Impact of Dams on Stream Fish Diversity: A Different Result
Abstract
:1. Introduction
2. Materials and Methods
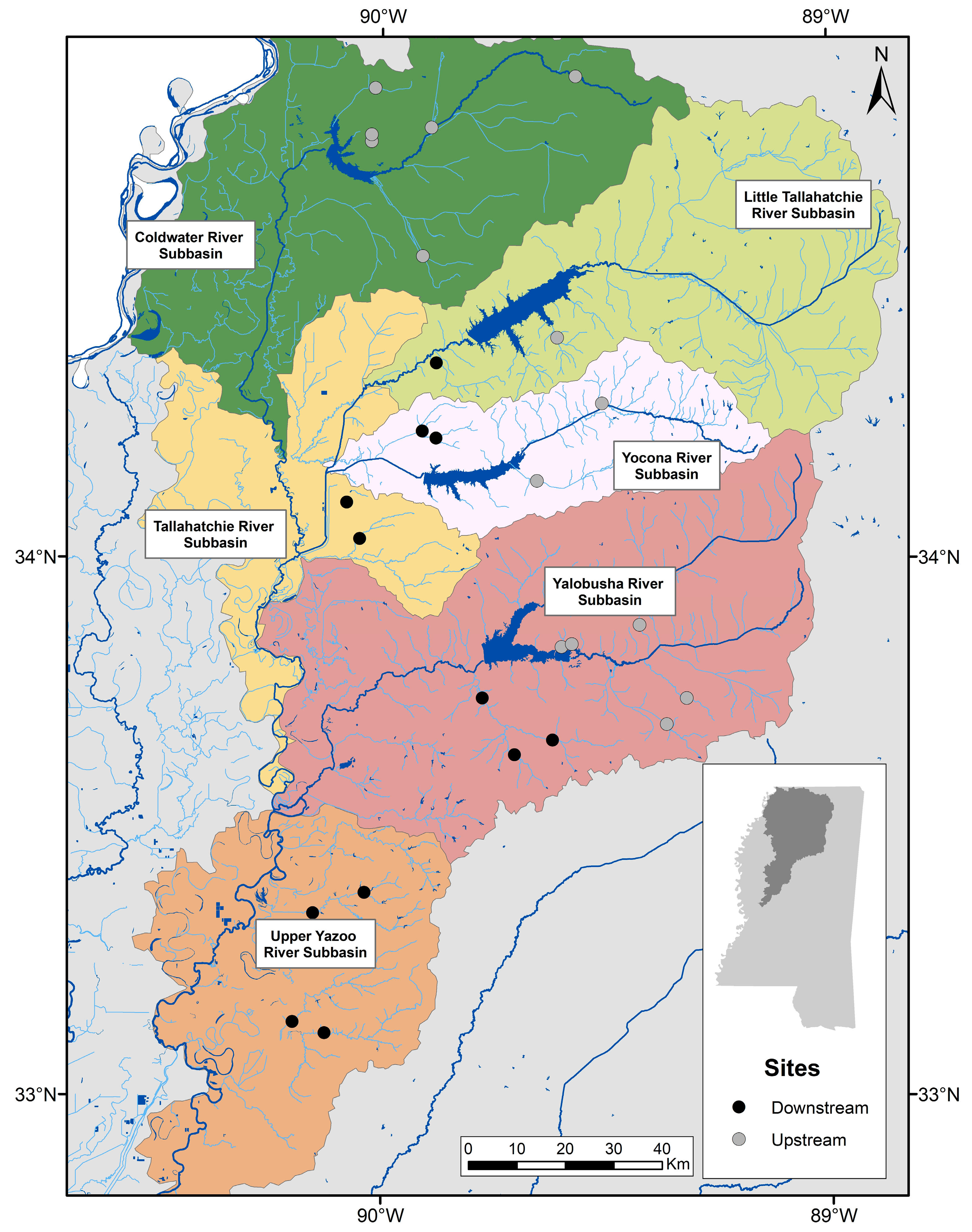
2.1. Study Area
2.2. Site Selection
2.3. Fish Sampling
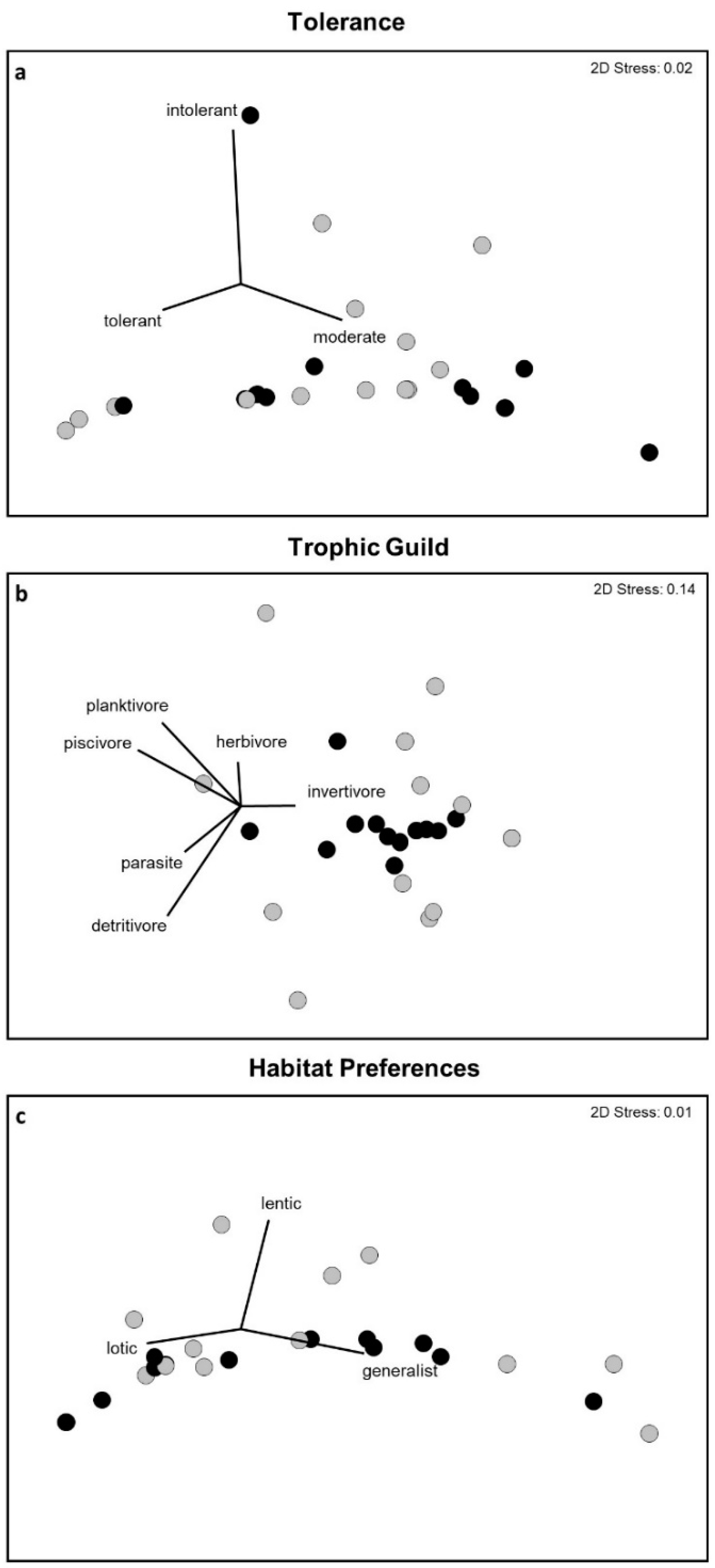
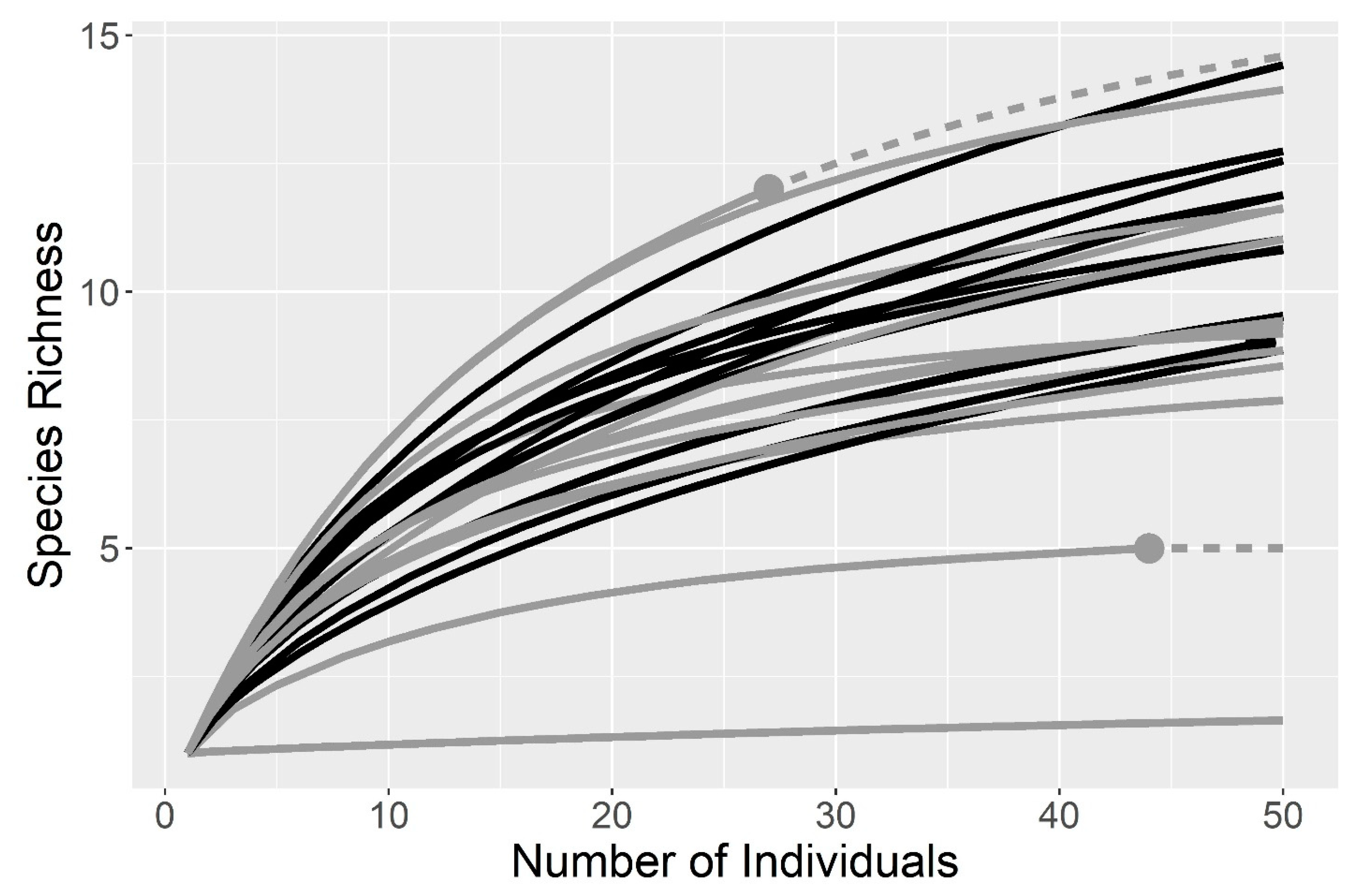
2.4. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McAllister, D.E.; Platania, S.P.; Schueler, F.W.; Baldwin, M.E.; Lee, D.S. Ichthyofaunal patterns on a geographic grid. In The Zoogeography of North American Freshwater Fishes; Hocutt, C.H., Wiley, E.O., Eds.; John Wiley and Sons: New York, NY, USA, 1986; pp. 17–52. ISBN 0-471-86419-6. [Google Scholar]
- Jelks, H.L.; Walsh, S.; Burkhead, N.M.; Contreras-Balderas, S.; Diaz-Pardo, E.; Hendrickson, D.A.; Lyons, J.; Mandrak, N.E.; McCormick, F.; Nelson, J.S.; et al. Conservation status of imperiled North American freshwater and diadromous fishes. Fisheries 2008, 33, 372–407. [Google Scholar] [CrossRef]
- Warren, M.L., Jr.; Haag, W.R.; Adams, S.B. Forest linkages to diversity and abundance in lowland stream fish communities. In Proceedings of the Conference on Sustainability of Wetlands and Water Resources: How Well Can Riverine Wetlands Continue to Support Society into the 21st Century? Oxford, MS, USA, 23–25 May 2000; Holland, M.M., Warren, M.L., Jr., Stanturf, J.A., Eds.; U.S. Department of Agriculture, Forest Service, Southern Research Station: Ashville, NC, USA, 2002. [Google Scholar]
- Bennet, S.J.; Rhoton, F.E. Linking upstream channel instability to downstream degradation: Grenada Lake and the Skuna and Yalobusha river basins, Mississippi. Ecohydrology 2009, 2, 235–247. [Google Scholar] [CrossRef]
- Graf, W.L. Dam nation: A geographic census of American dams and their large-scale hydrologic impacts. Water Resour. Res. 1999, 35, 1305–1311. [Google Scholar] [CrossRef]
- U.S. Army Corps of Engineers. National Inventory of Dams. 2018. Available online: https://nid.usace.army.mil (accessed on 18 August 2021).
- Hannan, H.H. Chemical modifications in reservoir-regulated streams. In The Ecology of Regulated Streams; Ward, J.V., Stanford, J.A., Eds.; Springer: Boston, MA, USA, 1979; pp. 75–94. ISBN 978-1-4684-8613-1. [Google Scholar]
- Whitten, C.B.; Patrick, D.M. Engineering Geology and Geomorphology of Stream-Bank Erosion; Report 2, Yazoo River Basin Uplands, Mississippi; Geotechnical Laboratory, US Army Corps of Engineers: Washington, DC, USA, 1981. [Google Scholar]
- Simon, A. A model of channel response in disturbed alluvial channels. Earth Surf. Process. Landf. 1989, 14, 11–26. [Google Scholar] [CrossRef]
- Biedenharn, D.S.; Elliott, C.M.; Watson, C.C. The WES Stream Investigation and Streambank Stabilization Handbook; US Army Engineer Waterways Experiment Station: Vicksburg, MS, USA, 1997. [Google Scholar]
- Schumm, S.A.; Harvey, M.D.; Watson, C.C. Incised Channels: Morphology, Dynamics, and Control; Water Resources Publications: Highlands Ranch, CO, USA, 1984; ISBN 978-1-887201-64-3. [Google Scholar]
- Simon, A. Gradation Processes and Channel Evolution in Modified West Tennessee Streams: Process, Response and Form; U.S. Geological Survey Professional Paper 1470: Washington, DC, USA, 1994. [Google Scholar]
- Junk, W.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. In Proceedings of the International Large River Symposium (LARS), Honey Harbour, ON, Canada, September 1989; Dodge, D.P., Ed.; Canadian Special Publication of Fisheries and Aquatic Sciences 106: Ottawa, ON, Canada, 1989; pp. 110–127. [Google Scholar]
- Winston, M.R.; Taylor, C.M.; Pigg, J. Upstream extirpation of four minnow species due to damming of a prairie stream. Trans. Am. Fish. Soc. 1991, 120, 98–105. [Google Scholar] [CrossRef]
- Herbert, M.E.; Gelwick, F.P. Spatial variation of headwater fish assemblages explained by hydrologic variability and upstream effects of impoundment. Copeia 2003, 2003, 273–284. [Google Scholar] [CrossRef]
- Falke, J.A.; Gido, K.B. Effects of reservoir connectivity on stream fish assemblages in the Great Plains. Can. J. Fish. Aquat. Sci. 2006, 63, 480–493. [Google Scholar] [CrossRef]
- Guenther, C.B.; Spacie, A. Changes in fish assemblage structure upstream of impoundments within the Upper Wabash River Basin, Indiana. Trans. Am. Fish. Soc. 2006, 135, 570–583. [Google Scholar] [CrossRef]
- Kashiwagi, M.T.; Miranda, L.E. Influence of small impoundments on habitat and fish communities in headwater streams. Southeast. Nat. 2009, 8, 23–36. [Google Scholar] [CrossRef]
- Sá-Oliveira, J.C.; Hawes, J.E.; Isaac-Nahuum, V.J.; Peres, C.A. Upstream and downstream responses of fish assemblages to an Eastern Amazonian hydroelectric dam. Freshw. Biol. 2015, 60, 2037–2050. [Google Scholar] [CrossRef]
- Hedden, S.C.; Renner, E.A.; Gido, K.B.; Hase, K.J. Impacts of small impoundments on an intermittent headwater stream community. Southwest. Nat. 2018, 63, 34–41. [Google Scholar] [CrossRef]
- Reuter, C.N.; Kaller, M.D.; Walsh, C.E.; Kelso, W.E. Fish assemblage response to altered dendritic connectivity in the Red River Basin, Central Louisiana. Am. Midl. Nat. 2019, 181, 63–80. [Google Scholar] [CrossRef]
- Barnett, Z.C.; Adams, S.B. Review of dam effects on native and invasive crayfishes illustrates complex choices for conservation planning. Front. Ecol. Evol. 2021, 8, 621723. [Google Scholar] [CrossRef]
- U.S. Geological Survey. National Hydrography Dataset (ver. USGS National Hydrography Dataset Best Resolution (NHD) for Hydrologic Unit (HU) 4–0803 (published 202110102)). 2021. Available online: https://www.usgs.gov/core-science-systems/ngp/national-hydrography/access-national-hydrography-products. (accessed on 5 January 2021).
- Hilgard, E.W. Report on the Geology and Agriculture of the State of Mississippi; E. Barksdale State Printer: Jackson, MS, USA, 1860. [Google Scholar]
- Dabney, S.M.; Shields, F.D.; Binger, R.L.; Kuhnle, R.A.; Rigby, J.R. Watershed management for erosion and sedimentation control case study Goodwin Creek, Panola County. In Advances in Soil Science, Soil Water and Agronomic Productivity; Lal, R., Stewart, B.A., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2012; pp. 539–568. ISBN 978-1439850794. [Google Scholar]
- Williston, H.L. The Yazoo-Little Tallahatchie Flood Prevention Project: A History of the Forest Service’s Role; Forgotten Books: London, UK, 1998; ISBN 978-0265065969. [Google Scholar]
- Bledsoe, B.P.; Watson, C.C.; Biedenharn, D.S. Quantification of incised channel evolution and equilibrium. J. Am. Water Resour. Assoc. 2002, 38, 861–870. [Google Scholar] [CrossRef]
- Knight, S.S.; Cooper, C.M. Fishes of Otoucalofa Creek, Mississippi prior to major channel modifications. J. Miss. Acad. Sci. 1987, 32, 31–38. [Google Scholar]
- Ross, S.T. Inland Fishes of Mississippi; University Press of Mississippi: Jackson, MS, USA, 2001; ISBN 1-57806-246-2. [Google Scholar]
- Faucheux, N.M. Assessing the Legacy of Erosion and Flood Control Management Efforts on the Fish Assemblages and Physical Conditions of Yazoo Basin Bluff Hill Streams. Doctoral Dissertation, Mississippi State University, Mississippi State, MS, USA, 2022. [Google Scholar]
- U.S. Geological Survey. The StreamStats Program v.4.3.11. 2020. Available online: http://streamstats.usgs.gov (accessed on 6 February 2020).
- Rabeni, C.F.; Lyons, J.; Mercado-Silva, N.; Patterson, J.T. Warmwater fish in wadeable streams. In Standard Methods for Sampling North American Freshwater Fishes; Bonar, S.A., Hubert, W.A., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 43–58. ISBN 978-1-934874-10-3. [Google Scholar]
- Price, A.L.; Peterson, J.T. Estimation and modeling of electrofishing capture efficiency for fishes in wadeable warmwater streams. N. Am. J. Fish. Manag. 2010, 30, 481–498. [Google Scholar] [CrossRef]
- Mahon, R. Accuracy of catch-effort methods for estimating fish density and biomass in streams. Environ. Biol. Fishes 1980, 5, 343–360. [Google Scholar] [CrossRef]
- Wagner, M.D.; Schumann, D.A.; Smith, B.J. Gear effectiveness and size selectivity for five cryptic madtom species (Noturus spp.). J. Appl. Ichthyol. 2019, 35, 673–682. [Google Scholar] [CrossRef]
- Poos, M.S.; Mandrak, N.E.; McLaughlin, R.L. The effectiveness of two common sampling methods for assessing imperiled freshwater fishes. J. Fish Biol. 2007, 70, 691–708. [Google Scholar] [CrossRef]
- Reid, S.M.; Yunker, G.; Jones, N.E. Evaluation of single-pass backpack electrofishing for stream fish community monitoring. Fish. Manag. Ecol. 2009, 16, 1–9. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, I.; Rinaldo, A. Fractal River Networks: Chance and Self-Organization; Cambridge University Press: New York, NY, USA, 1997; ISBN 0-521-00405-5. [Google Scholar]
- Altermatt, F. Diversity in riverine metacommunities: A network perspective. Aquat. Ecol. 2013, 47, 365–377. [Google Scholar] [CrossRef]
- Hoeinghaus, D.J.; Winemiller, K.O.; Birnbaum, J.S. Local and regional determinants of stream fish assemblage structure: Inferences based on taxonomic vs. functional groups. J. Biogeogr. 2007, 34, 324–338. [Google Scholar] [CrossRef]
- Smith, S.C.F.; Meiners, S.J.; Hastings, R.P.; Thomas, T.; Columbo, R.E. Low-head dam impacts on habitat and the functional composition of fish communities. River Res. Appl. 2017, 33, 680–689. [Google Scholar] [CrossRef]
- Meador, M.R.; Carlisle, D.M. Quantifying tolerance indicator values for common stream fish species of the United States. Ecol. Indic. 2007, 7, 329–338. [Google Scholar] [CrossRef]
- Frimpong, E.A.; Angermeier, P.L. FishTraits: A database of ecological and life-history traits of freshwater fishes of the United States. Fisheries 2009, 34, 487–495. [Google Scholar] [CrossRef]
- Goldstein, R.M.; Simon, T.P. Toward a united definition of guild structure for feeding ecology of North American freshwater fishes. In Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities; Simon, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 123–202. ISBN 978-0849340079. [Google Scholar]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Troia, M.J.; McManamay, R.A. Biogeographic classification of streams using fish community- and trait-environment relationships. Divers. Distrib. 2019, 26, 108–125. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Clark, K.R.; Gorley, R.N. Primer v7: User Manual/Tutorial; Primer-E: Plymouth, UK, 2015. [Google Scholar]
- U.S. Army Corps of Engineers. A comprehensive summary report on streambank erosion control demonstration projects constructed in eight watersheds of the Yazoo River basin. Appendix F. In The Final Report to Congress: The Streambank Erosion Control Evaluation and Demonstration Act of 1974 Section 32, Public Law 93-251; U.S. Army Corps of Engineers: Washington, DC, USA, 1981. [Google Scholar]
- Leech, J.R.; Biedenharn, D.S. Sediment Management at the Watershed Level. Coastal and Hydraulics Engineering Technical Note ERDC/CHL CHETN-VII-12; US Army Corps of Engineers, Defense Technical Information Center: Vicksburg, MS, USA, 2012. [Google Scholar]
- Harding, J.S.; Benfield, E.F.; Bolstad, P.V.; Helfman, G.S.; Jones, E.B.D. III. Stream biodiversity: The ghost of land use past. Proc. Natl. Acad. Sci. USA 1998, 95, 14843–14847. [Google Scholar] [CrossRef] [PubMed]
- Shields, F.D.; Knight, S.S.; Cooper, C.M. Use of the index of biotic integrity to assess physical habitat degradation in warmwater streams. Hydrobiologia 1995, 312, 191–208. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates, and Fish, 2nd ed.; EPA 841-B-99-002; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999. [Google Scholar]
- Adams, S.B.; Warren, M.L. Recolonization by warmwater fishes and crayfishes after severe drought in Upper Coastal Plain hill streams. Trans. Am. Fish. Soc. 2005, 134, 1173–1192. [Google Scholar] [CrossRef]
- Hubbell, J.P.; Schaefer, J.F.; Flood, P.; Warren, M.L., Jr.; Sterling, K.A. Fragmentation alters ecological gradients and headwater fish assemblage composition relative to land use in a dendritic river system. Can. J. Fish. Aquat. Sci. 2020, 77, 1281–1291. [Google Scholar] [CrossRef]
- Aadland, L.P.; Koel, T.M.; Franzin, W.G.; Stewart, K.W.; Nelson, P. Changes in fish assemblage structure of the Red River of the North. In Historical Changes in Large River Fish Assemblages of the Americas; Symposium 45; Rinne, J.R., Hughes, R.M., Calamussa, B., Eds.; American Fisheries Society: Bethesda, MD, USA, 2005; pp. 293–321. ISBN 978-1888569728. [Google Scholar]
- Agostinho, A.A.; Pelicice, F.M.; Gomes, L.C. Dams and the fish fauna of the Neotropical region: Impacts and management related to diversity and fisheries. Braz. J. Biol. 2008, 68, 1119–1132. [Google Scholar] [CrossRef]
- Cooney, P.B.; Kwak, T.J. Spatial extent and dynamics of dam impacts on tropical island freshwater fish assemblages. Bioscience 2013, 63, 176–190. [Google Scholar] [CrossRef]
- Agostinho, A.A.; Gomes, L.C.; Santos, N.L.C.; Ortega, J.C.G.; Pelicice, F.M. Fish assemblages in Neotropical reservoirs: Colonization patterns, impacts and management. Fish. Res. 2016, 173, 26–36. [Google Scholar] [CrossRef]
- Salmaso, N.; Naselli-Flores, L.; Padisák, J. Functional classifications and their application in phytoplankton ecology. Freshw. Biol. 2015, 60, 603–619. [Google Scholar] [CrossRef]

| Scientific Name | Common Name | Upstream Sites | Downstream Sites | Percentage of Catch | Habitat Preference | Trophic Guild | Tolerance |
|---|---|---|---|---|---|---|---|
| Ichthyomyzon castaneus | Chestnut Lamprey | 1 | 0 | 0.18 | lotic | parasite | intolerant |
| Atractosteus spatula | Alligator Gar | 1 | 0 | 0.02 | lentic | piscivore | tolerant |
| Lepisosteus oculatus | Spotted Gar | 1 | 4 | 0.27 | lentic | piscivore | tolerant |
| Dorosoma cepedianum | Gizzard Shad | 2 | 0 | 0.31 | generalist | herbivore | tolerant |
| Dorosoma petenense | Threadfin Shad | 2 | 0 | 0.47 | generalist | planktivore | tolerant |
| Campostoma anomalum | Central Stoneroller | 0 | 2 | 0.07 | lotic | herbivore | moderate |
| Cyprinella camura | Bluntface Shiner | 6 | 10 | 11.02 | lotic | invertivore | moderate |
| Cyprinella lutrensis | Red Shiner | 1 | 2 | 1.36 | generalist | invertivore | tolerant |
| Cyprinella venusta | Blacktail Shiner | 9 | 9 | 9.44 | lotic | invertivore | moderate |
| Cyprinus carpio | Common Carp | 2 | 0 | 0.04 | generalist | detritivore | tolerant |
| Luxilus chrysocephalus | Striped Shiner | 2 | 4 | 2.68 | lotic | invertivore | moderate |
| Lythrurus fumeus | Ribbon Shiner | 0 | 1 | 0.02 | lotic | invertivore | intolerant |
| Notemigonus crysoleucas | Golden Shiner | 3 | 0 | 0.09 | generalist | invertivore | moderate |
| Notropis ammophilus | Orangefin Shiner | 2 | 3 | 0.94 | lotic | invertivore | intolerant |
| Notropis atherinoides | Emerald Shiner | 4 | 0 | 2.05 | generalist | planktivore | tolerant |
| Notropis buchanani | Ghost Shiner | 1 | 0 | 0.29 | generalist | invertivore | tolerant |
| Notropis rafinesquei | Yazoo Shiner | 1 | 3 | 4.60 | lotic | invertivore | intolerant |
| Opsopoeodus emiliae | Pugnose Minnow | 1 | 0 | 0.04 | generalist | detritivore | moderate |
| Pimephales notatus | Bluntnose Minnow | 6 | 9 | 4.13 | generalist | detritivore | tolerant |
| Pimephales vigilax | Bullhead Minnow | 3 | 5 | 0.47 | generalist | invertivore | tolerant |
| Semotilus atromaculatus | Creek Chub | 8 | 8 | 5.38 | lotic | invertivore | tolerant |
| Erimyzon oblongus | Creek Chubsucker | 3 | 4 | 0.76 | lotic | invertivore | moderate |
| Ictiobus bubalus * | Smallmouth Buffalo | 0 | 4 | 0.20 | generalist | invertivore | tolerant |
| Moxostoma erythrurum | Golden Redhorse | 1 | 0 | 0.02 | generalist | invertivore | tolerant |
| Moxostoma poecilurum | Blacktail Redhorse | 0 | 5 | 0.31 | lotic | detritivore | intolerant |
| Ameiurus melas | Black Bullhead | 1 | 0 | 0.13 | generalist | invertivore | tolerant |
| Ameiurus natalis | Yellow Bullhead | 9 | 11 | 4.39 | lotic | invertivore | tolerant |
| Ameiurus nebulosus | Brown Bullhead | 0 | 1 | 0.02 | lentic | invertivore | tolerant |
| Ictalurus punctatus | Channel Catfish | 1 | 7 | 1.61 | generalist | piscivore | tolerant |
| Noturus gyrinus | Tadpole Madtom | 2 | 0 | 0.04 | lotic | invertivore | tolerant |
| Noturus hildebrandi | Least Madtom | 1 | 0 | 0.09 | lotic | invertivore | intolerant |
| Noturus nocturnus | Freckled Madtom | 2 | 0 | 0.20 | lotic | invertivore | tolerant |
| Pylodictis olivaris | Flathead Catfish | 0 | 2 | 0.13 | lotic | piscivore | tolerant |
| Esox americanus | Redfin Pickerel | 1 | 0 | 0.02 | lentic | piscivore | moderate |
| Aphredoderus sayanus | Pirate Perch | 4 | 0 | 1.03 | lentic | invertivore | moderate |
| Labidesthes sicculus | Brook Silverside | 1 | 1 | 0.42 | lentic | planktivore | tolerant |
| Fundulus chrysotus | Golden Topminnow | 1 | 1 | 0.33 | lentic | invertivore | moderate |
| Fundulus notatus | Blackstripe Topminnow | 1 | 7 | 1.96 | lotic | invertivore | tolerant |
| Fundulus olivaceus | Blackspotted Topminnow | 10 | 10 | 4.08 | lotic | invertivore | moderate |
| Gambusia affinis | Western Mosquitofish | 4 | 3 | 0.85 | generalist | invertivore | tolerant |
| Morone chrysops * | White Bass | 1 | 0 | 0.02 | generalist | piscivore | tolerant |
| Lepomis cyanellus | Green Sunfish | 12 | 11 | 11.40 | generalist | invertivore | tolerant |
| Lepomis gulosus | Warmouth | 4 | 1 | 0.18 | generalist | invertivore | tolerant |
| Lepomis macrochirus | Bluegill | 12 | 12 | 12.65 | generalist | invertivore | tolerant |
| Lepomis megalotis | Longear Sunfish | 4 | 10 | 8.48 | generalist | invertivore | tolerant |
| Lepomis miniatus | Redspotted Sunfish | 1 | 1 | 0.04 | generalist | invertivore | moderate |
| Micropterus punctulatus | Spotted Bass | 2 | 6 | 0.65 | generalist | piscivore | tolerant |
| Micropterus salmoides | Largemouth Bass | 6 | 9 | 3.86 | generalist | piscivore | tolerant |
| Pomoxis annularis | White Crappie | 2 | 0 | 0.11 | lentic | piscivore | tolerant |
| Etheostoma artesiae | Redspotted Darter | 1 | 2 | 0.11 | lotic | invertivore | moderate |
| Etheostoma histrio | Harlequin Darter | 1 | 0 | 0.04 | lotic | invertivore | intolerant |
| Etheostoma lynceum | Brighteye Darter | 0 | 2 | 0.13 | lotic | invertivore | intolerant |
| Etheostoma parvipinne | Goldstripe Darter | 1 | 1 | 0.07 | lotic | invertivore | intolerant |
| Etheostoma swaini | Gulf Darter | 1 | 1 | 0.13 | lotic | invertivore | intolerant |
| Percina caprodes | Logperch | 0 | 1 | 0.02 | lotic | invertivore | moderate |
| Percina maculata | Blackside Darter | 2 | 4 | 0.25 | lotic | invertivore | tolerant |
| Percina sciera | River Darter | 2 | 6 | 0.76 | lotic | invertivore | moderate |
| Aplodinotus grunniens | Freshwater Drum | 3 | 3 | 0.62 | generalist | piscivore | tolerant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faucheux, N.M.; Miranda, L.E.; Taylor, J.M.; Farris, J. Impact of Dams on Stream Fish Diversity: A Different Result. Diversity 2023, 15, 728. https://doi.org/10.3390/d15060728
Faucheux NM, Miranda LE, Taylor JM, Farris J. Impact of Dams on Stream Fish Diversity: A Different Result. Diversity. 2023; 15(6):728. https://doi.org/10.3390/d15060728
Chicago/Turabian StyleFaucheux, Nicky M., Leandro E. Miranda, Jason M. Taylor, and Jerry Farris. 2023. "Impact of Dams on Stream Fish Diversity: A Different Result" Diversity 15, no. 6: 728. https://doi.org/10.3390/d15060728
APA StyleFaucheux, N. M., Miranda, L. E., Taylor, J. M., & Farris, J. (2023). Impact of Dams on Stream Fish Diversity: A Different Result. Diversity, 15(6), 728. https://doi.org/10.3390/d15060728






