Biodiversity and Environmental Factors Structuring Diatom Assemblages of Mineral Saline Springs in the French Massif Central
Abstract
:1. Introduction
2. Materials and Methods
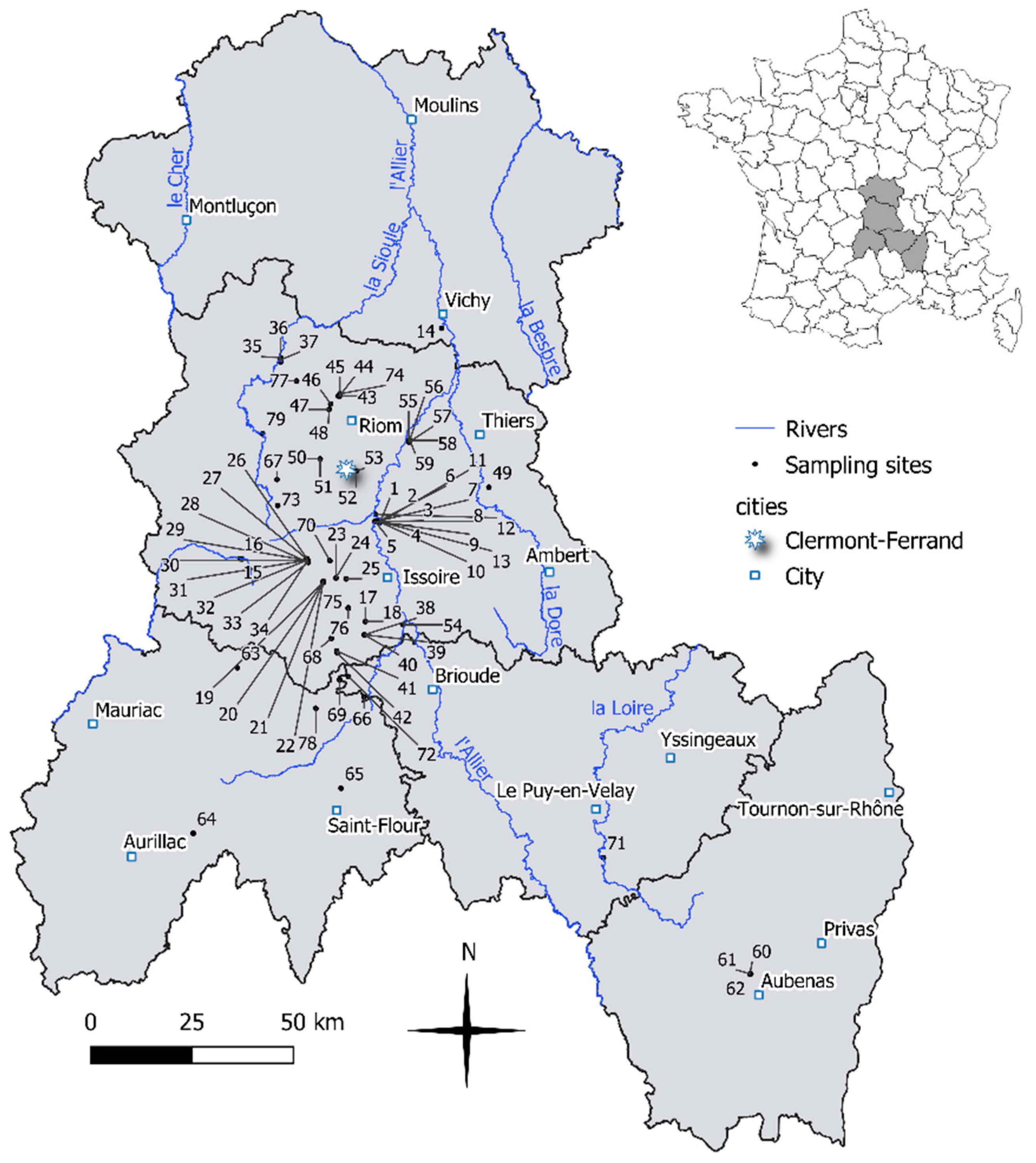
2.1. Study Site
2.2. Diatom Sampling and Physical and Chemical Analysis
2.3. Slide Preparation and Microscopy
2.4. Data Analysis
2.4.1. Statistical Analyses on the Physical and Chemical Variables
2.4.2. Diatoms Data Statistical Analyses
3. Results
3.1. Physical and Chemical Characteristics
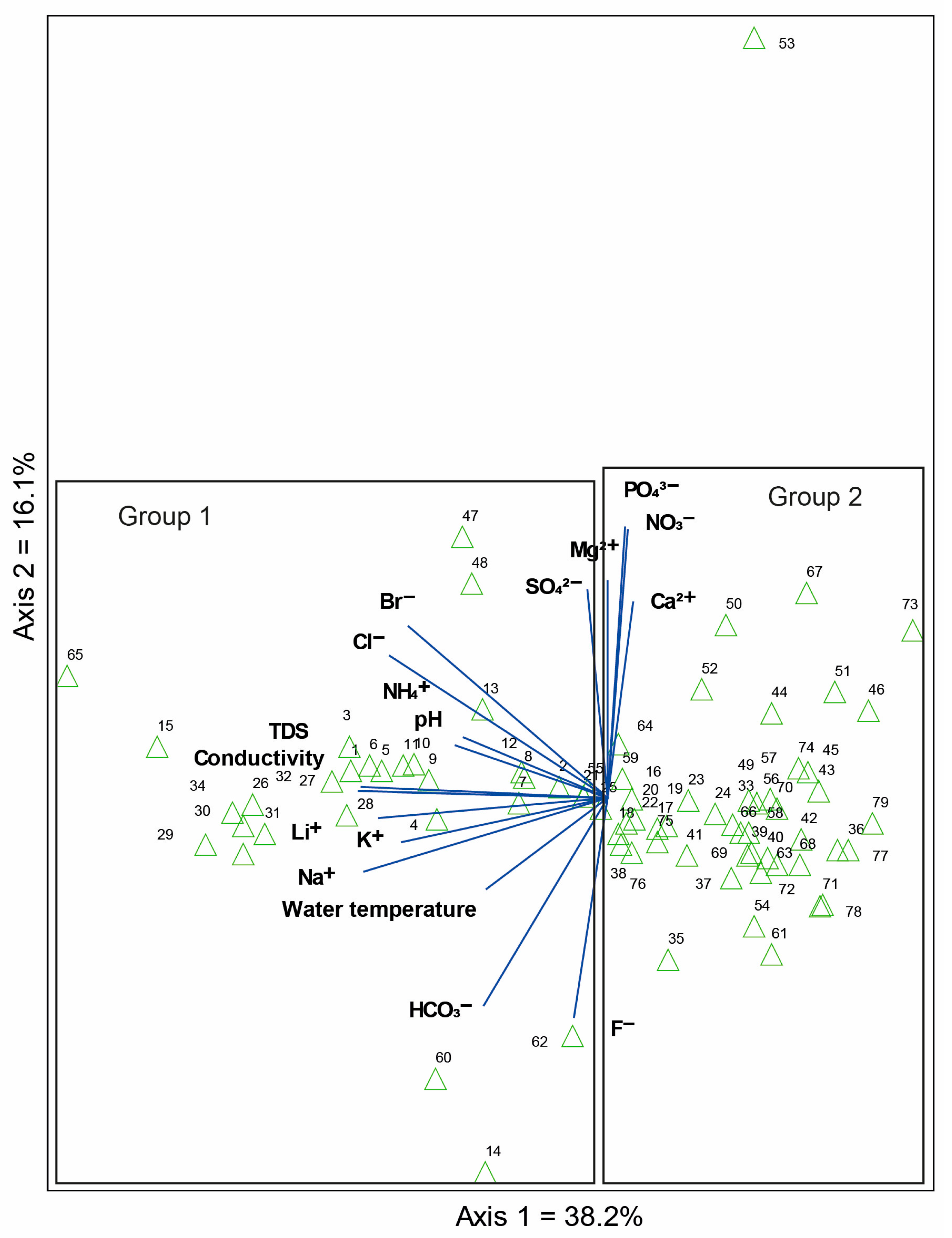
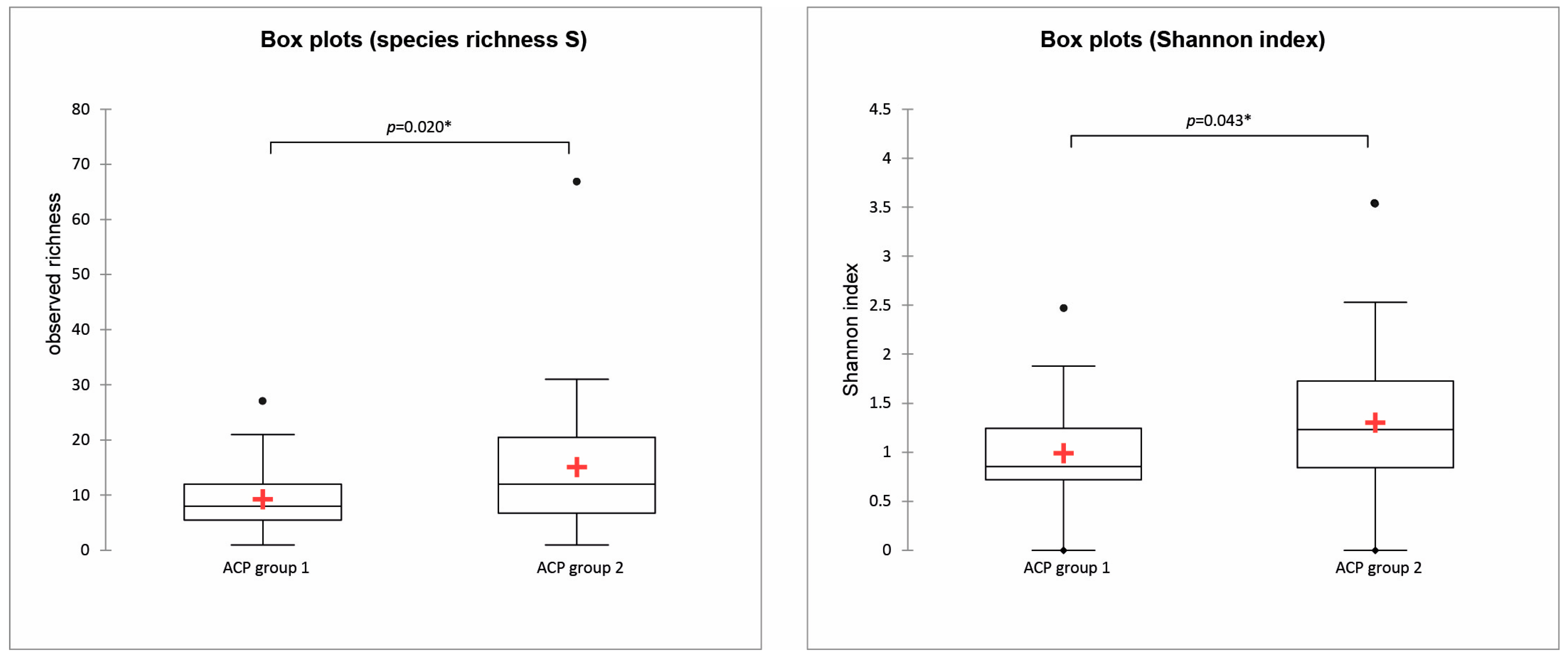
3.2. Diatom Composition and Diversity: Influence of the Environmental Variables
4. Discussion
4.1. Physical and Chemical Characteristics of the Saline Springs
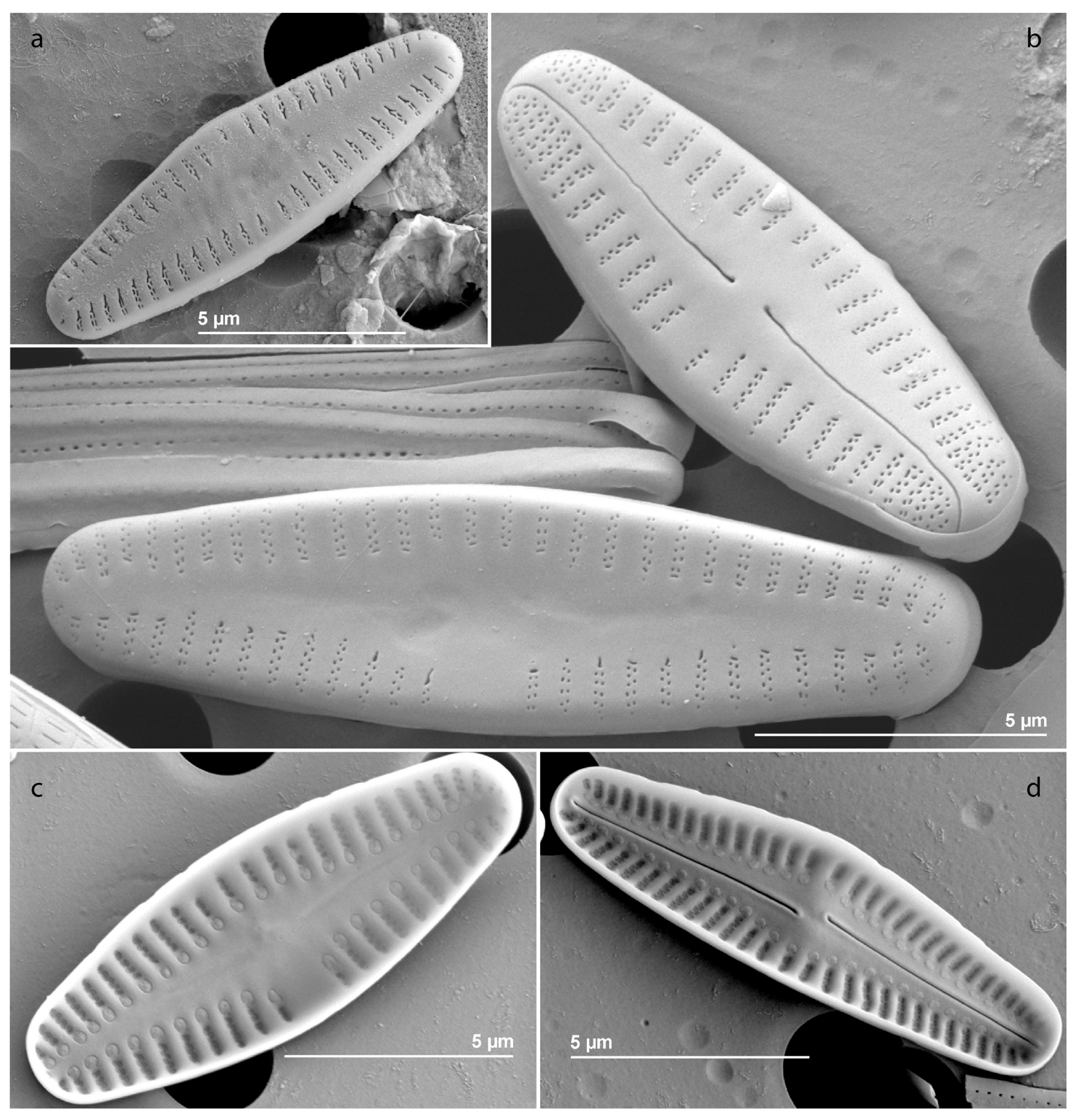
4.2. Diatom Structure and Composition
4.3. Environmental Variables Associated with Diatom Assemblages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, L.E.; Schenk, E.R.; Springer, A.E. Springs ecosystem classification. Ecol. Appl. 2021, 31, e2218. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press Elsevier: Oxford, UK, 2009; Volume 1, pp. 734–735. ISBN 978-0-1237-0626-3. [Google Scholar]
- Glazier, D.S. Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Ward, J.V. The four-dimensional nature of lotic ecosystems. J. N. Am. Benthol. Soc. 1989, 8, 2–8. [Google Scholar] [CrossRef]
- Risler, J.J. Description et classification géologique des sources minérales et thermales du Massif Central. BRGM Rep. 1974, 74, 24. [Google Scholar]
- Jonker, C.Z.; Van Ginkel, C.; Olivier, J. Association between Physical and Geochemical Characteristics of Thermal Springs and Algal Diversity in Limpopo Province, South Africa. Water SA 2013, 39, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Michard, G. Behaviour of major elements and some trace elements (Li, Rb, Cs, St, Fe, Mn, W, F) in deep hot waters from granitic areas. Chem. Geol. 1990, 89, 117–134. [Google Scholar] [CrossRef]
- Van der Kamp, G. The hydrogeology of springs in relation to the biodiversity of spring fauna: A review. J. Kans. Entomol. Soc. 1995, 68, 4–17. [Google Scholar]
- Cantonati, M.; Füreder, L.; Gerecke, R.; Jüttner, I.; Cox, E.J. Crenic habitats, hotspots for freshwater biodiversity conservation: Toward an understanding of their ecology. Freshw. Sci. 2012, 31, 463–480. [Google Scholar] [CrossRef]
- Kløve, B.; Ala-Aho, P.; Bertrand, G.; Boukalova, Z.; Ertürk, A.; Goldscheider, N.; Ilmonen, J.; Karakaya, N.; Kupfersberger, H.; Kvoerner, J.; et al. Groundwater dependent ecosystems. Part I: Hydroecological status and trends. Environ. Sci. Policy 2011, 14, 770–781. [Google Scholar] [CrossRef]
- Werum, M. Die Kieselalgen Gesellschaften in Quellen: Abhängigkeit von Geologie und Anthropogener Beeinfl Ussung in Hessen (Bunderepublik Deutschland); Hessische Landesamt für Umwelt und Geologie: Wiesbaden, Germany, 2001; ISBN 3-89026-331-3. [Google Scholar]
- Chaouite, J. Contribution à L’étude des Protistes des Eaux Minérales et Thermo-Minérales en Auvergne. Ph.D. Thesis, Université Blaise Pascal, Clermont-Ferrand, France, 1987. [Google Scholar]
- Segadelli, S.; Cantonati, M.; Bertoni, E.; Spitale, D.; Angeli, N.; Borsato, A. Can reference spring diatom communities be predicted from simple aquifer and emergence-site characteristics? In Abstract Book 9th Use of Algae for Monitoring Rivers and Comparable Habitats (UAMRIch, June 15th–17th 2015) and International Workshop on Benthic Algae Taxonomy (InBAT, June 17th–19th 2015); Cantonati, M., Kelly, M.G., Rott, E., Sabater, S., Stevenson, J.R., Whitton, B.A., Schneider, S., Shubert, L.E., Van de Vijver, B., Vis, M.L., et al., Eds.; Museo Delle Scienze—MUSE: Trento, Italy, 2015; p. 37. [Google Scholar]
- Beauger, A.; Voldoire, O.; Mertens, A.; Le Cohu, R.; Van de Vijver, B. Two new Navicula species (Bacillariophyceae) from Western Europe. Phytotaxa 2015, 230, 172–182. [Google Scholar] [CrossRef]
- Beauger, A.; Wetzel, C.E.; Voldoire, O.; Garreau, A.; Ector, L. Sellaphora labernardierei (Sellaphoraceae, Bacillariophyta), a new epilithic species from French spring and four new combinations within the genus Sellaphora. Phytotaxa 2016, 260, 235–246. [Google Scholar] [CrossRef]
- Beauger, A.; Wetzel, C.E.; Voldoire, O.; Garreau, A.; Ector, L. Morphology and ecology of Craticula lecohui sp. nov. (Bacillariophyceae) from hydrothermal springs (Puy-de-Dôme, Massif Central, France) and comparison with similar Craticula species. Nova Hedwig. Beih. 2017, 146, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Castenholz, R.W. The thermophilic cyanophytes of Iceland and the upper temperature limit. J. Phycol. 1969, 5, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Reimold, R.J.; Queen, W.H. Ecology of Halophytes; Academic Press: New York, NY, USA; London, UK, 1974; p. 607. [Google Scholar] [CrossRef]
- Krieger, R.A. The chemistry of saline waters. Groundwater 1963, 4, 7–12. [Google Scholar] [CrossRef]
- Poljakoff-Mayber, A.; Gale, J. Plants in Saline Environments; Springer: New York, NY, USA, 1975; Volume 15, p. 219. [Google Scholar] [CrossRef]
- Werum, M.; Lange-Bertalot, H. Diatoms in springs from Central Europe and elsewhere under the influence of hydrogeology and anthropogenic impacts. Iconogr. Diatomol. 2004, 13, 3–417. [Google Scholar]
- Żelazna-Wieczorek, J. Diatom flora in springs of Łódź Hills (Central Poland). Biodiversity, taxonomy, and temporal changes of epipsammic diatom assemblages in springs affected by human impact. Diatom Monogr. 2011, 13, 1–419. [Google Scholar]
- Solak, C.N.; Wojtal, A.Z. Diatoms in springs and streams of Türkmen Mt. (Sakarya river basin) common in Turkish inland waters. Pol. Bot. J. 2012, 57, 375–425. [Google Scholar]
- Wojtal, A.Z. Species Composition and Distribution of Diatom Assemblages in Spring Waters from Various Geological Formations in Southern Poland; Bibliotheca Diatomologica: Stuttgart, German, 2013; Volume 59, pp. 1–436. ISBN 978-3-443-57050-7. [Google Scholar]
- Lai, G.G.; Ector, L.; Lugliè, A.; Sechi, N.; Wetzel, C.E. Sellaphora gologonica sp. nov. (Bacillariophyta, Sellaphoraceae), a new diatom species from a Mediterranean karst spring (Sardinia, Italy). Phytotaxa 2018, 356, 145–157. [Google Scholar] [CrossRef]
- Delgado, C.; Gonçalves, V.; Blanco, S.; Almeida, S.F. A new diatom (Bacillariophyceae) species from a thermal spring in Azores archipelago (São Miguel island, Atlantic Ocean). Bot. Sci. 2020, 99, 169–181. [Google Scholar] [CrossRef]
- Angel, A.; Vila, I.; Díaz, C.; Molina, X.; Sepúlveda, P. Geothermal diatoms: Seasonal variability in the El Tatio geothermal field (Altiplano, Chile). Adv. Microbiol. 2018, 8, 211–234. [Google Scholar] [CrossRef] [Green Version]
- Ekins, L.; Rushforth, S.R. Diatom flora of Cowboy Hot Spring, Mono County, California. Great Basin Nat. 1986, 46, 612–624. [Google Scholar]
- Mpawenayo, B.; Cocquyt, C.; Nindorera, A. Les diatomées (Bacillariophyta) et autres algues des sources thermales du Burundi (Afrique Centrale) en relation avec les caractéristiques physiques et chimiques des eaux. Belg. J. Bot. 2005, 138, 152–164. [Google Scholar]
- Pumas, C.; Pruetiworanan, S.; Peerapornpisal, Y. Diatom diversity in some hot springs of northern Thailand. Botanica 2018, 24, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Nikulina, T.V.; Kociolek, J.P. Diatoms from Hot Springs from Kuril and Sakhalin Islands (Far East, Russia). In The Diatom World. Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Kociolek, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 19, ISBN 978-94-007-1326-0. [Google Scholar]
- Villeneuve, V.; Pienitz, R. Composition diatomifère de quatre sources thermales au Canada, en Islande et au Japon. Diatom Res. 1998, 13, 149–175. [Google Scholar] [CrossRef]
- Owen, R.B.; Renaut, R.W.; Jones, B. Geothermal diatoms: A comparative study of floras in hot spring systems of Iceland, New Zealand, and Kenya. Hydrobiologia 2008, 610, 175–192. [Google Scholar] [CrossRef]
- Héribaud, J. Les Diatomées d’Auvergne; Librairie des Sciences Naturelles: Clermont-Ferrand, France; Paris, France, 1893; p. 255. [Google Scholar]
- Chaouite, J.; Romagoux, J.C. La flore diatomique des eaux minérales de Vichy (Allier) et de Chaudes-Aigues (Cantal). Rev. Des Sci. Nat. D’auvergne 1989, 55, 7–23. [Google Scholar]
- Tudesque, L. Etude Hydrobiologique de 3 Sites Halophiles: Bard, Saint-Nectaire et Les Saladis. Master’s Thesis, (Diplôme Universitaire Supérieur) (mention Hydrobiol.), Université Blaise Pascal, Clermont-Ferrand, France, 1996; p. 71. [Google Scholar]
- Beauger, A.; Wetzel, C.E.; Voldoire, O.; Ector, L. Pseudostaurosira bardii (Fragilariaceae, Bacillariophyta), a new species from a saline hydrothermal spring of the Massif Central (France). Bot. Lett. 2019, 166, 3–13. [Google Scholar] [CrossRef]
- Beauger, A.; Wetzel, C.E.; Allain, E.; Bertin, C.; Voldoire, O.; Breton, V.; Baker, L.A.; Kolovi, S.; Biron, D.; Ector, L. Chamaepinnularia salina (Bacillariophyta), a new diatom species from French mineral springs (Massif Central). Phytotaxa 2022, 538, 55–73. [Google Scholar] [CrossRef]
- Beauger, A.; Wetzel, C.E.; Voldoire, O.; Allain, E.; Breton, V.; Miallier, D.; Ector, L. Fontina Gen. nov. (Bacillariophyta): A new diatom genus from a thermo-mineral spring of the French Massif Central (France). Diatom Res. 2022, 37, 51–61. [Google Scholar] [CrossRef]
- Beauger, A.; Voldoire, O.; Wetzel, C.E.; Allain, E.; Millan, F.; Breton, V.; Kolovi, S.; Ector, L. Biodiversité et écologie des diatomées dans les sources minérales de la région de Sainte Marguerite (Saint-Maurice-ès-Allier, Massif central, France). BIOM 2020, 1, 21–34. [Google Scholar] [CrossRef]
- Baker, L.A.; Beauger, A.; Wetzel, C.E.; Voldoire, O.; Blavignac, C.; Allain, E.; Ector, L.; Biron, D. Brackish diatom species observed at the bituminous Puy de la Poix spring: An island of curiosity. BIOM 2022, 3, 40–51. [Google Scholar] [CrossRef]
- Baker, L.A.; Biron, D.; Millan, F.; Voldoire, O.; Breton, V.; Allain, E.; Wetzel, C.E.; Ector, L.; Beauger, A. The substrate, a key factor or not, to explain the species diversity of diatom communities in mineral springs. Bot. Lett. 2022, 169, 155–165. [Google Scholar] [CrossRef]
- Prygiel, J.; Coste, M. Guide Méthodologique Pour la Mise en Œuvre de L’indice Biologique Diatomées NF T 90-354; Etude Agences de l’Eau-Cemagref Bordeaux, Agences de l’Eau: Douai, France, 2000; p. 134. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süsswasserflora von Mitteleuropa, 2nd ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1997; Volume 2, p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa, 2nd ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1997; Volume 2, p. 611. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiae. In Süsswasserflora von Mitteleuropa, 2nd ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 2000; Volume 2, p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 5. Teil: English and French translation of the keys. In Süsswasserflora von Mitteleuropa, 2nd ed.; Büdel, B., Gärtner, G., Krienitz, L., Lokhorst, G.M., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2000; Volume 2, p. 599. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae, kritische Ergänzungen zu Navicula (Lineoatae) und Gomphonema. In Süsswasserflora von Mitteleuropa, 2nd ed.; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2004; Volume 2, p. 468. [Google Scholar]
- Krammer, K. The genus Pinnularia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2000; Volume 1, p. 703. [Google Scholar]
- Krammer, K. Cymbella. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2002; Volume 3, p. 584. [Google Scholar]
- Krammer, K. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2003; Volume 4, p. 530. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto, 10 Genera separated from Navicula sensu lato Frustulia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2001; Volume 2, p. 526. [Google Scholar]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Witkowski, A.; Dorofeyuk, N.I.; Genkal, S.I. Diatom Assemblages from Sphagnum Bogs of the World. Part I: Nur bog in Northern Mongolia; Bibliotheca Diatomologica: Stuttgart, German, 2010; Volume 55, pp. 1–326. ISBN 978-3-443-57046-0. [Google Scholar]
- Lange-Bertalot, H.; Bąk, M.; Witkowski, A.; Tagliaventi, N. Eunotia and some related genera. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2011; Volume 6, p. 747. [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; English Edition with Updated Taxonomy and Added Species; Cantonati, M., Kelly, M.G., Lange-Bertalot, H., Eds.; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; p. 842. [Google Scholar]
- Wetzel, C.E.; Ector, L.; Van de Vijver, B.; Compère, P.; Mann, D.G. Morphology, typification and critical analysis of some ecologically important small naviculoid species (Bacillariophyta). Fottea 2015, 15, 203–234. [Google Scholar] [CrossRef] [Green Version]
- Levkov, Z.; Mitic-Kopanja, D.; Reichardt, E. The diatom genus Gomphonema from the Republic of Macedonia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2016; Volume 8, p. 552. [Google Scholar]
- Levkov, Z. Amphora sensu lato. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2009; Volume 5, p. 916. [Google Scholar]
- Piper, A.M. A Graphical Procedure in the Geochemical Interpretation of Water Analysis. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Simler, R. Software “Diagrammes”; Laboratoire d’Hydrologie d’Avignon, Université d’Avignon et Pays du Vaucluse: Avignon, France, 2018; Available online: http://www.lha.univ-avignon.fr (accessed on 18 January 2023).
- Soininen, J.; Heino, J.; Kokocinski, M.; Muotka, T. Local-regional diversity relationship varies with spatial scale in lotic diatoms. J. Biogeogr. 2009, 36, 720–727. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of the classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar] [CrossRef]
- Hill, M.O.; Gauch, H.G.J. Detrended correspondence analysis: An improved ordination technique. Vegetatio 1980, 42, 47–58. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Prentice, I.C. A theory of gradient analysis. Adv. Ecol. Res. 1988, 18, 271–317. [Google Scholar] [CrossRef]
- Birks, H.J.B. Quantitative palaeoenvironmental reconstructions. In Statistical Modelling of Quaternary Science Data; Maddy, D., Brew, J.J., Eds.; Quaternary Research Association: Cambridge, UK, 1995; pp. 161–254. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MJM Software Design: Gleneden Beach, OR, USA, 2002; p. 304. [Google Scholar]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution. Paris, France, 2022. Available online: https://www.xlstat.com (accessed on 18 January 2023).
- McCune, B.; Mefford, M.J. PC-ORD. In Multivariate Analysis of Ecological Data. Version 7; MJM Software Design: Gleneden Beach, OR, USA, 2016. [Google Scholar]
- Lange-Bertalot, H. Zur systematischen Bewertung der bandförmigen Kolonien bei Navicula und Fragilaria. Kriterien für die Vereinigung von Synedra (subgen. Synedra) Ehrenberg mit Fragilaria Lyngbye. In Nova Hedwigia; 1980; Volume 33, pp. 723–787. [Google Scholar]
- Kützing, F.T. Die Kieselschaligen Bacillarien Oder Diatomeen; Zu finden bei W. Köhne: Nordhausen, Germany,, 1844; p. 152. [Google Scholar]
- Rabenhorst, L. Die Algen Sachsens. Resp. Mittel-Europa’s Gesammelt und Herausgegeben von Dr. L. Rabenhorst, Dresden, Germay, 1848–1860, Dec. 1–100. No. 1–1000; Harvard University: Cambridge, MA, USA, 1848. [Google Scholar]
- Lange-Bertalot, H.; Simonsen, R. A taxonomic revision of the Nitzschiae lanceolatae Grunow. 2. European and related extra European freshwater and brackish water taxa. Bacillaria 1978, 1, 11–111. [Google Scholar]
- Krammer, K. Pinnularia: Eine Monographie der Europäischen Taxa; Bibliotheca Diatomologica: Berlin, Germany; Stuttgart, Germany, 1992; Volume 26, pp. 1–353. [Google Scholar]
- Lange-Bertalot, H. Neue Kombinationen von Taxa aus Achnanthes Bory (sensu lato). Iconogr. Diatomol. 1999, 6, 270–283. [Google Scholar]
- Czarnecki, D.B. The freshwater diatoms culture collection at Loras College, Dubuque, Iowa. In Proceedings of the 11th International Diatom Symposium, San Francisco, CA, USA, 12–17 August 1990; Memoirs of the California Academy of Sciences, 1994, Kockiolek, J.P., Eds.; Volume 17, pp. 155–174. [Google Scholar]
- Moser, G.; Lange-Bertalot, H.; Metzeltin, D. Insel der Endemiten. Geobotanisches Phänomen Neukaledonien. Island of Endemics New Caledonia—A Geobotanical Phenomenon. Bibliotheca Diatomologica: Berlin, Germany; Stuttgart, Germany, 1998; Volume 38, pp. 1–464. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; p. 747. [Google Scholar]
- Reichardt, E. Zur Revision der Gattung Gomphonema. Die Arten um G. affine/insigne, G. angustatum/micropus, G. acuminatum sowie gomphonemoide Diatomeen aus dem Oberoligozän in Böhmen. In Iconographia Diatomologica; A.R.G. Gantner: Ruggell, Liechtenstein, 1999; Volume 8, pp. 1–203. [Google Scholar]
- Schmidt, A. Atlas der Diatomaceen-Kunde; Series IV; Heft 47; O.R. Reisland: Leipzig, Germany, 1893; pp. 185–188. [Google Scholar]
- Cleve, P.T.; Grunow, A. Beiträge zur Kenntniss der arctischen Diatomeen. Kongliga Sven. Vetensk.-Akad. Handl. 1880, 17, 1–121. [Google Scholar]
- Williams, D.M.; Wetzel, C.E. Description of a new Pseudostaurosira based on “Fragilaria virescens f. parva” from Erbario Crittogamico Italiano. Bot. Lett. 2020, 167, 86–94. [Google Scholar] [CrossRef]
- Boineau, R.; Maisonneuve, J. Les sources minérales du Massif central français et leur cadre géologique. Rapp. BRGM 72-SGN-151-MCE 1972, 32. Available online: http://infoterre.brgm.fr/rapports/72-SGN-151-MCE.pdf (accessed on 18 January 2023).
- Gonnard, F. Sur le quartz du calcaire bitumineux de la Limagne. Bull. Société Française Minéralogie 1906, 29, 362–365. [Google Scholar] [CrossRef]
- Żelazna-Wieczorek, J.; Olszyński, R.M.; Nowicka-Krawczyk, P. Half a century of research on diatoms in athalassic habitats in central Poland. Oceanol. Hydrobiol. Stud. 2015, 44, 51–67. [Google Scholar] [CrossRef]
- Hoffman, L. Geographic distribution of blue-green algae. Hydrobiologia 1996, 336, 33–40. [Google Scholar] [CrossRef]
- Padisák, J. Phytoplankton. In The Lakes Handbook: Limnology and Limnetic Ecology; O’Sullivan, P.E., Reynolds, C.S., Eds.; Blackwell Science Ltd.: Singapore, 2003; Volume 1, pp. 251–308. ISBN 978-0-6320-4797-0. [Google Scholar]
- Coesel, P.F.M. Biogeography of desmids. Hydrobiologia 1996, 336, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Tyler, P.A. Endemism in freshwater algae: With special reference to the Australian region. Hydrobiologia 1996, 336, 127–135. [Google Scholar] [CrossRef]
- Lai, G.G.; Beauger, A.; Wetzel, C.E.; Padedda, B.M.; Voldoire, O.; Lugliè, A.; Allain, E.; Ector, L. Diversity, ecology and distribution of benthic diatoms in thermo-mineral springs in Auvergne (France) and Sardinia (Italy). PeerJ 2019, 7, e7238. [Google Scholar] [CrossRef]
- Lai, G.G.; Ector, L.; Wetzel, C.E.; Lugliè, A.; Padedda, B.M. Environmental factors structuring diatom assemblages in thermo-mineral springs of Sardinia, Italy. Freshw. Sci. 2022, 41, 45–61. [Google Scholar] [CrossRef]
- Kristiansen, J. Dispersal of freshwater algae—A review. Hydrobiologia 1996, 336, 151–157. [Google Scholar] [CrossRef]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Rakowska, B. Diatom communities in a salt spring at Pełczyska (Central Poland). Biologia 1997, 52, 489–493. [Google Scholar]
- Quintela, A.; Almeida, S.F.P.; Terroso, D.; Ferreira da Silva, E.; Forjaz, V.; Rocha, F. Diatom assemblages of thermal and mineral waters from volcanic environments in São Miguel Island, Azores. Diatom Res. 2013, 28, 407–417. [Google Scholar] [CrossRef]
- Niyatbekov, T.; Barinova, S. Diatom Species Richness in Algal Flora of Pamir, Tajikistan. Eur. Sci. J. 2018, 14, 301–323. [Google Scholar] [CrossRef] [Green Version]
- Economou-Amili, A. On Diatoms from Thermal Springs of Greece; Institute of Systematic Botany, University of Athens: Athens, Greece, 1976. [Google Scholar]
- Rehakova, Z. Diatoms from thermal waters and mud in Piestany Spa (Slovakia). Arch. Hydrobiol. 1976, 14, 1–175. [Google Scholar]
- Denys, L.; Oosterlynck, P. Diatom assemblages of non-living substrates in petrifying Cratoneurion springs from lower Belgium. Fottea 2015, 15, 123–138. [Google Scholar] [CrossRef]
- Musaabad, L.A.; Mirzahasanlou, J.P.; Mahmoodlu, M.G.; Bahlakeh, A. Diatom flora in three Springs of Golestan Province. J. Phycol. 2019, 3, 432–442. [Google Scholar] [CrossRef]
- Barinova, S.; Kukhaleishvili, L.; Nevo, E.; Janelidze, Z. Diversity and ecology of algae in the Algeti National Park as a part of the Georgian system of protected areas. Turk. J. Bot. 2011, 35, 729–774. [Google Scholar] [CrossRef]
- Leira, M.; Meijide-Failde, R.; Torres, E. Diatom communities in thermo-mineral springs of Galicia (NW Spain). Diatom Res. 2017, 32, 29–42. [Google Scholar] [CrossRef]
- Cantonati, M.; Fensham, R.J.; Stevens, L.E.; Gerecke, R.; Glazier, D.S.; Goldscheider, N.; Knight, R.L.; Richardson, J.S.; Springer, A.E.; Tockner, K. Urgent plea for global protection of springs. Conserv. Biol. 2020, 35, 378–382. [Google Scholar] [CrossRef]


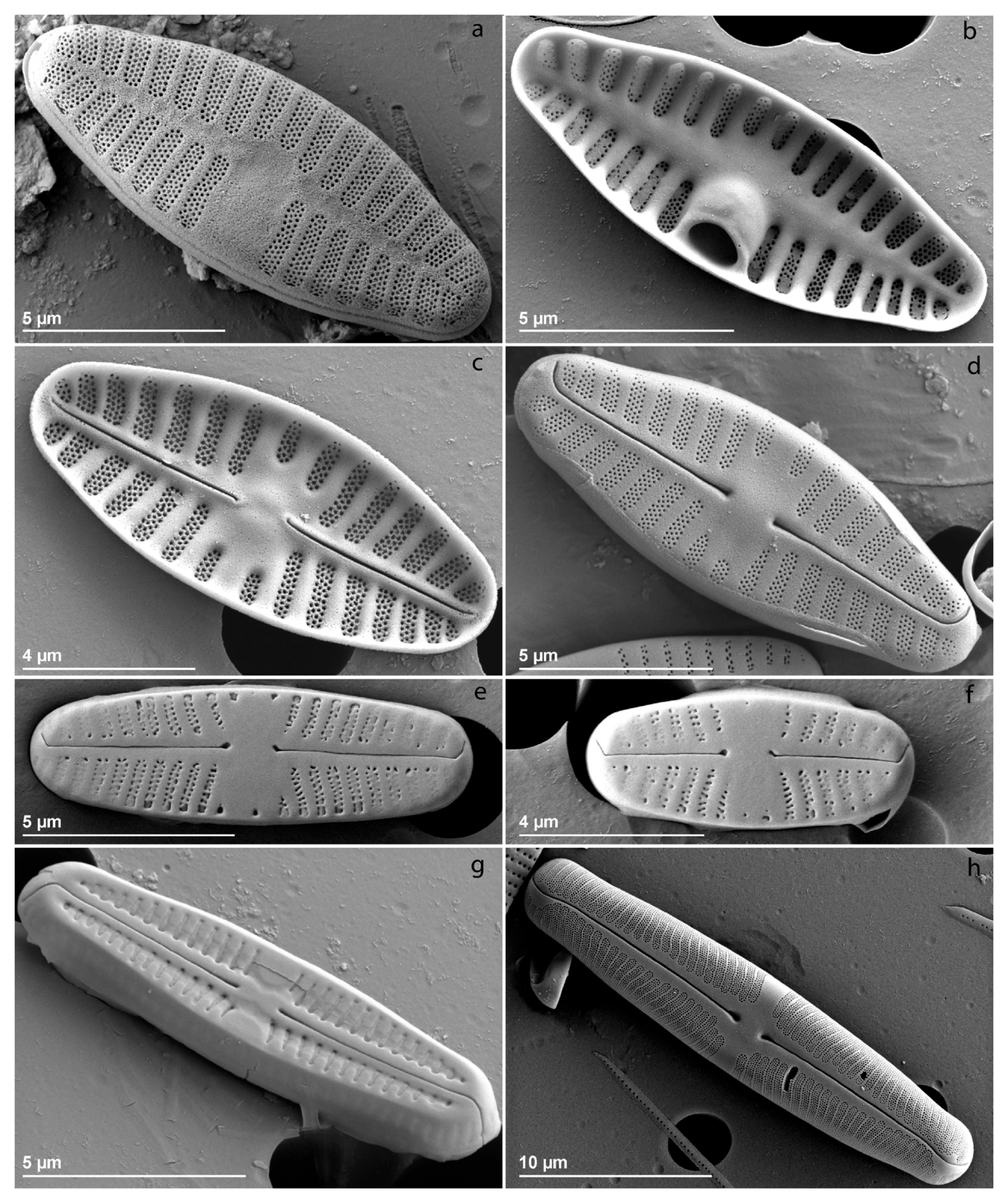

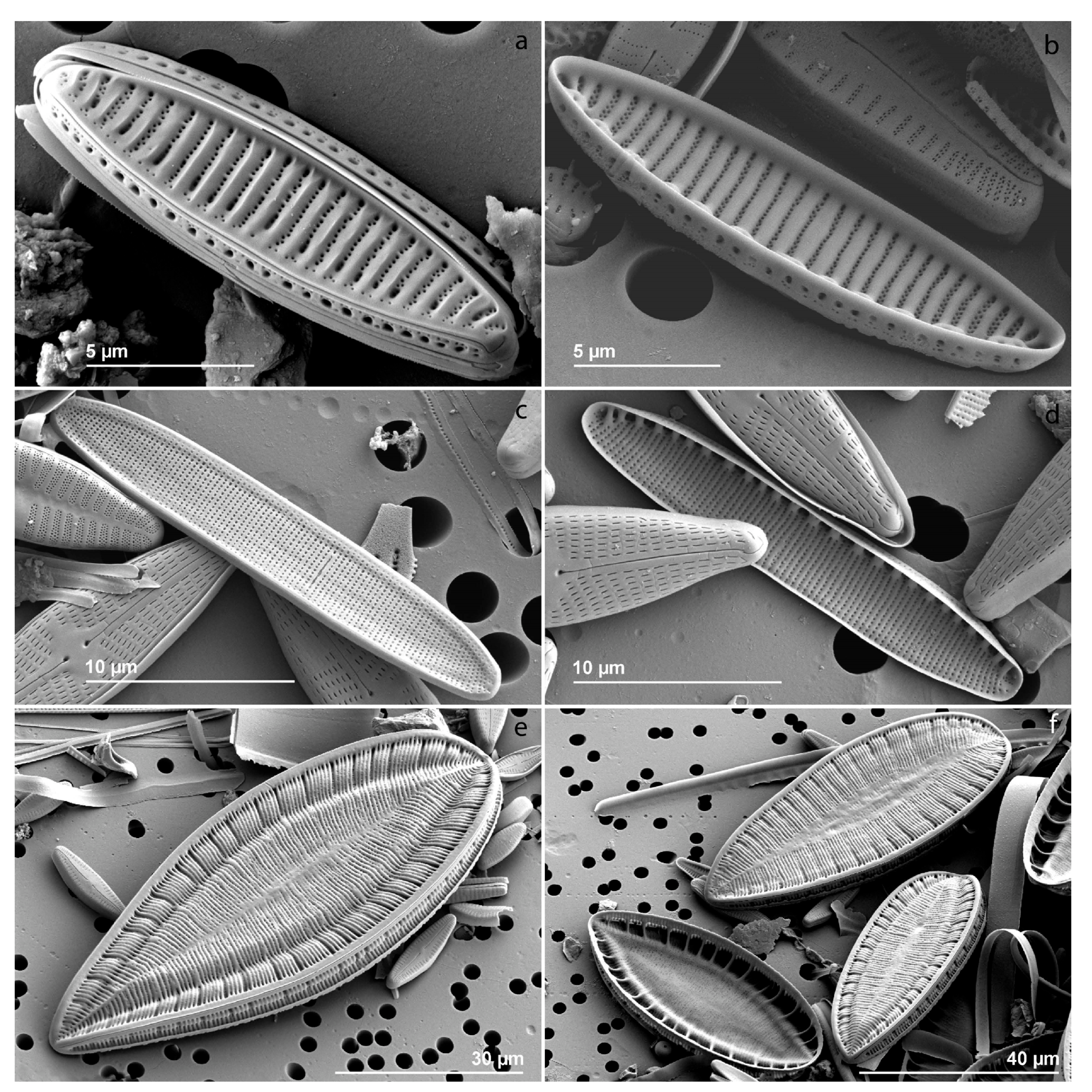

| Environmental Variables | Mean (±SD) |
|---|---|
| Conductivity (µS cm−1) | 6073.85 ± 2112.00 |
| pH (pH unit) | 6.87 ± 0.47 |
| Water temperature (°C) | 16.82 ± 8.24 |
| TDS (mg L−1) | 5025.80 ± 1665.47 |
| Li+ | 6.03 ± 4.08 |
| Na+ | 1065.66 ± 541.40 |
| NH4+ | 1.12 ± 0.78 |
| K+ | 125.52 ± 63.71 |
| Mg2+ | 124.63 ± 81.93 |
| Ca2+ | 209.73 ± 147.31 |
| F− | 1.18 ± 1.33 |
| Cl− | 996.88 ± 774.70 |
| Br- | 2.27 ± 1.88 |
| NO3− | 0.91 ± 2.92 |
| PO43− | 0.15 ± 0.59 |
| SO42− | 176.16 ± 243.02 |
| HCO3− | 2312.70 ± 749.37 |
| Variable | p-Value |
|---|---|
| Li+ | <0.0001 |
| Na+ | <0.0001 |
| NH4+ | 0.000 |
| K+ | <0.0001 |
| Mg2+ | 0.106 |
| Ca2+ | 0.896 |
| F− | 0.052 |
| Cl− | <0.0001 |
| Br− | <0.0001 |
| NO3− | 0.848 |
| PO43- | 0.009 |
| SO42− | 0.229 |
| HCO3− | <0.0001 |
| Conductivity | <0.0001 |
| pH | <0.0001 |
| Water temperature | <0.0001 |
| TDS | <0.0001 |
| Variables | Natural Springs | “Artificial” Springs |
|---|---|---|
| S | 18.94 ± 12.66 | 8.60 ± 6.21 |
| S.chao1 | 26.90 ± 24.02 | 10.73 ± 8.54 |
| Shannon index | 1.57 ± 0.76 | 0.91 ± 0.51 |
| Piélou index | 0.09 ± 0.03 | 0.12 ± 0.06 |
| Class 1 | Class 2 | Class 3 | |
|---|---|---|---|
| Li+ (mg L−1) | [1.04–5.80) | [5.8–11.30) | ≥11.3 |
| Na+ (mg L−1) | [185.77–1178.70) | [1178.7–1833.50) | ≥1833.5 |
| K+ (mg L−1) | [10.46–94.07) | [94.07–165.44) | ≥164.44 |
| Ca2+ (mg L−1) | [11.58–207.50) | [207.5–410.38) | ≥410.38 |
| Cl− (mg L−1) | [24.88–1118.89) | [1118.89–2066.06) | ≥2066.06 |
| Br− (mg L−1) | [0–1.44) | [1.44–3.81) | ≥3.81 |
| F− (mg L−1) | [0.092–3.49) | ≥3.49 | |
| TDS | [3018–5175) | [5175–7195) | ≥7195 |
| Conductivity (µS cm−1) | [3340-6350) | [6350–9220) | ≥9220 |
| pH | [6.12–6.82) | [6.82–7.56) | ≥7.56 |
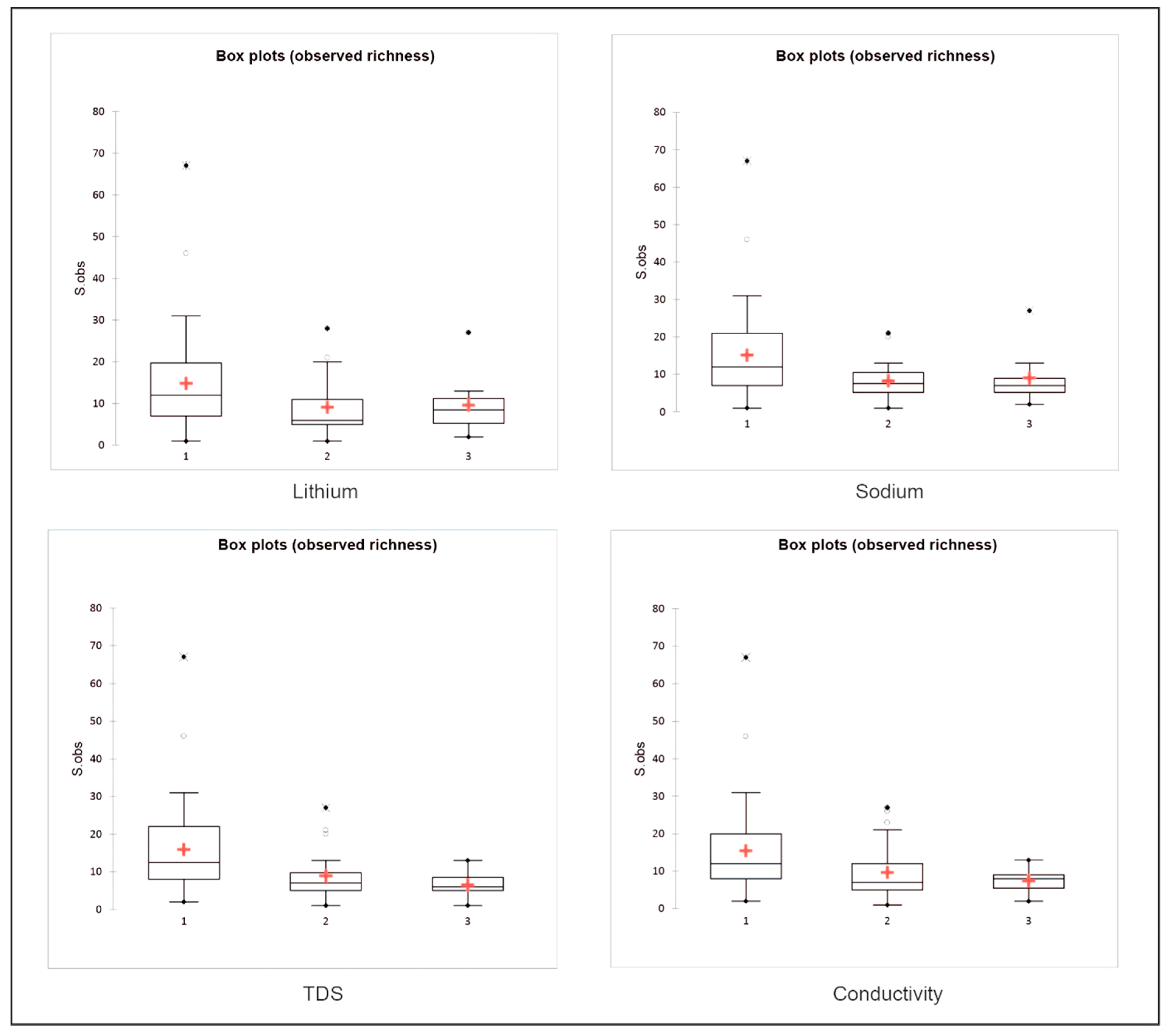
| Variables | Species Richness (p-Value) | Estimated Richness (p-Value) | Shannon Index (p-Value) | Piélou Index (p-Value) |
|---|---|---|---|---|
| Li+ | 0.048 | 0.118 | 0.036 | 0.003 |
| Na+ | 0.019 | 0.055 | 0.055 | 0.010 |
| K+ | 0.181 | 0.384 | 0.640 | 0.063 |
| Ca2+ | 0.578 | 0.525 | 0.335 | 0.617 |
| Cl− | 0.160 | 0.265 | 0.309 | 0.171 |
| Br− | 0.103 | 0.084 | 0.303 | 0.626 |
| F− | 0.139 | 0.089 | 0.094 | 0.911 |
| TDS | 0.001 | 0.003 | 0.054 | 0.035 |
| Conductivity | 0.020 | 0.054 | 0.045 | 0.005 |
| pH | 0.172 | 0.394 | 0.061 | 0.159 |
| Indicator Species | Observed Indicator Value (IV) | Mean | S.dev | p * |
|---|---|---|---|---|
| Lithium (mg L−1) | ||||
| [1.04–5.8) | ||||
| Crenotia angustior | 32.2 | 14.8 | 5.63 | 0.0150 |
| Planothidium frequentissimum | 44.2 | 29.3 | 4.81 | 0.0067 |
| ≥11.3 | ||||
| Fragilaria famelica | 27.3 | 17.9 | 5.51 | 0.0701 |
| Halamphora coffeaeformis | 61.6 | 11.6 | 5.04 | 0.0017 |
| Nitzschia communis | 28.7 | 14.0 | 5.36 | 0.0234 |
| Sodium (mg L−1) | ||||
| [185.77–1178.7) | ||||
| Crenotia angustior | 35.3 | 14.8 | 5.55 | 0.0117 |
| Planothidium frequentissimum | 49.4 | 29.6 | 5.11 | 0.0050 |
| ≥1833.5 | ||||
| Halamphora coffeaeformis | 47.6 | 12.4 | 5.61 | 0.0017 |
| Nitzschia valdecostata | 25.4 | 13.8 | 5.49 | 0.0484 |
| Potassium (mg L−1) | ||||
| ≥164.44 | ||||
| Halamphora coffeaeformis | 36.4 | 10.6 | 3.81 | 0.0010 |
| Navicula sanctamargaritae | 41.5 | 31.7 | 3.07 | 0.0080 |
| Calcium (mg L−1) | ||||
| ≥410.38 | ||||
| Crenotia angustior | 45.3 | 15.3 | 6.01 | 0.0030 |
| Sellaphora labernardierei | 33.1 | 15.0 | 5.63 | 0.0140 |
| Chloride (mg L−1) | ||||
| [1118.89–2066.06) | ||||
| Planothidium frequentissimum | 44.1 | 29.6 | 4.79 | 0.0140 |
| ≥2066.06 | ||||
| Halamphora coffeaeformis | 42.4 | 12.1 | 5.23 | 0.0020 |
| Bromine (mg L −1) | ||||
| [0–1.44) | ||||
| Planothidium frequentissimum | 44.0 | 28.0 | 3.77 | 0.0010 |
| Sellaphora labernardierei | 30.5 | 13.7 | 4.13 | 0.0030 |
| [1.44–3.81) | ||||
| Surirella patella | 45.8 | 21.1 | 4.28 | 0.0010 |
| ≥3.81 mg L−1 | ||||
| Halamphora coffeaeformis | 33.5 | 10.5 | 3.85 | 0.0020 |
| TDS (mg L−1) | ||||
| [3018–5175) | ||||
| Planothidium frequentissimum | 55.9 | 28.9 | 4.62 | 0.0010 |
| ≥7195 | ||||
| Halamphora coffeaeformis | 33.1 | 11.6 | 4.99 | 0.0050 |
| Conductivity (µS cm−1) | ||||
| [3340–6350) | ||||
| Crenotia angustior | 28.8 | 15.5 | 6.36 | 0.0451 |
| Planothidium frequentissimum | 55.1 | 30.0 | 5.47 | 0.0017 |
| ≥9220 µS cm−1 | ||||
| Halamphora coffeaeformis | 59.5 | 12.6 | 6.46 | 0.0017 |
| pH | ||||
| [6.12–6.82) | ||||
| Crenotia angustior | 41.2 | 15.4 | 6.58 | 0.0117 |
| Planothidium frequentissimum | 44.0 | 30.1 | 5.70 | 0.0351 |
| [6.82–7.56) | ||||
| Crenotia thermalis | 44.8 | 33.4 | 4.03 | 0.0017 |
| ≥7.56 | ||||
| Halamphora coffeaeformis | 31.7 | 12.8 | 6.37 | 0.0250 |
| Navicula veneta | 56.1 | 25.5 | 6.63 | 0.0033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beauger, A.; Voldoire, O.; Allain, E.; Gosseaume, P.; Blavignac, C.; Baker, L.-A.; Wetzel, C.E. Biodiversity and Environmental Factors Structuring Diatom Assemblages of Mineral Saline Springs in the French Massif Central. Diversity 2023, 15, 283. https://doi.org/10.3390/d15020283
Beauger A, Voldoire O, Allain E, Gosseaume P, Blavignac C, Baker L-A, Wetzel CE. Biodiversity and Environmental Factors Structuring Diatom Assemblages of Mineral Saline Springs in the French Massif Central. Diversity. 2023; 15(2):283. https://doi.org/10.3390/d15020283
Chicago/Turabian StyleBeauger, Aude, Olivier Voldoire, Elisabeth Allain, Pierre Gosseaume, Christelle Blavignac, Lory-Anne Baker, and Carlos E. Wetzel. 2023. "Biodiversity and Environmental Factors Structuring Diatom Assemblages of Mineral Saline Springs in the French Massif Central" Diversity 15, no. 2: 283. https://doi.org/10.3390/d15020283
APA StyleBeauger, A., Voldoire, O., Allain, E., Gosseaume, P., Blavignac, C., Baker, L.-A., & Wetzel, C. E. (2023). Biodiversity and Environmental Factors Structuring Diatom Assemblages of Mineral Saline Springs in the French Massif Central. Diversity, 15(2), 283. https://doi.org/10.3390/d15020283








