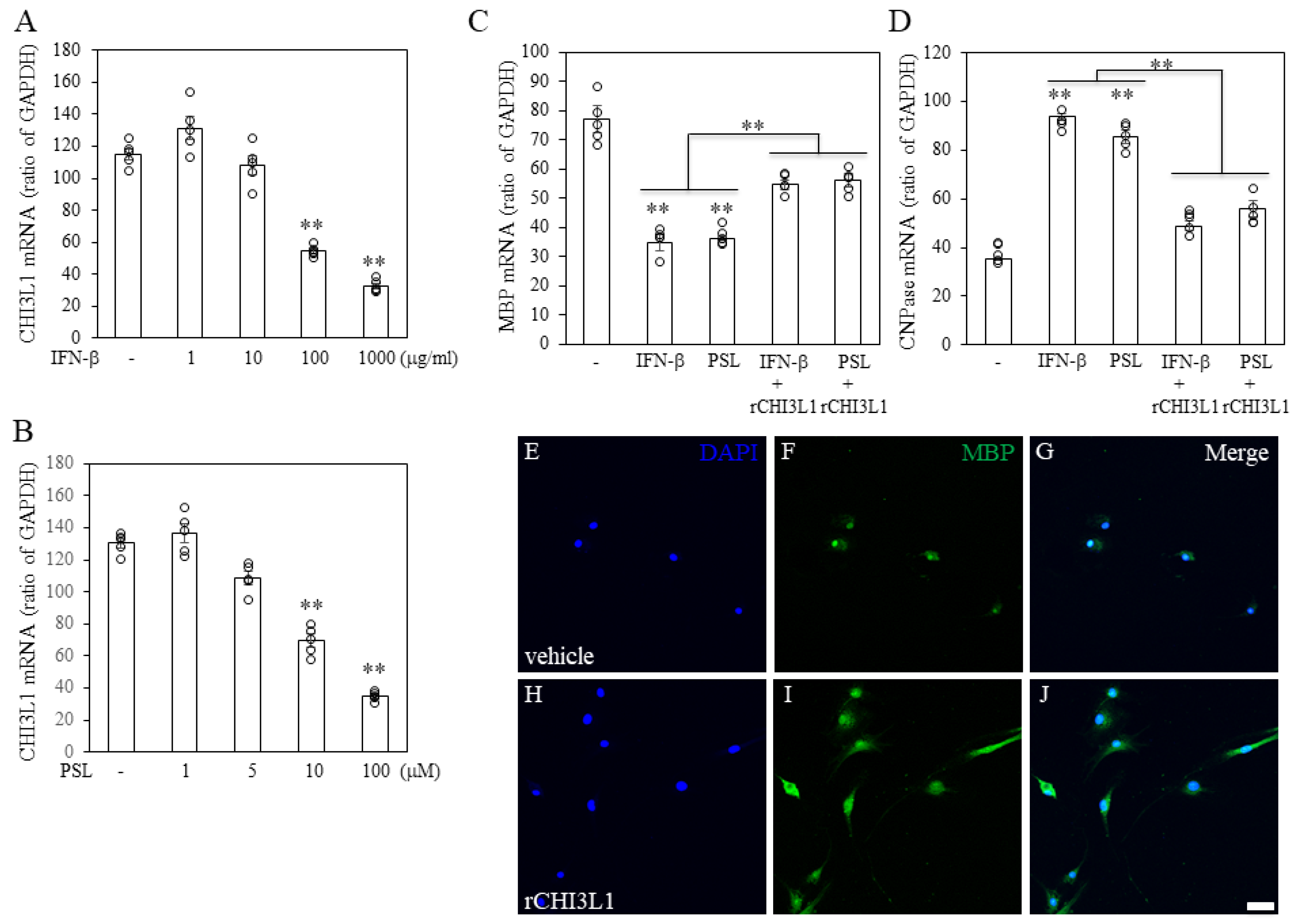
2.7. Effects of rCHI3L1 on EAE Development
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Preparation of CSF Samples
4.3. Proteomics Analysis Using the CSF from MS Patients
4.4. Determination of CHI3L1 Levels in CSF
4.5. Immunohistochemistry
4.6. Animals
4.7. Determination of CHI3L1 Expression by RT-PCR and Western Blot Analysis
4.8. Cell Culture
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EAE | experimental autoimmune encephalomyelitis |
| GFAP | glial fibrillary acid protein |
| Iba-1 | ionized calcium-binding adapter molecule 1 |
| MBP | myelin basic protein |
| MOG | myelin oligodendrocyte glycoprotein |
| MS | multiple sclerosis |
| OLs | oligodendrocytes |
| OPC | oligodendrocyte progenitor cell |
| PBS | phosphate-buffered saline |
| PFA | paraformaldehyde |
References
- Comabella, M.; Pappolla, A.; Monreal, E.; Fissolo, N.; Sao-Avilés, A.C.; Arrambide, G.; Carbonell-Mirabent, P.; Gutierrez, L.; Cobo-Calvo, Á.; Tur, C.; et al. Contribution of Blood Biomarkers to Multiple Sclerosis Diagnosis. Neurol. Neuroimmunol. Neuroinflamm. 2025, 2, e200370. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Keegan, B.M.; Noseworthy, J.H. Multiple sclerosis. Annu. Rev. Med. 2002, 53, 285–302. [Google Scholar] [CrossRef]
- Goodin, D.S.; Frohman, E.M.; Garmany, G.P., Jr.; Halper, J.; Likosky, W.H.; Lublin, F.D.; Silberberg, D.H.; Stuart, W.H.; van den Noort, S. Disease modifying therapies in multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002, 58, 169–178. [Google Scholar]
- Avasarala, J.R.; Cross, A.H. Oligoclonal band number as a marker for prognosis in multiple sclerosis. Arch. Neurol. 2001, 58, 2044–2045. [Google Scholar] [CrossRef]
- Martinez, M.A.; Olsson, B.; Bau, L.; Matas, E.; Cobo Calvo, Á.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult. Scler. 2015, 21, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Stoop, M.P.; Singh, V.; Stingl, C.; Martin, R.; Khademi, M.; Olsson, T.; Hintzen, R.Q.; Luider, T.M. Effects of natalizumab treatment on the cerebrospinal fluid proteome of multiple sclerosis patients. J. Proteome Res. 2013, 12, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Comabella, M.; Fernandez, M.; Martin, R.; Rivera-Vallvé, S.; Borrás, E.; Chiva, C.; Julià, E.; Rovira, A.; Cantó, E.; Alvarez-Cermeño, J.C.; et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain 2010, 133 Pt 4, 1082–1093. [Google Scholar] [CrossRef]
- Gómez-Cardona, E.E.; Hernández-Domínguez, E.E.; Velarde-Salcedo, A.J.; Pacheco, A.B.; Diaz-Gois, A.; De León-Rodríguez, A.; Barba de la Rosa, A.P. 2D-DIGE as a strategy to identify serum biomarkers in Mexican patients with Type-2 diabetes with different body mass index. Sci. Rep. 2017, 7, 46536. [Google Scholar]
- Sotgiu, S.; Barone, R.; Arru, G.; Fois, M.L.; Pugliatti, M.; Sanna, A.; Rosati, G.; Musumeci, S. Intrathecal chitotriosidase and the outcome of multiple sclerosis. Mult. Scler. 2006, 12, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Fusetti, F.; Pijning, T.; Kalk, K.H.; Bos, E.; Dijkstra, B.W. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J. Biol. Chem. 2003, 278, 37753–37760. [Google Scholar] [CrossRef]
- Yeo, I.J.; Lee, C.K.; Han, S.B.; Yun, J.; Hong, J.T. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol. Ther. 2019, 203, 107394. [Google Scholar] [CrossRef]
- Liu, D.; Hu, X.; Ding, X.; Li, M.; Ding, L. Inflammatory Effects and Regulatory Mechanisms of Chitinase-3-like-1 in Multiple Human Body Systems: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 13437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Sig. Transduct. Target Ther. 2020, 5, 201. [Google Scholar] [CrossRef]
- Floro, S.; Carandini, T.; Pietroboni, A.M.; De Riz, M.A.; Scarpini, E.; Galimberti, D. Role of Chitinase 3–like 1 as a biomarker in multiple sclerosis: A systematic review and meta-analysis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1164. [Google Scholar] [CrossRef] [PubMed]
- Cantó, E.; Tintoré, M.; Villar, L.M.; Costa, C.; Nurtdinov, R.; Álvarez-Cermeño, J.C.; Arrambide, G.; Reverter, F.; Deisenhammer, F.; Hegen, H.; et al. Chitinase 3-like 1: Prognostic biomarker in clinically isolated syndromes. Brain 2015, 138 Pt 4, 918–931. [Google Scholar] [CrossRef]
- Jatczak-Pawlik, I.; Jurewicz, A.; Domowicz, M.; Ewiak-Paszyńska, A.; Stasiołek, M. CHI3L1 in Multiple Sclerosis-From Bench to Clinic. Cells 2024, 13, 2086. [Google Scholar] [CrossRef]
- Talaat, F.; Abdelatty, S.; Ragaie, C.; Dahshan, A. Chitinase-3-like 1-protein in CSF: A novel biomarker for progression in patients with multiple sclerosis. Neurol. Sci. 2023, 44, 3243–3252. [Google Scholar] [CrossRef]
- Hinsinger, G.; Galéotti, N.; Nabholz, N.; Urbach, S.; Rigau, V.; Demattei, C.; Lehmann, S.; Camu, W.; Labauge, P.; Castelnovo, G.; et al. Chitinase 3-like Proteins as Diagnostic and Prognostic Biomarkers of Multiple Sclerosis. Mult. Scler. 2015, 21, 1251–1261. [Google Scholar] [CrossRef]
- Cantó, E.; Reverter, F.; Morcillo-Suárez, C.; Matesanz, F.; Fernández, O.; Izquierdo, G.; Vandenbroeck, K.; Rodríguez-Antigüedad, A.; Urcelay, E.; Arroyo, R.; et al. Chitinase 3-like 1 Plasma Levels Are Increased in Patients with Progressive Forms of Multiple Sclerosis. Mult. Scler. 2012, 18, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Cubas-Núñez, L.; Gil-Perotín, S.; Castillo-Villalba, J.; López, V.; Solís Tarazona, L.; Gasqué-Rubio, R.; Carratalá-Boscá, S.; Alcalá-Vicente, C.; Pérez-Miralles, F.; Lassmann, H.; et al. Potential Role of CHI3L1+ Astrocytes in Progression in MS. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e972. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jiang, W.; Afridi, S.K.; Wang, T.; Zhu, F.; Xu, H.; Nazir, F.H.; Liu, C.; Wang, Y.; Long, Y.; et al. Astrocyte-derived CHI3L1 signaling impairs neurogenesis and cognition in the demyelinated hippocampus. Cell Rep. 2024, 43, 114226. [Google Scholar] [CrossRef]
- Bonneh-Barkay, D.; Bissel, S.J.; Kofler, J.; Starkey, A.; Wang, G.; Wiley, C.A. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012, 22, 530–546. [Google Scholar] [CrossRef] [PubMed]
- Bonneh-Barkay, D.G.; Wang, G.; Starkey, A.; Hamilton, R.L.; Wiley, C.A. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J. Neuroinflamm. 2010, 7, 34. [Google Scholar] [CrossRef]
- Mizoguchi, E.; Sadanaga, T.; Nanni, L.; Wang, S.; Mizoguchi, A. Recently Updated Role of Chitinase 3-like 1 on Various Cell Types as a Major Influencer of Chronic Inflammation. Cells 2024, 13, 678. [Google Scholar] [CrossRef]
- Blazevic, N.; Rogic, D.; Pelajic, S.; Miler, M.; Glavcic, G.; Ratkajec, V.; Vrkljan, N.; Bakula, D.; Hrabar, D.; Pavic, T. YKL-40 as a biomarker in various inflammatory diseases: A review. Biochem. Med. 2024, 34, 010502. [Google Scholar]
- Kim, E.G.; Kim, M.N.; Hong, J.Y.; Lee, J.W.; Kim, S.Y.; Kim, K.W.; Lee, C.G.; Elias, J.A.; Song, T.W.; Sohn, M.H. Chitinase 3-Like 1 Contributes to Food Allergy via M2 Macrophage Polarization. Allergy Asthma Immunol. Res. 2022, 12, 1012–1028. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, X.; Shi, Y.; Zhu, X.; Dai, Y.; Qian, X.; Gu, J. CHI3L1 alleviate acute liver injury by inhibiting Th1 cells differentiation through STAT3 signaling pathway. Ann. Transl. Med. 2021, 9, 529. [Google Scholar] [CrossRef]
- Kwak, E.J.; Hong, J.Y.; Kim, M.N.; Kim, S.Y.; Kim, S.H.; Park, C.O.; Kim, K.W.; Lee, C.G.; Elias, J.A.; Jee, H.M.; et al. Chitinase 3-like 1 drives allergic skin inflammation via Th2 immunity and M2 macrophage activation. Clin. Exp. Allergy 2019, 49, 1464–1474. [Google Scholar] [CrossRef]
- Lee, C.M.; He, C.H.; Nour, A.M.; Zhou, Y.; Ma, B.; Park, J.W.; Kim, K.H.; Dela Cruz, C.; Sharma, L.; Nasr, M.L.; et al. IL-13Rα2 uses TMEM219 in chitinase 3-like-1-induced signalling and effector responses. Nat. Commun. 2016, 7, 12752. [Google Scholar] [CrossRef] [PubMed]
- He, C.H.; Lee, C.G.; Dela Cruz, C.S.; Lee, C.M.; Zhou, Y.; Ahangari, F.; Ma, B.; Herzog, E.L.; Rosenberg, S.A.; Li, Y.; et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor alpha2. Cell Rep. 2013, 4, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Hartl, D.; Lee, G.R.; Koller, B.; Matsuura, H.; Da Silva, C.A.; Sohn, M.H.; Cohn, L.; Homer, R.J.; Kozhich, A.A.; et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J. Exp. Med. 2009, 206, 1149–1166. [Google Scholar] [CrossRef]
- van Sleen, Y.; Jiemy, W.F.; Pringle, S.; van der Geest, K.S.M.; Abdulahad, W.H.; Sandovici, M.; Brouwer, E.; Heeringa, P.; Boots, A.M.H. A Distinct Macrophage Subset Mediating Tissue Destruction and Neovascularization in Giant Cell Arteritis: Implication of the YKL-40/Interleukin-13 Receptor α2 Axis. Arthritis Rheumatol. 2021, 73, 2327–2337. [Google Scholar] [CrossRef]
- Ni, M.; Lee, A.S. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007, 581, 3641–3651. [Google Scholar] [CrossRef] [PubMed]
- Starossom, S.C.; Garcia, J.C.; Woelfle, T.; Romero-Suarez, S.; Olah, M.; Watanabe, F.; Cao, L.; Yeste, A.; Tukker, J.J.; Quintana, F.J.; et al. Chi3l3 induces oligodendrogenesis in an experimental model of autoimmune neuroinflammation. Nat. Commun. 2019, 10, 217. [Google Scholar] [CrossRef]
- Im, J.H.; Yeo, I.J.; Park, P.H.; Choi, D.Y.; Han, S.B.; Yun, J.; Hong, J.T. Deletion of Chitinase-3-like 1 accelerates stroke development through enhancement of neuroinflammation by STAT6-dependent M2 microglial inactivation in Chitinase-3-like 1 knockout mice. Exp. Neurol. 2020, 323, 113082. [Google Scholar] [CrossRef]
- Ling, H.; Recklies, A.D. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem. J. 2004, 380 Pt 3, 651–659. [Google Scholar] [CrossRef]
- Novakova, L.; Axelsson, M.; Khademi, M.; Zetterberg, H.; Blennow, K.; Malmeström, C.; Piehl, F.; Olsson, T.; Lycke, J. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult. Scler. 2017, 23, 62–71. [Google Scholar] [CrossRef]
- Bando, Y.; Hagiwara, Y.; Suzuki, Y.; Yoshida, K.; Aburakawa, Y.; Kimura, T.; Murakami, C.; Ono, M.; Tanaka, T.; Jiang, Y.P.; et al. Kallikrein 6 secreted by oligodendrocytes regulates the progression of experimental autoimmune encephalomyelitis. Glia 2018, 66, 359–378. [Google Scholar] [CrossRef]
- Takano, C.; Takano, T.; Masumura, M.; Nakamura, R.; Koda, S.; Bochimoto, H.; Yoshida, S.; Bando, Y. Involvement of Degenerating 21.5 kDa Isoform of Myelin Basic Protein in the Pathogenesis of the Relapse in Murine Relapsing-Remitting Experimental Autoimmune Encephalomyelitis and MS Autopsied Brain. Int. J. Mol. Sci. 2023, 24, 8160. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Takano, C.; Funakoshi, H.; Bando, Y. Impact of Neuron-Derived HGF on c-Met and KAI-1 in CNS Glial Cells: Implications for Multiple Sclerosis Pathology. Int. J. Mol. Sci. 2024, 25, 11261. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.; Geisler, J.G. Disease modifying mitochondrial uncouplers, MP101, and a slow release ProDrug, MP201, in models of Multiple Sclerosis. Neurochem. Int. 2019, 131, 104561. [Google Scholar] [CrossRef]
- Ohse, T.; Inagi, R.; Tanaka, T.; Ota, T.; Miyata, T.; Kojima, I.; Ingelfinger, J.R.; Ogawa, S.; Fujita, T.; Nangaku, M. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int. 2006, 70, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Mwale, P.F.; Hsieh, C.T.; Yen, T.L.; Jan, J.S.; Taliyan, R.; Yang, C.H.; Yang, W.B. Chitinase-3-like-1: A multifaceted player in neuroinflammation and degenerative pathologies with therapeutic implications. Mol. Neurodegener. 2025, 20, 7. [Google Scholar] [CrossRef]
- Horiuchi, M.; Tomooka, Y. An oligodendroglial progenitor progenitor cell line FBD-102b possibly secretes a radial glia-inducing factor. Neurosci. Res. 2006, 56, 213–219. [Google Scholar] [CrossRef]

| Age/Sex | Diagnosis | AQP-4 |
|---|---|---|
| 25/F | RRMS | ND |
| 28/F | RRMS | ND |
| 29/F | RRMS | ND |
| 18/F | headache | - |
| 35/F | headache | - |
| 20/F | headache | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bando, Y.; Suzuki, Y.; Murakami, C.; Kimura, T.; Yahara, O. Implications of Chitinase 3-like 1 Protein in the Pathogenesis of Multiple Sclerosis in Autopsied Brains and a Murine Model. Int. J. Mol. Sci. 2025, 26, 4160. https://doi.org/10.3390/ijms26094160
Bando Y, Suzuki Y, Murakami C, Kimura T, Yahara O. Implications of Chitinase 3-like 1 Protein in the Pathogenesis of Multiple Sclerosis in Autopsied Brains and a Murine Model. International Journal of Molecular Sciences. 2025; 26(9):4160. https://doi.org/10.3390/ijms26094160
Chicago/Turabian StyleBando, Yoshio, Yasuhiro Suzuki, Chisato Murakami, Takashi Kimura, and Osamu Yahara. 2025. "Implications of Chitinase 3-like 1 Protein in the Pathogenesis of Multiple Sclerosis in Autopsied Brains and a Murine Model" International Journal of Molecular Sciences 26, no. 9: 4160. https://doi.org/10.3390/ijms26094160
APA StyleBando, Y., Suzuki, Y., Murakami, C., Kimura, T., & Yahara, O. (2025). Implications of Chitinase 3-like 1 Protein in the Pathogenesis of Multiple Sclerosis in Autopsied Brains and a Murine Model. International Journal of Molecular Sciences, 26(9), 4160. https://doi.org/10.3390/ijms26094160





