Abstract
Seed banks are fundamentally important components of the wetland ecosystem. Water levels on germination in soil seed banks have been documented in many ecosystems. However, there is a lack of knowledge about water levels on seed banks in freshwater wetlands, especially in those buried by sedimentation. Three burial depths (0–5 cm, 5–10 cm and 10–15 cm) within five water level gradient zones along the elevation in Poyang Lake, Eastern China, were sampled. The seedling-emergence method in a greenhouse under moist conditions and submersion was applied to allow all active seeds to be germinated. The experiment continued over an eight-week period in late spring up to early summer. A total of 7090 seedlings emerged, belonged to 20 families, 36 genera and 49 species. In terms of results, an average active soil seed density of 17,328 ± 1675 seeds/m2 was found in 0–15 cm in Poyang Lake, and the greatest average seed density was found at 0–5 cm with 7607 ± 790 seeds/m2, along with 5–10 cm and 10–15 cm with 5419 ± 589 seeds/m2 and 3855 ± 790 seeds/m2, respectively. An obvious difference in composition was found in the species composition of the seed bank at different water levels and burial depths. The highest water level and top layer of soil had the highest diversity index, with a Shannon–Wiener value of 2.011. Seed density, species richness and diversity decreased with the water level gradient zone from low elevation to high elevation and burial depth from surface to deepness. However, there was no interaction between inundation duration and burial depth, indicating that the water level fluctuation and sediment buried had a separate impact on the seed bank composition and diversity index. The present findings can be directly applied to the ecosystem conservation of healthy wetlands, and the ecological restoration of devastated and degraded wetlands in Poyang Lake.
1. Introduction
Seed banks are fundamentally important components of wetland ecosystems [1,2,3]. At the species level, seed banks act as an ecological ‘bet-hedging’ strategy, providing many opportunities for germination and decreasing the levels of extinction risk [4,5]. At the community level, seed banks hold important proportions of “hidden and dark diversity”, which is absent from sampled vegetation, and these can contribute to abundance and species richness [6,7]. At the ecosystem level, seed banks increase the resilience of plant communities to physical disturbance by allowing rapid regeneration [8]. Consequently, seed banks play a vital role in wetland ecosystems, especially in vegetation dynamics.
Flooding duration plays an important role in seed bank composition and diversity [9,10]. It is a key factor in controlling wetland zonation through the effect of the seed bank composition, and the zonation manifests as wetland vegetation zonation [11,12]. Previous studies have assumed that flooding duration greatly affected seed banks in three Tibetan Plateau wetlands, in which the seed bank species’ richness decreased as the water depth increased without changes in seed density [13]. However, some researchers have concluded that the inundation duration affects both the seed bank species’ richness and density for those species that have different responses to flooding frequency [14,15,16]. Therefore, the effects of flooding duration on seed banks may differ in distinguished types of wetlands. Few studies have been conducted on the seed bank’s spatial distribution in high-amplitude inundation fluctuation wetlands.
Deeply buried seeds, which act as a persistent seed bank, play important roles in determining the resilience of vegetation and aiding in the restoration of wetlands [17,18]. However, germinable seed banks in terrestrial environments are often concentrated with shallow seed banks, which are assumed to linearly decrease with soil depth [1,19]. For example, the study conducted by Nicholson and Keddy (1983) revealed that 81% of seedlings emerged from the top of 2 cm of soil, while 11% of seedlings emerged from the next 2–4 cm, and both seedling and species richness rapidly declined with sediment depth in Matchedash Lake in Canada [20]. However, most authors rarely extended beyond depths of 10 cm [19]. Whether the seed bank can be seedlings after germination is not only determined by their characteristics, but also by their location in the soil [21,22]. The decline that resulted from the distribution of the seeds set in the sedimentation or disturbance of the substrate indicates that the longevity of the seeds in deeper layers is higher compared to species with more seeds in the surface layer [23,24]. In any regard, the impact of seed burial on germination and the recruitment of seedlings is probably important.
In this research, the seed bank in the freshwater wetlands of Poyang Lake was investigated. The Poyang Lake is an inland natural wetland with an annual high-amplitude water level that fluctuates from 7.12 m a.s.l. (as the Yellow Sea’s mean sea level) in the dry season (winter) to 18.96 m a.s.l. in the flood season (summer) [25]. In addition, the vegetation of Poyang Lake is mainly distributed between 10–15 m a.s.l. [26] Furthermore, the vegetation in the lake has zonation patterns in the dry season: from low water level gradient zones (low altitude zone) to high water level gradient zones (high altitude zone). These are established as a sparse vegetation belt (dominated by Polygonum criopolitanum, Cardamine lyrata, etc.), a sedge vegetation belt (dominated by several Carex species, especially C. cinerascens), a tall grass vegetation belt (dominated by Miscanthus sacchariflorus ssp. lutarioriparius and Phragmites australis), and a mesophytic grassland belt (dominated by Cynodon dactylon, Artemisia vulgaris, Carex argyi, etc.) with elevation from low to high, respectively [27]. As for the vegetation distribution pattern along the elevation gradients, it remains to be determined whether the corresponding soil seed bank produces a similar distribution pattern. Furthermore, it remains unknown whether there is a vertical pattern of soil seed banks along the burial depth. The aim of the present study was to quantify the role of the water level gradient and depth of burial on the germinable seed bank spatial distribution pattern. Hence, the following were predicted: (1) Seed bank species have higher abundance and richness in high water level gradient zones compared to low water level gradient zones, because the plant community in high water level gradient zones has higher seed production and diversity. This is because more surface soil is flushed out by water in low water level gradient zones compared to high zones on the lakeshore, which makes it harder to preserve the soil seed bank. (2) Seed bank species’ abundance and richness become minor as the sediment is buried deeper. That is, the seed lifespan becomes longer as the soil becomes deeper, but activated seed richness declines. (3) There is an additive effect between the inundation duration and soil depth. Because flooding duration is shorter as the elevation becomes higher, the harder it is to preserve the seed bank in soil. This difficulty in seed bank persistence becomes more severe according to burial depth.
2. Materials and Methods
2.1. Study Area
Poyang Lake is the largest freshwater lake in China, which is situated in the middle-to-lower reaches of the Yangtze River in northern Jiangxi Province, eastern China (115°49′ to 116°46′ E, 28°24′ to 29°26′ N). Based on the area below 21 m a.s.l., the lake covers an area of 3447 km2 [27,28]. It receives water from five main branches: Ganjiang River, Fuhe River, Xinjiang River, Raohe River and Xiushui River. The water is discharged to the Yangtze River through a channel in the north, and the sedimentation rate in the littoral zone is about 0.12 cm/a to 0.31 cm/a [29]. The water level fluctuates naturally with the flooding–drought cycle inter-annually in different seasons. In early May, the water levels of the lake rise as the watershed reaches the rainy season. This inundation lasts for five months until late September. The area of open water exceeds to 3000 km2 in this period, and the vegetation is dominated by submerged plants. In October, flooding declines as the lake approaches the dry season. In this period, the lake water surface area shrinks to 1000 km2, and the wetland vegetation develops a belt vegetation pattern along the shoreline at different elevations, as previously described in the present study. For the periodic wet–dry cycle, the lake water level fluctuates with a nearly 16-m difference between the highest and lowest mean monthly water levels. Thus, the lake’s water level’s inter-annual fluctuations create various heterogeneous habitats for approximately 500,000 migratory birds during the winter period, which makes Poyang Lake a world-famous wetland for winter migratory birds [30].
2.2. Data Collection
Three sites in Poyang Lake were selected for replication, and these three sites were located at similar elevations and had similar vegetation patterns (Figure 1): site 1, in Banghu at the Poyang Lake National Nature Reserve; site 2, in Donghu at the Nanji Wetland National Nature Reserve; site 3, in the east of the Nanji Wetland National Nature Reserve, which adjoins the Kangshan River. The elevation of all sites changes from 11 m to nearly 15 m. The dominant species of standing vegetation include Cynodon dactylon, Artemisia vulgaris, Miscanthus sacchariflorus ssp. lutarioriparius, Phragmites australis, Carex cinerascens, etc. Five water level gradient zones could be distinguished according to wetland vegetation and water level fluctuation features (Figure 2, Table 1). These five water level gradient zones also represent a set of inundation duration gradients in Poyang Lake. Among these gradients, the water level in gradient zone 1 had the longest inundation duration, while the water level in gradient zone 5 had the shortest inundation duration, and the location and inundation duration of other three gradient zones were between these two gradient zones.
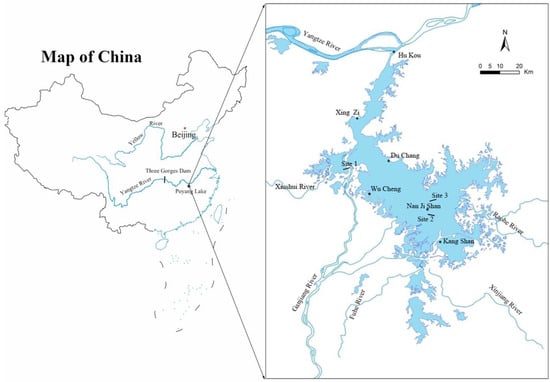
Figure 1.
Poyang Lake study area and locations of the three sites: site 1 in Banghu at Poyang Lake National Nature Reserve; site 2 in Donghu at Nanji Wetland National Nature Reserve; site 3 in east of Nanji Wetland National Nature Reserve, which adjoins the Kangshan River. The blue line indicates the five main rivers. The blue area indicates the range of Poyang Lake.
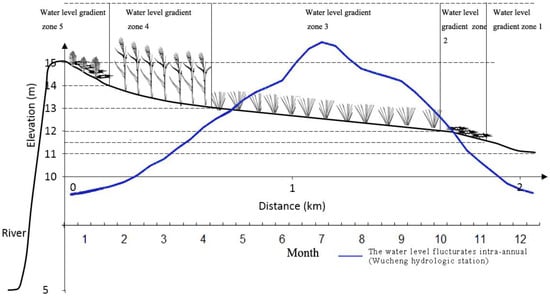
Figure 2.
Diagram of a typical cross-section containing the five water level gradient zones along the distance to the lakeshore and annual hydrological regime variation of Poyang Lake. Gradient 1 was a bare soil belt; gradient 2 was a sparse grassland belt; gradient 3 was a sedge grassland belt; gradient 4 was a tall grass land belt; and gradient 5 was a weed grassland belt. The blue solid line indicates that the water level fluctuates intra-annually.

Table 1.
The elevation range and vegetation characteristics of the five water level gradient zones in the present study.
In late October, before the migratory birds arrived, the soil seed bank samples were collected along the water level gradients at three sites. At each site, three transects were selected along the water level gradient zone as replications, and the distance of these three transects were approximately 100 m. At each transect, one 1 × 1 m2 sampling plot was randomly selected in every water level gradient zone. Thus, the total number of sampling plots was 42, because site 3 did not have a water level gradient zone 5. In each plot, five soil cores were randomly collected using a steel bulk density ring (Φ20 cm × 5 cm) and mixed as one soil sample. Three soil layers were collected at depths of 0–5, 5–10 and 10–15 cm, respectively. Hence, a total of 126 soil seed bank samples were collected.
A seedling emergence study was conducted in the Hu Hsien-Hsu Garden glasshouse facility at Nanchang University to estimate the seed bank composition and density. The glasshouse temperature ranged between 18 °C and 25 °C. Coarse debris and visible rhizomes were removed from the soil samples, and the soil was mixed thoroughly before the seedling emergence experiment. Then, each soil sample was spread onto 3 cm of vermiculite in a 22.5 × 15.0 × 7.0 cm3 deep plastic container, and the soil sample depth was approximately 1 cm. The trays in the greenhouse were randomly distributed to avoid any bias in the placement. The experiments lasted for four months, in which the soil samples were kept moist during the first two months, while 2–3 cm of water was maintained above the soil surface in the subsequent two months to promote more submersed macrophyte sprouting. The emerging seedlings were tallied by species and removed as they were identified to prevent crowding. The nomenclature was based on WFO [31].
2.3. Data Analysis
Soil seed bank species rank abundance curves (RAC) were established to compare the abundance of seeds at the five water levels and the total seedlings that emerged. In order to assess the overall difference in species richness, one- and two-way ANOVA constructed the diversity index and the density of the germinable seed bank between different water level gradients and sediment depths, followed by the post hoc Holm–Sidak method [32]. Seedling number data were Box–Cox transformed (λ = 0.85) for analysis to meet the ANOVA normality assumptions. All ANOVA tests were conducted using Sigmaplot 11.0 from Systat Software, San Jose, CA. In order to investigate species diversity changes at different water level gradients and burial depths, Shannon–Wiener diversity index (H = −∑pilnpi) was used [33], where pi was the abundance of the ith species. Meanwhile, species–area curves were used to illustrate the cumulative species richness as a function of the area sampled, and these varied among sediment depths. Sample-based calculations were used for the species–area curves, which also provided the expected species richness based on the mean number of individuals in a random sample without replacement [34,35]. Expected species richness was estimated following 1000 randomizations using bias-corrected Chao2 in EstimateS version 9.1.0 [36]. These results were expressed in seeds/m2 for convenience.
3. Results
3.1. Composition of the Soil Seed Bank
A total of 7090 individual seedlings emerged in all soil seed bank samples obtained within 0–15 cm in depth, representing 49 different species from 21 families and 36 genera (Table S1). Most of the germinated seedlings were identified according to species, except for some true sedge seedlings that could be from several Carex species. Among these, one fern species from Ceratopteridaceae emerged, with the other 48 species from angiosperm. Among these species, nine families represented ≥2 taxa: Asteraceae (seven species), Polygonaceae (seven species), Cyperaceae (five species), Brassicaceae (four species), Poaceae (four species), Scrophulariaceae (four species), Apiaceae (two species), Hydrocharitaceae (two species), and Rosaceae (two species). Most of the seedlings germinated from six species, which were Cardamine yrate, Laphangium affine, Eleocharis valleculosa var. setosa, Rumex dentatus, Carex spp., and Phalaris arundinacea. These six species contributed 67.38% of all seedlings, and emerged in most of the samples. Among all the germinated species, 40.82% were annuals, 12.24% were biennials, and 46.94% were perennial herbs. In these seedlings, three invasive species were found, including Soliva anthemifolia, Cotula anthemoides, Daucus carota, etc. A complete list of all species identified within the seed bank across all samples, including the information on their families and life form, is provided in the supporting material.
3.2. Rank Abundance Relationship
In terms of the results (Figure 3), the RAC had similar steepness for all five water levels, and the total showed the seed banks were dominated by few abundant species. Water level gradient zone 1 was dominated by Cardamine lyrata, Limosella aquatica and Carex spp., which accounted for 55.07% of the total number of seeds in that gradient zone. Water level gradient zone 2 was dominated by C. lyrata, L. aquatica and Vallisneria natans, which accounted for 62.21% of the total number of seeds in this gradient zone. Water level gradient zone 3 was dominated by C. lyrata, Eleocharis valleculosa var. setosa and Alopecurus aequalis, which accounted for 61.37% of the total number of seeds in this gradient zone. Water level gradient zone 4 was dominated by Laphangium affine, C. lyrata, E. valleculosa var. setosa and Carex cinerascens, which accounted for 62.08% of the total number of seeds in this gradient zone. Water level gradient zone 5 was dominated by L. affine, Phalaris arundinacea and Carex argyi, which accounted for 66.47% of the total number of seeds in this gradient zone. In addition, Rumex dentatus, A. aequalis, V. natans, C. lyrata and Carex spp. occurred in all water level gradient zones and sediment depths. And the C. lyrata, Carex cinerascens, Carex argyi, A. aegualis and A. vulgaris were the dominant species of the standing vegetation.
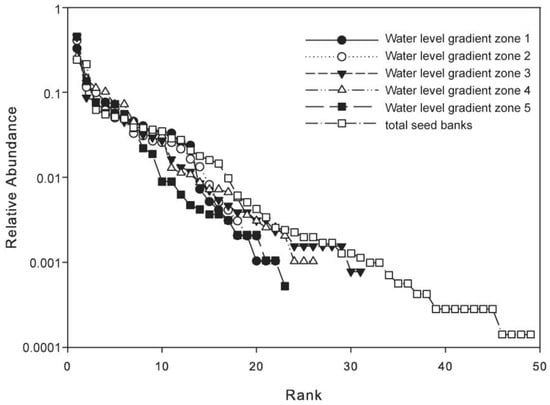
Figure 3.
Rank abundance distribution curves of seed bank species in the five water level gradient zones.
3.3. Seed Bank Density
In the greenhouse experiment, the estimated mean seed bank density in soil at a depth of 0–15 cm was 16,881 ± 1616 (M ± SE) seeds/m2, and the mean seed bank density per m2 increased with the increase in water level in soil at a depth of 0–15 cm (F4,37 = 8.68, p < 0.001; Figure 4D). The lowest density was water level gradient zone 1, which had a density of 10,733 ± 1765 seeds/m2, while the highest density was 31,867 ± 4358 seeds/m2 in water level gradient zone 5. The highest density was nearly three times the lowest density. When different soil layers were separately analyzed, the seed density per m2 significantly differed among the five water level gradient zones in the three soil layers (Figure 4A–C), showing an increasing trend as the elevation increased along each water level gradient zone. In addition, the seed density significantly differed in these three soil layers (F2,123 = 8.48, p < 0.001), showing that the seed density exhibited a decreasing trend according to the sediment depth. The highest density was the top 0–5 cm soil layer, which had a density of 7607 ± 790 seeds/m2. This was nearly two times that of the 10–15 cm soil layer, along with the density of the 5–10 cm and 10–15 cm soil layers, which were 5419 ± 589 seeds/m2 and 3855 ± 790 seeds/m2, respectively. However, there were no interaction effects observed between water level and sediment depth (Table 2).
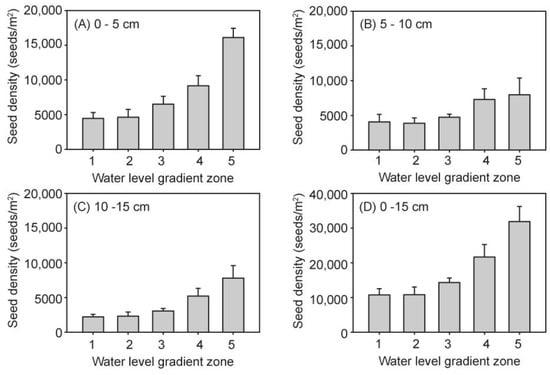
Figure 4.
Seed bank density in the five water level gradients of each soil layer (A–D).

Table 2.
Two-way ANOVA for seed bank density.
3.4. Diversity of the Seed Bank
The species richness and Shannon–Wiener index of the seed banks complexly changed with the increase in water level, while exhibiting a declining trend with the sediment depth. However, there were no interactions between the water level and soil layers (Table 3; Figure 5 and Figure 6). In the 0–15 cm soil layers, the highest in species richness was at water level 5 (8.67 ± 1.17), while the lowest was at water level 2 (6.00 ± 0.75) (Figure 5D). Similarly, the lowest Shannon–Wiener index was 0.85 ± 0.14 at water level 2, but there was no significant differences among the other four water level gradients. When three soil layers at the different water level gradients were separately analyzed, the species richness of all samples exhibited an increasing trend with the increase in water level. However, the comparison of the different seed banks of these three soil layers revealed that the highest was the top 0–5 cm soil seed bank, which had a species richness of 5.50 ± 0.35. This was followed by the 5–10 cm soil layer with a species richness of 4.3 ± 0.26, and the lowest of species richness was the 10–15 cm soil layer (3.67 ± 0.27). In the same manner, the significantly effects of the buried sediment Shannon–Wiener index revealed different soil layers, in which the maximum Shannon–Wiener index was 1.18 ± 0.05 in 0–5 cm, while the minor index was 0.88 ± 0.07 in 10–15 cm.

Table 3.
Comparison via two-way ANOVA to understand the effects of water level and burial depth on species richness and Shannon–Wiener index.
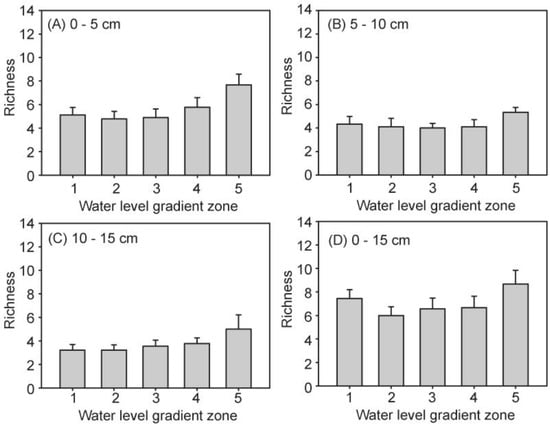
Figure 5.
Seed bank species richness in the five water level gradients of the three soil layers (A–D).
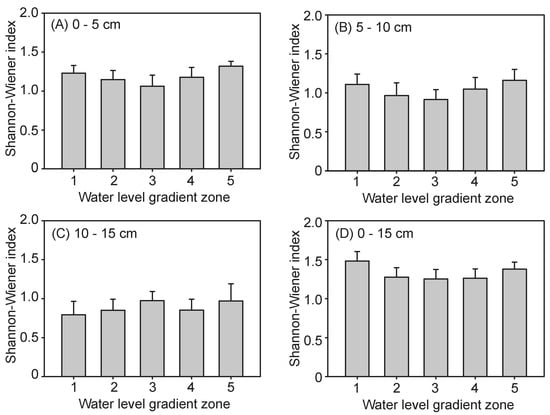
Figure 6.
Seed bank Shannon–Wiener index at the five water level gradients of each soil layer (A–D).
Patterns of cumulative species richness with sediment depth were established to compare the species–area relationship (Figure 7). Across the wetland landscape, the number of species increased with the area at a faster rate in the 0–5 cm soil layer compared to the following two layers. Ultimately, within a cumulative sampling area of 42 m2, a total of 43, 31 and 26 species emerged from the seed bank at the 0–5, 5–10 and 10–15 cm soil layers, respectively. This suggests that the composition of the germinable seed bank is quite variable with depth.
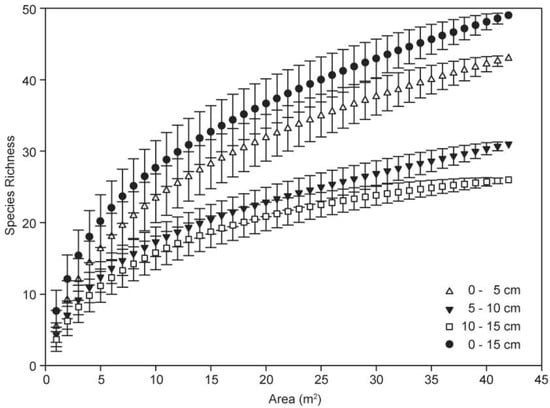
Figure 7.
Species–area curves of seed banks depending on burial depth.
4. Discussion
4.1. Seed Bank Related to Inundation Duration Gradients
Wetland seed bank composition can be greatly affected by seasonal flooding [1,36]. The distribution of the soil seed bank was examined from a range of water level gradients with different inundation durations and seasonal flooding regimes. The seed density and species richness of the seed bank were found to increase with increasing elevation, which largely supports the hypothesis in the introduction. The seed bank input and output usually determine the composition and density of the seed bank. The input of a local seed bank is determined by seed rain and hydrochory, in which local dispersal predominates may make a major contribution [16,36]. But the dominant species of seed bank and standing vegetation were different (Table 1 and Table S1). Water level fluctuation may regulate the species composition and spatial distribution of the seed bank, along with their similarities with existing vegetation [12,37]. Seed rain induces a correlation between seed bank and aboveground vegetation, and the hydrochory allows some species to be distributed in all water level gradients, such as Cardamine lyrata, Alopecurus aequalis, Rumex dentatus, etc. Seed bank size and composition may be determined through several environmental factors, especially through the hydrological regime. In hydrologically stable wetlands, seed bank species richness is minor compared to hydrologically instable wetlands [38]. In wetlands with fluctuating water level conditions, hydrological variations act as flooding disturbances affecting the seed bank composition and structure. That is, the richness of the seed bank species in the Tibetan Plateau Wetland is minor [13] compared to that of the seed bank species in the present study. In the present study, water level gradients 1 and 5 had more frequent fluctuations in different periods. The frequent fluctuation of gradient 1 occurred in low water level periods between October to early May, but gradient 5 occurred in high water level periods between May to late September. Hence, the seed rain produced before the flood season may be delivered to the gradient 5 zone and preserved. In contrast, few seed rain occurs after the flood season in Poyang Lake. Hence, the seed enters in addition to the frequent water fluctuation. Furthermore, the top soil may be regularly flushed by water, and the seed bank in the top soil may be carried off with the water.
4.2. Seed Bank Related to Soil Depth
Many studies have examined the vertical distribution of soil seed banks, and some authors have considered shallow seed banks. In any case, a steady decrease in the density and richness of seeds with depth has been found in various environments, including lakeshore, marshes and so on [1,17,20]. In the present results, seed density and species richness decreased as the sediment depth increased, supporting the prediction in the introduction. As previously discussed, several factors influence the vertical development of seed banks in these wetland systems. However, these seeds were deeply buried in swamps and prairie marshes, with only 20–50% occurring in the top 5 cm [1,20]. Meanwhile, O’Donnell et al. (2014) found that seed abundance and species richness were highly variable with depth, and the greatest seed abundance was preserved at the depth of 20–30 cm in the bar and bench of riparian wetlands [39]. Furthermore, Espinar et al. (2005) assumed bimodal seed abundance and species richness in depth distribution [40]. Seed bank size, composition and depth distribution are determined by external environmental and internal biological factors. In terms of external environmental factors, the vertical distribution of seeds in shorelines may be determined through hydrologically related factors. The inundation of shorelines may result in sediment deposition, reworking and erosion. Sediment motion results in the delivery of seed banks via hydrochory, such as seeds brought by deposition, while reworking disturbs seeds and erosion removes them. Meanwhile, seeds may also be lost due to seed germination, predation and mortality. In addition, plant growth at high elevations may have time to complete an entire lifecycle with the production of seeds, but this is not possible to the same extent at lower elevations. Some species have shorter seed longevity, which means they would not be preserved in deep soil. Hence, the seed bank density and diversity in deep soil were lower than in the top soil.
4.3. Non-Additive Effect of Water Level and Soil Depth
Most studies have respectively investigated soil bank spatial distributions according to environmental gradients and vertical depth. However, few studies have examined the interaction effects between water level gradients and burial depths. Eager et al. (2013) developed a mechanistic seed bank model that assumed there is no relationship between disturbance frequency and vertical distribution [41]. When we considered inundation and sediment burial as natural stochastic disturbances, the result supports a density-dependent stochastic integral projection model [42]. The seed bank density increased along with the water level gradient (Figure 4), where water level gradient zones 1 to 5 embodied a rise in flooding inundation frequency [43]. Meanwhile, seed abundance, species richness and the diversity index analyzed using two-way ANOVA revealed that there was no additive effect from water level and burial depth in seed bank distribution. Hence, the inundation duration and sediment burial separately affects these wetland soil seed banks. Once these seed banks are buried by sediment, these seeds are independently preserved without the influence of the water level. Then, the fate of the seed in the soil is determined by seed longevity [44]. However, the top soil seed bank is influenced by the fluctuation in surface water level, leading to the distribution of the seed bank according to the water level gradients. Indeed, the deep soil seed was the historically preserved top soil seed bank, but the interaction between water level and buried depth was effectively under-detected.
5. Conclusions
Understanding how seed banks distribute along a water level gradient and burial depth is an important step in understanding wetland vegetation dynamics and ecosystem resilience. The Poyang Lake wetlands provided an opportunity to see how water level gradient zone changes and buried sediments influence below-ground seed banks. The present findings show that the density and species richness of germinable seed banks increased with the increase in water level and decrease in burial depth, but without diversity. It was also found that there was no additive effect from water level gradient zone and soil burial depth on the distribution of seed banks, and these two factors separately influenced these soil seed banks. These findings add a regional case in the study of worldwide wetlands soil seed banks, which can be directly applied to the ecosystem management and ecological restoration of Poyang Lake.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d16010003/s1, Table S1: Seeds/m2 of species in soil seed banks of the five water level gradients in Poyang Lake.
Author Contributions
Conceptualization, Y.L. and G.G.; methodology, N.L.; investigation, N.L. and C.J.; writing—original draft preparation, Y.L., C.J. and Q.C.; writing—review and editing, Y.L. and Q.C.; visualization, Y.L., N.L. and C.J.; funding acquisition, Y.L. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32260290, 32360285) and the Youth Science Fund Project in Jiangxi Province (2018BA214004).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
We are extremely grateful to Binhua Hu, Huicai Guo, Songxian Wan and Jiandong Wu, Managers of the Poyang Lake wetlands, for access to the sample sites and site information. We also thank Guoqing Zou for helping in the greenhouse facility.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leck, M.A. Wetland seed banks. In Ecology of Soil Seed Banks; Leck, M.A., Parker, V.T., Simpson, R.L., Eds.; Academic Press Inc.: San Diego, CA, USA, 1989; pp. 283–305. [Google Scholar]
- Wambugu, P.W.; Nyamongo, D.O.; Kirwa, E.C. Role of seed banks in supporting ecosystem and biodiversity conservation and restoration. Diversity 2023, 15, 896. [Google Scholar] [CrossRef]
- Zhu, T.; Fang, Q.; Jia, L.; Zou, Y.; Wang, X.; Qu, C.; Yu, J.; Yang, J. Diversity of soil seed bank and influencing factors in the nascent wetland of the Yellow River Delta. Front. Plant. Sci. 2023, 14, 1249139. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.K.J.; Auld, T.D.; Denham, A.J. Climate change and bet-hedging: Interactions between increased soil temperatures and seed bank persistence. Global Chang. Biol. 2009, 15, 2375–2386. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.; Pyšek, P. Soil seed banks under a warming climate. In Plant Regeneration from Seeds: A Global Warming Perspective; Baskin, C., Baskin, J., Eds.; Academic Press: London, UK, 2022; pp. 285–298. [Google Scholar]
- Pärtel, M.; Szava-Kovats, R.; Zobel, M. Dark diversity: Shedding light on absent species. Trends. Ecol. Evol. 2011, 26, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Pärtel, M. Community ecology of absent species: Hidden and dark diversity. J. Veg. Sci. 2014, 25, 1154–1159. [Google Scholar] [CrossRef]
- Tracey, A.; Aarssen, L. Resident species with larger size metrics do not recruit more offspring from the soil seed bank in old-field meadow vegetation. J. Ecol. 2019, 107, 1067–1078. [Google Scholar] [CrossRef]
- Keddy, P.A.; Reznicek, A.A. Great lakes vegetation dynamics: The role of fluctuating water levels and buried seeds. J. Great Lakes Res. 1986, 12, 25–36. [Google Scholar] [CrossRef]
- Čížková, E.; Navrátilová, J.; Martinát, S.; Navrátil, J.; Frazier, R.J. Impact of water level on species quantity and composition grown from the soil seed bank of the inland salt marsh: An ex-situ experiment. Land 2020, 9, 533. [Google Scholar] [CrossRef]
- Leck, M.A.; Simpson, R.L. Tidal freshwater wetland zonation: Seed and seedling dynamics. Aquat. Bot. 1994, 47, 61–75. [Google Scholar] [CrossRef][Green Version]
- Greulich, S.; Chevalier, R.; Villar, M. Soil seed banks in the floodplain of a large river: A test of hypotheses on seed bank composition in relation to flooding and established vegetation. J. Veg. Sci. 2019, 30, 732–745. [Google Scholar] [CrossRef]
- Ma, M.; Ma, Z.; Du, G. Effects of water level on three wetlands soil seed banks on the Tibetan Plateau. PLoS ONE 2014, 9, e101458. [Google Scholar] [CrossRef] [PubMed]
- Elsy-Quirk, T.; Leck, M.A. Patterns of seed bank and vegetation diversity along a tidal freshwater river. Am. J. Bot. 2015, 102, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Padonou, E.A.; Akakpo, B.A.; Tchigossou, B.; Djossa, B. Methods of soil seed bank estimation: A literature review proposing further work in Africa. iForest 2022, 15, 121–127. [Google Scholar] [CrossRef]
- Li, T.; Zhu, Z.; Shao, Y.; Chen, Z.; Roß-Nickoll, M. Soil seedbank: Importance for revegetation in the water level fluctuation zone of the reservoir area. Sci. Total. Environ. 2022, 829, 154686. [Google Scholar] [CrossRef] [PubMed]
- Bonis, A.; Lepart, J. Vertical structure of seed banks and the impact of depth of burial on recruitment in two temporary marshes. Vegetatio 1994, 112, 127–139. [Google Scholar] [CrossRef]
- Tóth, Á.; Deák, B.; Tóth, K.; Kiss, R.; Lukács, K.; Rádai, Z.; Godó, L.; Borza, S.; Kelemen, A.; Miglécz, T.; et al. Vertical distribution of soil seed bank and the ecological importance of deeply buried seeds in alkaline grasslands. PeerJ 2022, 10, e13226. [Google Scholar] [CrossRef]
- Ling, J.E.; Wen, L.; Ellis, B.; Krogh, M. Spatial heterogeneity of the seed bank at a peat lake in Australia. Mar. Freshw. Res. 2022, 73, 774–791. [Google Scholar] [CrossRef]
- Nicholson, A.; Keddy, P.A. The depth profile of a shoreline seed bank in Matchedash Lake, Ontario. Can. J. Bot. 1983, 61, 3293–3296. [Google Scholar] [CrossRef]
- Jahantab, E.; Yazdanshenas, H.; Saray, A.A.; Matinkhah, S.; Khazaei, M. Sediment burial depth, seedling emergence, and height as affected by animal trampling in marl soils. Plant Ecol. 2022, 223, 493–506. [Google Scholar] [CrossRef]
- Ren, A.; Hu, D.-Y.; Qi, P.-X.; Zhang, S.-C.; Gao, H.-M.; Mickan, B.S.; Xiong, Y.-C.; Yuan, L.-Y. Buffering effects of the soil seed bank on annual plant community composition after wetland drying. Land Degrad. Dev. 2022, 34, 1601–1611. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, J.G. Seed bank has potential for the restoration of insectivorous plants in Janggun montane wetland. Ecol. Eng. 2022, 182, 106728. [Google Scholar] [CrossRef]
- Mašková, T.; Poschlod, P. Soil seed bank persistence across time and burial depth in calcareous grassland habitats. Front. Plant Sci. 2022, 12, 790867. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lai, X.; Wu, X.; Chen, Y. Dry-season water level shift induced by channel change of the river–lake system in China’s largest freshwater lake, Poyang Lake. Wetlands 2022, 42, 112. [Google Scholar] [CrossRef]
- Huang, W.; Hu, T.; Mao, J.; Montzka, C.; Bol, R.; Wan, S.; Li, J.; Yue, J.; Dai, H. Hydrological drivers for the spatial distribution of wetland herbaceous communities in Poyang Lake. Remote Sens. 2022, 14, 4870. [Google Scholar] [CrossRef]
- Ji, W.; Ge, G.; Li, H.; Zhan, Y. Poyang Lake: Topography, Hydrography and Vegetation; Science Press: Beijing, China, 2017. [Google Scholar]
- Mei, X.; Dai, Z.; Fagherazzi, S.; Chen, J. Dramatic variations in emergent wetland area in China’s largest freshwater lake, Poyang Lake. Adv. Water Resour. 2016, 96, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Liu, Y.; Guo, H.; Chen, G.; Fu, Z.; Fu, Y.; Ge, G. Effects of sediment types on the distribution and diversity of plant communities in the Poyang Lake Wetlands. Diversity 2022, 14, 491. [Google Scholar] [CrossRef]
- Debela, M.T.; Wu, Q.; Chen, L.; Sun, X.; Xu, Z.; Li, Z. Composition and diversity of over-wintering aquatic bird community on Poyang Lake, China. Diversity 2020, 12, 308. [Google Scholar] [CrossRef]
- WFO. World Flora Online. 2023. Available online: http://www.worldfloraonline.org (accessed on 21 October 2023).
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Gotelli, N.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples Version 9. User’s Guide and Application. 2013. Available online: https://www.robertkcolwell.org/pages/1407-estimates (accessed on 21 October 2023).
- Gaudichet, C.; Greulich, S.; Grellier, S.; Rodrigues, S. Effect of flooding gradient on soil seedbank and standing vegetation in a disconnecting side channel of the Loire River (France). Hydrobiologia 2022, 849, 1383–1396. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Wu, Y.; Huang, Y. Seed rain and seed bank of a draw-down zone and their similarities to vegetation under the regulated water-level fluctuation in Xiangxi River. J. Freshw. Ecol. 2020, 35, 57–71. [Google Scholar] [CrossRef]
- Nielsen, D.L.; Podnar, K.; Watts, R.J.; Wilson, A.L. Empirical evidence linking increased hydrologic stability with decreased biotic diversity within wetlands. Hydrobiologia 2013, 708, 81–96. [Google Scholar] [CrossRef]
- O’Donnell, J.; Fryirs, K.; Leishman, M.R. Digging deep for diversity: Riparian seed bank abundance and species richness in relation to burial depth. Freshw. Biol. 2014, 59, 100–113. [Google Scholar] [CrossRef]
- Espinar, J.L.; Thompson, K.; Garcia, L.V. Timing of seed dispersal generates a bimodal seed bank depth distribution. Am. J. Bot. 2005, 92, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Eager, E.A.; Haridas, C.V.; Pilson, D.; Rebarber, R.; Tenhumberg, B. Disturbance frequency and vertical distribution of seeds affect long-term population dynamics: A mechanistic seed bank model. Am. Nat. 2013, 182, 180–190. [Google Scholar] [CrossRef]
- Eager, E.A.; Rebarber, R.; Tenhumberg, B. Modeling and Analysis of a Density-Dependent Stochastic Integral Projection Model for a Disturbance Specialist Plant and Its Seed Bank. Bull. Math. Biol. 2014, 76, 1809–1834. [Google Scholar] [CrossRef]
- Mu, S.; Yang, G.; Xu, X.; Wan, R.; Li, B. Assessing the inundation dynamics and its impacts on habitat suitability in Poyang Lake based on integrating Landsat and MODIS observations. Sci. Total Environ. 2022, 834, 154936. [Google Scholar] [CrossRef]
- Abbas, A.M.; Pickart, A.J.; Goldsmith, L.M.; Davenport, D.N.; Newby, B.; Muñoz-Rodríguez, A.F.; Grewell, B.J.; Castillo, J.M. Seed bank persistence of a South American cordgrass in invaded northern Atlantic and Pacific Coast estuaries. AoB Plants 2021, 13, plab014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).