Abstract
An aza-Michael/Michael cascade reaction of 2-((E)-2-nitrovinyl)-N-tosylbenzenamine with 3-phenylpropiolaldehyde catalyzed by pyrrolidine has produced a new compound, 1,4-dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde. The structure of the newly synthesized compound was determined using 1H, 13C-NMR, IR, and mass spectral data.
1. Introduction
Hydroquinoline is structurally essential unit in biologically active natural products [1,2]. The hydroquinoline is widely used as a pharmacophore in drug discovery and exhibits a broad range of biological activities such as anti-HIV, antibacterial, antifungal, antimalarial, antitumor, and cardiovascular effects [3,4,5]. In view of the significance of the hydroquinoline structure in medicinal and organic chemistry, numerous synthetic methods for hydroquinline scaffolds have been developed [6]. Based on our previous results of the cascade reaction for the synthesis of hydroquinoline compounds [7,8,9,10], we have successfully obtained a novel compound: 1,4-dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde.
2. Results
The synthesis of 1,4-dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde (3) was achieved in one step, as presented in Scheme 1, which was performed via an aza-Michael/Michael cascade reaction of 2-((E)-2-nitrovinyl)-N-tosylbenzenamine (1) with 3-phenylpropiolaldehyde (2). The reaction was carried out in toluene in the presence of 20 mol % pyrrolidine as a catalyst and 20 mol % 2-nitrobenzoic acid as an additive. The desired product 3 was obtained in moderate yield via an aza-Michael/Michael cascade reaction. The structure of compound 3 was confirmed via 1H- and 13C-NMR, IR, and mass spectral data, and all data are in accordance with the proposed structure.
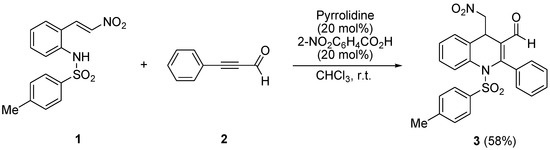
Scheme 1.
Synthesis of 1,4-dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde (3).
3. Experimental Section
3.1. General Information
All reagents were used as received without further purification. Organic solutions were concentrated under reduced pressure using a Büchi rotary evaporator. Chromatographic purification of the title compound 3 was accomplished using forced-flow chromatography on ICN 60 32–64 mesh silica gel 63. Thin-layer chromatography (TLC) was performed on EM Reagents 0.25 mm silica gel 60-F plates. Developed chromatograms were visualized by fluorescence quenching and anisaldehyde staining. 1H- and 13C-NMR spectra were recorded on a Bruker Avance 400 spectrometer (Bruker BioSpin GmbH, Karlsruhe, Germany) in CDCl3. Chemical shifts are internally referenced to residual protio solvent signals (δ 7.26 ppm for 1H; δ77.16 ppm for 13C). Data for 1H-NMR are reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, m = multiplet), integration, coupling constant (Hz), and assignment. Data for 13C-NMR are reported in terms of chemical shift. IR spectra were recorded on an ALPHA FT-IR spectrometer (Bruker Optics GmbH, Ettlingen, Germany) and reported in terms of frequency of absorption (cm−1). High-resolution mass spectrometry data was recorded on a JEOL JMS-700 MStation mass spectrometer (JEOL, Tokyo, Japan).
3.2. Syntheis of 1,4-Dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde (3)
2-((E)-2-Nitrovinyl)-N-tosylbenzenamine (1) (63 mg, 0.2 mmol) was added to a solution of pyrrolidine (3.4 μL, 0.04 mmol) and 2-nitrobenzoic acid (6.7 mg, 0.04 mmol) in CHCl3 (0.7 mL) at room temperature. The solution was stirred for 5 min before the addition of 3-phenylpropiolaldehyde (2) (30 μL, 0.24 mmol). The resulting mixture was stirred for 48 h until complete consumption of 2-((E)-2-nitrovinyl)-N-tosylbenzenamine (1) was observed as determined by TLC. The resulting mixture was directly purified by flash silica gel column chromatography using EtOAc/hexane (1/10) as eluent to afford the desired title compound 3 (58%, 52 mg).
White solid; m.p. 215–217 °C; 1H-NMR (400 MHz, CDCl3) δ 9.31 (s, 1H), 8.06–7.95 (m, 1H), 7.64–7.42 (m, 6H), 7.33 (ddd, J = 10.5, 8.6, 4.7 Hz, 3H), 7.29–7.19 (m, 3H), 4.72 (dd, J = 9.7, 5.8 Hz, 1H), 3.91 (dd, J = 11.8, 5.8 Hz, 1H), 3.15 (dd, J = 11.8, 9.7 Hz, 1H), 2.40 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 190.37, 157.50, 145.87, 136.36, 134.81, 133.12, 131.22, 130.94, 130.42, 130.10, 129.27, 128.37, 128.35, 127.92, 127.82, 125.18, 124.83, 78.46, 34.56, 21.64; IR (film) 3235, 2863, 1714, 1592, 1512, 1440, 1398, 1318, 1185, 1125, 1084 cm−1; HRMS (EI) m/z calcd for [M]+ C24H20N2O5S: 448.1093 Found: 448.1084.
Supplementary Materials
1H- and 13C-NMR spectra for compound 3 are available online at www.mdpi.com/1422-8599/2016/4/M918.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
This work was supported by Kyonggi University's Graduate Research Assistantship 2015.
Author Contributions
Both authors contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kouznetsov, V.; Palma, A.; Ewert, C.; Varlamov, A. Some aspects of reduced quinoline chemistry. J. Heterocycl. Chem. 1998, 35, 761–785. [Google Scholar] [CrossRef]
- Zhou, Y.-G. Asymmetric Hydrogenation of Heteroaromatic Compounds. Acc. Chem. Res. 2007, 40, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, E.; Manian, R.D.R.S.; Raghunathan, R.; Sainath, S.; Raghunathan, M. Synthesis and antibacterial property of quinolines with potent DNA gyrase activity. Bioorg. Med. Chem. 2009, 17, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.Z.; Hunt, J.T.; Ricca, C.; Manne, V. 3-Imidazolylmethylaminophenylsulfonyl- tetrahydroquinolines, a novel series of farnesyltransferase inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 273–275. [Google Scholar] [CrossRef]
- Fotie, J.; Kaiser, M.; Delfin, D.A.; Manley, J.; Reid, C.S.; Paris, J.-M.; Wenzler, T.; Maes, L.; Mahasenan, K.V.; Li, C.; Werbovetz, K.A. Antitrypanosomal Activity of 1,2-Dihydroquinolin-6-ols and Their Ester Derivatives. J. Med. Chem. 2010, 53, 966–982. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Suryavanshi, P.A.; Menendez, J.C. Advances in the Chemistry of Tetrahydroquinolines. Chem. Rev. 2011, 111, 7157–7259. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Heo, S.; Kim, S.-G. Asymmetric one-pot synthesis of 1,4-dihydroquinolines via an organocatalytic aza-Michael/Michael cascade strategy. Adv. Synth. Catal. 2015, 357, 1545–1550. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.-G. One-pot organocatalytic enantioselective Michael addition and aza-cyclization/dehydration cascade reaction strategy: Asymmetric synthesis of highly functionalized 1,4-dihydroquinolines. Tetrahedron Lett. 2015, 56, 4819–4823. [Google Scholar] [CrossRef]
- Yu, M.; Kim, S.-G. Asymmetric organocatalytic Michael addition/aza-cyclization coupled with sequential Michael addition for synthesizing densely polycyclic-fused dihydroquinolines. Tetrahedron Lett. 2015, 56, 4159–4162. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.-G. Asymmetric organocatalytic cascade reaction of aldehydes with 2-amino-β-nitrostyrenes: Synthesis of chiral tetrahydroquinolines and dihydroquinolines. J. Org. Chem. 2014, 79, 8234–8243. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).