Integrative Analysis of Retusa pertenuis (Heterobranchia: Cephalaspidea) from Arctic and Russian Far East Seas with Discussion of Its Morphology, Validity and Population Structure
Abstract
:1. Introduction
2. Material and Methods
2.1. Collection of Material
2.2. Morphological Methods
2.3. Molecular Methods
2.3.1. Taxon Sampling
2.3.2. DNA Extraction, Amplification and Sequencing
2.3.3. Phylogenetic Analysis
2.3.4. Population Analysis
3. Results
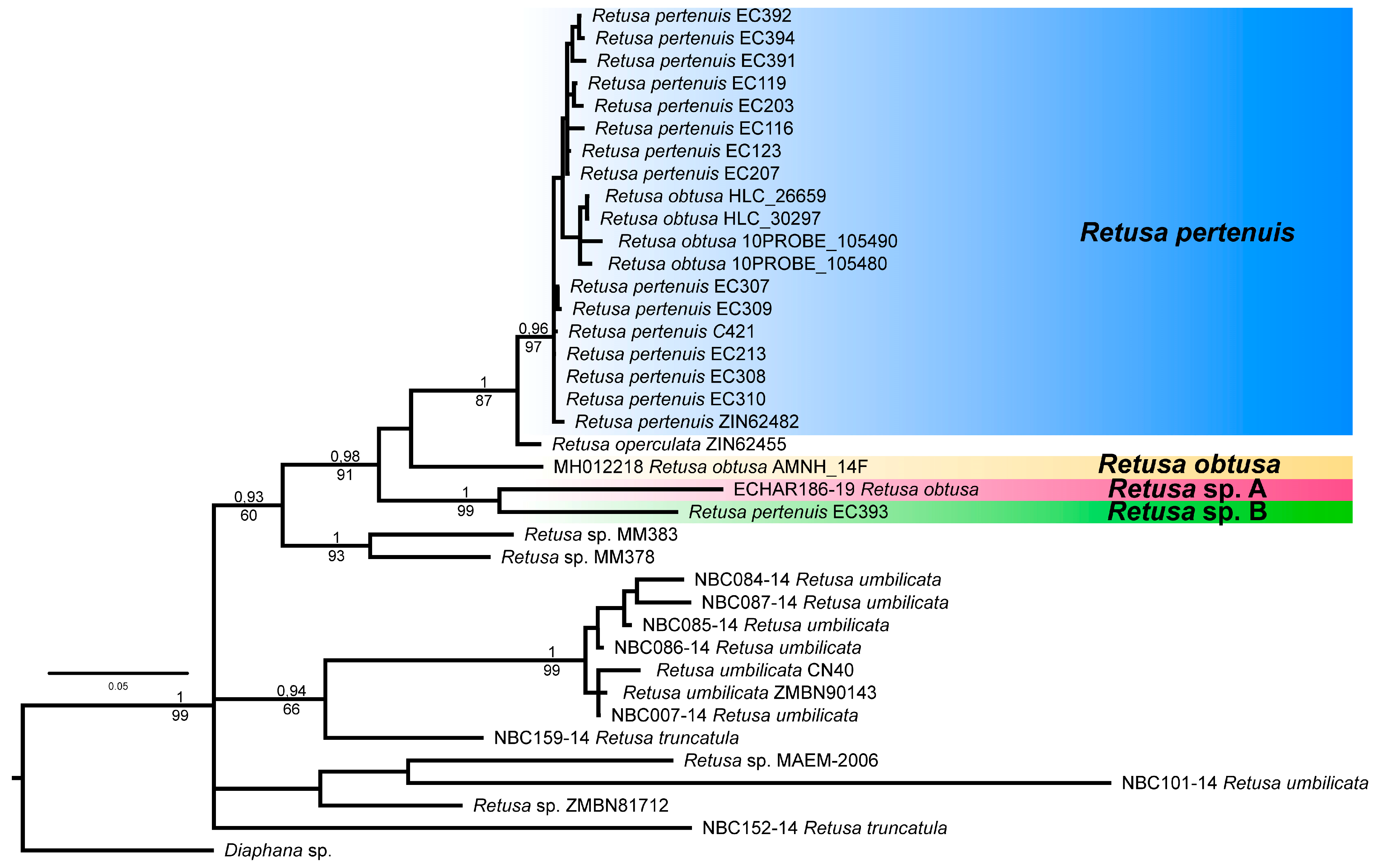
3.1. Molecular Analysis
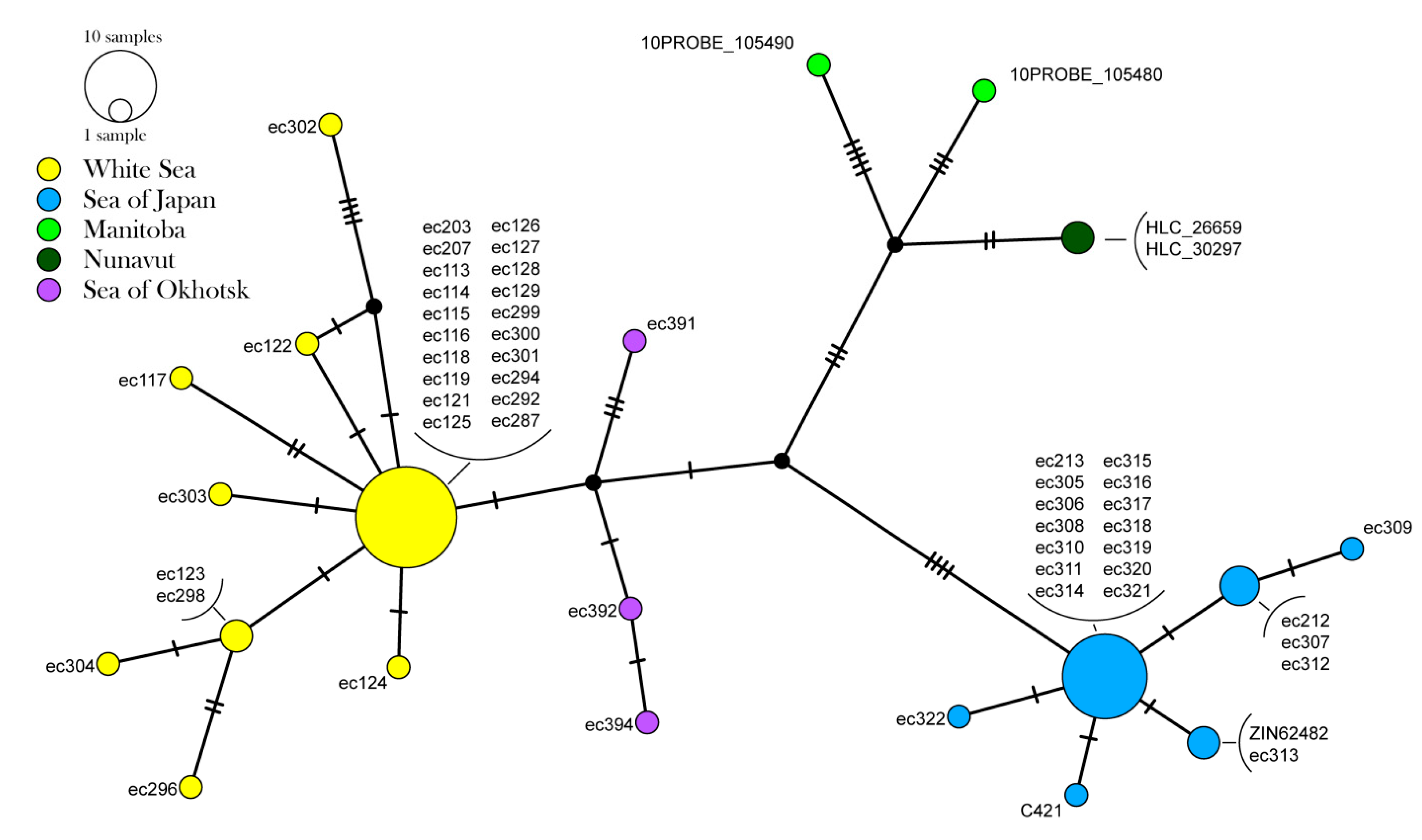
3.2. Population Genetic Data
3.3. Morphology
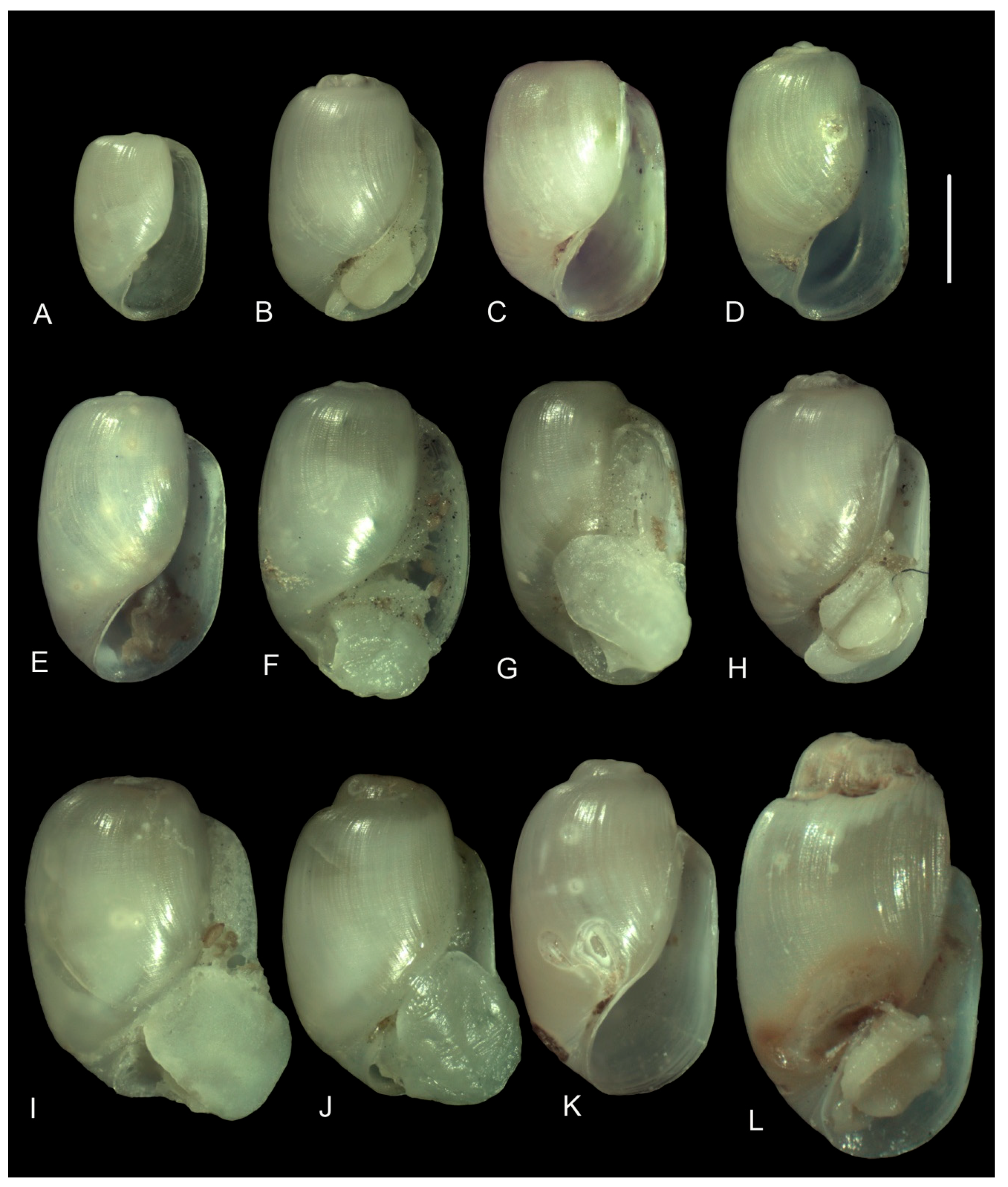
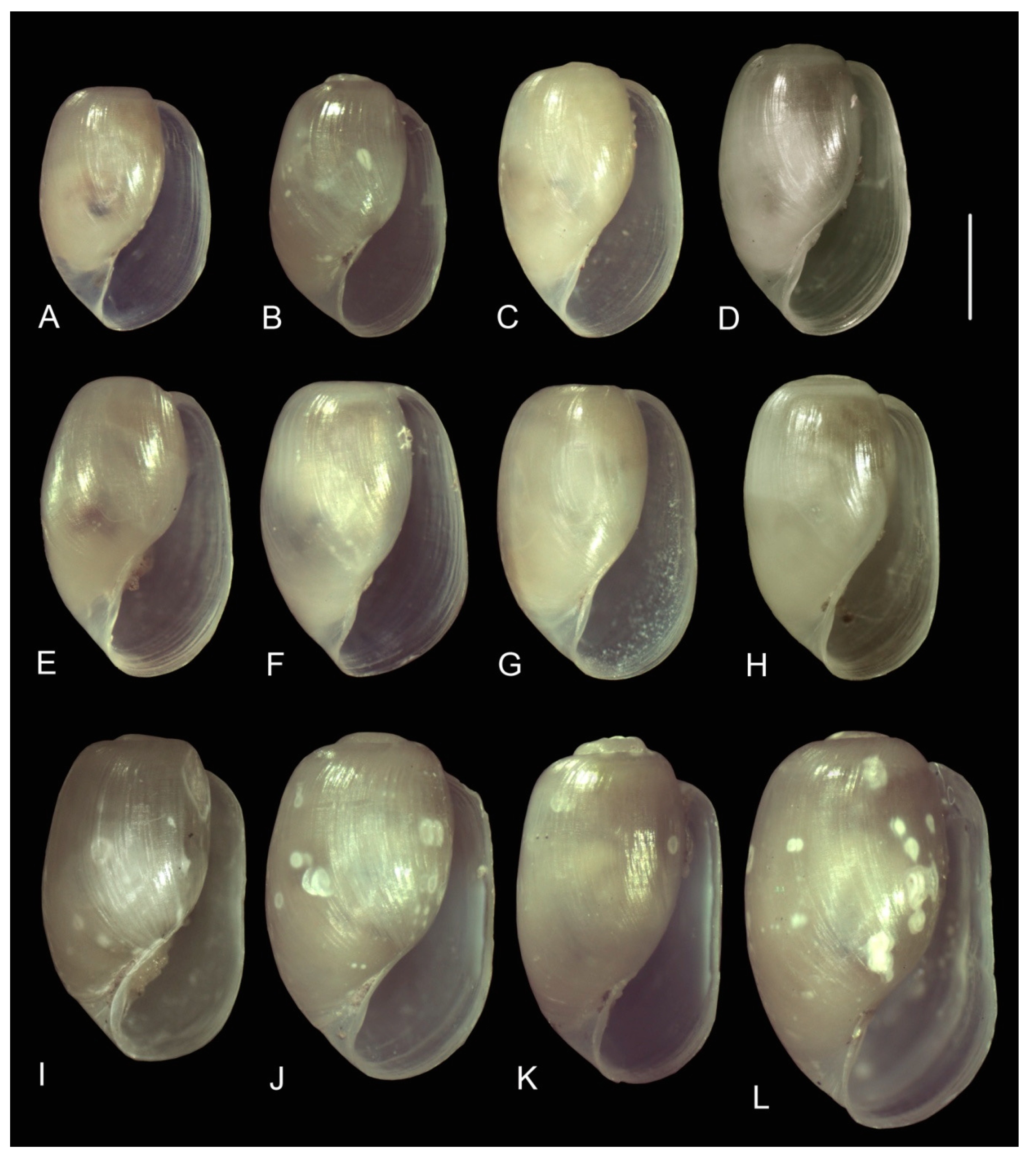
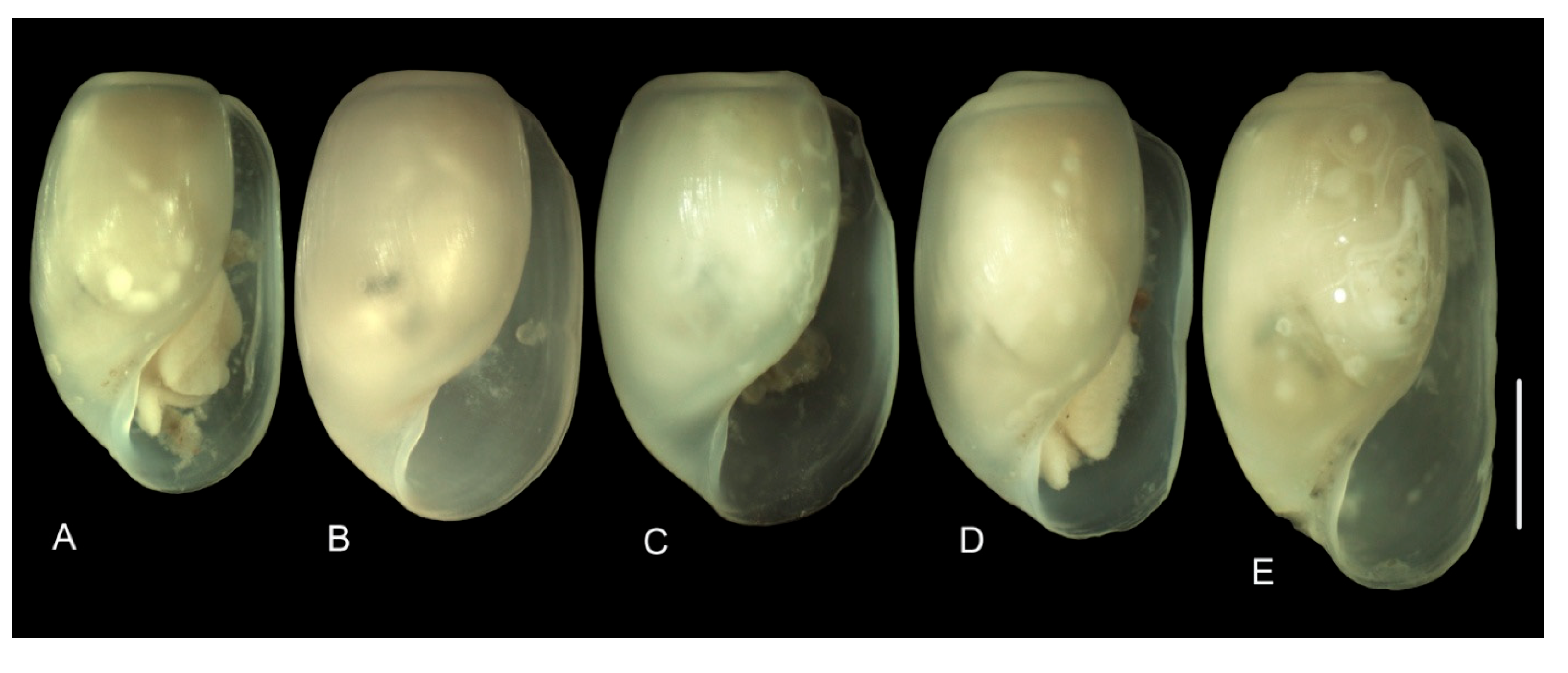
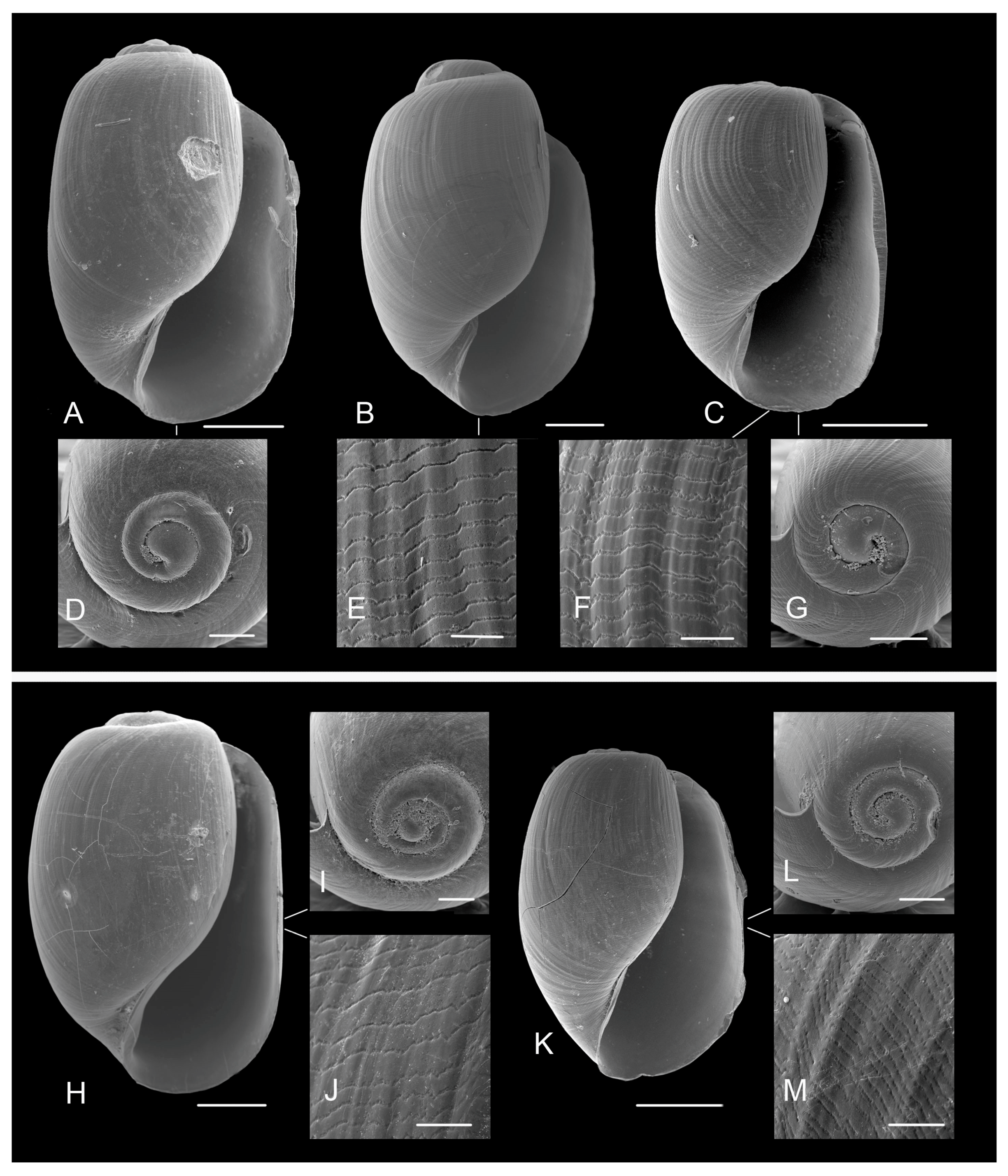
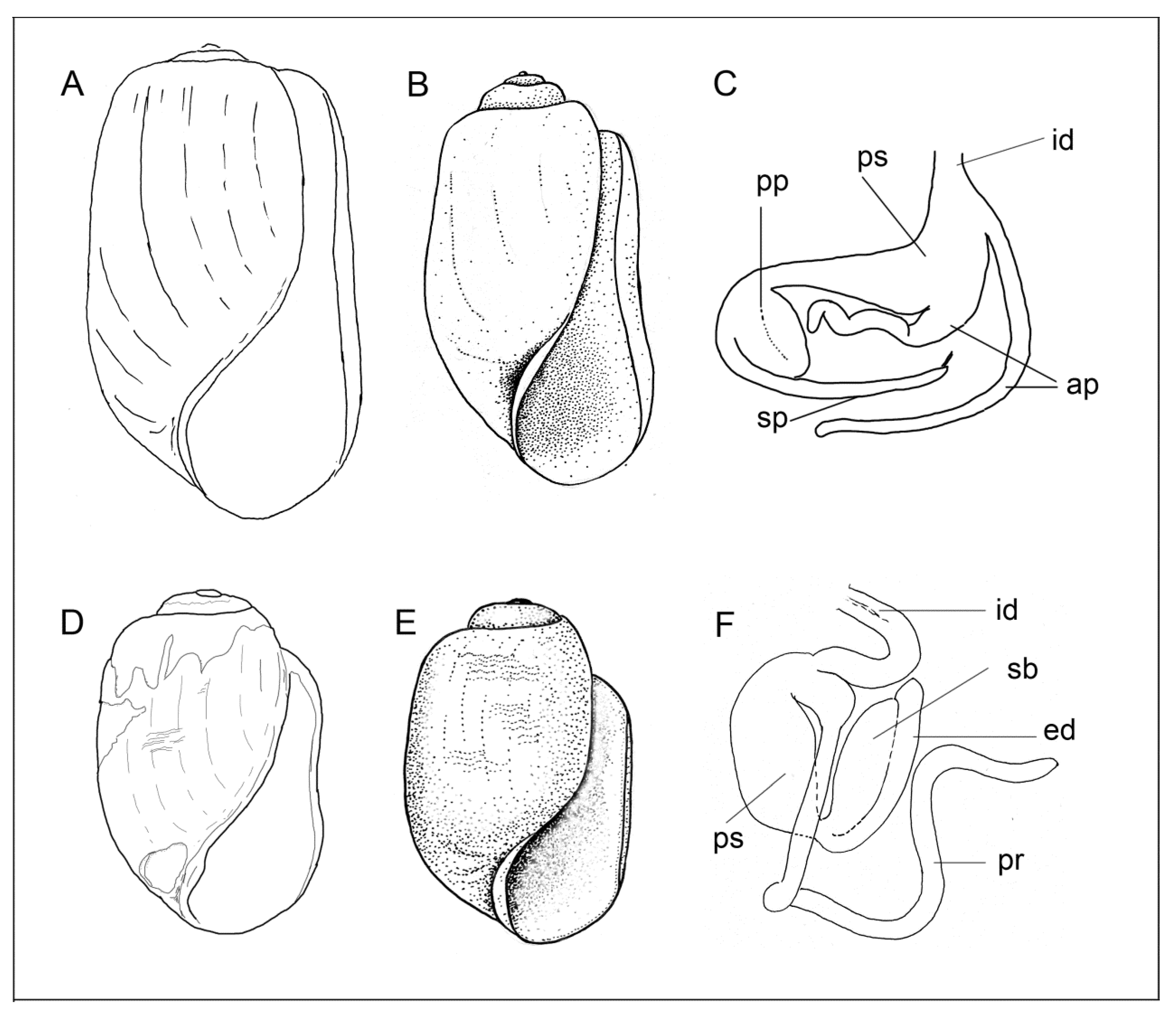
3.3.1. External Morphology
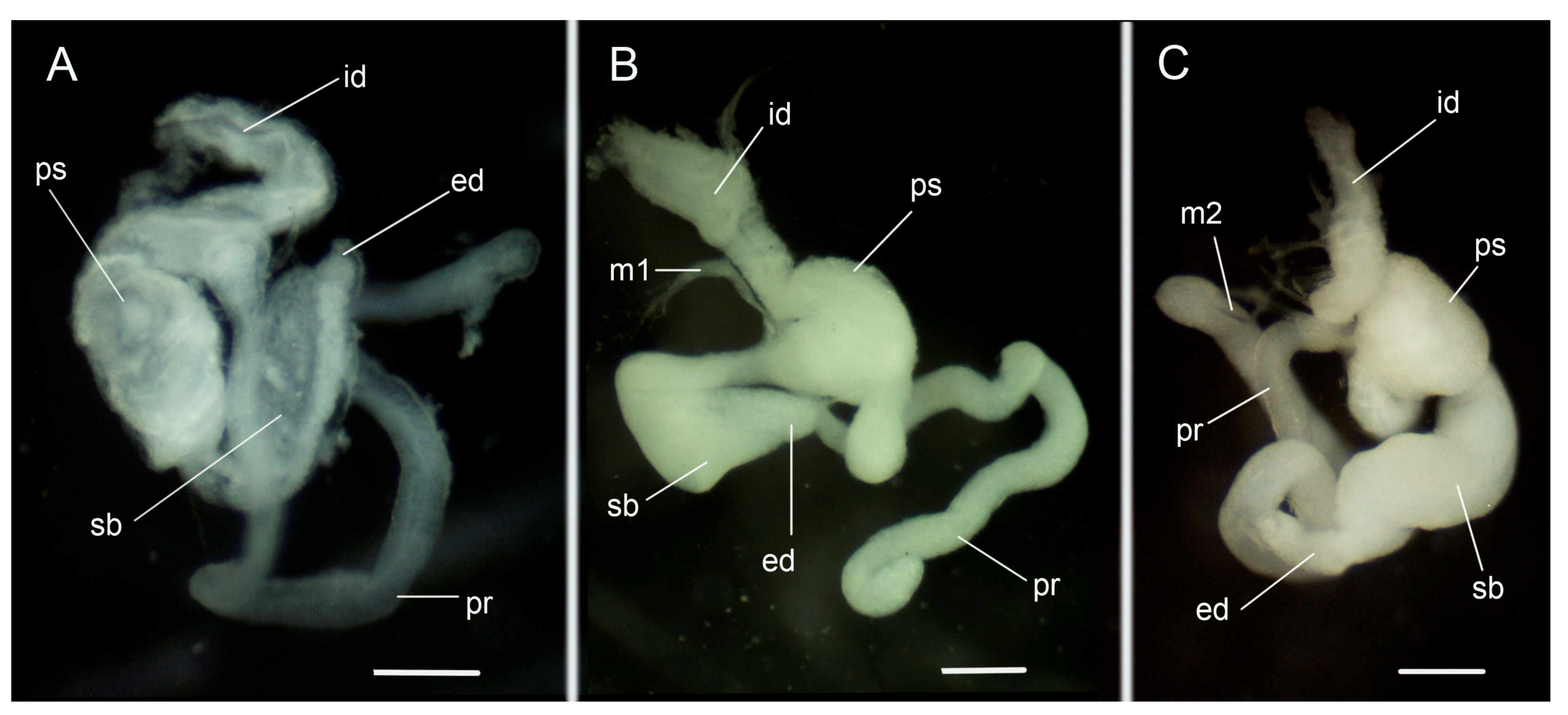
3.3.2. Internal Morphology
Gizzard Plate Morphology
Copulatory Apparatus Morphology
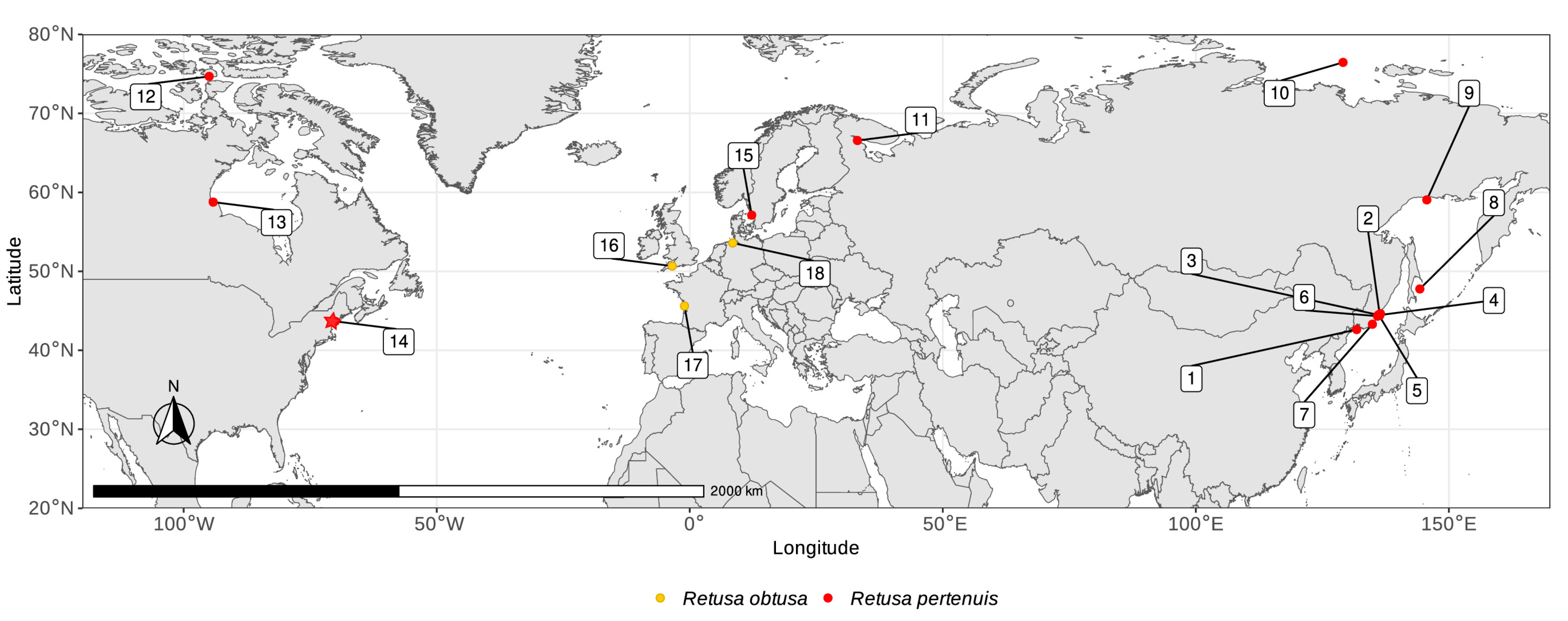
3.4. Distribution
3.5. Ecology
4. Discussion
4.1. Taxonomy
4.2. Variability in the Morphological Characteristics across Distant Populations
4.3. Phylogeography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golikov, A.N. Shell-bearing gastropod molluscs of the Laptev Sea. Explor. Fauna Seas 1990, 37, 365–384. (In Russian) [Google Scholar]
- Golikov, A.N. Shell-bearing gastropod molluscs of the East-Siberian Sea. Explor. Fauna Seas 1994, 49, 67–122. (In Russian) [Google Scholar]
- Lubin, P.V. Fauna and ecology of shell-bearing gastropod mollusks of the Kara Sea. In Invertebrate Fauna of the Kara, Barents and White Seas (Informatics, Ecology, Biodiversity); Matishov, G.G., Ed.; Kola Scientific Center Russian Academy of Sciences: Apatity, Russia, 2003; pp. 130–195. (In Russian) [Google Scholar]
- Nekhaev, I.O.; Kroll, E.N. Diversity of shell-bearing gastropods along the western coast of the Arctic archipelago Novaya Zemlya: An evaluation of modern and historical data. Polar Biol. 2017, 40, 2279–2289. [Google Scholar] [CrossRef]
- Chaban, E.M. Shell-bearing mollusks of the order Cephalaspidea (Gastropoda: Heterobranchia) of the Kara Sea. Proc. Zool. Inst. RAS 2021, 325, 197–216. (In Russian) [Google Scholar] [CrossRef]
- Thompson, T.E. Biology of Opisthobranch Molluscs; The Ray Society: London, UK, 1976; Volume I, pp. 1–207. [Google Scholar]
- Berry, A.J.; Thompson, D.R. Changing prey size preferences in the animal cycle of Retusa obtusa (Montagu) (Opisthobranchia) feeding on Hydrobia ulvae (Pennant) (Prosobranchia). J. Exp. Mar. Biol. Ecol. 1990, 141, 145–158. [Google Scholar] [CrossRef]
- Mighels, J.W. Descriptions of six species of shells regarded as new. Boston J. Nat. Hist. 1843, 4, 345–350. [Google Scholar]
- Sars, G.O. Bidrag Til Kundskaben om Norges Arktiske Fauna. I. Mollusca Regions Arcticae Norvegiae; Brøgger: Christiania, Denmark, 1878; 426p. [Google Scholar]
- Herzenstein, S. Beiträge zur Kenntnis der Fauna der Murmanküste und des Weissen Meeres. 1. Mollusks. Trudy Sankt-Peterburgskogo Obshchetstva Estestvoispytatelei 1885, 16, 635–814. (In Russian) [Google Scholar]
- Friele, H. Zoologi. Mollusca, II. In Den Norske Nordhavs—Ekspedition 1876/78; Bd. XVI; Christiania Grøndahl & Sons: Oslo, Norway, 1886; pp. 1–44. [Google Scholar]
- Aurivillius, C.W.S. Öfversigt öfver de af Vega-Expeditionen insamlade Arktiska Hafsmollusker. II. Placophora och Gastropoda. Vega-Exp. Vetenskapliga Jakffagelser. Stockh. 1887, 4, 313–383. [Google Scholar]
- Knipowitsch, N. Eine zoologische Excursion im nordwestlichen Theile des Weissen Meeres im Sommer 1895. Ann. Mus. Zool. Acad. Imp. Sci. 1896, 1, 278–326. [Google Scholar]
- Knipowitsch, N. Zur Kenntniss der Geologischen Geschichte der Fauna des Weissen und des Murman Meeres. (Postpliocaene Mollusken und Brachiopoden). Verhan. K. Russ. Miner. Gesell. 1900, 38, 1–169. [Google Scholar]
- Knipowitsch, N. Zoologische Ergebnisse der Russischen Expeditionen nach Spitzbergen. Mollusca und Brachiopoda. I. Ann. Mus. Zool. Acad. Imp. Sci. 1901, 6, 435–558. [Google Scholar]
- Odhner, N. Northern and Arctic invertebrates in the collection of the Swedish State Museum (Riksmuseum). III. Opisthobranchia and Pteropoda. K. Sven. Vetensk. Akad Handl. 1907, 41, 1–114. [Google Scholar]
- Odhner, N. Die Molluskenfauna des Eisfjords, pt. 2, N 1. Zoologische Ergebnisse der Schwedischen Expedition nach Spitzbergen 1908. K. Sven. Vetensk. Akad. Handl. 1915, 54, 1–274. [Google Scholar]
- Oldroyd, I.S. The Marine Shells of the West Coast of North America; Stanford University Press: Stanford, CA, USA, 1927; Volume 2, pp. 1–300. [Google Scholar]
- Lemche, H. Gastropoda, Opisthobranchia. Zool. Iceland 1938, 4, 1–54. [Google Scholar]
- Pergament, T.S. Benthos of the Kara Sea. Problemy Arctiki. 1; Izdatelstvo Glavsevmorputi: Moscow, Russia, 1945; pp. 102–130. (In Russian) [Google Scholar]
- Filatova, Z.A.; Zatsepin, V.I. Class Gastropoda. In Key to Identification of Fauna and Flora of the Northern Seas of USSR; Gaevskaya, N.S., Ed.; Sovetskaya Nauka: Moscow, Russia, 1948; pp. 358–401. (In Russian) [Google Scholar]
- Golikov, A.N. Shell-Bearing Gastropod Molluscs of the Arctic Ocean; Tropa: Moscow, Russia, 1995; pp. 1–185. [Google Scholar]
- Chaban, E.M. Subclass Opisthobranchia: Cephalaspidea, Anaspidea, Acochlidiida, Sacoglossa, Thecosomata, Gymnosomata. In List of Species of Free-Living Invertebrates of Eurasian Arctic Seas and Adjacent Deep Waters; Russian Academy of Science, Zoological Institute: Moscow, Russia, 2001; Volume 51, pp. 108–109. [Google Scholar]
- Chaban, E.M. Cephalaspidean mollusks (Mollusca, Opisthobranchia) of the Laptev Sea. Expl. Fauna Seas 2004, 54, 71–87. (In Russian) [Google Scholar]
- Chaban, E.M. Opisthobranch mollusks of the orders Cephalaspidea, Thecosomata, Gymnosomata (Mollusca, Opisthobranchia) of the Chukchi Sea and Bering Strait. Expl. Fauna Seas 2008, 61, 149–162. (In Russian) [Google Scholar]
- Chaban, E.M. Cephalaspidean mollusks (Gastropoda, Opisthobranchia) of the East Siberian Sea. Expl. Fauna Seas 2010, 66, 71–88. (In Russian) [Google Scholar]
- Chaban, E.M.; Martynov, A.V. Acteonoidea, Cephalaspidea. In Marine and Brackish Water Gastropoda of Russia and Adjacent Countries: An Illustrated Catalogue; Kantor, Y.I., Sysoev, A.V., Eds.; KMK Scientific Press Ltd.: Moscow, Russia, 2006; Volume 249–261, pp. 124–129. [Google Scholar]
- Martynov, A.V.; Korshunova, T.A.; Savinkin, O.V. Shallow-water opisthobranch molluscs of the Murman coast of the Barents Sea, with new distributional data and remarks on biology. Ruthenica 2006, 16, 59–72. [Google Scholar]
- Nekhaev, I.O. Marine shell-bearing Gastropoda of Murman (Barents Sea): An annotated check-list. Ruthenica 2014, 24, 75–121. [Google Scholar]
- Gould, A.A. Report on the Invertebrata of Massachusetts, 2nd ed.; Wright and Potter, State Printers: Boston, MA, USA, 1870; pp. 1–524. [Google Scholar]
- Dall, W.H. A Preliminary Catalogue of the Shell-Bearing Marine Mollusks and Brachiopods of the Southeastern Coast of the United States, with Illustrations of Many of the Species; No 37; Government Printing Office: Washington, DC, USA, 1889; pp. 1–232.
- La Rocque, A. Catalogue of the recent Mollusca of Canada. Nat. Mus. Can. Bull. 1953, 129, 1–405. [Google Scholar]
- Nordsieck, F. Die Europaischen Meeresschnecken. (Opisthobranchia, mit Pyramidellidae, Rissoacea); Gustav Fischer Verlag: Stuttgart, Germany, 1972; pp. 1–327. [Google Scholar]
- Lee, R.S.; Foster, N.R. A distributional list with range extentions of the opisthobranch gastropods of Alaska. Veliger 1985, 27, 440–448. [Google Scholar]
- Chaban, E.M.; Martynov, A.V. Clade Cephalaspidea, Clade Sacoglossa. In Check-List of Species of Free-Living Invertebrates of the Russian Far-Eastern Seas; Sirenko, B.I., Ed.; Russian Academy of Sciences-Zoological Institute: St. Petersburg, Russia, 2013; Volume 75, pp. 166–167. [Google Scholar]
- MolluscaBase. Retusa T.Brown, 1827. World Register of Marine Species. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=138432 (accessed on 22 March 2023).
- Lemche, H. Northern and Arctic tectibranch gastropods. Det K. Dan. Vidensk. Selsk. Biol. Skr. 1948, 5, 1–136. [Google Scholar]
- Chaban, E.M. Opisthobranch molluscs of the family Diaphanidae (Gastropoda, Opisthobranchia) of the Russian seas. Ruthenica 1996, 6, 127–148. (In Russian) [Google Scholar]
- Chaban, E.M. Opisthobranch molluscs of “Cylichna occulta group” (Gastropoda: Opisthobranchia: Cylichnidae) from the Chukchi Sea and adjacent waters. Bull. Russ. Far East Malacol. Soc. 2016, 20, 5–26. [Google Scholar]
- Schiøtte, T. A taxonomic revision of the genus Diaphana Brown, 1827, including a discussion of the phylogeny and zoogeography of the genus (Mollusca: Opisthobranchia). Steenstrupia 1998, 24, 77–140. [Google Scholar]
- Hain, S.; Wägele, H.; Willan, R.C. Tomthompsonia spiroconchalis Wägele & Hain, 1991 (Opisthobranchia, Notaspidea): A junior synonym of Adeorbis antarcticus Thiele, 1912 (Prosobranchia: Truncatelloidea) with notes on diet and histology. J. Moll. Stud. 1993, 59, 366–370. [Google Scholar]
- Lemche, H. Gastropoda Opisthobranchiata. The zoology of East Greenland. Meddelelser Om Grønland 1941, 121, 1–22. [Google Scholar]
- Lemche, H. Gastropoda Opisthobranchiata (excl. Pteropoda). The Godthaab expedition 1928. Meddelelser Om Grønland 1941, 80, 1–65. [Google Scholar]
- Abbott, R.T. American seashells. In The Marine Mollusca of the Atlantic and Pacific Coasts of North America, 2nd ed.; Van Nostrand Reinhold Company: New York, NY, USA, 1974; 663p. [Google Scholar]
- Thompson, T.E. Molluscs: Benthic Opisthobranchs. In Synopsis of British Fauna (New Series); No. 8; The Linnean Society of London and the Estuarine & Bracjish-water Sciences Association: Leiden, The Netherlands; New York, NY, USA; København, Denmark; Köln, Germany, 1988; 356p. [Google Scholar]
- Feder, H.M.; Foster, N.R.; Jewett, S.C.; Weingartner, T.J.; Baxter, R. Mollusks in the Northeastern Chukchi Sea. Arctic 1993, 47, 145–163. [Google Scholar] [CrossRef]
- Høisæter, T. Distribution of marine, benthic, shell bearing gastropods along the Norwegian coast. Fauna Nor. 2009, 28, 5–106. [Google Scholar] [CrossRef]
- Valdés, Á. Northeast Pacific benthic shelled sea slugs. Zoosympozia 2019, 13, 242–304. [Google Scholar] [CrossRef]
- Golikov, A.N. Class Gastropoda. In Molluscs of the White Sea. Opredeliteli po Faune SSSR, Izdavaemye ZIN AN SSSR; Starobogatov, Y.I., Naumov, A.D., Eds.; Nauka: Leningrad, Russia, 1987; Volume 151, pp. 41–148. (In Russian) [Google Scholar]
- Lubin, P.V. Quantitative distribution of gastropods (Gastropoda) in the Kara Sea. In Modern Benthos of the Barents and Kara Seas; Matishov, G.G., Ed.; Kola Scientific Center Russian Academy of Sciences: Apatity, Russia, 2000; pp. 189–203. (In Russian) [Google Scholar]
- Martynov, A.; Korshunova, T. Opisthobranch Molluscs of the Seas of Russia: A Colour Guide to Their Taxonomy and Biology; Fiton+: Moscow, Russia, 2011; pp. 1–232. [Google Scholar]
- Chaban, E.M. Shelled Opisthobranchiate Mollusks of the Orders Cephalaspidea and Anaspidea of Northern and Far-East Seas of Russia. Ph.D. Thesis, Zoological Institute of the Russian Academy of Sciences, St. Petersburg, Russia, 1999; pp. 1–256. (In Russian). [Google Scholar]
- Chaban, E.M. Some materials for revision of opisthobranchs of the family Retusidae (Mollusca: Cephalaspidea). Procc. Zool. Inst. RAS 2000, 286, 23–28. [Google Scholar]
- Ablett, J.; Brown, C.; Gallichan, J.; Gordon, D.; Holmes, A.M.; Hunter, T.; Machin, R.; Morgenroth, H.; Oliver, P.G.; Petts, R.; et al. Mollusca Types in Great Britain. Amgueddfa Cymru-National Museum Wales, Natural History Museum. 2019. Available online: https://gbmolluscatypes.ac.uk (accessed on 18 July 2023).
- Ivanova, N.V.; deWaard, J.; Hebert, P.D.N. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol. Ecol. Notes 2006, 6, 998–1002. [Google Scholar] [CrossRef]
- Chaban, E.M.; Ekimova, I.A.; Schepetov, D.M.; Kohnert, P.C.; Schrodl, M.; Chernyshev, A.V. Euopisthobranch mollusks of the order Cephalaspidea (Gastropoda: Heterobranchia) of the Kuril-Kamchatka Trench and the adjacent Pacific abyssal plain with descriptions of three new species of the genus Spiraphiline (Philinidae). Progr. Oceanol. 2019, 178, 102185. [Google Scholar] [CrossRef]
- Chaban, E.M.; Ekimova, I.A.; Schepetov, D.M.; Chernyshev, A.V. The new genus Aglaona: The first abyssal aglajid (Heterobranchia: Cephalaspidea: Aglajidae) with a description of two new species from the north-western Pacific Oceans. Zool. J. Linn. Soc. 2022, 196, 198–214. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Drummond, A. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Chaban, E.M.; Ekimova, I.A.; Schepetov, D.M.; Chrnyshev, A.V. Meloscaphander grandis (Heterobranchia: Cephalaspidea), a deep-water species from the North Pacific: Redescription and taxonomic remarks. Zootaxa 2019, 4646, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Clement, M.; Snell, Q.; Walker, P.; Posada, D.; Crandall, K. TCS: Estimating gene genealogies. In Parallel and Distributed Processing Symposium, Ft. Lauderdale, FL, USA, 15–19 April 2002; International Proceedings: Lauderdale, FL, USA, 2002; Volume 2, p. 184. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.J.; Purvis, J.; Radhakrishnan, K.V. Reproductive system and spermatogenesis in the opisthobranch gastropod Retusa obtusa (Montagu). J. Moll. Stud. 1992, 58, 357–367. [Google Scholar] [CrossRef]
- Minichev, Y.S. Morphological peculiarities of abyssal Cephalaspidea (Gastropoda, Opisthobranchia). Zool. Zh. 1966, 45, 509–515. (In Russian) [Google Scholar]
- Rudman, W.B. On a new genus for “Tornatina” murdochi Suter, 1913 (Retusidae, Opisthobranchia). J. Malac. Soc. Aust. 1971, 2, 187–193. [Google Scholar]
- Ghiselin, M. Reproductive function and the phylogeny of opisthobranch gastropods. Malacologia 1966, 3, 327–378. [Google Scholar]
- Knipowitsch, N. Zoologische Ergebnisse der Russischen Expeditionen nach Spitzbergen. Mollusca und Brachiopoda. II und III. Ann. Mus. Zool. Acad. Imp. Sci. 1902, 7, 355–459. [Google Scholar]
- Golikov, A.N.; Sirenko, B.I.; Gulbin, V.V.; Chaban, E.M. Checklist of shell-bearing gastropods of the northwestern Pacific. Ruthenica 2001, 11, 153–173. [Google Scholar]
- Golikov, A.N.; Scarlato, O.A.; Averintcev, V.G.; Menshutkina, T.V.; Novikov, O.K.; Sheremetevsky, A.M. Ecosystems of the New Siberian shoals, their distribution and functioning. Expl. Fauna Seas 1990, 37, 4–79. (In Russian) [Google Scholar]
- Montagu, G. Testacea Britannica or Natural History of British Shells; Part 1–2; J.S.Hollis: London, UK, 1803; pp. 1–606. [Google Scholar]
- Oliver, P.G.; Morgenroth, H.; Salvador, A. Type specimens of Mollusca described by Col. George Montagu in the Royal Albert Memorial Museum & Art Gallery, Exeter and the Natural History Museum, London. Zoosys. Evol. 2017, 93, 363–412. [Google Scholar]
- Leche, W. Ofversigt ofver de af Svenska expeditionerna till Novaja Semlja och Jenissej 1875 och 1876 insamlade hafs—Mollusker. K. Svens. Vetens. Akad. Handl. 1878, 16, 1–86. [Google Scholar]
- Pilsbry, H.A. Manual of Conchology; Structural and Systematic; Tryon, G.W., Ed.; Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA, 1893; Volume 15, 436p. [Google Scholar]
- Dall, W.H. The mollusca of the Arctic coast of America collected by the Canadian Arctic Expedition west from Bathurst Inlet with an appended report on a collection of Pleistocene fossil molluska. In Volume VIII: Mollusks, Echinoderms, Coelenterates, etc. Part A: Mollusks, Recent and Pleistocene; Report of the Canadian Arctic Expedition 1913–1918; F.A. Acland: Ottawa, Canada, 1919; pp. 3A–29A. [Google Scholar]
- Reeve, L. Account of the shells collected by Capitain Sir Edward Belcher C.B., North of Beechey Island. In The Last of the Arctic Voyages; Being a Narrative of the Expedition in H.M.S. Assistance, under the Command of Captain Sir Edward Belcher; John Edward Taylor: London, UK, 1985; Volume 2, pp. 392–399. [Google Scholar]
- Shimizu, K.; Noshita, K.; Kimoto, K.; Saski, T. Phylogeography and shell morphology of the pelagic snail Limacina helicina in the Okhotsk Sea and western North Pacific. Mar. Biol. 2021, 51, 22. [Google Scholar] [CrossRef]
- Iguchi, A.; Takai, S.; Ueno, M.; Maeda, T.; Minami, T.; Hayashi, I. Comparative analysis on the genetic population structures of the deep-sea whelks Buccinum tsubai and Neptunea constrictain in the Sea of Japan. Mar. Biol. 2007, 151, 31–39. [Google Scholar] [CrossRef]
- Orlova, S.Y.; Smirnova, M.A.; Stroganov, A.N.; Mukhametov, I.N.; Smirnov, A.A.; Tok, K.S.; Park, J.H.; Orlov, A.M. Population structure and microevolution of Pacific cod Gadus macrocephalus based on the analysis of the control region (mtDNA) polymorphism. Russ. J. Genet. 2019, 55, 580–591. [Google Scholar] [CrossRef]
- Hardy, S.M.; Carr, C.M.; Hardman, M.; Steinke, D.; Corstorphine, E.; Mah, C. Biodiversity and phylogeography of Arctic marine fauna: Insights from molecular tools. Mar. Biodiv. 2011, 41, 195–210. [Google Scholar] [CrossRef]
- Laakkonen, H.M.; Hardman, M.; Strelkov, P.; Väinölä, R. Cycles of trans-Arctic dispersal and vicariance, and diversification of the amphi-boreal marine fauna. J. Evol. Biol. 2021, 34, 73–96. [Google Scholar] [CrossRef]
- Smith, S.T. The ecology and life history of Retusa obtusa (Montagu) (Gastropoda, Opisthobranchia). Can. J. Zool. 1967, 45, 397–405. [Google Scholar] [CrossRef]
- Smith, S.T. The development of Retusa obtusa (Montagu) (Gastropoda, Opisthobranchia). Can. J. Zool. 1967, 45, 737–764. [Google Scholar] [CrossRef]
- Selkoe, K.A.; Toonen, R.J. Marine connectivity: A new look at pelagic larval duration and genetic metrics of dispersal. Mar. Ecol. Prog. Ser. 2011, 436, 291–305. [Google Scholar] [CrossRef]
- Melroy, L.M.; Smith, R.J.; Cohen, C.S. Phylogeography of direct-developing sea stars in the genus Leptasterias in relation to San Francisco Bay outflow in central California. Mar. Biol. 2017, 164, 1–14. [Google Scholar] [CrossRef]
- Sherman, C.D.; Hunt, A.; Ayre, D.J. Is life history a barrier to dispersal? Contrasting patterns of genetic differentiation along an oceanographically complex coast. Biol. J. Linn. Soc. 2008, 95, 106–116. [Google Scholar] [CrossRef]
- Fleming, A.M.; Dohner, M.M.; Phillips, N.E.; Ritchie, P.A. Genetic connectivity among populations of two congeneric direct-developing whelks varies across spatial scales. N. Z. J. Mar. Freshw. Res. 2018, 52, 100–117. [Google Scholar] [CrossRef]
- Ekimova, I.A.; Mikhlina, A.L.; Vorobyeva, O.A.; Antokhina, T.I.; Tambovtseva, V.G.; Schepetov, D.M. Young but distinct: Description of Eubranchus malakhovi sp. n. a new, recently diverged nudibranch species (Gastropoda: Heterobranchia) from the Sea of Japan. Inv. Zool. 2021, 18, 197–222. [Google Scholar] [CrossRef]
- Ekimova, I.; Valdés, Á.; Malaquias, M.A.E.; Rauch, C.; Chichvarkhin, A.; Mikhlina, A.; Antokhina, T.; Chichvarkhina, O.; Schepetov, D. High-level taxonomic splitting in allopatric taxa causes confusion downstream: A revision of the nudibranch family Coryphellidae. Zool. J. Linn. Soc. 2022, 196, 215–249. [Google Scholar] [CrossRef]
- Kolbasova, G.; Schmidt-Rhaesa, A.; Syomin, V.; Bredikhin, D.; Morozov, T.; Neretina, T. Cryptic species complex or an incomplete speciation? Phylogeographic analysis reveals an intricate Pleistocene history of Priapulus caudatus Lamarck, 1816. Zool. Anz. 2023, 302, 113–130. [Google Scholar] [CrossRef]
- Kienberger, K.; Carmona, L.; Pola, M.; Padula, V.; Gosliner, T.M.; Cervera, J.L. Aeolidia papillosa (Linnaeus, 1761) (Mollusca: Heterobranchia: Nudibranchia), single species or a cryptic species complex? A morphological and molecular study. Zool. J. Linn. Soc. 2016, 177, 481–506. [Google Scholar] [CrossRef]
- Ekimova, I.; Valdés, Á.; Chichvarkhin, A.; Antokhina, T.; Lindsay, T.; Schepetov, D. Diet-driven ecological radiation and allopatric speciation result in high species diversity in a temperate-cold water marine genus Dendronotus (Gastropoda: Nudibranchia). Molec. Phyl. Evol. 2019, 141, 106609. [Google Scholar] [CrossRef]
- Korshunova, T.; Fletcher, K.; Picton, B.; Lundin, K.; Kashio, S.; Sanamyan, N.; Sanamyan, K.; Padula, V.; Schrödl, M.; Martynov, A. The Emperor’s Cadlina, hidden diversity and gill cavity evolution: New insights for the taxonomy and phylogeny of dorid nudibranchs (Mollusca: Gastropoda). Zool. J. Linn. Soc. 2020, 189, 762–827. [Google Scholar] [CrossRef]
- Ekimova, I.; Nikitenko, E.; Stanovova, M.; Schepetov, D.; Antokhina, T.; Malaquias, M.A.E.; Valdés, Á. Unity in diversity: Morphological and genetic variability, integrative systematics, and phylogeography of the widespread nudibranch mollusk Onchidoris muricata. System. Biodiv. 2023, 21, 1. [Google Scholar] [CrossRef]


| Retusa pertenuis | Retusa obtusa | Retusa sp. A | Retusa sp. B | |
|---|---|---|---|---|
| Retusa pertenuis | 0.1–1.2 | |||
| Retusa obtusa | 7.2–7.5 | n/a | ||
| Retusa sp. A | 10.6–11.8 | 9.8 | n/a | |
| Retusa sp. B | 9.9–10.7 | 9.4 | 9.2 | n/a |
| White Sea | Sea of Japan | Sea of Okhotsk | Canada | |
|---|---|---|---|---|
| White Sea | 0.00175 | |||
| Sea of Japan | 0.86721 | 0.00112 | ||
| Sea of Okhotsk | 0.37828 | 0.72393 | 0.00893 | |
| Canada | 0.68634 | 0.75161 | 0.65631 | 0.00734 |
| Species | h (mm) | Shell Sculpture | Shell Sutures | Copulatory System | Prey | Distribution | References |
|---|---|---|---|---|---|---|---|
| R. obtusa | up to 10 | lines of growth | channeled | with four outgrowths | Hydrobia ulvae | Atlantic, boreal, shallow-water species | [7,25,67] |
| R. pertenuis | up to 4 | spiral striations | pressed | with three outgrowths | foraminifera | boreal–arctic, circumpolar, 3–1300 m | [24,52], this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaban, E.; Ekimova, I.; Stanovova, M.; Schepetov, D. Integrative Analysis of Retusa pertenuis (Heterobranchia: Cephalaspidea) from Arctic and Russian Far East Seas with Discussion of Its Morphology, Validity and Population Structure. Diversity 2023, 15, 974. https://doi.org/10.3390/d15090974
Chaban E, Ekimova I, Stanovova M, Schepetov D. Integrative Analysis of Retusa pertenuis (Heterobranchia: Cephalaspidea) from Arctic and Russian Far East Seas with Discussion of Its Morphology, Validity and Population Structure. Diversity. 2023; 15(9):974. https://doi.org/10.3390/d15090974
Chicago/Turabian StyleChaban, Elena, Irina Ekimova, Maria Stanovova, and Dimitry Schepetov. 2023. "Integrative Analysis of Retusa pertenuis (Heterobranchia: Cephalaspidea) from Arctic and Russian Far East Seas with Discussion of Its Morphology, Validity and Population Structure" Diversity 15, no. 9: 974. https://doi.org/10.3390/d15090974
APA StyleChaban, E., Ekimova, I., Stanovova, M., & Schepetov, D. (2023). Integrative Analysis of Retusa pertenuis (Heterobranchia: Cephalaspidea) from Arctic and Russian Far East Seas with Discussion of Its Morphology, Validity and Population Structure. Diversity, 15(9), 974. https://doi.org/10.3390/d15090974









