Effects of Forest Composition and Disturbance on Arbuscular Mycorrhizae Spore Density, Arbuscular Mycorrhizae Root Colonization and Soil Carbon Stocks in a Dry Afromontane Forest in Northern Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
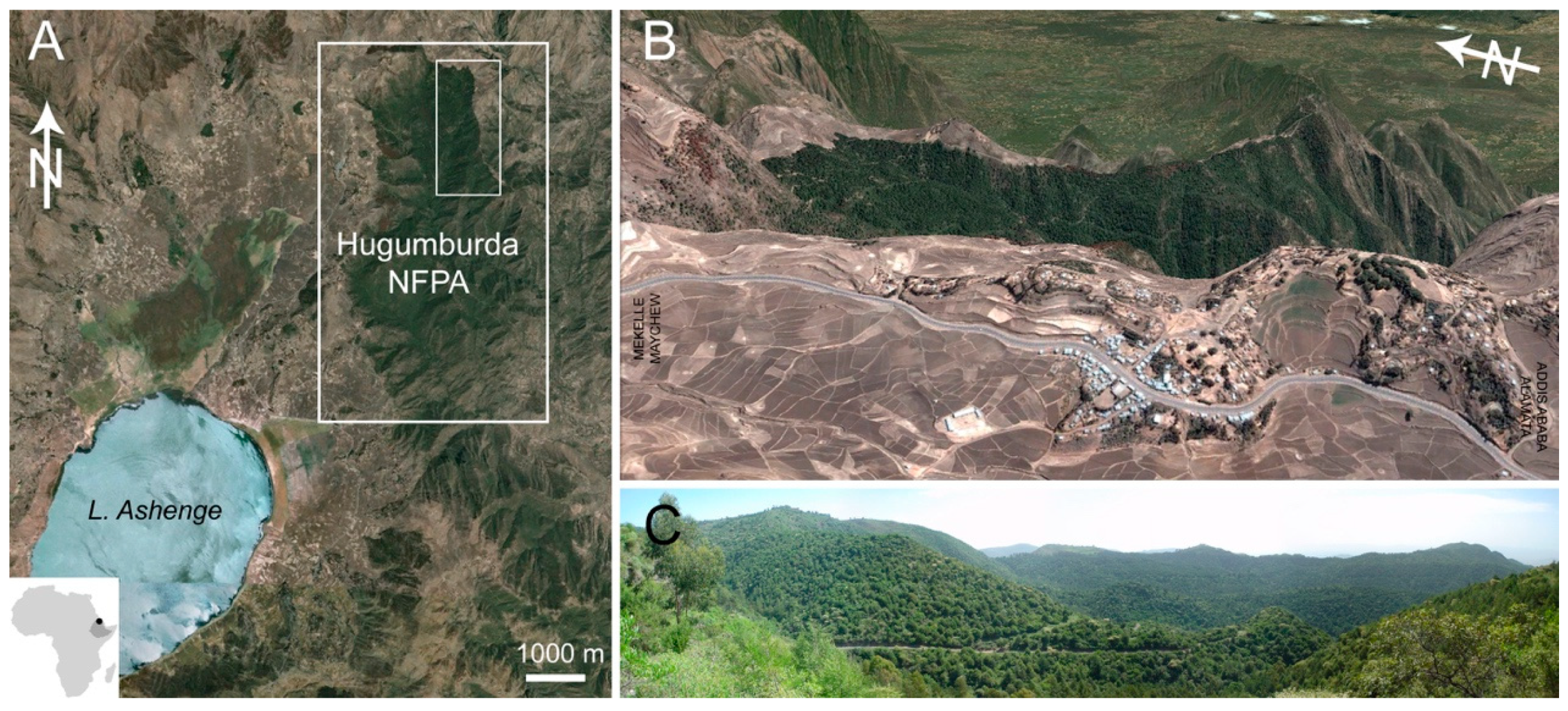
2.1. Study Area
2.2. Experimental Design and Layout
2.3. Data Collection
Root and Rhizosphere Soil Sampling
2.4. Soil Sampling
2.5. Laboratory Analysis
2.5.1. Soil Laboratory Analysis for AMF Spore Density
2.5.2. Arbuscular Mycorrhiza Fungi Root Colonization Analysis
2.5.3. Analysis of Soil Chemical Properties
2.5.4. Soil Organic Carbon Stock Estimation
2.6. Statistical Analysis
3. Results
3.1. Assessment of Plant Communities and Levels of Disturbance
3.2. Differences in AMF Spore Density, Root Colonization and AMF Structures between Plant Communities and at Different Soil Depths
3.3. Differences in Soil Properties between Plant Communities and at Different Depths
3.4. Effect of the Interactions between Plant Communities and Soil Depth on Spore Density and root Colonization of AMF
3.5. Correlation between Spore Density and Root Colonization of AMF, with Soil Chemical Property and Soil Carbon Stock
4. Discussion
4.1. Differences in AMF Spore Density, Root Colonization and AMF Structures, between Plant Communities and at Different Soil Depths
4.2. Correlation of AMF Spore Density and Root Colonization with Soil Property and Soil Carbon Stock
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parry, M.L.; Canziani, O.F.; Palutikof, J.P.; van der Linden, P.J.; Hanson, C.E. (Eds.) Climate Change 2007: Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Van der Werf, G.R.; Morton, D.C.; DeFries, R.S.; Olivier, J.G.; Kasibhatla, P.S.; Jackson, R.B.; Randerson, J.T. CO2 emissions from forest loss. Nat. Geosci. 2009, 2, 737–738. [Google Scholar] [CrossRef]
- Aynekulu, E.; Denich, M.; Tsegaye, D.; Aerts, R.; Neuwirth, B.; Boehmer, H.J. Dieback affects forest structure in a dry Afromontane forest in northern Ethiopia. J. Arid Environ. 2011, 75, 499–503. [Google Scholar] [CrossRef]
- Birhane, E.; Kuyper, T.W.; Sterck, F.J.; Bongers, F. Arbuscular mycorrhizal associations in Boswellia papyrifera (frankincense-tree) dominated dry deciduous woodlands of northern Ethiopia. For. Ecol. Manag. 2010, 260, 2160–2169. [Google Scholar] [CrossRef]
- Wassie, A.; Sterck, F.J.; Bongers, F. Species and structural diversity of church forests in a fragmented Ethiopian Highland landscape. J. Veg. Sci. 2010, 21, 938–948. [Google Scholar] [CrossRef]
- Mokria, M.; Gebrekirstos, A.; Aynekulu, E.; Bräuning, A. Tree dieback affects climate change mitigation potential of a dry afromontane forest in northern Ethiopia. For. Ecol. Manag. 2015, 344, 73–83. [Google Scholar] [CrossRef]
- Herre, E.A.; Kyllo, D.; Mangan, S.A.; Husband, R.; Mejia, L.C.; Eom, A.H. An overview of arbuscular mycorrhizal fungi composition, distribution, and host effects from a tropical moist forest. In Biotic interactions in the tropics: Their role in the maintenance of species diversity; Burslem, D.F.R.P., Pinard, M.A., Hartley, S.E., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 201–225. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press Ltd.: Cambridge, UK, 2008. [Google Scholar]
- Allen, M.F.; Moore, T.S.; Christensen, M. Phytohormone changes in Bouteloua gracilis infected by vesicular-arbuscular mycorrhizae. I. Cytokinin increases in the host plant. Can. J. Botany. 1980, 58, 371–374. [Google Scholar] [CrossRef]
- Newsham, K.K.; Fitter, A.H.; Watkinson, A.R. Arbuscular mycorrhiza protect an annual grass from root pathogenic fungi in the field. J. Ecol. 1995, 83, 991–1000. [Google Scholar] [CrossRef]
- Kyllo, D.A.; Velez, V.; Tyree, M.T. Combined effects of arbuscular mycorrhizas and light on water uptake of the neotropical understory shrubs, Piper and Psychotria. New Phytol. 2003, 160, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Andrade, G.; Linderman, R.G.; Bethlenfalvay, G.J. Bacterial associations with the mycorrhizosphere and hyphosphere of the arbuscular mycorrhizal fungus Glomus mosseae. Plant. Soil 1998, 202, 79–87. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Allen, M.F.; Field, C.B. Rise in carbon dioxide changes soil structure. Nature 1999, 400, 628. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Li, M.; Cha, D.J.; Lai, Y.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 2007, 66, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Sha, L.; Yang, X.; Cao, M.; Tang, J.; Zheng, Z. Distribution of roots and arbuscular mycorrhizal associations in tropical forest types of Xishuangbanna, southwest China. Appl. Soil Ecol. 2003, 22, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Bainard, L.D.; Bainard, J.D.; Hamel, C.; Gan, Y. Spatial and temporal structuring of arbuscular mycorrhizal communities is differentially influenced by abiotic factors and host crop in a semi-arid prairie agroecosystem. FEMS Microbiol. Ecol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Bainard, L.D.; Dai, M.; Gomez, E.F.; Torres-Arias, Y.; Bainard, J.D.; Sheng, M.; Eilers, W.; Hamel, C. Arbuscular mycorrhizal fungal communities are influenced by agricultural land use and not soil type among the Chernozem great groups of the Canadian Prairies. Plant. Soil 2015, 387, 351–362. [Google Scholar] [CrossRef]
- Bennett, A.E.; Daniell, T.J.; Öpik, M.; Davison, J.; Moora, M.; Zobel, M.; Evans, D. Arbuscular mycorrhizal fungal networks vary throughout the growing season and between successional stages. PLoS ONE 2013, 8, e83241. [Google Scholar] [CrossRef] [Green Version]
- Brundrett, M.C.; Ashwath, N. Glomeromycotan mycorrhizal fungi from tropical Australia III. Measuring diversity in natural and disturbed habitats. Plant. Soil 2013, 370, 419–433. [Google Scholar] [CrossRef]
- Soteras, F.; Grilli, G.; Cofré, M.N.; Marro, N.; Becerra, A. Arbuscular mycorrhizal fungal composition in high montane forests with different disturbance histories in central Argentina. Appl. Soil Ecol. 2015, 85, 30–37. [Google Scholar] [CrossRef]
- Jasper, D.A.; Abbott, L.K.; Robson, A.D. Hyphae of a vesicular-arbuscular mycorrhizal fungus maintain infectivity in dry soil, except when the soil is disturbed. New Phytol. 1989, 112, 101–107. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bosch, R.; van der Heijden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; de Carvalho, M.; Chatagnier, O.; van Tuinen, D. Impact of tillage system on arbuscular mycorrhiza fungal communities in the soil under Mediterranean conditions. Soil Tillage Res. 2012, 121, 63–67. [Google Scholar] [CrossRef]
- Chaudhary, V.B.; O’Dell, T.E.; Rillig, M.C.; Johnson, N.C. Multiscale patterns of arbuscular mycorrhizal fungal abundance and diversity in semiarid shrublands. Fungal Ecol. 2014, 12, 32–43. [Google Scholar] [CrossRef]
- Gai, J.P.; Tian, H.; Yang, F.Y.; Christie, P.; Li, X.L.; Klironomos, J.N. Arbuscular mycorrhizal fungal diversity along a Tibetan elevation gradient. Pedobiologia 2012, 55, 145–151. [Google Scholar] [CrossRef]
- Coutinho, E.S.; Fernandes, G.W.; Berbara, R.L.L.; Valério, H.M.; Goto, B.T. Variation of arbuscular mycorrhizal fungal communities along an altitudinal gradient in rupestrian grasslands in Brazil. Mycorrhiza 2015, 25, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, A.; Lisanework, N.; Friis, I.B. Impacts of tree plantations in the Ethiopian highland on soil fertility, shoot and root growth, nutrient utilisation and mycorrhizal colonisation. For. Ecol. Manag. 1993, 61, 299–324. [Google Scholar] [CrossRef]
- Wubet, T.; Weiß, M.; Kottke, I.; Teketay, D.; Oberwinkler, F. Molecular diversity of arbuscular mycorrhizal fungi in Prunus africana, an endangered medicinal tree species in dry Afromontane forests of Ethiopia. New Phytol. 2004, 161, 517–528. [Google Scholar] [CrossRef]
- Aynekulu, E. Forest diversity in fragmented landscapes of northern Ethiopia and implications for conservation. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germany, 2011. [Google Scholar]
- Friis, I.B. Forests and forest trees of northeast tropical Africa: Their natural habitats and distribution patterns in Ethiopia, Djibouti and Somalia. Kew Bulletin Additional Series XV; Her Majesty’s Stationery Office: London, UK, 1992. [Google Scholar]
- Parent, G. Manual for woody biomass inventory. In Woody Biomass Inventory and Strategic Planning Project; Ministry of Agriculture: Addis Ababa, Ethiopia, 2000. [Google Scholar]
- Woldemichael, L.K.; Bekele, T.; Nemomissa, S. Vegetation composition in Hugumbirda-Gratkhassu national forest priority area, South Tigray. Momona Ethiop. J. Sci. 2010, 2, 27–48. [Google Scholar] [CrossRef]
- Sewnet, T.C.; Tuju, F.A. Arbuscular mycorrhizal fungi associated with shade trees and Coffea arabica L. in a coffee-based agroforestry system in Bonga, Southwestern Ethiopia. Afr. Focus 2013, 26, 111–131. [Google Scholar] [CrossRef] [Green Version]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Walker, C.; Mize, C.W.; McNabb, H.S. Population of endogonaceous fungi at two locations in central Iowa. Can. J. Bot. 1982, 60, 2518–2529. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Das, P.; Kayang, H. Stamp pad ink, an effective stain for observing arbuscular mycorrhizal structure in roots. World J. Agric. Sci. 2008, 4, 58–60. [Google Scholar]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with mycorrhizas in forestry and agriculture. In ACIAR Monograph 32; Australian Centre for International Agricultural Research: Canberra, ACT, Australia, 1996. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part. 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison WI, USA, 1982; pp. 403–430. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of soil analysis, Part 1. Physical and mineralogical methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison WI, USA, 1986; pp. 383–411. [Google Scholar]
- Pearson, T.R.H.; Brown, S.L.; Birdsey, R.A. Measurement guidelines for the sequestration of forest carbon; Gen Tech Rep NRS-18; USDA Forest Service: Newtown Square, PA, USA, 2007.
- Blake, G.R. Bulk density 1. In Methods of Soil Analysis. Part. 1. Physical and Mineralogical Properties, including Statistics of Measurement and Sampling; Black, C.A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; pp. 374–390. [Google Scholar]
- Berhane, A.; Totland, Ø.; Haile, M.; Moe, S.R. Intense use of woody plants in a semiarid environment of Northern Ethiopia: Effects on species composition, richness and diversity. J. Arid Environ. 2015, 114, 14–21. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, Y.; Qin, X.; Li, X.; Cheng, L.; Sha, T.; Wang, G. Arbuscular mycorrhizal status of plants and the spore density of arbuscular mycorrhizal fungi in the tropical rain forest of Xishuangbanna, southwest China. Mycorrhiza 2001, 11, 159–162. [Google Scholar] [CrossRef]
- Birhane, E.; Gebremedihin, K.M.; Tadesse, T.; Hailemariam, M.; Solomon, N. Exclosures restored the density and root colonization of arbuscular mycorrhizal fungi in Tigray, Northern Ethiopia. Ecol. Process. 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Dobo, B.; Asefa, F.; Asfaw, Z. Diversity of arbuscular mycorrhizal fungi of different plant species grown in three land use types in Wensho and Shebidino Districts of Sidama in Southern Ethiopia. Adv. Biosci. Bioeng. 2016, 4, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Merryweather, J.; Fitter, A. The arbuscular mycorrhizal fungi of Hyacinthoides non-scripta I. Diversity of fungal taxa. New Phytol. 1998, 138, 117–129. [Google Scholar]
- Öpik, M.; Moora, M.; Liira, J.; Zobel, M. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 2006, 94, 778–790. [Google Scholar] [CrossRef]
- Sarkar, U.; Choudhary, B.K.; Sharma, B.K. Vascular arbuscular mycorrhizal (VAM) spore diversity and density across the soil of degraded forest and rubber plantation in Tripura, India. Am. Eurasian J. Agric. Environ. Sci 2014, 14, 1080–1088. [Google Scholar]
- Boddington, C.L.; Dodd, J.C. The effect of agricultural practices on the development of indigenous arbuscular mycorrhizal fungi. II. Studies in experimental microcosms. Plant. Soil 2000, 218, 145–157. [Google Scholar] [CrossRef]
- Oechel, W.C.; Vourlitis, G.L.; Hastings, S.J.; Zulueta, R.C. Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Nature 2000, 406, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Ris, E.A.; Boller, T.; Wiemken, A. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively managed agroecosystems. New Phytol. 2005, 165, 273–283. [Google Scholar] [CrossRef]
- Mohammad, M.J.; Hamadt, S.R.; Malkawit, H.I. Population of arbuscular mycorrhizal fungi in semi-arid environment of Jordan as influenced by biotic and abiotic factors. J. Arid Environ. 2003, 53, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Souza, M.; Trufem, S.F.B.; Gomes-da-Costa, S.M.; Cardoso, E.J.B.N. Arbuscular mycorrhizal fungi associated with Araucaria angustifolia (Bert.) O. Ktze. Mycorrhiza 2003, 13, 211–215. [Google Scholar] [CrossRef]
- Belay, Z.; Vestberg, M.; Assefa, F. Diversity and abundance of arbuscular mycorrhizal fungi associated with acacia trees from different land use systems in Ethiopia. Afr. J. Microbiol. Res. 2013, 7, 5503–5515. [Google Scholar]
- Shukla, A.; Vyas, D.; Jha, A. Soil depth: An overriding factor for distribution of arbuscular mycorrhizal fungi. J. Soil Sci. Plant. Nutr. 2013, 13, 23–33. [Google Scholar]
- Cuenca, G.; Lovera, M. Seasonal variation and distribution at different soil depths of arbuscular mycorrhizal fungi spores in a tropical sclerophyllous shrubland. Botany 2010, 88, 54–64. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Prentice-Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Verma, N.; Tarafadar, J.C.; Shrivastava, K.K. Periodic changes in Prosopis cineraria associated AM population at different soil depth and its relationship with organic carbon and soil moisture. Afr. J. Microbiol. 2010, 4, 115–121. [Google Scholar]
- Adugna, A.; Abegaz, A.; Cerdà, A. Soil erosion assessment and control in Northeast Wollega, Ethiopia. Solid Earth Discuss. 2015, 7, 3511–3540. [Google Scholar] [CrossRef]
- Mekuria, W. Changes in regulating ecosystem services following establishing exclosures on communal grazing lands in Ethiopia: A synthesis. J. Ecosyst. 2013. [Google Scholar] [CrossRef] [Green Version]
- Sagar, R.; Singh, A.; Singh, J.S. Differential effect of woody plant canopies on species composition and diversity of ground vegetation: A case study. Trop. Ecol. 2008, 49, 189–197. [Google Scholar]
- Yohannes, Y.; Webb, P. Classification and regression trees, CART. A user manual for identifying indicators of vulnerability to famine and chronic food insecurity. In Microcomputers in Policy Research No. 3; International Food Policy Research Institute: Washington, DC, USA, 1999. [Google Scholar]
- Girmay, G.; Singh, B.R.; Mitiku, H.; Borresen, T.; Lal, R. Carbon stocks in Ethiopian soils in relation to land use and soil management. Land. Degrad. Dev. 2008, 19, 351–367. [Google Scholar] [CrossRef]
- Abbott, L.K.; Robson, A.D. Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric. Ecosyst. Environ. 1991, 35, 121–150. [Google Scholar] [CrossRef]
- Wuen, K.; Saito, K.; Sato, S.; Sugawara, K. Arbuscular mycorrhizal colonization and sporulation in rhizosphere of common species on native and sown grasslands. Grassl. Sci. 2002, 48, 248–253, (In Japanese with English abstract). [Google Scholar]
- Sivakumar, N. Effect of edaphic factors and seasonal variation on spore density and root colonization of arbuscular mycorrhizal fungi in sugarcane fields. Ann. Microbiol. 2013, 63, 151–160. [Google Scholar] [CrossRef]
- Songachan, L.S.; Kayang, H.; Lyngdoh, I. Colonization of arbuscular mycorrhizal fungi in moderately degraded sub-tropical forest stands of Meghalaya, Northeast India. J. Agric. Technol. 2014, 7, 1673–1684. [Google Scholar]
- Ministry of Agriculture (MOA), Agricultural Transformation Agency (ATA). Soil Fertility Status and Fertilizer Recommendation Atlas for Tigray Regional State, Ethiopia; Agricultural Transformation Agency, Ministry of Agriculture: Addis Ababa, Ethiopia, 2014.
- An, G.-H.; Miyakawa, S.; Kawaharu, A.; Osaki, M.; Ezawa, T. Community structure of arbuscular mycorrhizal fungi associated with pioneer grass species Miscanthus sinensis in acid sulfate soils: Habitat segregation along pH gradients. Soil Sci. Plant. Nutr. 2008, 4, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, M.; Khara, J.; Abbaspour, N. Effects of season and soil conditions on the mycorrhizal status and colonization of seven grass species. Iran. J. Plant. Physiol. 2012, 2, 387–393. [Google Scholar]
- Khakpour, O.; Khara, J. Spore density and root colonization by arbuscular mycorrhizal fungi in some species in the northwest of Iran. Int. Res. J. Appl. Basic. Sci. 2012, 3, 977–982. [Google Scholar]
- Gaur, S.; Kaushik, P. Medicinal plants in Indian Central Himalayas. J. Biol. Sci. 2011, 11, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Khanam, D.; Mridha, M.; Solaiman, A.; Hossain, T. Effect of edaphic factors on root colonization and spore population of arbuscular mycorrhizal fungi. Bull. Inst. Trop. Agric. Kyushu. Univ. 2006, 29, 97–104. [Google Scholar]
- Khade, S.W.; Rodrigues, B.F. Arbuscular mycorrhizal fungi associated with varieties of Carica papaya L. in tropical agro-based ecosystem of Goa, India. Trop. Subtrop. Agroecosyst. 2009, 10, 369–381. [Google Scholar]
- Gaur, A.; Adholeya, A. Arbuscular-mycorrhizal inoculation of five tropical fodder crops and inoculum production in marginal soil amended with organic matter. Biol. Fertil. Soils 2002, 35, 214–218. [Google Scholar] [CrossRef]
- Vogel-Mikus, K.; Pongrac, P.; Kump, P.; Neceman, M.; Regvar, M. Colonization of a Zn, Cd and Pb hyper accumulator Thlaspi praecox Wulfen with indigenous arbuscular mycorrhizal fungi mixture induces changes in heavy metal and nutrient uptake. Environ. Pollut. 2005, 139, 362–371. [Google Scholar] [CrossRef]
- Ardestani, N.K.; Zare-Maivan, H.; Ghanati, F. Effect of different concentrations of potassium and magnesium on mycorrhizal colonization of maize in pot culture. Afr. J. Biotech. 2011, 10, 16548–16550. [Google Scholar]
- Alghamdi, A.M.; Jais, H.M. Interaction between soil textural components, flavonoids in the roots and mycorrhizal colonization in Juniperus procera in Saudi Arabia. Afr. J. Microbiol. Res. 2013, 7, 996–1001. [Google Scholar]
- Don-Rodrigue, R.B.V.; Jacob, N.; Jean-Marc, D.S.; Beaulys, F.; Jesus, A.A.; Marie-Stephanie, A.K.; Seydou, O.; Sebastien, N.; Adolphe, Z. Abundance and diversity of Arbuscular mycorrhizal fungal (AMF) communities associated with cassava (Manihot esculenta Crantz) rhizosphere in Abengourou, East Côte d’Ivoire. J. Ecol. Nat. Environ. 2013, 5, 360–370. [Google Scholar]
- Deepak, V.; Vyas, R.; Giri, V.; Karanth, K.P. A taxonomic mystery for more than 180 years: The identity and systematic position of Brachysaura minor. Vertebr. Zool. 2015, 65, 371–381. [Google Scholar]
- Bago, B.; Cano, C.; Azcón-Aguilar, C.; Samson, J.; Coughlan, A.P.; Piché, Y. Differential morphogenesis of the extraradical mycelium of an arbuscular mycorrhizal fungus grown monoxenically on spatially heterogeneous culture media. Mycologia 2004, 96, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.J.; Li, X.L. Arbuscular Mycorrhizal Research and Application; Science Press: Beijing, China, 2000; p. 224. [Google Scholar]
- Muthukumar, T.; Udaiyan, K. Arbuscular mycorrhizas of plants growing in the Western Ghats region, Southern India. Mycorrhiza 2000, 9, 297–313. [Google Scholar] [CrossRef]
- Wilson, G.W.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Iversen, C.M.; Keller, J.K.; Garten, C.T.; Norby, R.J. Soil carbon and nitrogen cycling and storage throughout the soil profile in a sweetgum plantation after 11 years of CO2-enrichment. Glob. Chang. Biol. 2012, 18, 1684–1697. [Google Scholar] [CrossRef]
- Drigo, B.; Pijl, A.S.; Duyts, H.; Kielak, A.; Gamper, H.A.; Houtekamer, M.J.; Boschker, H.T.S.; Bodelier, P.L.E.; Whiteley, A.S.; van Veen, J.A.; et al. Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proc. Natl. Acad. Sci. USA 2010, 107, 10938–10942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.G.; Miller, R.M. Carbon cycling by arbuscular mycorrhizal fungi in soil–plant systems. Trends Plant. Sci. 2003, 8, 407–409. [Google Scholar] [CrossRef]



| Disturbance Levels | Description |
|---|---|
| No disturbance (0) | No grazing observed, no human trails, no cut stems or droppings |
| Slightly disturbed (1) | 1–2 animal dung patches and the presence of cut stem within the plot |
| Moderately disturbed (2) | 3–5 grazing animals observed; human trails, cut stems and droppings present |
| Highly disturbed (3) | > 6 grazing animals observed; human trails, cut stems present |
| Structure Colonization | Soil Depth (cm) | Plant Communities | 1-way ANOVA | |||
|---|---|---|---|---|---|---|
| Jupr-Mase (n = 78) | Ptst-Ceaf (n = 26) | Capu-Opfi (n = 26) | F2,127 | p | ||
| HC | 0–25 | 68.89 ± 1.07 a | 70.04 ± 0.73 a | 64.29 ± 0.93 b | 4.26 | < 0.018 |
| 26–50 | 70.19 ± 0.58 a | 50.05 ± 0.86 b | 63.59 ± 2.03c | 98.53 | < 0.001 | |
| MHC | 0–25 | 64.18 ± 1.03 a | 65.73 ± 1.09 a | 54.88 ± 1.78 b | 13.67 | < 0.001 |
| 26–50 | 65.20 ± 0.89 a | 65.10 ± 1.53 a | 53.18 ± 1.94 b | 21.92 | < 0.001 | |
| VC | 0–25 | 44.81 ±0.79 a | 45.75 ± 0.82 a | 41.07 ± 1.28 b | 4.18 | 0.020 |
| 26–50 | 42.59 ± 0.65 a | 43.96 ± 1.04 b | 39.80 ± 1.17a,b | 3.73 | 0.029 | |
| AC | 0–25 | 38.68 ± 0.51 a | 34.15 ± 0.97 b | 32.07 ± 1.24 b | 20.7 | < 0.001 |
| 26–50 | 33.09 ± 0.82 a | 26.56 ± 1.07 b | 35.51 ± 2.27 a | 9.17 | < 0.001 | |
| Spore density | 0–25 | 570.45 ± 39.43 a | 581.41 ± 45.15 a | 512.14 ± 36.02 b | 12.49 | < 0.001 |
| 26–50 | 559.71 ± 50.15 a | 515.44 ± 47.99 a | 514.83 ±135.48 a | 2.80 | 0.069 | |
| Total root colonization | 0–25 | 54.14 ± 0.48 a | 53.92 ± 0.55 a | 48.08 ± 0.82 b | 23.7 | < 0.001 |
| 26–50 | 52.77 ± 0.42 a | 46.42 ± 0.56a,b | 48.02 ± 0.58 b | 42.07 | < 0.001 | |
| Soil Variable | Unit | Soil Depth (cm) | Plant Communities | 1-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Jupr-Mase (n = 78) | Ptst-Ceaf (n = 26) | Capu-Opfi (n = 26) | F2,127 | p | |||
| pH | 0–25 | 6.75 ± 0.06 a | 7.04 ± 0.51 a | 6.91 ± 0.14 a | 0.67 | 0.104 | |
| 26–50 | 7.39 ± 0.09 a | 6.94 ± 0.15 b | 7.43 ± 0.12a,b | 3.78 | 0.028 | ||
| Electrical conductivity (EC) | dSm−1 | 0–25 | 0.33 ± 0.06 a | 0.37 ± 0.13 a | 0.31 ± 0.08 a | 0.11 | 0.894 |
| 26–50 | 0.33 ± 0.05 a | 0.23 ± 0.07 a | 0.46 ± 0.08 a | 2.02 | 0.141 | ||
| Available P | ppm | 0–25 | 10.89 ± 0.47 a | 10.78 ± 0.76 a | 9.46 ± 0.70 a | 1.29 | 0.282 |
| 26–50 | 6.66 ± 0.70 a | 7.66 ± 0.59 a | 7.46 ± 0.68 a | 0.46 | 0.631 | ||
| Exch K | ppm | 0–25 | 28.65 ± 1.53 a | 134.76 ± 27.13 b | 38.41 ± 4.73 a | 28.7 | < 0.001 |
| 26–50 | 19.38 ± 1.36 a | 114.80 ± 23.52 b | 33.67 ± 3.93 a | 30.41 | < 0.001 | ||
| Total N | % | 0–25 | 0.39 ± 0.03 a | 0.23 ± 0.03 b | 0.16 ± 0.01 b | 17.32 | < 0.001 |
| 26–50 | 0.19 ± 0.01 a | 0.17 ± 0.03a,b | 0.12 ± 0.01 b | 5.52 | 0.006 | ||
| SOC | % | 0–25 | 4.34 ± 0.30 a | 2.78 ± 0.35 a | 1.75 ± 0.17 b | 14.41 | < 0.001 |
| 26–50 | 1.88 ± 0.12 a | 2.20 ± 0.36 a | 1.24 ± 0.11 b | 4.63 | 0.013 | ||
| SOC stocks | Mg ha−1 | 0–25 | 126.77 ± 3.01 a | 124.72 ± 12.68 a | 79.38 ± 5.22 b | 16.62 | < 0.001 |
| 26–50 | 89.23 ± 3.78 a | 87.61 ± 6.60a,b | 71.36 ± 3.78 b | 3.29 | 0.044 | ||
| AMF Spore Density (100 g−1 Dry Soil) | AMF Root Colonization (%) | ||||
|---|---|---|---|---|---|
| Source | d.f. | F | p | F | p |
| Plant communities | 2, 127 | 4.387 | 0.014 | 46.802 | < 0.001 |
| Soil depth | 1, 128 | 2.868 | 0.093 | 32.121 | < 0.001 |
| Plant community*Soil depth | 2, 124 | 0.803 | 0.450 | 16.268 | < 0.001 |
| Variables | AMF Spore Density (100 g−1 Dry Soil) | AMF Root Colonization (%) | ||
|---|---|---|---|---|
| Pearson r | p | Pearson r | p | |
| Root colonization | 0.215 | 0.014 | -- | |
| pH | −0.021 | 0.810 | −0.159 | 0.071 |
| EC | −0.048 | 0.584 | −0.004 | 0.965 |
| Avail. P | 0.007 | 0.938 | 0.156 | 0.076 |
| Exch. K | −0.053 | 0.546 | −0.085 | 0.337 |
| SOC content | 0.256 | 0.003 | 0.404 | < 0.001 |
| TN | 0.288 | 0.001 | 0.403 | < 0.001 |
| SOC stocks | 0.263 | 0.003 | 0.373 | < 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birhane, E.; Gebretsadik, K.F.; Taye, G.; Aynekulu, E.; Rannestad, M.M.; Norgrove, L. Effects of Forest Composition and Disturbance on Arbuscular Mycorrhizae Spore Density, Arbuscular Mycorrhizae Root Colonization and Soil Carbon Stocks in a Dry Afromontane Forest in Northern Ethiopia. Diversity 2020, 12, 133. https://doi.org/10.3390/d12040133
Birhane E, Gebretsadik KF, Taye G, Aynekulu E, Rannestad MM, Norgrove L. Effects of Forest Composition and Disturbance on Arbuscular Mycorrhizae Spore Density, Arbuscular Mycorrhizae Root Colonization and Soil Carbon Stocks in a Dry Afromontane Forest in Northern Ethiopia. Diversity. 2020; 12(4):133. https://doi.org/10.3390/d12040133
Chicago/Turabian StyleBirhane, Emiru, Kbrom Fissiha Gebretsadik, Gebeyehu Taye, Ermias Aynekulu, Meley Mekonen Rannestad, and Lindsey Norgrove. 2020. "Effects of Forest Composition and Disturbance on Arbuscular Mycorrhizae Spore Density, Arbuscular Mycorrhizae Root Colonization and Soil Carbon Stocks in a Dry Afromontane Forest in Northern Ethiopia" Diversity 12, no. 4: 133. https://doi.org/10.3390/d12040133
APA StyleBirhane, E., Gebretsadik, K. F., Taye, G., Aynekulu, E., Rannestad, M. M., & Norgrove, L. (2020). Effects of Forest Composition and Disturbance on Arbuscular Mycorrhizae Spore Density, Arbuscular Mycorrhizae Root Colonization and Soil Carbon Stocks in a Dry Afromontane Forest in Northern Ethiopia. Diversity, 12(4), 133. https://doi.org/10.3390/d12040133





