Toxic Effect of Anionic Surfactants on Freshwater Sponge Lubomirskia baikalensis and Its Endosymbiotic Microalgae Chlorella sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sponge Sampling
2.1.1. Choosing the Sponge Target Species for the Experiments
2.1.2. Lubomirskia baikalensis Sampling for In Vitro Aquarian Experiments
2.1.3. Lubomirskia baikalensis Sampling for In Situ Biochemical Analysis
2.2. Conditions for Cultivation of L. baikalensis
2.3. Cell Viability Test Procedure
2.4. Blank Samples–The “Control Sponges”
2.5. Linear Alkylbenzene Sulfonate Toxicity Studies
2.6. Fatty Acid Qualitative and Quantitative Analysis
2.6.1. Lipid Extraction and Fatty Acid Derivatization
2.6.2. Fatty Acid Analysis by Gas Chromatography Coupled with Mass-Spectrometry
2.7. Malondialdehyde Content Determination
2.8. Total Antioxidant Activity (AOA) of Lubomirskia baikalensis
2.8.1. Extraction of Antioxidants
2.8.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.8.3. TEAC Data Presentation
3. Results
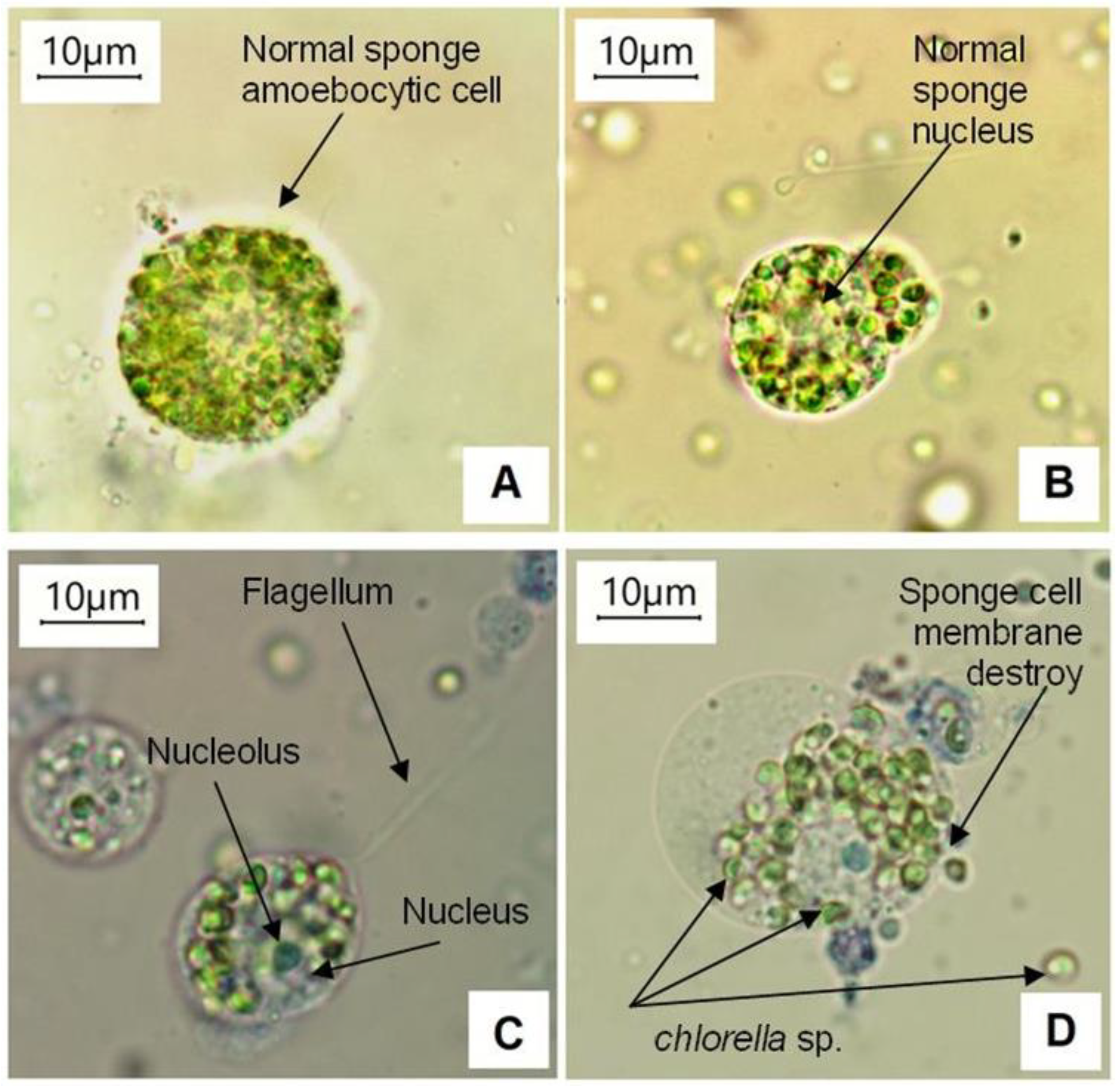
3.1. Classification of L. baikalensis Dominant Cell Types
- Sponge Amoebocytes of the first type named as SA1-cells (Figure 2B1) contained predominantly eukaryotic Chlorophyta (Figure 2B4). These spherical sponge cells were characterized by relatively large size from 10 to 25 μm and large central globular nuclei with prominent nucleoli of ~2.8 μm. The flexible cell membranes of L. baikalensis SA1-cells provide structural strength, regular spherical shape, as well as counteracting the osmotic pressure of the cytoplasm. Ectoplasm and endoplasm of different viscosity were found within the plasma membrane. The outside membrane was characterized by the singular and branching strands (Figure 2B) of different lengths. The cells of the similar structure are known for marine sponge Microciona prolifera (Ellis and Solander, 1786) (phylum Porifera, class Demospongiae, order Poecilosclerida, family Microcionidae) [46]. We suppose that the SA1-cells present nucleolar archeocytes, also called sponge stem-like cells or an intermediate cell type between the archeocytes and gray cells [46]. These cells might have different functions, including the function of phagocytes or amoebocytes [17], immunocytes [46], as well as potentially representing evolutionary precursors to a true nervous system [47,48], and allow the sponge cells to communicate with one another by passing electrical or chemical signals.
- Amoebocytic single cells of the third type contained both prokaryotic symbionts and small amount of eukaryotic symbionts (SA3-cells) (Figure 2E8).
- Amoebocytic cells with moving flagella containing predominantly prokaryotic symbionts were also noticed (SA4-cells) (Figure 2E3).
3.2. Lubomirskia baikalensis SA1-Cells as an Indicator of LAS Pollution of Water
- The SA1-cells of the healthy control sponges L. baikalensis did not dye with methylene blue. Cells of symbiotic microalgae Chlorella sp. were not stained with methylene blue either. On the contrary, the amoebocytic cells of other types as well as endosymbiotic prokaryotes Cyanophyta stained with methylene blue. Instant staining of SA2-, SA3-, SA4-, and Cyanophyta cells occurs due to absence of enzymes that suppress the methylene blue effect.
- The SA1-cells and Chlorella sp. cells exposed to 10 µg L−1 LAS solution for two weeks also did not stain as well as the control sponge cells. On the contrary, the amoebocytes of other types and blue–green algae cells stained immediately.
- The SA1-cells and endosymbiotic Chlorella sp. cells exposed to 20 µg L−1 LAS solution for 48 and 72 h, accordingly, became blue in color. The SA2-, SA3-, SA4-, and Cyanophyta cells stained immediately. Flagellum motility of SA4-cells and endosymbiotic flagellates was noted during the first minutes after LAS being added into the aquariums.
3.3. Control Sponges Cell Viability
3.4. Cellular Stress as a Response to Toxic Effect of Linear Alkylbenzene Sulfonates
3.5. Acute Toxicity of Linear Alkylbenzene Sulfonates to Lubomirskia baikalensis
3.5.1. Cell Size and Shape Changes: Cell Viability
3.5.2. Lubomirskia baikalensis Fatty Acid Composition and Content Changes
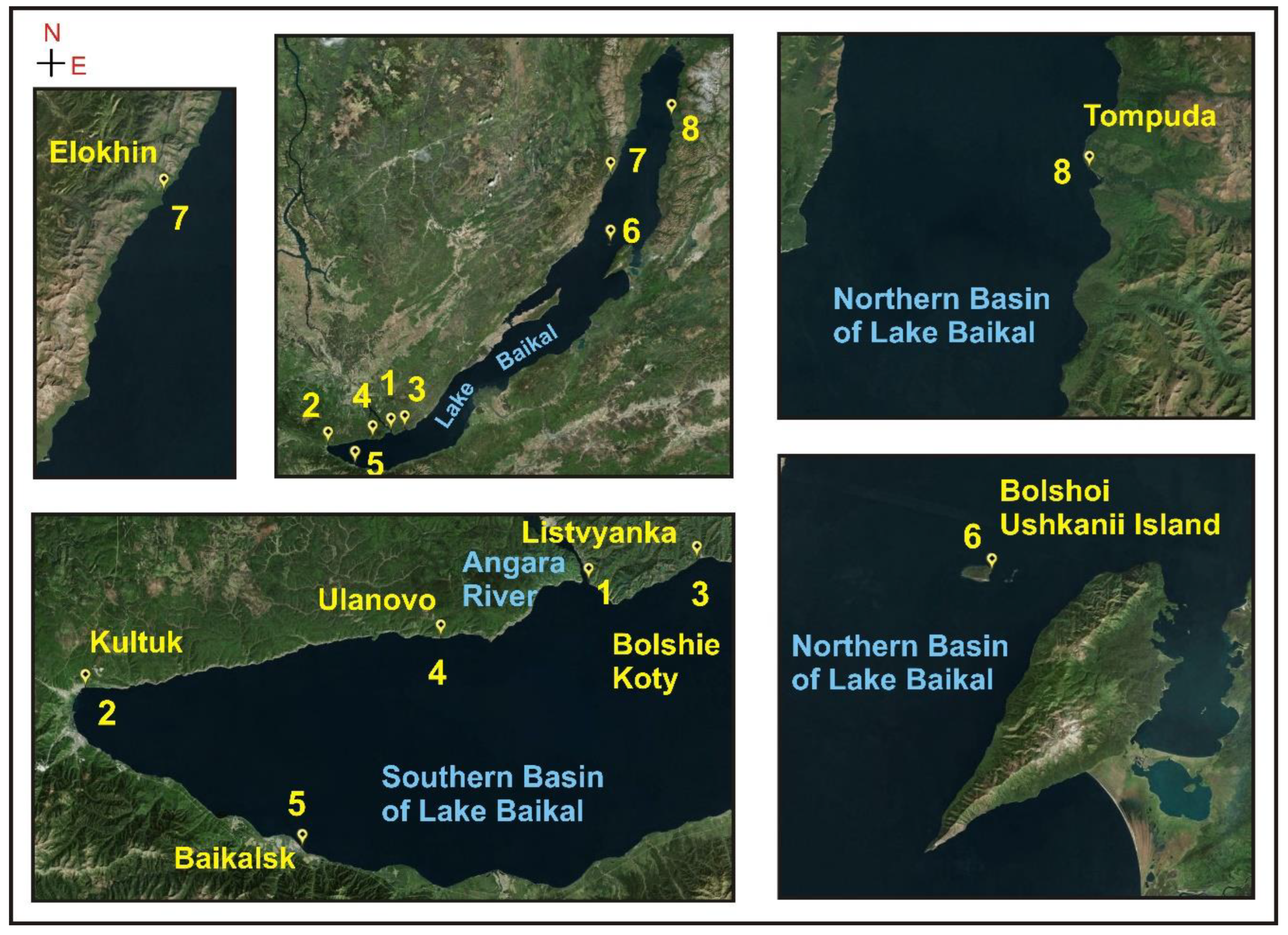
3.6. In Situ Experiments: Analysis of the Environmental Samples
3.6.1. Oxidative Stress of Lubomirskia baikalensis in Lake Baikal
3.6.2. Antioxidant Capacity of Lubomirskia baikalensis
3.7. Statistical Data
4. Discussion
4.1. Toxicity Test Protocol
4.2. Linear Alkylbenzene Sulfonate Acute Toxicity Effect
4.3. Oxidative Stress as a Response to LAS Toxic Effect: In Situ and In Vitro Investigations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmid, M.; De Batist, M.; Granin, N.G.; Kapitanov, V.A.; McGinnis, D.F.; Mizandrontsev, I.B.; Obzhirov, A.I.; Wuest, A. Sources and sinks of methane in Lake Baikal: A synthesis of measurements and modeling. Limnol. Oceanogr. 2007, 52, 1824–1837. [Google Scholar] [CrossRef] [Green Version]
- De Batist, M.; Canals, M.; Sherstyankin, P.; Alekseev, S. A New Bathymetric Map of Lake Baikal. The INTAS Project 99-1669 Team. 2002. Available online: http://www.lin.irk.ru/intas/index.htm (accessed on 4 January 2023).
- Vadeboncoeur, Y.; McIntyre, P.B.; Vander Zanden, M.J. Borders of Biodiversity: Life at the Edge of the World’s Large Lakes. BioScience 2011, 61, 526–537. [Google Scholar] [CrossRef]
- Lut, B.F. Morfologiya i morfometriya Baikalskoi vpadiny [Morphology and Morphometry of Baikal Depression]. Chapter II. In The Way for Knowledge of Baikal; Afanasieva, E.L., Beckman, M.Y., Bezrukova, E.V., Verbolov, V.I., Votintsev, K.K., Galasii, G.I., Goldyrev, G.S., Granina, L.Z., Dryukker, V.V., Ladeischikov, N.P., et al., Eds.; Nauka Publ.: Novosibirsk, Russia, 1987; pp. 34–47. (In Russian) [Google Scholar]
- Votintsev, K.K.; Meshcheryakova, A.I.; Popovskya, G.I. Krugovorot Organicheskogo Veshchestva v Ozere Baikal [Organic Substance Cycle in Lake Baikal]; Nauka Publ.: Novosibirsk, Russia, 1975; 190p. (In Russian) [Google Scholar]
- Manconi, R.; Pronzato, R. Phylum Porifera Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J., Rogers, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 133–157. [Google Scholar]
- Erpenbeck, D.; Weier, T.; de Voogd, J.N.; Wörheide, G.; Sutcliffe, P.; Todd, J.A.; Michel, E. Insights into the evolution of freshwater sponges (Porifera: Demospongiae: Spongillina): Barcoding and phylogenetic data from Lake Tanganyika endemics indicate multiple invasions and unsettle existing taxonomy. Mol. Phylogenet. Evol. 2011, 61, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Itskovich, V.; Kaluzhnaya, O.; Ostrovsky, I.; McCormack, G. The number of endemic species of freshwater sponges (Malawispongiidae; Spongillina; Porifera) from Lake Kinneret is overestimated. J. Zool. Syst. Evol. Res. 2013, 51, 252–257. [Google Scholar] [CrossRef]
- Laurer, T.E.; Spacie, A. New Records of Freshwater Sponges (Porifera) for Southern Lake Michigan. J. Great Lakes Res. 1996, 22, 77–82. [Google Scholar] [CrossRef]
- Khanaev, I.V.; Kravtsova, L.S.; Maikova, O.O.; Bukshuk, N.A.; Sakirko, M.V.; Kulakova, N.V.; Butina, T.V.; Nebesnykh, I.A.; Belikov, D.I. Current state of the sponge fauna (Porifera: Lubomirskiidae) of Lake Baikal: Sponge disease and the problem of conservation of diversity. J. Great Lakes Res. 2018, 44, 77–85. [Google Scholar] [CrossRef]
- Maikova, O.; Bukshuk, N.; Kravtsova, L.; Nebesnyh, I.; Yakhnenko, A.; Butina, T.; Khanaev, I. Baikal endemic sponge disease and anthropogenic factor. IOP Conf. Ser. Earth Environ. Sci. 2021, 937, 022071IOP. [Google Scholar] [CrossRef]
- Itskovich, V.; Belikov, S.; Kaluzhnaya, O. Study of the biodiversity of deep-water sponges of Lake Baikal by molecular methods. Proceedings of The Materials of the VIII World Sponge Conference “Ancient animals, new challenges”, Girona, Spain, 20–24 September 2010; p. 222. [Google Scholar]
- Kozhova, O.M.; Izmest’eva, L.R. Lake Baikal. Evolution and Biodiversity; Backhuys Publishers: Leiden, The Netherlands, 1998; 447p. [Google Scholar]
- Rusinek, O.T.; Takhteev, V.V.; Khodzher, T.V.; Pleshanov, A.S.; Voronin, V.I.; Arov, I.V.; Azovskii, M.G.; Goryunova, O.I.; Dryukker, V.V.; Zadonina, N.V.; et al. Baikalogy. Book 2: In 2 Books; Nauka: Novosibirsk, Russia, 2012; 644p. (In Russian) [Google Scholar]
- Kulakova, N.V.; Kashin, S.A.; Bukin, Y.S. The genetic diversity and phylogeny of green microalgae in the genus Choricystis (Trebouxiophyceae, Chlorophyta) in Lake Baikal. Limnology 2020, 21, 15–24. [Google Scholar] [CrossRef]
- Kulakova, N.V.; Denikina, N.N.; Belikov, S.I. Diversity of bacterial photosymbionts in Lubomirskiidae sponges from Lake Baikal. Int. J. Biodivers. 2014, 2014, 152097. [Google Scholar] [CrossRef] [Green Version]
- Ereskovsky, A.V.; Chernogor, L.I.; Belikov, S.I. Ultrastructural description of development and cell composition of primmorphs in the endemic Baikal sponge Lubomirskia Baicalensis. Zoomorphology 2016, 135, 1–17. [Google Scholar] [CrossRef]
- Gladkikh, A.S.; Kalyuzhnaya, O.V.; Belykh, O.I.; Ahn, T.S.; Parfenova, V.V. Analysis of Bacterial Communities of Two Lake Baikal Endemic Sponge Species. Mikrobiologiya 2014, 83, 682–693. [Google Scholar] [CrossRef]
- Belykh, O.I.; Fedorova, G.A.; Kuzmin, A.V.; Tikhonova, I.V.; Timoshkin, O.A.; Sorokovikova, E.G. Microcystins in Cyanobacterial Biofilms from the Littoral Zone of Lake Baikal. Mosc. Univ. Biol. Sci. Bull. 2017, 72, 225–231. [Google Scholar] [CrossRef]
- Mohamed, N.M.; Rao, V.; Hamann, M.T.; Kelly, M.; Hill, R.T. Monitoring bacterial diversity of the marine sponge Ircinia strobilina upon transfer into aquaculture. Appl. Environ. Microbiol. 2008, 74, 4133–4143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernogor, L.; Denikina, N.; Kondratov, I.; Solovarov, I.; Khanaev, I.; Belikov, S.; Ehrlich, H. Isolation and identification of the microalgal symbiont from primmorphs of the endemic freshwater sponge Lubomirskia baikalensis (Lubomirskiidae, Porifera). Eur. J. Phycol. 2013, 48, 497–508. [Google Scholar] [CrossRef]
- Timoshkin, O.A.; Samsonov, D.P.; Yamamuro, M.; Moore, M.V.; Belykh, O.I.; Malnik, V.V.; Sakirko, M.V.; Shirokaya, A.A.; Bondarenko, N.A.; Domysheva, V.M.; et al. Rapid ecological change in the coastal zone of Lake Baikal (East Siberia): Is the site of the world’s greatest freshwater biodiversity in danger? J. Great Lakes Res. 2006, 42, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Nikonova, A.A.; Shishlyannikov, S.M.; Volokitina, N.A.; Galachyants, Y.P.; Bukin, Y.S.; Blinov, V.V.; Gnatovsky, R.Y.; Vorobyeva, S.S. Fatty Acid Changes in Nearshore Phytoplankton under Anthropogenic Impact as a Biodiversity Risk Factor for the World’s Deepest Lake Baikal. Diversity 2022, 14, 55. [Google Scholar] [CrossRef]
- Maikova, O.O.; Kravtsova, L.S.; Khanaev, I.V. Baikal endemic sponges in the system of ecological monitoring. Limnol. Freshw. Biol. 2020, 1, 364–367. [Google Scholar] [CrossRef] [Green Version]
- Belikov, S.I.; Feranchuk, S.I.; Butina, T.V.; Chernogor, L.I.; Khanaev, I.V.; Maikova, O.O. Mass disease and mortality of Baikal sponges. Limnol. Freshw. Biol. 2018, 1, 36–42. [Google Scholar] [CrossRef]
- Webster, N.S. Sponge disease: A global threat? Environ. Microbiol. 2007, 9, 1363–1375. [Google Scholar] [CrossRef]
- Denikina, N.N.; Dzyuba, E.V.; Bel’kova, N.L.; Khanaev, I.V.; Feranchuk, S.I.; Makarov, M.M.; Granin, N.G.; Belikov, S.I. Pervyi sluchai zabolevaniya gubki Lubomirskia baikalensis: Issledovanie microbioma [The first case of Lubomirskia baikalensis sponge decease. Microbiome investigation]. Izv. RAN Biol. Ser. Ecol. 2016, 3, 315–322. (In Russian) [Google Scholar]
- Granin, N.G.; Mizandrontsev, I.B.; Obzhirov, A.I.; Vereshchagina, O.F.; Gnatovskii, R.Y.; Zhdanov, A.A. Okislenie metana v vodnoi tolshe ozera Baikal [Oxidation of methane in water column of Lake Baikal]. Dokl. Akad. Nauk. Earth Sci. 2013, 451, 332–335. [Google Scholar]
- Butina, T.V.; Bukin, Y.S.; Petrushin, I.S.; Tupikin, A.E.; Kabilov, M.R.; Belikov, S.I. Extended evaluation of viral diversity in Lake Baikal. Microorganisms 2021, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Nikonova, A.A. Organic synthetic anionic surfactants as persistent organic pollutants of water ecosystems. Limnol. Freshw. Biol. 2020, 4, 620–621. [Google Scholar] [CrossRef]
- Belanger, S.E.; Bowling, J.W.; Lee, D.M.; Le Blank, E.M.; Kerr, K.M.; McAvoy, D.C.; Christman, S.C.; Davidson, D.H. Integration of aquatic fate and ecological responses to linear alkyl benzene sulfonate (LAS) in model stream ecosystems. Ecotoxicol. Environ. Saf. 2002, 52, 150–171. [Google Scholar] [CrossRef]
- Hon-Nami, H.; Hanya, T. Linear alkylbenzene sulfonates in river, estuary and bay water. Water Res. 1980, 14, 1251–1256. [Google Scholar] [CrossRef]
- Terzic, S.; Ahel, M. Input and behaviour of linear alkylbenzenesulphonates (LAS) in a stratified estuary. Mar. Pollut. Bull. 1994, 28, 735–740. [Google Scholar] [CrossRef]
- Okbah, M.A.; Ibrahim, A.M.A.; Gamal, M.N.M. Environmental monitoring of linear alkylbenzene sulfonates and physicochemical characteristics of seawater in El-Mex Bay (Alexandria, Egypt). Environ. Monit. Assess. 2013, 185, 3103–3115. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.A. Chronic and sublethal toxicities of surfactants to aquatic animals: A review and risk assessment. Water Res. 1991, 25, 101–113. [Google Scholar] [CrossRef]
- Jorgensen, E.; Christoffersen, K. Short-term effects of linear alkylbenzene sulfonate on freshwater Plankton studied under field conditions. Environ. Toxicol. Chem. 2000, 19, 904–911. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Pakou, C.; Lyberatos, G. Linear alkylbenzene sulfonates (LAS). Occurrence, toxicity, and biodegradation of selected emerging priority pollutants in municipal sewage sludge. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2011; Volume 6, pp. 473–484. [Google Scholar]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA; San Francisco, CA, USA; New York, NY, USA; Boston, MA, USA; London, UK; Sydney, Australia; Tokyo, Japan, 2001; 1006p. [Google Scholar]
- Mizandrontsev, I.B.; Mizandrontseva, K.N. Kislorodnyi obmen mezhdu vodnoi poverhnost’yu I atmosferoi nad Baikalom [The oxygen exchange between Lake Baikal water surface and the atmosphere]. Russ. Meteorol. Hydrol. 1993, 2, 108–113. (In Russian) [Google Scholar]
- Mizandrontsev, I.B.; Domysheva, V.M.; Mizandrontseva, K.N.; Tomas, K. O sutochnoi dinamike koncentracii svobodnoi uglekisloty I kisloroda v vode Baikala [About daily dynamics of free carbonic acid and oxygen in Baikal water]. Geogr. Nat. Resour. 2002, 1, 73–78. (In Russian) [Google Scholar]
- Rakita, S.M.; Colovic, D.S.; Levart, A.R.; Banjac, V.V.; Colovic, R.R.; Dragojlovic, D.M.; Duragic, O.M. Rapid spectrophotometric method for determination of thiobarbituric acid reactive substances in rainbow trout feed. Food Feed. Res. 2020, 47, 43–53. [Google Scholar] [CrossRef]
- Nikonova, A.A.; Shishlyannikov, S.M.; Shishlyannikova, T.A.; Avezova, T.N.; Babenko, T.A.; Belykh, O.I.; Glyzina, O.Y.; Obolkin, V.A.; Pavlova, O.N.; Smagunova, A.N.; et al. Determination of Free and Esterified Fatty Acids in Hydrocoles of Different Content of Polyunsaturated Fatty Acids by Gas–Liquid Chromatography. J. Anal. Chem. 2020, 75, 1310–1321. [Google Scholar] [CrossRef]
- Al-Rashed, S.A.; Ibrahim, M.M.; El-Gaaly, G.A.; Al-Shehri, S.; Mostafa, A. Evaluation of radical scavenging system in two microalgae in response to interactive stresses of UV-B radiation and nitrogen starvation. Saudi J. Biol. Sci. 2016, 23, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [Green Version]
- Potts, E. Freshwater sponges; A monograph. Proc. Acad. Nat. Sci. Phila. 1887, 1887, 157–279. [Google Scholar]
- Sabella, S.; Faszewski, E.; Himic, L.; Colpitts, K.M.; Kaltenbach, J.; Burger, M.M.; Fernàndez-Busquets, X. Cyclosporin A suspends transplantation reactions in the marine sponge Microciona prolifera1. J. Immunol. 2007, 179, 5927–5935. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, M. Sponge cells hint at origins of nervous system. Synapse genes help cells to communicate in the digestive chambers of sponges. Nature 2021, 559, 193. [Google Scholar] [CrossRef]
- Musser, J.M.; Schippers, K.J.; Kohn, A.B.; Pape, C.; Ronchi, P.; Arendt, D. Profiling cellular diversity in sponges informs animal cell type and nervous system evolution. Science 2021, 374, 717–723. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Kashin, A.G. Comparative study of the endemic freshwater fauna of Lake Baikal. Unusual lipid composition of two sponge species Baicalospongia bacillifera and Baicalospongia intermedia (family Lubomirskiidae, class Demospongiae). Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 106, 825–831. [Google Scholar] [CrossRef]
- Sharga, B.M.; Pylypiv, D.B.; Feketa, V. Cytology. Practical. 4. Differentiation between living and dead eukaryotic cells by staining. In Medical Biology Practicals; Uzhorod National University Publ.: Uzhorod, Ukraine, 2017. [Google Scholar]
- Debelius, B.; Forja, J.M.; Del Valls, A.; Lubian, L.M. Effect of linear alkylbenzene sulfonate (LAS) and atrazine on marine microalgae. Mar. Pollut. Bull. 2008, 57, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.A.; Suk, K.; Lee, C.S.; Lee, C.S. The effects of surfactants on the biosynthesis of galactolipid and the composition of fatty acids in chloroplast envelope and thylakoid membrane of Chlorella ellipsoidea. Korean J. Biol. Sci. 1998, 2, 341–349. [Google Scholar] [CrossRef]
- Lewis, M.A. Chronic Toxicities of Surfactants and Detergent Builders to Algae: A Review and Risk Assessment. Ecotoxicol. Environ. Saf. 1990, 20, 123–140. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acidreactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 424, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Parshi, K. Interaction of detergent sclerosants with cell membranes. Phlebology 2015, 30, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Ebnesajjad, S. Surface treatment of materials for adhesive bonding. In Surface Tension and Its Measurement, 2nd ed.; Elsevier: New York, NY, USA, 2014; pp. 7–24. [Google Scholar]
- Aguirre-Ramírez, M.; Silva-Jiménez, H.; Banat, I.M.; De Rienzo, M.A.D. Surfactants: Physicochemical interactions with biological macromolecules. Biotechnol. Lett. 2021, 43, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Vega-López, A.; Ayala-López, G.; Posadas-Espadas, B.P.; Olivares-Rubio, H.F.; Dzul-Caamal, R. Relations of oxidative stress in freshwater phytoplankton with heavy metals and polycyclic aromatic hydrocarbon. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 498–507. [Google Scholar] [CrossRef]
- Srivastava, S.; Tripathi, G.; Mishra, S.; Gupta, G. Copper-induced oxidative stress and responses of antioxidants and phyto-chelatins in Hydrilla verticillata (L.f.). Royle Aquatic Toxicol. 2006, 8, 405–415. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Z.; Yu, D.; Pang, Y.; Cai, H.; Liu, Y. Toxicity of linear alkylbenzene sulfonate to aquatic plant Potamogeton perfoliatus L. Environ. Sci. Pollut. Res. 2018, 25, 32303–32311. [Google Scholar] [CrossRef]
- Liu, N.; Wu, Z. Toxic effects of linear alkylbenzene sulfonate on Chara vulgaris L. Environ. Sci. Pollut. Res. 2018, 25, 4934–4941. [Google Scholar] [CrossRef]
- Montaño-Castañeda, M.C.; Santafé-Patiño, G.G. Evaluation of antioxidant activity of marine sponges from the Colombian Caribbean. Actual Biol. 2011, 33, 173–181. [Google Scholar] [CrossRef]
- Quiroz-Lobo, Y.; Santafe-Patino, G.; Quiros-Rodriguez, J.-A. Bioactivity and identification of fatty acids of the marine sponge Tetilla rodriguesi (Tetractinellida: Tetillidae) in the Colombian Caribbean. Rev. Biol. Trop. 2022, 70, 20–29. [Google Scholar] [CrossRef]



| Organisms | |||||
|---|---|---|---|---|---|
| L. baikalensis SA-1 Cells | Endosymbiotic Algae Chlorella sp. Cells | ||||
| Cell Size, μm | Cell Percentages (of the Sum) | Cell Size, μm | Cell Percentages (of the Sum) | ||
| Control | 48 h LAS Exposure | Control | 48 h LAS Exposure | ||
| 10.0–15.0 | 17.9 | 0.0 | 2.0–2.5 | 27.5 | 3.6 |
| 15.0–20.0 | 38.5 | 0.5 | 2.5–3.0 | 20.0 | 35.7 |
| 20.0–25.0 | 38.5 | 1.0 | 3.0–3.5 | 30.0 | 28.6 |
| 25.0–30.0 | 5.1 | 0.8 | 3.5–4.0 | 17.5 | 32.1 |
| 30.0–35.0 | 0.0 | 0.5 | 4.0–4.5 | 5.0 | 0.0 |
| 35.0–40.0 | 0.0 | 0.2 | 4.5–5.0 | 0.0 | 0.0 |
| Lysed cells | 0.0 | 97 | Lysed cells | 0.0 | 0.0 |
| Sample Site No. | Depth of Sampling in Lake Baikal, m | Lipid Peroxidation Markers | |
|---|---|---|---|
| CMDA, μg g−1 of d.w. | PUFA, % | ||
| 2 | 5 | from 0.05 to 0.53 average 0.18 ± 0.06 | 33 (n = 7) |
| 20 | not detected | 43 (n = 3) | |
| 3 | 5–15 | from 0.01 to 0.41 average 0.20 ± 0.07 | 32 (n = 4) |
| 5–15 | not detected | 42 (n = 6) | |
| Control sponges after 10 days cultivation in clean water | 5–15 | not detected | 42–55 (n = 12) |
| Sample Site No. | Sampling Sites | Sample (Sponge) No. | I, % | IC50, mg L−1 | AOC, TE |
|---|---|---|---|---|---|
| 4 | Ulanovo Cape | 1 | 0.0064 ± 0.0003 | 7808 ± 390 | 0.00040 |
| 2 | 0.0059 ± 0.0003 | 8542 ± 427 | 0.00031 | ||
| 5 | Region of inoperative Baikalsk City paper mill | 3 | 0.0140 ± 0.0007 | 3574 ± 179 | 0.00075 |
| 4 | 0.0133 ± 0.0007 | 3770 ± 189 | 0.00064 | ||
| 5 | 0.0084 ± 0.0004 | 5972 ± 299 | 0.00044 | ||
| 6 | Bolshoi Ushkanii Island | 6 | 0.0129 ± 0.0006 | 3889 ± 194 | 0.00071 |
| 7 | 0.0110 ± 0.0006 | 4529 ± 226 | 0.00065 | ||
| 7 | Elokhin Cape | 8 | 0.0149 ± 0.0007 | 3360 ± 168 | 0.00077 |
| 9 | 0.0111 ±0.0006 | 4491 ± 225 | 0.00062 | ||
| 8 | Tompuda Bight | 10 | 0.0140 ± 0.0007 | 3580 ± 179 | 0.00071 |
| 11 | 0.0089 ±0.0004 | 5628 ± 281 | 0.00046 | ||
| 2 | Control healthy sponge after 6 months exposure in clean aquarian water | 12 | 0.0160 ± 0.0008 | 3125 ± 156 | 0.00062 |
| The same sponge treated with 20 μg L−1 LAS for 72 h | 12 | 0.0122 ± 0.0006 | 4098 ± 205 | 0.00081 | |
| Trolox (standard solution) | 19.7 ± 1.0 | 2.53 ± 0.13 | 1.00000 | ||
| Sponge [Ref.] | Type: Marine/Freshwater | IC50, μg mL−1 | I (%) | TEAC |
|---|---|---|---|---|
| Lubomirskia baikalensis with maximal TEAC (present investigation) | freshwater | 3125 | 0.0160 | very low |
| Lubomirskia baikalensis with minimal TEAC (present investigation) | freshwater | 8542 | 0.0059 | very low |
| Tedania ignis [63] | marine | no data | 0.135 * | moderate |
| Niphates erecta [63] | marine | no data | 0.184 * | moderate |
| Callyspongia vaginalis [63] | marine | no data | 0.265 * | moderate |
| Lissodendoryx carolinensis [63] | marine | no data | 0.325 * | moderate |
| Tetilla rodriguesi [64] | marine | 297 | 0.327 * | moderate |
| Amorphinopsis atlantica [63] | marine | 88 | 0.531 * | high |
| Ircinia felix [63] | marine | 89 | 0.608 * | high |
| Mycale microsigmatosa [63] | marine | 60 | 0.669 * | high |
| Standard (Trolox) | 2.5 | 19.733 * | very high | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikonova, A.A.; Mizandrontsev, I.B.; Bazhenov, B.N.; Khanaev, I.V.; Shabalina, O.V.; Afanasyeva, A.A.; Avezova, T.N.; Chindyavskaya, A.N.; Bityutsky, A.N.; Kan, A.Y.; et al. Toxic Effect of Anionic Surfactants on Freshwater Sponge Lubomirskia baikalensis and Its Endosymbiotic Microalgae Chlorella sp. Diversity 2023, 15, 77. https://doi.org/10.3390/d15010077
Nikonova AA, Mizandrontsev IB, Bazhenov BN, Khanaev IV, Shabalina OV, Afanasyeva AA, Avezova TN, Chindyavskaya AN, Bityutsky AN, Kan AY, et al. Toxic Effect of Anionic Surfactants on Freshwater Sponge Lubomirskia baikalensis and Its Endosymbiotic Microalgae Chlorella sp. Diversity. 2023; 15(1):77. https://doi.org/10.3390/d15010077
Chicago/Turabian StyleNikonova, Alyona Alexandrovna, Igor Borisovich Mizandrontsev, Boris Nikolaevich Bazhenov, Igor Veniaminovich Khanaev, Olesya Viktorovna Shabalina, Alexandra Alexandrovna Afanasyeva, Tatiana Nikolaevna Avezova, Anna Nikolaevna Chindyavskaya, Alexander Nikolaevich Bityutsky, Andrey Yurievich Kan, and et al. 2023. "Toxic Effect of Anionic Surfactants on Freshwater Sponge Lubomirskia baikalensis and Its Endosymbiotic Microalgae Chlorella sp." Diversity 15, no. 1: 77. https://doi.org/10.3390/d15010077
APA StyleNikonova, A. A., Mizandrontsev, I. B., Bazhenov, B. N., Khanaev, I. V., Shabalina, O. V., Afanasyeva, A. A., Avezova, T. N., Chindyavskaya, A. N., Bityutsky, A. N., Kan, A. Y., Karikh, L. G., Dubrova, K. S., Vorobyeva, S. S., & Glyzina, O. Y. (2023). Toxic Effect of Anionic Surfactants on Freshwater Sponge Lubomirskia baikalensis and Its Endosymbiotic Microalgae Chlorella sp. Diversity, 15(1), 77. https://doi.org/10.3390/d15010077






