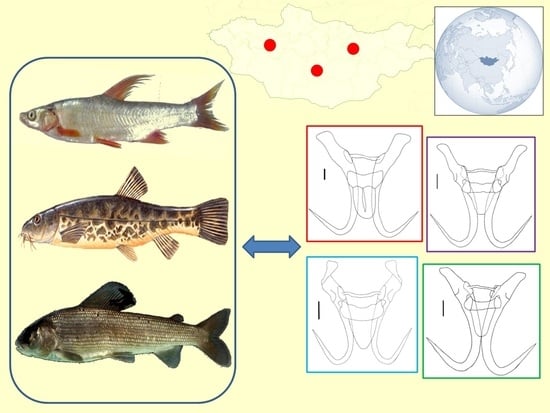
Survivors from a Pliocene Climatic Catastrophe: Gyrodactylus (Platyhelminthes, Monogenea) Parasites of the Relict Fishes in the Central Asian Internal Drainage Basin of Mongolia
Abstract
1. Introduction
2. Material and Methods
2.1. Fish and Parasite Sampling
2.2. Morphological Methods
2.3. Molecular Analysis
3. Results
4. Discussion
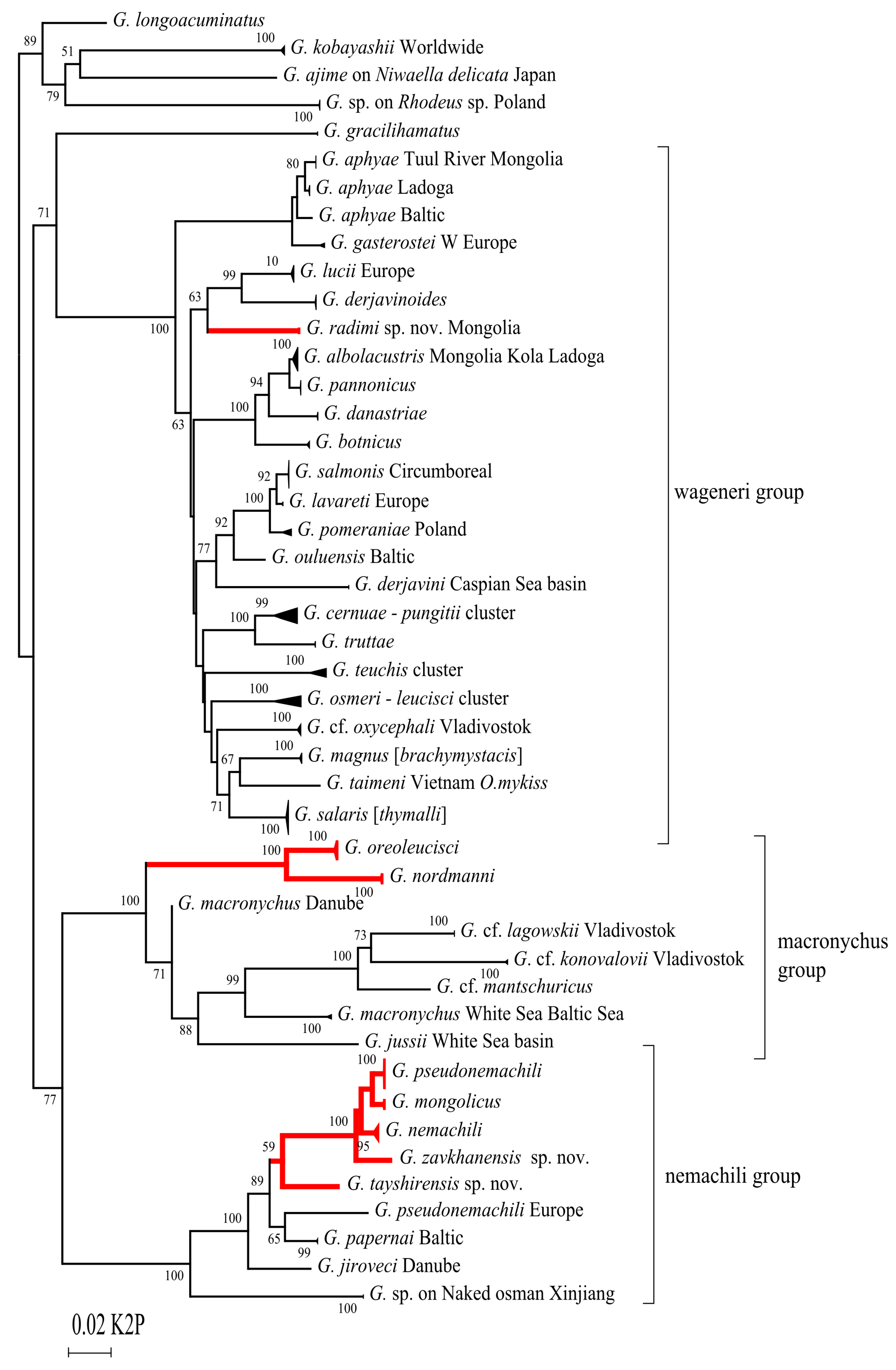
4.1. The Two Freshwater Subgenera of Gyrodactylus Were Defined by Malmberg 1970
4.2. The Faunal Poverty of The Hollow
4.3. Endemic Diversification
4.4. Host Specificity Lost in Hard Times in the Compressed Ecosystem
“[I]t is most difficult to explain the occurrence of G. mongolicus on the host Oreoleuciscus humilis, O. pewzowi and O. potanini, because this parasite is a typical member of the morphological group of the species G. nemachili, comprising parasites which are characteristic of several Cobitidae (sic) (Nemachilus, Lefua). It may only be suggested that some of these parasites started gradually to exchange their hosts living under the same ecological conditions and that this process led finally to a definitive adaptation to the new host both physiologically and morphologically. Never, however, did not find G. mongolicus on any species of the genus Nemachilus or possibly on any other genus of Cobitidae (sic).”
4.5. A Conclusive Endnote
“Clearly, the best guesses are not at all scientific results but can be considered as scientific hypotheses”. Kottelat in “Fishes of Mongolia”[10].
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dulmaa, A. Fish and fisheries in Mongolia. Fish and Fisheries at Higher Altitudes: Asia; FAO Fisheries Technical Paper No. 385; FAO: Rome, Italy, 1999; Volume 385. [Google Scholar]
- Sytchevskaya, K.E. History of the formation of the ichthyofauna of Mongolia and the problem of faunistic complexes. In Fishes of the Mongolian People’s Republic; Nauka: Moscow, Russia, 1985; pp. 225–249. (In Russian) [Google Scholar]
- Sytchevskaya, E.K. Freshwater Palaeogene Ichthyofauna of the Soviet Union and Mongolia; Nauka: Moscow, Russia, 1986. (In Russian) [Google Scholar]
- Sytchevskaya, E.K. Presnovodnaya Ikhtiofauna Neogena Mongolii; Nauka: Moscow, Russia, 1989. (In Russian) [Google Scholar]
- Dgebuadze, Y.; Mendsaikhan, B.; Dulmaa, A. Erforschung biologischer Ressourcen der Mongolei/Exploration into the Biological Resources of Mongolia, ISSN 0440-1298. In Diversity and Distribution of Mongolian Fish: Recent State, Trends and Studies; University of Nebraska: Lincoln, NE, USA, 2012; Volume 23, pp. 219–230. Available online: http://digitalcommons.unl.edu/biolmongol/23 (accessed on 1 January 2023).
- Slynko, Y.V.; Borovikova, E.A. Phylogeography of Altai Osmans Fishes (Oreoleuciscus sp.; Cyprinidae, Pisces) inferred from nucleotide variation of the mitochondrial DNA Cytochrome b gene. Russ. J. Genet. 2012, 48, 618–627. [Google Scholar] [CrossRef]
- Kartavtsev, Y.P.; Batischeva, N.M.; Bogutskaya, N.G.; Katugina, A.O.; Hanzawa, N. Molecular systematics and DNA barcoding of Altai osmans, Oreoleuciscus (Pisces, Cyprinidae, and Leuciscinae), and their nearest relatives, inferred from sequences of cytochrome b (Cyt-b), cytochrome oxidase c (Co-1), and complete mitochondrial genome. Mitochondrial DNA Part A 2017, 28, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Grimm, J.; Gonçalves, D.V.; Secci-Petretto, G.; Englmaier, G.K.; Baimukanov, M.; Froufe, E. Comparative genetic analysis of grayling (Thymallus spp. Salmonidae) across the paleohydrologically dynamic river drainages of the Altai-Sayan mountain region. Hydrobiologia 2020, 847, 2823–2844. [Google Scholar] [CrossRef]
- Weiss, S.J.; Gonçalves, D.V.; Secci-Petretto, G.; Englmaier, G.K.; Gomes-Dos-Santos, A.; Denys, G.P.; Froufe, E. Global systematic diversity, range distributions, conservation and taxonomic assessments of graylings (Teleostei: Salmonidae; Thymallus spp.). Org. Divers. Evol. 2021, 21, 25–42. [Google Scholar] [CrossRef]
- Kottelat, M. Fishes of Mongolia. A Check-List of the Fishes Known to Occur in Mongolia with Comments on Systematics and Nomenclature; The International Bank for Reconstruction and Development; The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Kottelat, M. Conspectus cobitidum: An inventory of the loaches of the world (Teleostei: Cypriniformes: Cobitoidei). Raffles Bull. Zool. 2012, (Suppl. 26), 1–199. [Google Scholar]
- Prokofiev, A.M. Loaches of the genus Barbatula (Nemacheilinae) of the Zavkhan River basin (Western Mongolia). J. Ichthyol. 2016, 56, 818–831. [Google Scholar] [CrossRef]
- Ergens, R. Two new species of Gyrodactylus (Monogenoidea) from Mongolian Brachymystaxlenok (Pallas). Folia Parasitol. 1978, 25, 79–81. [Google Scholar]
- Ergens, R. Rediscription of Gyrodactylus latus Bychowsky, 1933 and other two new species of the genus Gyrodactylus from fishes of the genus Cobitis, L. Folia Parasitol. 1978, 25, 333–337. [Google Scholar]
- Ergens, R. Six new species of Gyrodactylus from freshwater fishes of the Palearctic region. Folia Parasitol. 1980, 27, 303–307. [Google Scholar]
- Ergens, R. Gyrodactylus from Eurasian freshwater Salmonidae and Thymallidae. Folia Parasitol. 1983, 30, 15–26. [Google Scholar]
- Ergens, R.; Dulmaa, A. Monogenoidea from genus Phoxinus from Mongolia. Folia Parasitol. 1967, 14, 321–333. [Google Scholar]
- Ergens, R.; Dulmaa, A. Monogenoidea from fishes of the genus Oreoleuciscus (Cyprinidae) from Mongolia. Folia Parasitol. 1970, 17, 1–11. [Google Scholar]
- Ergens, R.; Dulmaa, A. Monogenoidea in Cobitis taenia sibirica from Mongolia. Folia Parasitol. 1968, 15, 317–321. [Google Scholar]
- Ergens, R.; Dulmaa, A. Monogenoidea from Cyprinus carpio haematopterus and Carassius auratus gibelio (Cyprinidae) from Mongolia. Folia Parasitol. 1969, 16, 201–206. [Google Scholar]
- Ergens, R. Gyrodactylus konovalovi sp.n. (Gyrodactylidae: Monogenoidea) from Phoxinus lagowskii Dubowski. Folia Parasitol. 1976, 23, 87–89. [Google Scholar]
- Gusev, A.V.; Bauer, O.N. Key to Parasites of Freshwater Fishes of the Fauna of the USSR; Nauka: Moscow, Russia, 1985; Volume 2. (In Russian) [Google Scholar]
- Pugachev, O.N.; Gerasev, P.I.; Gussev, A.V.; Ergens, R.; Khotenowsky, I. Guide to Monogenoidea of Freshwater Fish of Palaearctic and Amur Regions; Ledizione-Ledi Publishing: Milan, Italy, 2009. [Google Scholar]
- Ziętara, M.S.; Lumme, J. Speciation by host switch and adaptive radiation in a fish parasite genus Gyrodactylus (Monogenea: Gyrodactylidae). Evolution 2002, 56, 2445–2458. [Google Scholar] [CrossRef]
- Lumme, J.; Ziętara, M.S.; Lebedeva, D. Ancient and modern genome shuffling: Reticulate mito-nuclear phylogeny of four related allopatric species of Gyrodactylus von Nordmann, 1832 (Monogenea: Gyrodactylidae), ectoparasites on the Eurasian minnow Phoxinus phoxinus (L.) (Cyprinidae). Syst. Parasitol. 2017, 94, 183–200. [Google Scholar] [CrossRef]
- Ziętara, M.S.; Huyse, T.; Lumme, J.; Volckaert, F.A. Deep divergence among subgenera of Gyrodactylus inferred from rDNA ITS region. Parasitology 2002, 124, 39–52. [Google Scholar] [CrossRef]
- Ziętara, M.S.; Kuusela, J.; Veselov, A.; Lumme, J. Molecular faunistics of accidental infections of Gyrodactylus Nordmann, 1832 (Monogenea) parasitic on salmon Salmo salar L. and brown trout Salmo trutta L. in NW Russia. Syst. Parasitol. 2008, 69, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Matĕjusová, I.; Gelnar, M.; McBeath, A.J.; Collins, C.M.; Cunningham, C.O. Molecular markers for gyrodactylids (Gyrodactylidae: Monogenea) from five fish families (Teleostei). Int. J. Parasitol. 2001, 31, 738–745. [Google Scholar] [CrossRef]
- Matejusová, I.; Gelnar, M.; Verneau, O.; Cunningham, C.O.; Littlewood, D.T.J. Molecular phylogenetic analysis of the genus Gyrodactylus (Platyhelminthes: Monogenea) inferred from rDNA ITS region: Subgenera versus species groups. Parasitology 2003, 127, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Přikrylová, I.; Matĕjusová, I.; Jarkovský, J.; Gelnar, M. Morphometric comparison of three members of the Gyrodactylus nemachili-like species group (Monogenea: Gyrodactylidae) on Barbatulabarbatula L. in the Czech Republic, with a reinstatement of G. papernai Ergens&Bychowsky, 1967. Syst. Parasitol. 2008, 69, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Přikrylová, I.; Blažek, R.; Vanhove, M.P. An overview of the Gyrodactylus (Monogenea: Gyrodactylidae) species parasitizing African catfishes, and their morphological and molecular diversity. Parasitol. Res. 2012, 110, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Ziętara, M.; Rokicka, M.; Stojanovski, S.; Lumme, J. Introgression of distant mitochondria into the genome of Gyrodactylus salaris: Nuclear and mitochondrial markers are necessary to identify parasite strains. ActaParasitologica 2010, 55, 20–28. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Hancock, J.M.; Tautz, D.; Dover, G.A. Evolution of the secondary structures and compensatory mutations of the ribosomal RNAs of Drosophila melanogaster. Mol. Biol. Evol. 1988, 5, 393–414. [Google Scholar] [CrossRef]
- Dixon, M.T.; Hillis, D.M. Ribosomal RNA secondary structure: Compensatory mutations and implications for phylogenetic analysis. Mol. Biol. Evol. 1993, 10, 256–267. [Google Scholar] [CrossRef]
- Coleman, A.W. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Res. 2007, 35, 3322–3329. [Google Scholar] [CrossRef]
- Gundrizer, A.N. On the study of the parasite fauna of fish in Tuva. In New Data on the Nature of Siberia; Tomsk State University: Tomsk, Russia, 1981; pp. 43–55. (In Russian) [Google Scholar]
- Leis, E.; King, S.; Leis, S.; Cone, D. Infections of Gyrodactylus crysoleucas and Gyrodactylus sp. (Monogenea) at a Golden Shiner (Notemigonus crysoleucas) Farm in Minnesota. Comp. Parasitol. 2016, 83, 105–110. [Google Scholar] [CrossRef]
- Leis, E.; Chi, T.K.; Lumme, J. Global phylogeography of salmonid ectoparasites of the genus Gyrodactylus, with an emphasis on the origin of the circumpolar Gyrodactylus salmonis (Platyhelminthes; Monogenea). Comp. Parasitol. 2021, 88, 130–143. [Google Scholar] [CrossRef]
- Nitta, M. A new monogenean species, Gyrodactylus ajime n. sp. (Gyrodactylidae), parasitic on Niwaella delicata (Niwa), an endemic loach of Japan. Syst. Parasitol. 2021, 98, 307–319. [Google Scholar] [CrossRef]
- Ergens, R. Dactylogyridae and Gyrodactylidae (Monogenoidea) from some fishes from Mongolia. Folia Parasitol. 1971, 18, 241–254. [Google Scholar]
- Hansen, H.; Bakke, T.A.; Bachmann, L. Mitochondrial haplotype diversity of Gyrodactylus thymalli (Platyhelminthes, Monogenea): Extended geographic sampling in United Kingdom, Poland and Norway reveals further lineages. Parasitol. Res. 2007, 100, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Ergens, R.; Bykhovsky, B.E. Revision of the species Gyrodactylus nemachili Bychowsky, 1936 (Monogenoidea). Folia Parasitol. 1967, 14, 225–238. [Google Scholar]
- Pugachev, O.N. Checklist of the Freshwater Fish Parasites of Northern Asia. Cnidaria, Monogenoidea, Cestoda; Zoological Institute RAS: Saint Petersburg, Russia, 2002. (In Russian) [Google Scholar]
- Paranlejamts, J. Helminths and Other Groups of Fish Parasites of Mongolia (Fauna, Ecological-Faunistic Characteristic, Zoogeography). Ph.D. Thesis, Abstract: All-Russian Scientific Research Institute of Helminthology after K.I. Skrjabin, Moscow, Russia, 1993. (In Russian). [Google Scholar]
- Boeger, W.A.; Kritsky, D.C.; Patella, L.; Bueno-Silva, M. Phylogenetic status and historical origins of the oviparous and viviparous gyrodactylids (Monogenoidea, Gyrodactylidea). ZoologicaScripta 2021, 50, 112–124. [Google Scholar] [CrossRef]
- Reyda, F.B.; Wells, S.M.; Ermolenko, A.V.; Ziętara, M.S.; Lumme, J.I. Global parasite trafficking: Asian Gyrodactylus (Monogenea) arrived to the USA via invasive fish Misgurnus anguillicaudatus as a threat to amphibians. Biol. Invasions 2020, 22, 391–402. [Google Scholar] [CrossRef]
- Ergens, R. Monogenoidea from fishes of the genus Neomacheilus (Cobitidae) from Mongolia. Folia Parasitol. 1970, 17, 285–290. [Google Scholar]
- Ermolenko, A.V. Parasites of Fish in Freshwater Reservoirs of the Continental Part of the Sea of Japan Basin; Far East branch of RAS: Vladivostok, Russia, 1992. (In Russian) [Google Scholar]
- Zhao, X.; Hu, S.; Xie, P.; Ao, M.; Cai, L.; Niu, J.; Ma, X. The complete mitochondrial genome of Barbatula nuda (Cypriniformes: Nemacheilidae). Mitochondrial DNA 2015, 26, 692–693. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.; Chen, Y. The complete mitochondrial genome of Barbatula nuda and B. toni (Teleostei: Nemacheilidae). Mitochondrial DNA Part B 2019, 4, 2585–2587. [Google Scholar] [CrossRef]
- Akhmerov, A.K. New species of fish monogeneans of the Amur River. Parasitol. Collect. ZIN RAS 1952, 14, 181–212. (In Russian) [Google Scholar]
- Rumyantsev, E.A.; Ieshko, E.P. Parasites of Fishes of Water Bodies of Karelia: A Systematic Catalogue; KRC RAS: Petrozavodsk, Russia, 1997. (In Russian) [Google Scholar]
- Gundrizer, A.N. Studies of fishes of the Western Mongolian ichthyological province (within the USSR boundaries). In Siberian Aquatic Resources and Their Prospective Fishery Utilization; Ioganzen, B.G., Ed.; Tomsk University: Tomsk, Russia, 1973; pp. 77–78. (In Russian) [Google Scholar]
- Prokofiev, A.M. Morphology, Systematics and Origin of Whiskered Char of the Genus Orthrias (Teleostei: Balitoridae: Nemacheilinae); KMK Scientific Press: Moscow, Russia, 2007. (In Russian) [Google Scholar]
- Svendsen, J.I.; Alexanderson, H.; Astakhov, V.I.; Demidov, I.; Dowdeswell, J.A.; Funder, S.; Gataullin, V.; Henriksen, M.; Hjort, C.; Houmark-Nielsen, M.; et al. Late Quaternary ice sheet history of northern Eurasia. Quat. Sci. Rev. 2004, 23, 1229–1271. [Google Scholar] [CrossRef]
- Mangerud, J.; Jakobsson, M.; Alexanderson, H.; Astakhov, V.; Clarke, G.K.C. Ice-dammed lakes and rerouting of the drainage of northern Eurasia during the Last Glaciation. Quat. Sci. Rev. 2004, 23, 1313–1332. [Google Scholar] [CrossRef]
- Hughes, A.L.; Gyllencreutz, R.; Lohne, Ø.S.; Mangerud, J.; Svendsen, J.I. The last Eurasian ice sheets–a chronological database and time-slice reconstruction, DATED-1. Boreas 2016, 45, 1–45. [Google Scholar] [CrossRef]
- Vucić, M.; Jelić, D.; Žutinić, P.; Grandjean, F.; Jelić, M. Distribution of Eurasian minnows (Phoxinus: Cypriniformes) in the Western Balkans. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 11. [Google Scholar] [CrossRef]
- Lebedeva, D.I.; Chrisanfova, G.G.; Ieshko, E.P.; Guliaev, A.S.; Yakovleva, G.A.; Mendsaikhan, B.; Semyenova, S.K. Morphological and molecular differentiation of Diplostomum spp. metacercariae from brain of minnows (Phoxinus phoxinus L.) in four populations of Northern Europe and East Asia. Infect. Genet. Evol. 2021, 92, 104911. [Google Scholar] [CrossRef]
- Imoto, J.M.; Saitoh, K.; Sasaki, T.; Yonezawa, T.; Adachi, J.; Kartavtsev, Y.P.; Hanzawa, N. Phylogeny and biogeography of highly diverged freshwater fish species (Leuciscinae, Cyprinidae, Teleostei) inferred from mitochondrial genome analysis. Gene 2013, 514, 112–124. [Google Scholar] [CrossRef]
- Schönhuth, S.; Vukić, J.; Šanda, R.; Yang, L.; Mayden, R.L. Phylogenetic relationships and classification of the Holarctic family Leuciscidae (Cypriniformes: Cyprinoidei). Mol. Phylogenetics Evol. 2018, 127, 781–799. [Google Scholar] [CrossRef]
- Sakai, H.; Watanabe, K.; Goto, A. A revised generic taxonomy for Far East Asian minnow Rhynchocypris and dace Pseudaspius. Ichthyol. Res. 2020, 67, 330–334. [Google Scholar] [CrossRef]
- Froufe, E.; Alekseyev, S.; Knizhin, I.; Alexandrino, P.; Weiss, S. Comparative phylogeography of salmonid fishes (Salmonidae) reveals late to post-Pleistocene exchange between three now-disjunct river basins in Siberia. Divers. Distrib. 2003, 9, 269–282. [Google Scholar] [CrossRef]
- Koskinen, M.T.; Knizhin, I.; Primmer, C.R.; Schlötterer, C.; Weiss, S. Mitochondrial and nuclear DNA phylogeography of Thymallus spp. (grayling) provides evidence of ice-age mediated environmental perturbations in the world’s oldest body of freshwater, Lake Baikal. Mol. Ecol. 2002, 11, 2599–2611. [Google Scholar] [CrossRef]
- Perdices, A.; Bohlen, J.; Šlechtová, V.; Doadrio, I. Molecular Evidence for Multiple Origins of the European Spined Loaches (Teleostei, Cobitidae). PLoS ONE 2016, 11, e0144628. [Google Scholar] [CrossRef]
- Kontula, T.; Kirilchik, S.V.; Väinölä, R. Endemic diversification of the monophyletic cottoid fish species flock in Lake Baikal explored with mtDNAsequencing. Mol. Phylogenet. Evol. 2003, 27, 143–155. [Google Scholar] [CrossRef]
- Sember, A.; Bohlen, J.; Šlechtová, V.; Altmanová, M.; Symonová, R.; Ráb, P. Karyotype differentiation in 19 species of river loach fishes (Nemacheilidae, Teleostei): Extensive variability associated with rDNA and heterochromatin distribution and its phylogenetic and ecological interpretation. BMC Evol. Biol. 2015, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Fang, B.; Shikano, T.; Momigliano, P.; Wang, C.; Kravchenko, A.; Merilä, J. A phylogenomic perspective on diversity, hybridization and evolutionary affinities in the stickleback genus Pungitius. Mol. Ecol. 2019, 28, 4046–4064. [Google Scholar] [CrossRef] [PubMed]
- Skog, A.; Vøllestad, L.A.; Stenseth, N.C.; Kasumyan, A.; Jakobsen, K.S. Circumpolar phylogeography of the northern pike (Esoxlucius) and its relationship to the Amur pike (E. reichertii). Front. Zool. 2014, 11, 67. [Google Scholar] [CrossRef]
- Haponski, A.E.; Stepien, C.A. Phylogenetic and biogeographical relationships of the Sanderpikeperches (Percidae: Perciformes): Patterns across North America and Eurasia. Biol. J. Linn. Soc. 2013, 110, 156–179. [Google Scholar] [CrossRef]
- Stepien, C.A.; Haponski, A.E. Taxonomy, distribution, and evolution of the Percidae. In Biology and Culture of Percid Fishes; Kestemont, P., Dabrowski, K., Summerfelt, R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 3–60. [Google Scholar] [CrossRef]
- Van Houdt, J.K.J.; De Cleyn, L.; Perretti, A.; Volckaert, F.A.M. A mitogenic view on the evolutionary history of the Holarctic freshwater gadoid, burbot (Lota lota). Mol. Ecol. 2005, 14, 2445–2457. [Google Scholar] [CrossRef]
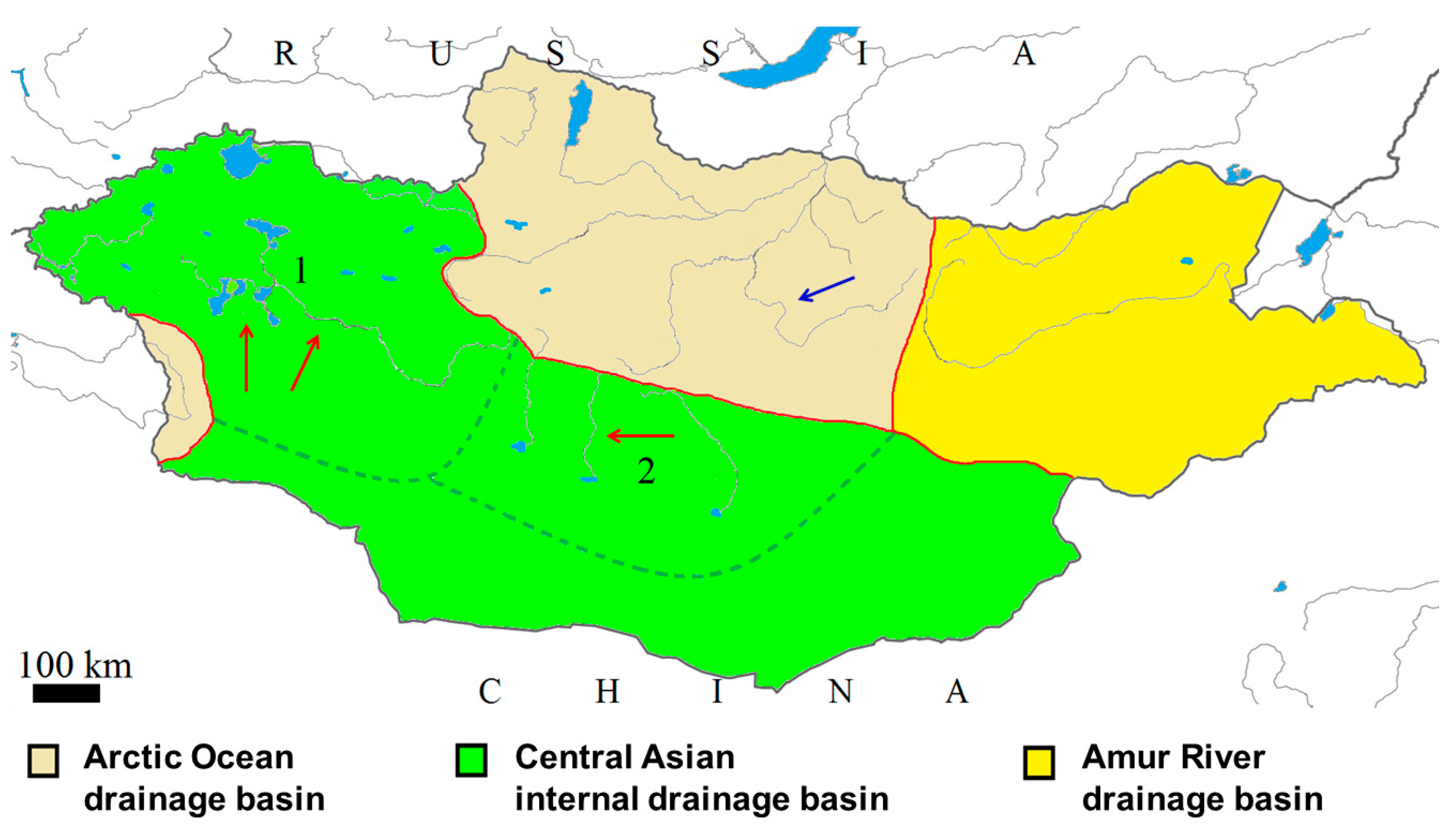
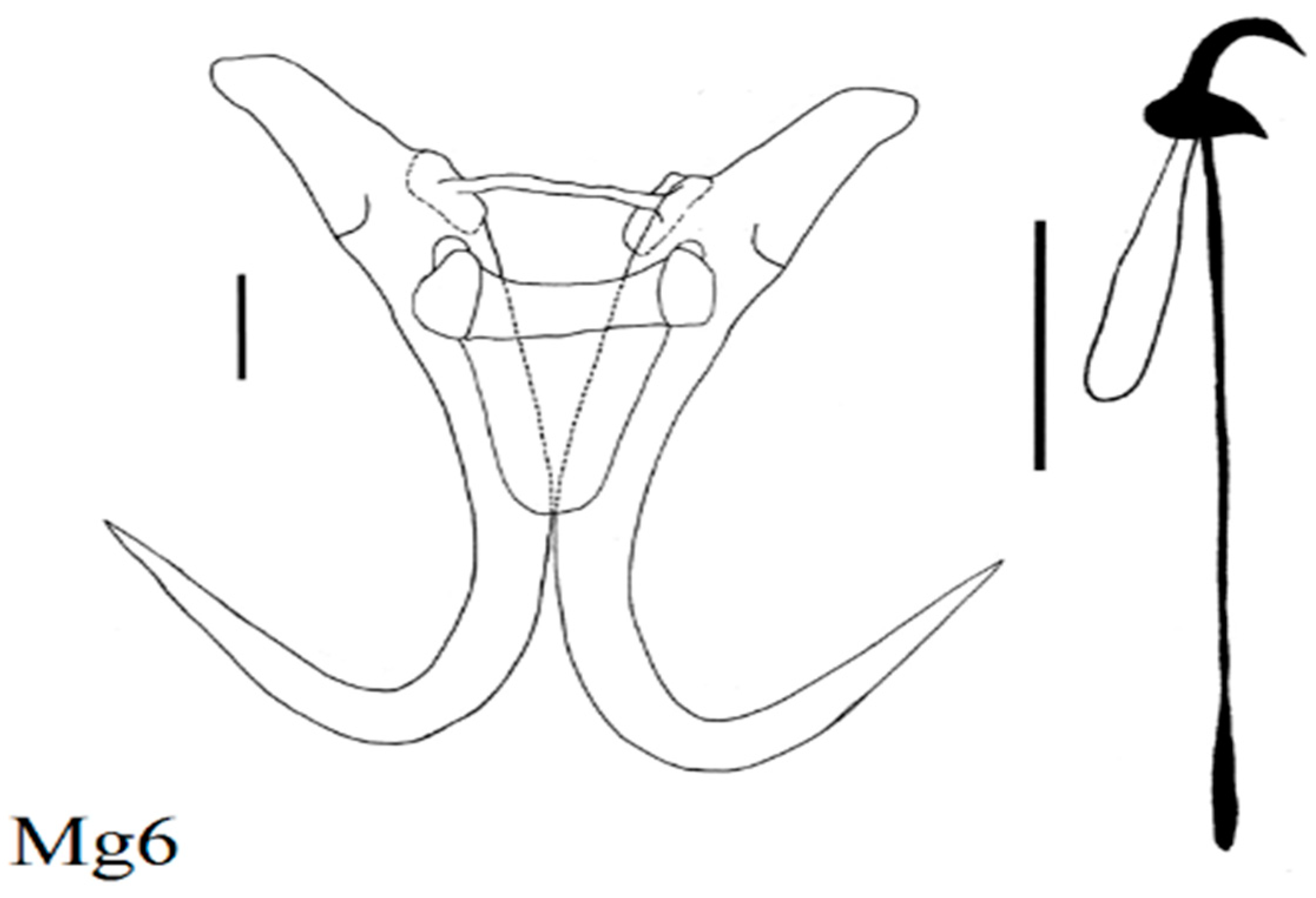
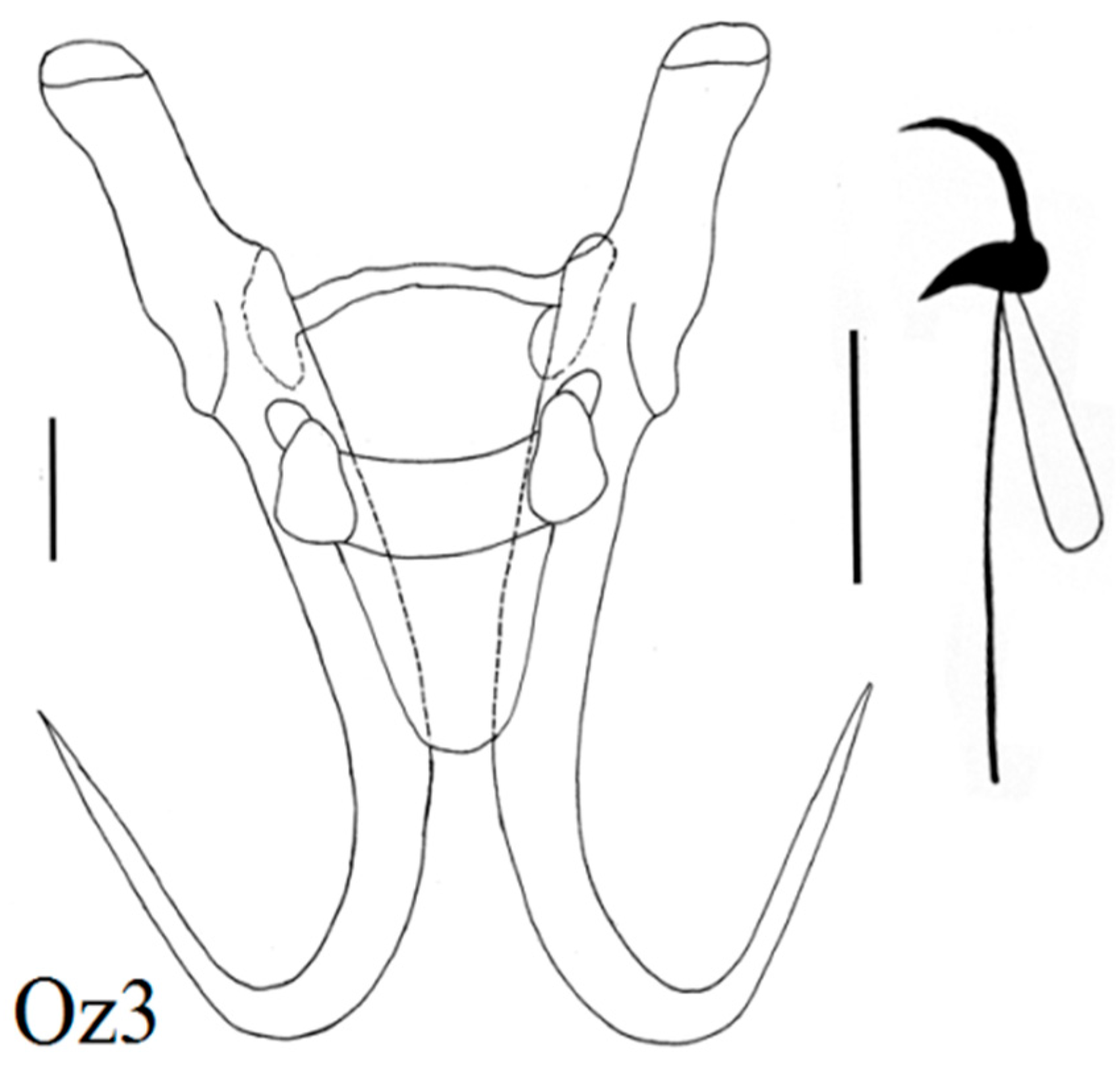

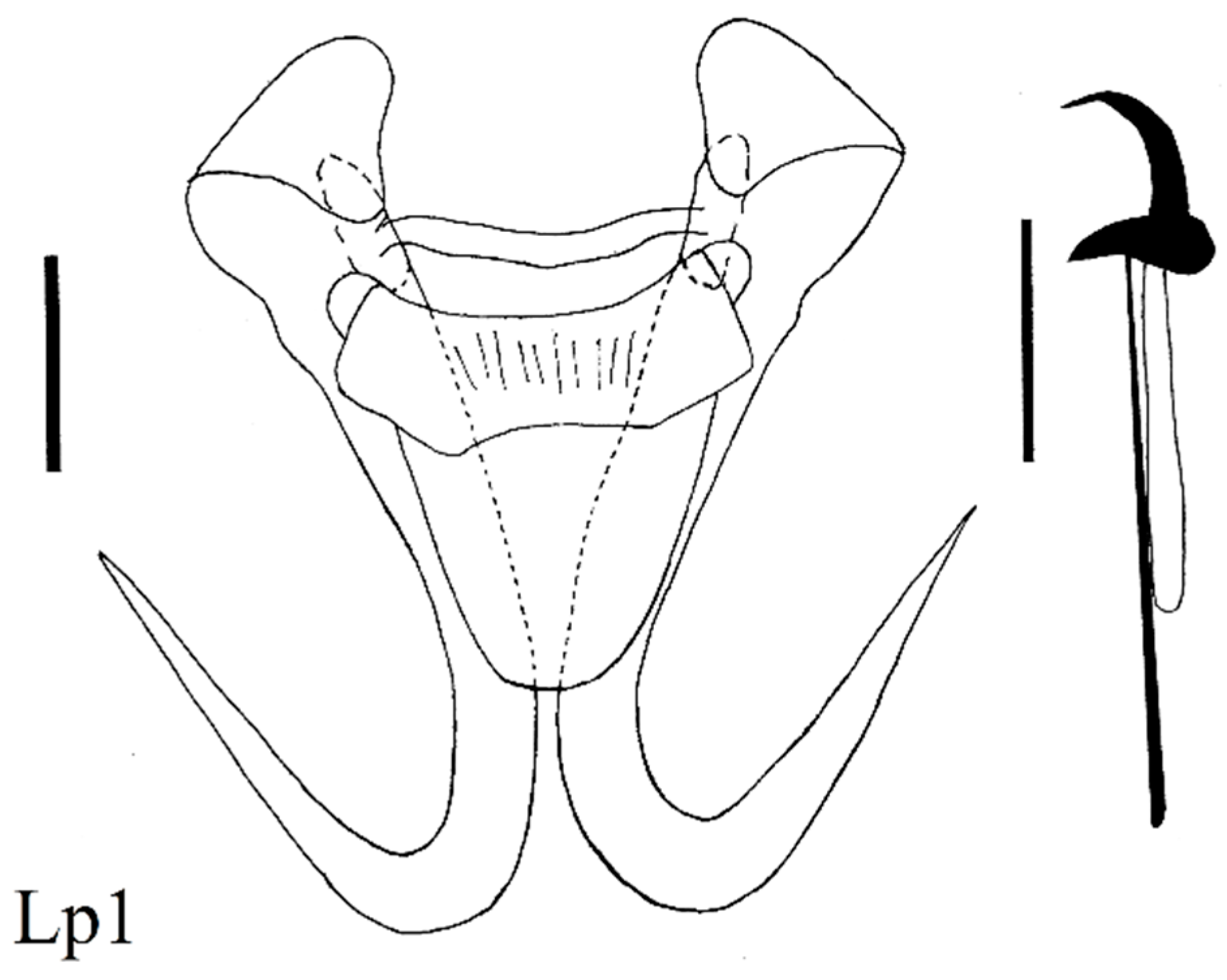
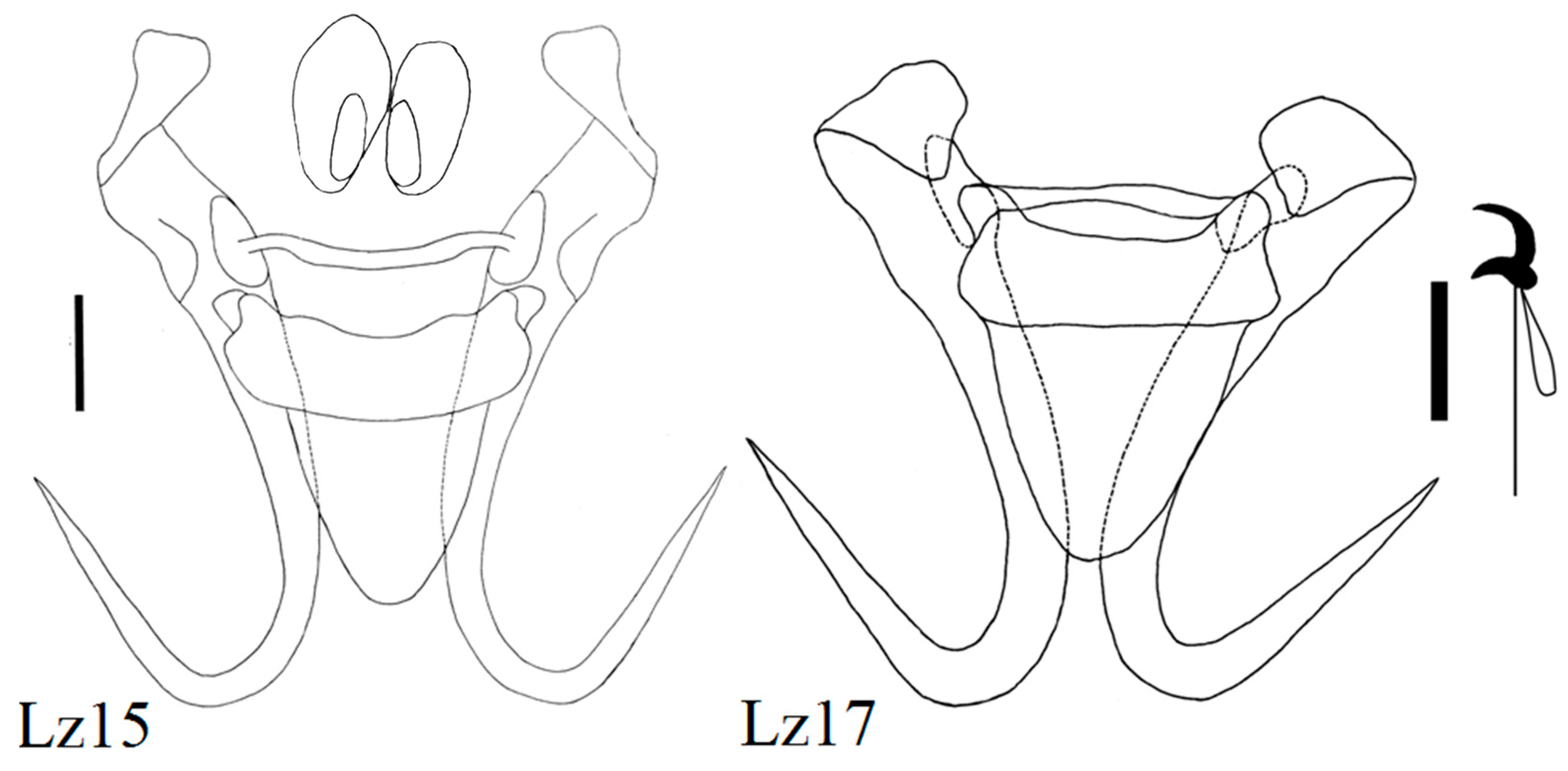
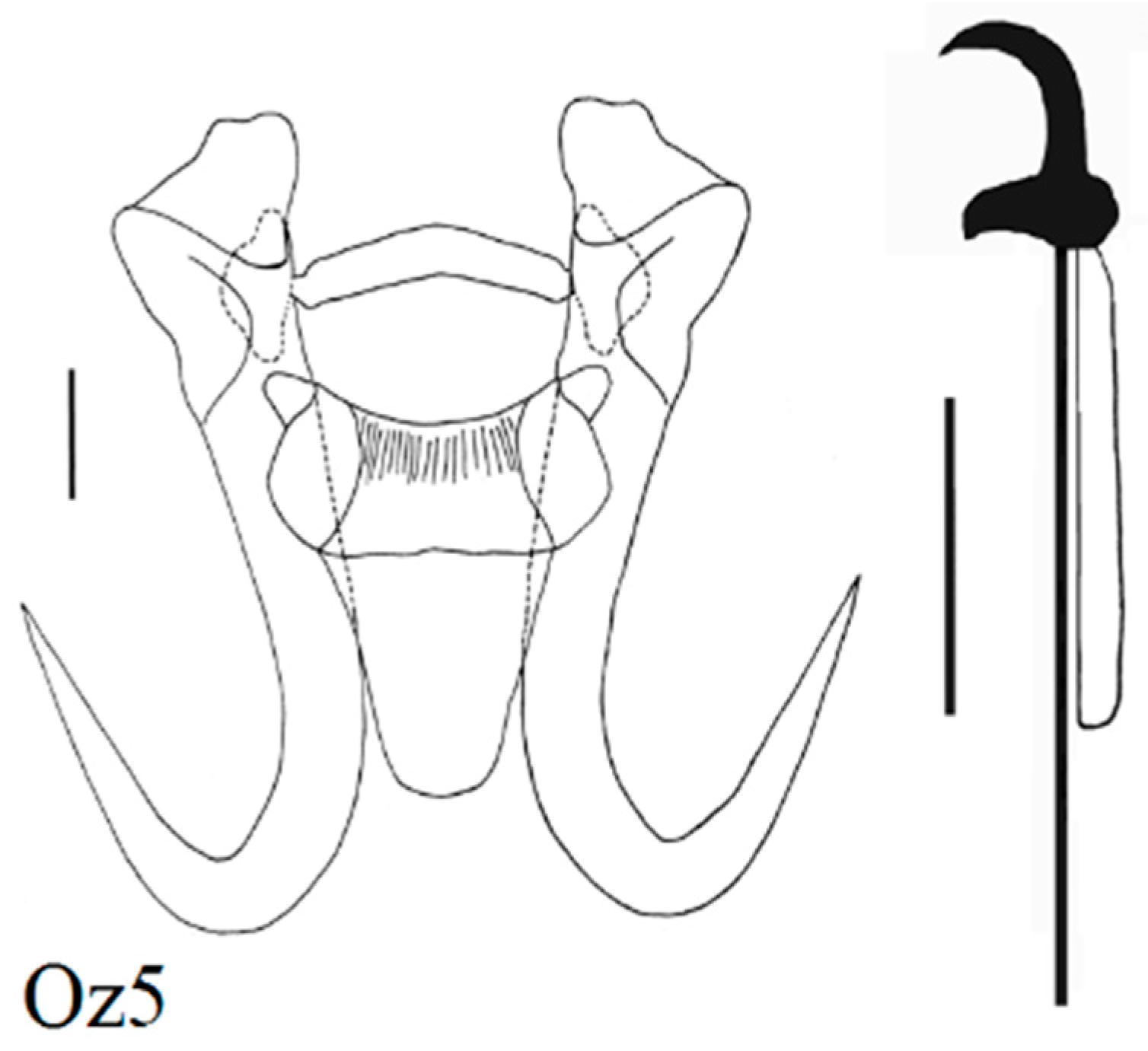


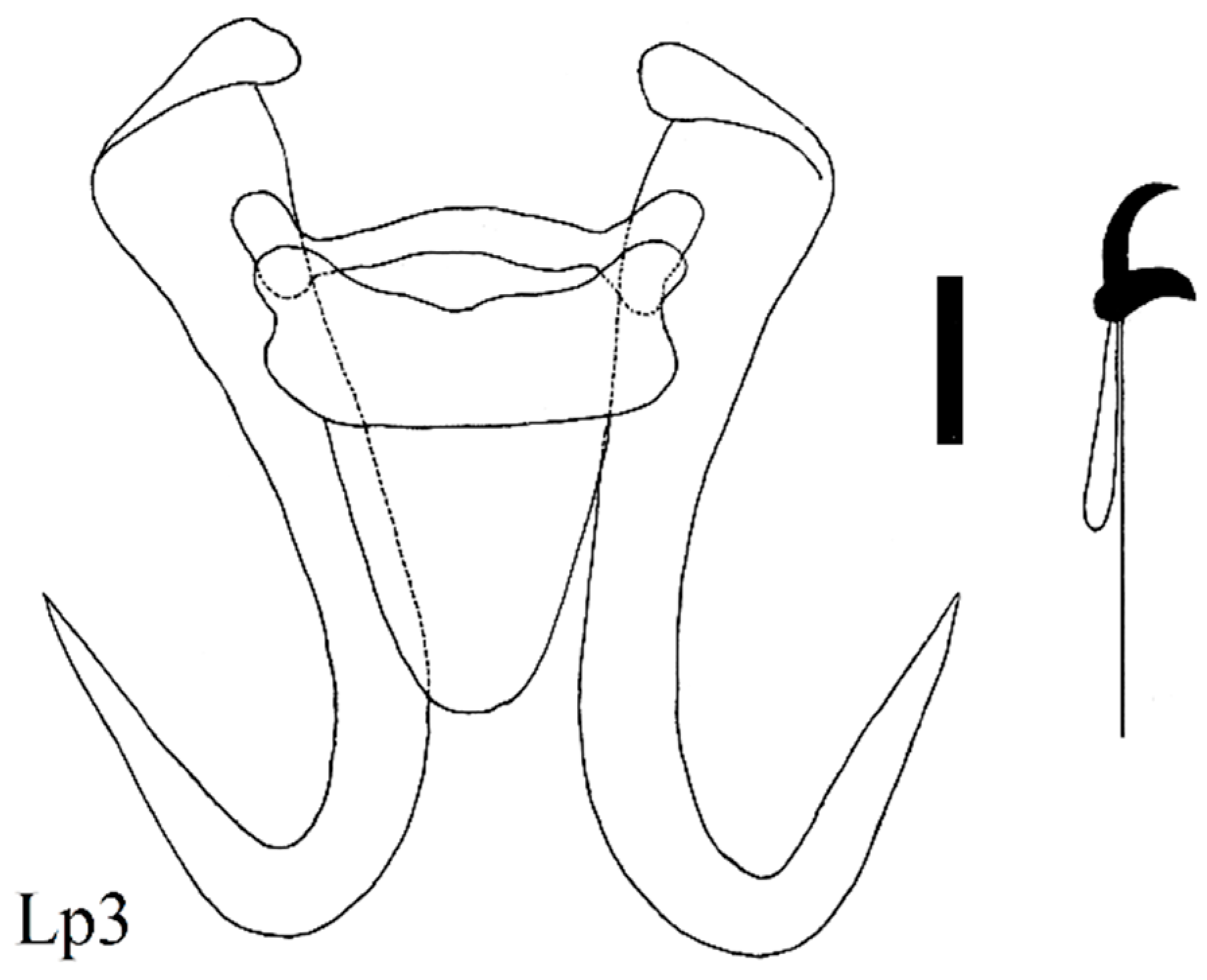
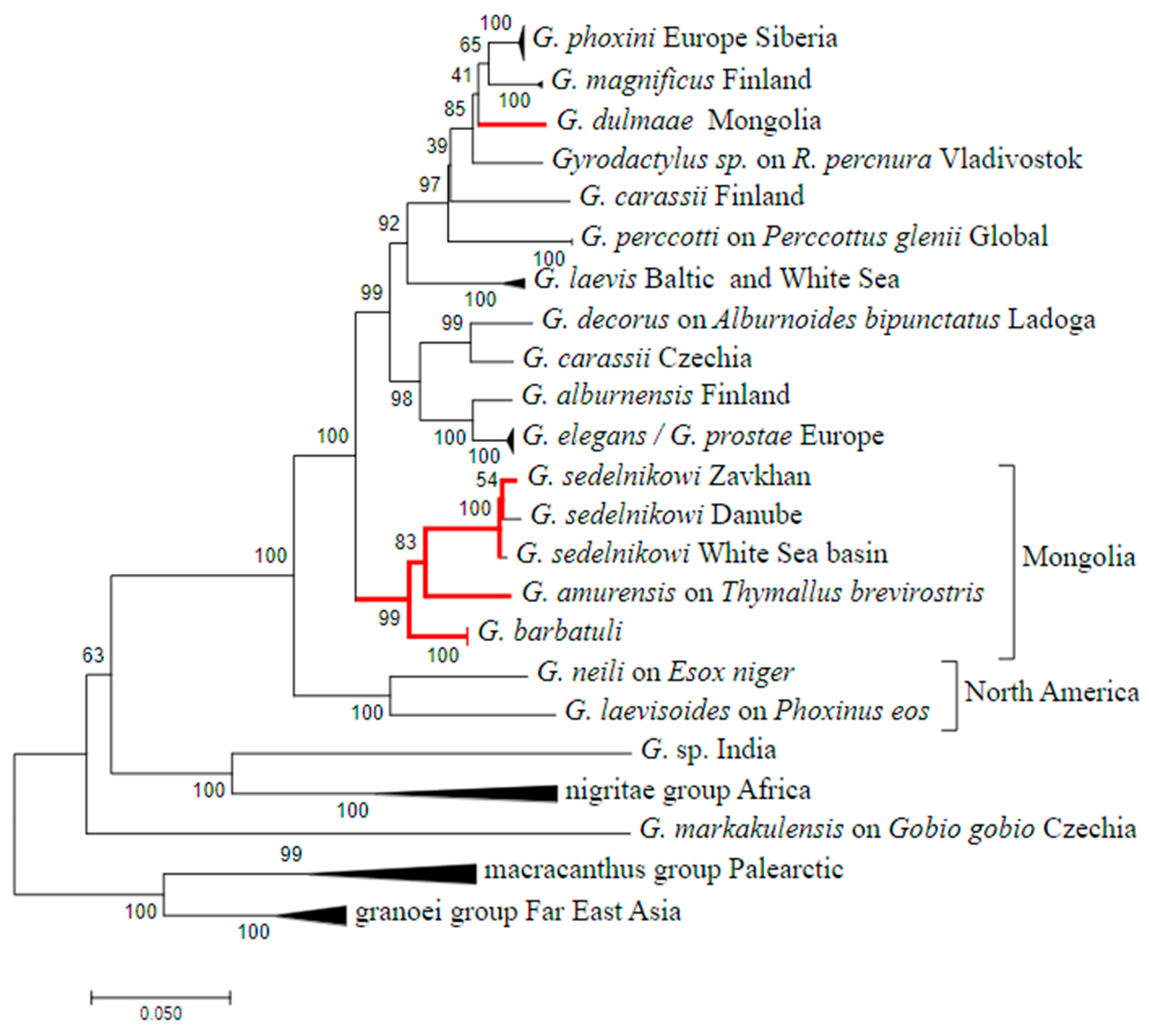


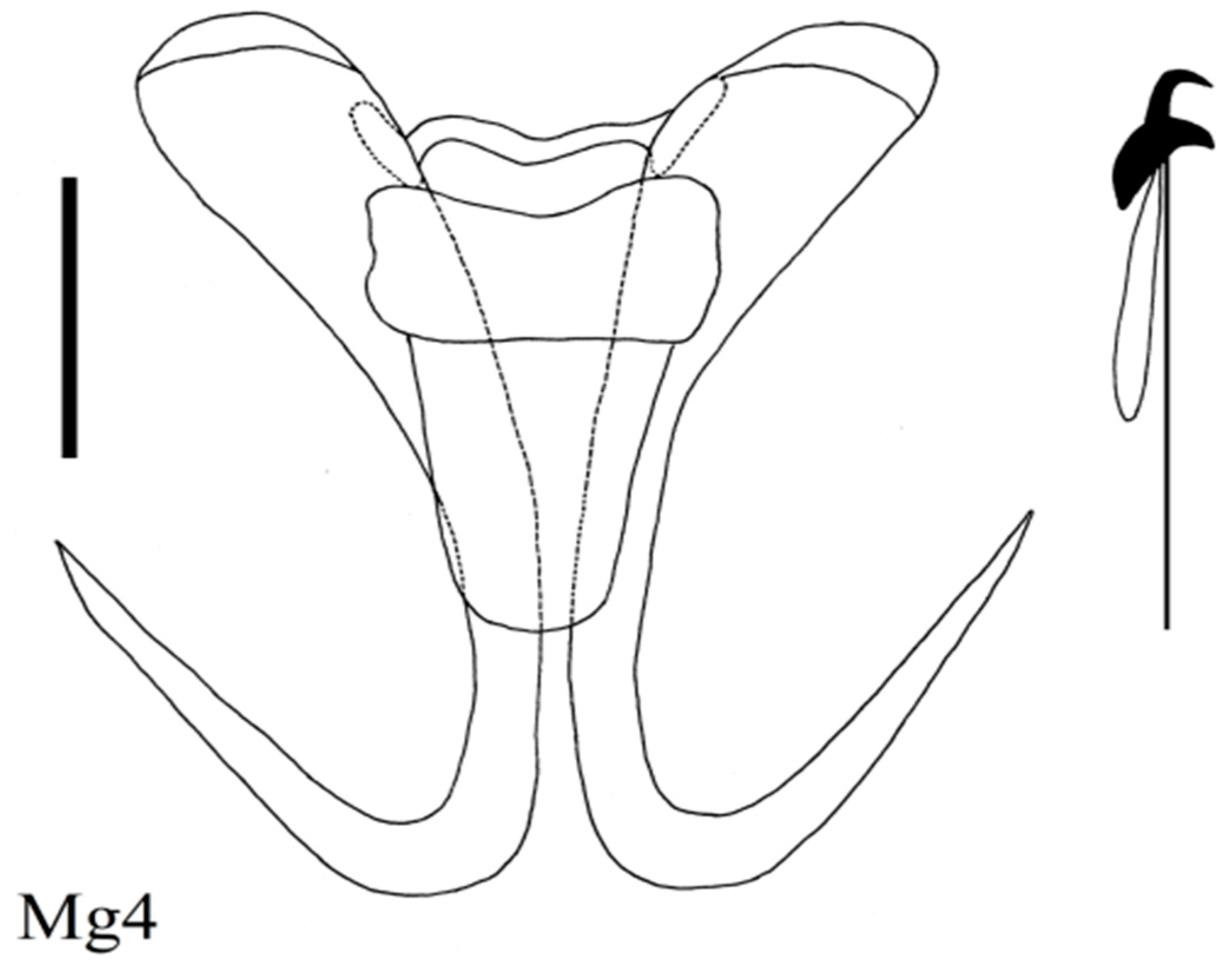
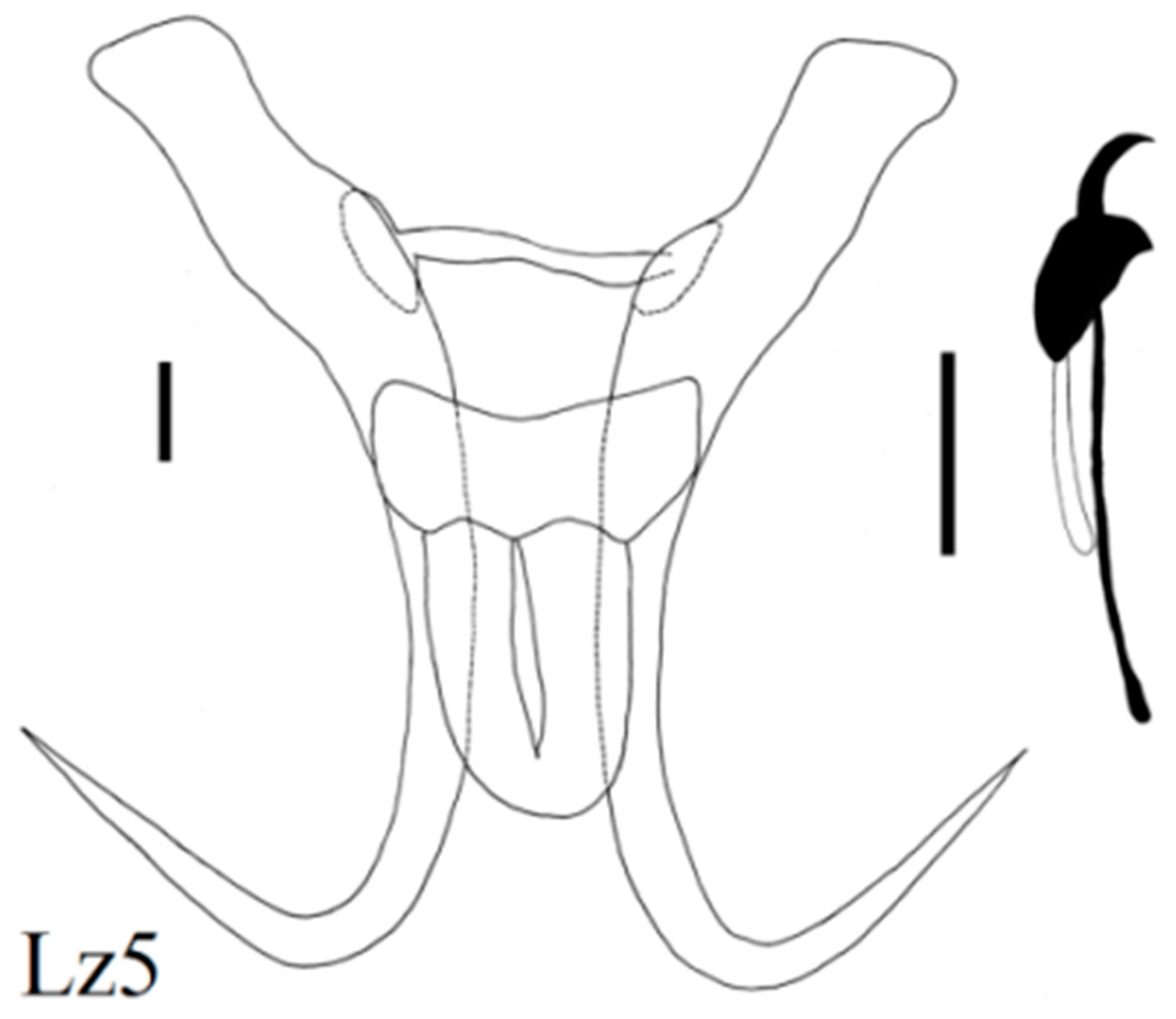
| Locality | Fish Species | Lab Code of Parasite | Gyrodactylus Species | Subgenus * |
|---|---|---|---|---|
| Tuin River | Little Altai Osman (N = 10) Oreoleuciscus humilis | Ot2 Ot1, 3, 4, 5, 6 | G. pseudonemachili Ergens and Bychowsky, 1967 G. nordmanni Ergens and Dulmaa, 1970 | L n L m |
| Zavkhan River above dam | Altai Osman (N = 3) Oreoleuciscus potanini | Oz6 Oz2,5 Oz1, 3, 4 | G. radimi sp. nov. G. mongolicus Ergens and Dulmaa, 1970 G. oreoleucisci Ergens and Dulmaa, 1970 | L w L n L m |
| Mongolian grayling (N = 15) Thymallus brevirostris | Mg2 Mg1, 6 Mg5, 7, 8 Mg4 | G. zavkhanensis sp. nov. G. radimi sp. nov. G. pseudonemachili Ergens and Bychowsky, 1967 G. amurensis Akhmerov, 1952 | L n L w L n G | |
| Stone loach (N = 11) Barbatula conilobus | Lz12 | G. oreoleucisci | L m | |
| Zavkhan River below dam | Stone loach (N = 15) Barbatula conilobus | Lz2 Lz4, 5, 6, 8, 9, 10 Lz11, 13, 15, 16, 17, 18 Lz7 | G. sedelnikowi Gvozdev, 1950 G. barbatuli Akhmerov, 1952 G. pseudonemachili Ergens and Bychowsky, 1967 G. tayshirensis sp. nov. | G G L n L n |
| Chono Kharaikh river | Altai Osman (N = 14) Oreoleuciscus potanini | Op4, 5, 6, 8, 9 Op1 Op2, 3, 7 | G. oreoleucisci G. dulmaae Ergens, 1970 G. mongolicus | L m G L n |
| Stone loach (N = 9) Barbatula cobdonensis | Lp1, 2, 4, 5, 6 Lp3 | G. nemachili Bychowsky, 1936 hybrid G. nemachili × G. mongolicus | L n L n | |
| Tuul River, Yenisei basin | Minnow (N = 15) Phoxinus cf. phoxinus | Ph4, 5, 7, 9, 10 Ph, Ph2 Ph1, 6 | G. aphyae Malmberg, 1957 G. albolacustris Lebedeva, Ziętara and Lumme, 2017 G. phoxini Malmberg, 1957 | L w L w G |
| Species | Populations | mtDNA | Distance | Source | |
|---|---|---|---|---|---|
| G. albolacustris | Tuul | Ladoga | cox1 | 8.4% | [61] |
| G. aphyae | Tuul | Ladoga | cox1 | 11.8% | Present study |
| G. nordmanni vs. G. oreoleucisci | Tuin | Zavkhan | cox1 | 19.8% | Present study |
| O. humilis vs. O. potanini | Tuin | Zavkhan | cyt B | 4.2% | [6] |
| P. phoxinus | Tuul | Ladoga | cyt B | 8.9–9.1% | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedeva, D.; Ziętara, M.; Mendsaikhan, B.; Ermolenko, A.; Lumme, J. Survivors from a Pliocene Climatic Catastrophe: Gyrodactylus (Platyhelminthes, Monogenea) Parasites of the Relict Fishes in the Central Asian Internal Drainage Basin of Mongolia. Diversity 2023, 15, 860. https://doi.org/10.3390/d15070860
Lebedeva D, Ziętara M, Mendsaikhan B, Ermolenko A, Lumme J. Survivors from a Pliocene Climatic Catastrophe: Gyrodactylus (Platyhelminthes, Monogenea) Parasites of the Relict Fishes in the Central Asian Internal Drainage Basin of Mongolia. Diversity. 2023; 15(7):860. https://doi.org/10.3390/d15070860
Chicago/Turabian StyleLebedeva, Daria, Marek Ziętara, Bud Mendsaikhan, Alexey Ermolenko, and Jaakko Lumme. 2023. "Survivors from a Pliocene Climatic Catastrophe: Gyrodactylus (Platyhelminthes, Monogenea) Parasites of the Relict Fishes in the Central Asian Internal Drainage Basin of Mongolia" Diversity 15, no. 7: 860. https://doi.org/10.3390/d15070860
APA StyleLebedeva, D., Ziętara, M., Mendsaikhan, B., Ermolenko, A., & Lumme, J. (2023). Survivors from a Pliocene Climatic Catastrophe: Gyrodactylus (Platyhelminthes, Monogenea) Parasites of the Relict Fishes in the Central Asian Internal Drainage Basin of Mongolia. Diversity, 15(7), 860. https://doi.org/10.3390/d15070860