Butterfly Diversity in a Rapidly Developing Urban Area: A Case Study on a University Campus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Definition of Seasons
2.3. Transect Settings
2.4. Butterfly Observation and Sampling
2.5. Data Analyses
2.5.1. Species Assemblage and Conservation Status
2.5.2. Diversity and Seasonal Dynamics
3. Results
3.1. Species Assemblage and Conservation Status
3.1.1. Species Assemblage
3.1.2. Species Diversity
3.1.3. Conservation Status of Surveyed Species
3.2. Spatial Variation in Butterfly Community
3.2.1. Species Assemblage and Habitat Associations
3.2.2. Differences in Habitat Diversity
3.3. Temporal Variation in Butterfly Community
3.3.1. Species Assemblage and Seasonal Associations
3.3.2. Differences in Seasonal Diversity
3.3.3. Temporal Dynamics of Butterfly Communities
4. Discussion
4.1. Status of Urban/Campus Butterfly Composition
4.2. Urban/Campus Disturbance and Butterfly Diversity
4.3. Citizen Science and Butterfly Biodiversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, M.; Garcia, J.; Thomas, F.; Howard, S.; Chua, J.; Tscheulin, T.; Dorin, A.; Nielsen, A.; Dyer, A. Insects in the City: Does Remnant Native Habitat Influence Insect Order Distributions? Diversity 2021, 13, 148. [Google Scholar] [CrossRef]
- Park, S.C.; Han, B.H. Using the City Biodiversity Index as a Method to Protect Biodiversity in Korean Cities. Sustainability 2021, 13, 11284. [Google Scholar] [CrossRef]
- Parker, S.S. Incorporating critical elements of city distinctiveness into urban biodiversity conservation. Biodivers. Conserv. 2015, 24, 683–700. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- Lacoeuilhe, A.; Prévot, A.; Shwartz, A. The social value of conservation initiatives in the workplace. Landsc. Urban Plan. 2017, 157, 493–501. [Google Scholar] [CrossRef]
- Simkin, R.D.; Seto, K.C.; McDonald, R.I.; Jetz, W. Biodiversity impacts and conservation implications of urban land expansion projected to 2050. Proc. Natl. Acad. Sci. USA 2022, 119, e2117297119. [Google Scholar] [CrossRef]
- Bashan, D.; Colléony, A.; Shwartz, A. Urban versus rural? The effects of residential status on species identification skills and connection to nature. People Nat. 2021, 3, 347–358. [Google Scholar] [CrossRef]
- Chawla, L. Childhood nature connection and constructive hope: A review of research on connecting with nature and coping with environmental loss. People Nat. 2020, 2, 619–642. [Google Scholar] [CrossRef]
- Di Cecco, G.; Barve, V.; Belitz, M.; Stucky, B.; Robert, G.; Hurlbert, A. Observing the Observers: How Participants Contribute Data to iNaturalist and Implications for Biodiversity Science. BioScience 2021, 71, 1179–1188. [Google Scholar] [CrossRef]
- Rochat, E.; Manel, S.; Deschamps-Cottin, M.; Widmer, I.; Joost, S. Persistence of butterfly populations in fragmented habitats along urban density gradients: Motility helps. Heredity 2017, 119, 328–338. [Google Scholar] [CrossRef]
- Weisser, W.W.; Hensel, M.; Barath, S.; Culshaw, V.; Grobman, Y.J.; Hauck, T.E.; Joschinski, J.; Ludwig, F.; Mimet, A.; Perini, K.; et al. Creating ecologically sound buildings by integrating ecology, architecture and computational design. People Nat. 2023, 5, 4–20. [Google Scholar] [CrossRef]
- Zambrano, L.; Aronson, M.F.J.; Fernandez, T. The Consequences of Landscape Fragmentation on Socio-Ecological Patterns in a Rapidly Developing Urban Area: A Case Study of the National Autonomous University of Mexico. Front. Environ. Sci. 2019, 7, 152. [Google Scholar] [CrossRef]
- Chandler, M.; See, L.; Buesching, C.D.; Cousins, J.A.; Gillies, C.; Kays, R.W.; Newman, C.; Pereira, H.M.; Tiago, P. Involving Citizen Scientists in Biodiversity Observation. In The GEO Handbook on Biodiversity Observation Networks; Walters, M., Scholes, R.J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 211–237. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Si, X.; Feng, G.; Slik, F.; Zhang, J. University campuses as valuable resources for urban biodiversity research and conservation. Urban For. Urban Green. 2021, 64, 127255. [Google Scholar] [CrossRef]
- Moerman, D.E.; Estabrook, G.F. The botanist effect: Counties with maximal species richness tend to be home to universities and botanists. J. Biogeogr. 2006, 33, 1969–1974. [Google Scholar] [CrossRef]
- Bagnolini, G.; Da Costa, G.; Gerino, M.; Mathias, R.; Cecile, T. Multidisciplinarity for Biodiversity Management on Campus through Citizen Sciences; IEEE: San Francisco, CA, USA, 2017. [Google Scholar] [CrossRef]
- Nautiyal, S. Plant Biodiversity and Its Conservation in Institute for Social and Economic Change (ISEC) Campus, Bangalore: A Case Study. J. Biodivers. 2011, 2, 9–26. [Google Scholar] [CrossRef]
- Wan, H.; Lin, Z.; Wu, M.; Zhang, M.; He, H.; Wu, C.; Xu, N.; Ma, Z. Realization of a Controlled Campus Ecosystem. Acta Ecol. Sin. 2015, 35, 7172–7181. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Poore, A.G.B.; Hofmann, M.; Roberts, C.J.; Pereira, H.M. Large-bodied birds are over-represented in unstructured citizen science data. Sci. Rep. 2021, 11, 19073. [Google Scholar] [CrossRef]
- Garcia-Cegarra, A.M.; Toro, F.; Gonzalez-Borasca, V. Citizen science as a tool to assess cetacean diversity in the Atacama Desert coast. Ocean. Coast. Manag. 2021, 213, 105858. [Google Scholar] [CrossRef]
- Guthula, V.B.; Shrotriya, S.; Nigam, P.; Goyal, S.P.; Mohan, D.; Habib, B. Biodiversity significance of small habitat patches: More than half of Indian bird species are in academic campuses. Landsc. Urban Plan. 2022, 228, 104552. [Google Scholar] [CrossRef]
- Wang, W.L.; Suman, D.O.; Zhang, H.H.; Xu, Z.B.; Ma, F.Z.; Hu, S.J. Butterfly Conservation in China: From Science to Action. Insects 2020, 11, 661. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Wang, W.L.; Yu, Q.; Xing, D.H.; Xu, Z.B.; Duan, K.; Zhu, J.Q.; Zhang, X.; Li, Y.P.; Hu, S.J. Spatial Distribution of Pollinating Butterflies in Yunnan Province, Southwest China with Resource Conservation Implications. Insects 2020, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Kremer, P.; Hamstead, Z.; Haase, D.; McPhearson, T.; Frantzeskaki, N.; Andersson, E.; Kabisch, N.; Larondelle, N.; Rall, E.L.; Voigt, A.; et al. Key insights for the future of urban ecosystem services research. Ecol. Soc. 2016, 21, 29. [Google Scholar] [CrossRef]
- Ouin, A.; Holland, G.J.; Tessier, M.; Clarke, R.H.; Bennett, A.F. Do butterfly communities benefit from woodland restoration in rural environments? A landscape perspective from south-eastern Australia. Restor. Ecol. 2022, 30, e13478. [Google Scholar] [CrossRef]
- Hajibayova, L.; Coladangelo, L.P.; Soyka, H.A. Exploring the invisible college of citizen science: Questions, methods and contributions. Scientometrics 2021, 126, 6989–7003. [Google Scholar] [CrossRef]
- Oberhauser, K.; LeBuhn, G. Insects and plants: Engaging undergraduates in authentic research through citizen science. Front. Ecol. Environ. 2012, 10, 318–320. [Google Scholar] [CrossRef]
- Lehmann, I. Inspiration from the Kunming-Montreal Global Biodiversity Framework for SDG 15. Int. Environ. Agreem. Politics Law Econ. 2023, 23, 207–214. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Z.F.; Wang, R.X. Research of land cover classification based on ecognition using obeject-oriented method: A case study in Chenggong Campus of Yunnan University. Yunnan Geogr. Environ. Res. 2013, 25, 76–79. [Google Scholar] [CrossRef]
- Marselle, M.R.; Hartig, T.; Cox, D.T.C.; de Bell, S.; Knapp, S.; Lindley, S.; Triguero-Mas, M.; Böhning-Gaese, K.; Braubach, M.; Cook, P.A.; et al. Pathways linking biodiversity to human health: A conceptual framework. Environ. Int. 2021, 150, 106420. [Google Scholar] [CrossRef]
- Habel, J.C.; Gossner, M.M.; Schmitt, T. Just beautiful?! What determines butterfly species for nature conservation. Biodivers. Conserv. 2021, 30, 2481–2493. [Google Scholar] [CrossRef]
- Daniels, J.; Hill, G.; Rossetti, K.; Sanchez, S.; Hornfeldt, J. At-Risk Butterfly Captive Propagation Programs to Enhance Life History Knowledge and Effective Ex Situ Conservation Techniques. J. Vis. Exp. 2020, 156, e60591. [Google Scholar] [CrossRef]
- Liang, H.; He, Y.-D.; Theodorou, P.; Yang, C.-F. The effects of urbanization on pollinators and pollination: A meta-analysis. Ecol. Lett. 2023, 26, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.T.C.; Gaston, K.J. Human–nature interactions and the consequences and drivers of provisioning wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170092. [Google Scholar] [CrossRef] [PubMed]
- Jaturas, N.; Sing, K.W.; Wilson, J.J.; Dong, H. Butterflies in urban parks in the Bangkok Metropolitan Region, Thailand. Biodivers. Data J. 2020, 8, e56317. [Google Scholar] [CrossRef]
- Lindemann-matthies, P. The Influence of an Educational Program on Children’s Perception of Biodiversity. J. Environ. Educ. 2002, 33, 22–31. [Google Scholar] [CrossRef]
- Pilgrim, S.; Smith, D.; Pretty, J. A cross-regional assessment of the factors affecting ecoliteracy: Implications for policy and practice. Ecol. Appl. 2007, 17, 1742–1751. [Google Scholar] [CrossRef]
- Jimenez, M.F.; Pejchar, L.; Reed, S.E. Tradeoffs of using place-based community science for urban biodiversity monitoring. Conserv. Sci. Pract. 2021, 3, e338. [Google Scholar] [CrossRef]
- Karmakar, P.; Mishra, A.; Borah, C.; Deka, A. Diversity and spatial distribution of butterflies in different macrohabitat of North East India. Int. J. Trop. Insect Sci. 2022, 42, 3671–3686. [Google Scholar] [CrossRef]
- Ternisien, M.; Deschamps-Cottin, M.; Lizée, M.-H.; March, L.; Robles, C.; Vila, B. How butterfly communities are structured and have changed in urbanized areas of Marseille: A 12-year monitoring survey. Urban Ecosyst. 2023, 26, 1427–1438. [Google Scholar] [CrossRef]
- Soanes, K.; Taylor, L.; Ramalho, C.E.; Maller, C.J.; Parris, K.M.; Bush, J.; Mata, L.; Williams, N.S.G.; Threlfall, C.G. Conserving urban biodiversity: Current practice, barriers, and enablers. Conserv. Lett. 2023, 16, e12946. [Google Scholar] [CrossRef]
- Zhu, L.A. Landscape Design of Mountain Campus in Chenggong New Campus of Yunnan University. Mod. Landsc. Archit. 2013, 10, 55–59. [Google Scholar]
- Yi, Z.Y. Analysis of campus landscape resources and tourism development prospects driven by cultural tourism—Taking Chenggong Campus of Yunnan University as an example. Sustain. Dev. 2023, 13, 699–709. [Google Scholar] [CrossRef]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araújo, M.B. Multiple Dimensions of Climate Change and Their Implications for Biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, B.; Jia, L. Spatio-temporal variation in China’s climatic seasons from 1951 to 2017. J. Geogr. Sci. 2020, 30, 1387–1400. [Google Scholar] [CrossRef]
- Trenberth, K.E. What are the Seasons? Bull. Am. Meteorol. Soc. 1983, 64, 1276–1282. [Google Scholar] [CrossRef]
- China Meteorological Administration. Division of Climatic Seasons. Available online: https://www.cma.gov.cn/zfxxgk/gknr/flfgbz/bz/202307/t20230712_5642647.html (accessed on 10 May 2023).
- Pollard, E. A method for assessing changes in the abundance of butterflies. Biol. Conserv. 1977, 12, 115–134. [Google Scholar] [CrossRef]
- Royer, R.A.; Austin, J.E.; Newton, W.E. Checklist and “Pollard Walk” Butterfly Survey Methods on Public Lands. Am. Midl. Nat. 1998, 140, 358–371. [Google Scholar] [CrossRef]
- Technical Guidelines for Biodiversity Monitoring—Butterflies. Available online: https://english.mee.gov.cn/Resources/standards/Eco_Environment/201605/t20160512_337611.shtml (accessed on 7 October 2021).
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. circlize Implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Handcock, M.S.; Hunter, D.R.; Butts, C.T.; Goodreau, S.M.; Morris, M. statnet: Software Tools for the Representation, Visualization, Analysis and Simulation of Network Data. J. Stat. Softw. 2008, 24, 1–11. [Google Scholar] [CrossRef]
- Turbelin, A.J.; Malamud, B.D.; Francis, R.A. Mapping the global state of invasive alien species: Patterns of invasion and policy responses. Glob. Ecol. Biogeogr. 2017, 26, 78–92. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y. China Species Red List (Vol3—Inveterbrate); Higher Education Press: Beijing, China, 2005. [Google Scholar]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Kubota, Y.; Zelený, D.; Chiu, C.H.; Li, C.F.; Kusumoto, B.; Yasuhara, M.; Thorn, S.; Wei, C.L.; Costello, M.J.; et al. Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 2020, 35, 292–314. [Google Scholar] [CrossRef]
- Arjona, J.M.; Ibáñez-Álamo, J.D.; Sanllorente, O. Mediterranean university campuses enhance butterfly (Lepidoptera) and beetle (Coleoptera) diversity. Front. Ecol. Evol. 2023, 11, 1130557. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Villéger, S.; Sun, I.-F.; Thorn, S.; Lin, Y.-C.; Chiang, J.-M.; Sherwin, W.B. An attribute-diversity approach to functional diversity, functional beta diversity, and related (dis)similarity measures. Ecol. Monogr. 2019, 89, e01343. [Google Scholar] [CrossRef]
- Leinster, T.; Cobbold, C.A. Measuring diversity: The importance of species similarity. Ecology 2012, 93, 477–489. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Wilkinson, L. ggplot2: Elegant graphics for data analysis by H. Wickham. Biometrics 2011, 67, 678–679. [Google Scholar] [CrossRef]
- Lang, Y.; Yakun, D.; Baige, M.; Yanqiong, P. Diversity of butterfly communities in Gaoligong region of Yunnan. Biodivers. Sci. 2021, 29, 950–959. [Google Scholar] [CrossRef]
- Dixon, P. Vegan, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, S. Assemblages and seasonal patterns in butterflies across different ecosystems in a sub-tropical zone of Jammu Shiwaliks, Jammu and Kashmir, India. Trop. Ecol. 2021, 62, 261–278. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J. Analysis of the main elements and implications of the Kunming-Montreal Global Biodiversity Framework. Biodivers. Sci. 2023, 31, 23020. [Google Scholar] [CrossRef]
- Jiu, H.Q.; Hui, Y.C.; Shan, W.; Wang, Z.Z. A Preliminary Study on Community Diversity of Butterfly in Kunming. J. Northwest For. Univ. 2008, 23, 147–150. [Google Scholar]
- Zhang, Y.; Tu, X.Y.; Chen, Y.S.; Liu, N. Investigation on butterflies diversity inside and outside a new university campus in Jiangxi. J. Anhui Agric. Sci. 2011, 39, 4682–4683. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yuan, X.Z. Preliminary study on butterfly species diversity in Chongqing downtown area. Resour. Dev. Mark. 2008, 24, 815–819. [Google Scholar]
- Xu, Z.B.; Zhang, X. Investigation on Butterfly Resource in Binhu Campus of Huaibei Normal University. Anhui Agric. Sci. Bull. 2017, 23, 22–25. [Google Scholar] [CrossRef]
- Hu, S.J. Preliminary field survey of butterflies on Xishan Hill (Kunming, Yunnan Province, China). J. Res. Lepid. 2009, 41, 60–69. [Google Scholar] [CrossRef]
- Huang, Q.X.; Zhang, Z.; Ma, H.Y. Characterization of butterfly diversity in urban parks in Kunming under multiple landscape dimensions. Chin. J. Ecol. 2023, 42, 2193–2203. [Google Scholar] [CrossRef]
- Wang, L.M.; Li, C.Y.; YIn, J.; Shi, W.; Wang, Y.H.; Yi, C.H.; He, Q.J. Structure and species diversity of butterfly community in Wenshan city. China J. Agric. Sci. 2023, 36, 1336–1345. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Yang, C.; Zhu, E.J.; Ren, X.P.; Zhang, Z.W.; He, Q.J.; Yi, C.H. Study on the diversity of butterflies in Xichou County, Yunnan Province. J. Sichuan Agric. Univ. 2019, 37, 404–410. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Yang, K.H.; Li, L.; Zeng, W.B.; Cai, M.F.; Liu, Y.; Zhang, K.; Xu, Y. Butterfly diversity and nestedness on fragmented woodlots in the Huaxi University Town, Guizhou. Sichuan J. Zool. 2023, 42, 579–585. [Google Scholar] [CrossRef]
- Lin, H.W.; Wang, C.J.; Yang, L.; Zhao, D.Y.; Zhou, K.L.; Ying, M.H.; Pan, Z.X. Effects of urbanization on butterfly diversity of Taizhou. Sichuan J. Zool. 2018, 37, 541–547. [Google Scholar] [CrossRef]
- Sing, K.W.; Dong, H.; Wang, W.Z.; Wilson, J.J. Can butterflies cope with city life? Butterfly diversity in a young megacity in southern China. Genome 2016, 59, 751–761. [Google Scholar] [CrossRef] [PubMed]
- She, J.Y.; HAn, D.; Wang, C.; Yin, L.Q.; Sun, Z.K.; Han, C.H. Butterfly diversity in Pocket Parks at urban core of Beijing. J. Chin. Urban For. 2022, 20, 1–6. [Google Scholar] [CrossRef]
- Sing, K.W.; Luo, J.S.; Wang, W.Z.; Jaturas, N.; Soga, M.; Yang, X.Z.; Dong, H.; Wilson, J.J. Ring roads and urban biodiversity: Distribution of butterflies in urban parks in Beijing city and correlations with other indicator species. Sci. Rep. 2019, 9, 7653. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhang, C.; Wang, C.; She, J.; Sun, Z.; Zhao, D.; Bian, Q.; Han, W.; Yin, L.; Sun, R.; et al. Differences in response of butterfly diversity and species composition in urban parks to land cover and local habitat variables. Forests 2021, 12, 140. [Google Scholar] [CrossRef]
- Glatz-Jorde, S.; Jungmeier, M.; Hradetzky, R.; Berger, D. The IUCN Green List as a tool for protected area management—The example of City meets Nature. J. Prot. Mt. Areas Res. Manag. 2020, 12, 50–54. [Google Scholar] [CrossRef]
- Verma, A.; Arya, M.K. Proposed multipurpose project at pancheshwar in the western Himalaya affects rich butterfly diversity: A conservation concern. J. Insect Conserv. 2021, 25, 89–107. [Google Scholar] [CrossRef]
- Cabette, H.S.R.; Souza, J.R.; Shimano, Y.; Juen, L. Effects of changes in the riparian forest on the butterfly community (Insecta: Lepidoptera) in Cerrado areas. Rev. Bras. Entomol. 2017, 61, 43–50. [Google Scholar] [CrossRef]
- Sharma, K.; Acharya, B.K.; Sharma, G.; Valente, D.; Pasimeni, M.R.; Petrosillo, I.; Selvan, T. Land use effect on butterfly alpha and beta diversity in the Eastern Himalaya, India. Ecol. Indic. 2020, 110, 105605. [Google Scholar] [CrossRef]
- Fox, R.; Harrower, C.A.; Bell, J.R.; Shortall, C.R.; Middlebrook, I.; Wilson, R.J. Insect population trends and the IUCN Red List process. J. Insect Conserv. 2019, 23, 269–278. [Google Scholar] [CrossRef]
- Lin, F.M.; Yuan, X.Z.; Wu, Y.Y.; Liu, H. Diversity of butterfly in different habitat types in rapid urbanization area. Chin. J. Ecol. 2012, 31, 2579–2584. [Google Scholar] [CrossRef]
- Ohwaki, A.; Tanabe, S.; Nakamura, K. Effects of anthropogenic disturbances on the butterfly assemblage in an urban green area: The changes from 1990 to 2005 in Kanazawa Castle Park, Japan. Ecol. Res. 2008, 23, 697–708. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, S.J.; Fang, W.Q.; Zhao, Y.J.; Huang, Z.L.; Zheng, R.X.; Huang, J.K.; Dong, J.Y.; Fu, W.C. Butterfly communities vary under different urbanization types in city parks. Animals 2023, 13, 1775. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Tiwari, C.; Diwakar, S. Butterfly diversity and effect of temperature and humidity gradients on butterfly assemblages in a sub-tropical urban landscape. Trop. Ecol. 2019, 60, 150–158. [Google Scholar] [CrossRef]
- Ducatez, S.; Sayol, F.; Sol, D.; Lefebvre, L. Are urban vertebrates city specialists, artificial habitat exploiters, or environmental generalists? Integr. Comp. Biol. 2018, 58, 929–938. [Google Scholar] [CrossRef]
- Kuussaari, M.; Toivonen, M.; Heliölä, J.; Pöyry, J.; Mellado, J.; Ekroos, J.; Hyyryläinen, V.; Vähä-Piikkiö, I.; Tiainen, J. Butterfly species’ responses to urbanization: Differing effects of human population density and built-up area. Urban Ecosyst. 2021, 24, 515–527. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Nylin, S.; Wiklund, C.; Gotthard, K. Evolution of butterfly seasonal plasticity driven by climate change varies across life stages. Ecol. Lett. 2023, 26, 1548–1558. [Google Scholar] [CrossRef]
- Paul, M.; Sultana, A. Studies on butterfly (Insecta: Lepidoptera) diversity across different urban landscapes of Delhi, India. Curr. Sci. 2020, 118, 819–827. [Google Scholar] [CrossRef]
- Crossley, M.S.; Smith, O.M.; Berry, L.L.; Phillips-Cosio, R.; Glassberg, J.; Holman, K.M.; Holmquest, J.G.; Meier, A.R.; Varriano, S.A.; McClung, M.R.; et al. Recent climate change is creating hotspots of butterfly increase and decline across North America. Glob. Chang. Biol. 2021, 27, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Garbuzov, M.; Ratnieks, F.L.W. Quantifying variation among garden plants in attractiveness to bees and other flower-visiting insects. Funct. Ecol. 2014, 28, 364–374. [Google Scholar] [CrossRef]
- Feber, R.E.; Smith, H.L.; Macdonald, D.W. The effects on butterfly abundance of the management of uncropped edges of arable fields. J. Appl. Ecol. 1996, 33, 1191–1205. [Google Scholar] [CrossRef]
- Kaiser, A.; Merckx, T.; Van Dyck, H. The urban heat island and its spatial scale dependent impact on survival and development in butterflies of different thermal sensitivity. Ecol. Evol. 2016, 6, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Wang, Z.H.; Liu, M.Y.; Zhou, Y.; Leng, T.T. Urban heat island effect of Chenggong district in Kunming, China. Environ. Eng. Manag. J. 2019, 18, 1591–1598. [Google Scholar] [CrossRef]
- Ao, S.C.; Ye, L.; Liu, X.Y.; Cai, Q.H.; He, F.Z. Elevational patterns of trait composition and functional diversity of stream macroinvertebrates in the Hengduan Mountains region, Southwest China. Ecol. Indic. 2022, 144, 109558. [Google Scholar] [CrossRef]
- Miao, B.G.; Peng, Y.Q.; Yang, D.R.; Kubota, Y.; Economo, E.P.; Liu, C. Climate and land-use interactively shape butterfly diversity in tropical rainforest and savanna ecosystems of southwestern China. Insect Sci. 2021, 28, 1109–1120. [Google Scholar] [CrossRef]
- Baldock, K.C.R. Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect Sci. 2020, 38, 63–71. [Google Scholar] [CrossRef]
- Semeraro, T.; Scarano, A.; Buccolieri, R.; Santino, A.; Aarrevaara, E. Planning of urban green spaces: An ecological perspective on human benefits. Land 2021, 10, 105. [Google Scholar] [CrossRef]
- Nason, L.D.; Eason, P.K. Urban yards as potential conservation space: Large, diverse gardens may be valuable resource patches for butterflies. Urban Ecosyst. 2023, 26, 1573–1588. [Google Scholar] [CrossRef]
- Qiu, L.; Lindberg, S.; Nielsen, A.B. Is biodiversity attractive?—On-site perception of recreational and biodiversity values in urban green space. Landsc. Urban Plan. 2013, 119, 136–146. [Google Scholar] [CrossRef]
- Lang, B.J.; Dixon, P.M.; Klaver, R.W.; Thompson, J.R.; Widrlechner, M.P. Characterizing urban butterfly populations: The case for purposive point-count surveys. Urban Ecosyst. 2019, 22, 1083–1096. [Google Scholar] [CrossRef]
- Yin, J.; Fu, P.; Cheshmehzangi, A.; Li, Z.; Dong, J. Investigating the Changes in Urban Green-Space Patterns with Urban Land-Use Changes: A Case Study in Hangzhou, China. Remote Sens. 2022, 14, 5410. [Google Scholar] [CrossRef]
- Ries, L.; Debinski, D.M.; Wieland, M.L. Conservation Value of Roadside Prairie Restoration to Butterfly Communities. Conserv. Biol. 2001, 15, 401–411. [Google Scholar] [CrossRef]
- He, M.Y.; Ran, N.; Jiang, H.Q.; Han, Z.M.; Dian, Y.Y.; Li, X.X.; Xie, D.; Bowler, P.A.; Wang, H. Effects of landscape and local factors on the diversity of flower-visitor groups under an urbanization gradient, a case study in Wuhan, China. Diversity 2022, 14, 208. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, L.; Liu, H.; Xu, B.; Zou, Z. The effects of habitat network construction and urban block unit structure on biodiversity in semiarid green spaces. Environ. Monit. Assess. 2020, 192, 179. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.C.; Bonebrake, T.C. Butterfly diversity, habitat and vegetation usage in Hong Kong urban parks. Urban Ecosyst. 2016, 19, 721–733. [Google Scholar] [CrossRef]
- Saarinen, K.; Valtonen, A.; Jantunen, J.; Saarnio, S. Butterflies and diurnal moths along road verges: Does road type affect diversity and abundance? Biol. Conserv. 2005, 123, 403–412. [Google Scholar] [CrossRef]
- Sweaney, N.; Lindenmayer, D.B.; Driscoll, D.A. Is the matrix important to butterflies in fragmented landscapes? J. Insect Conserv. 2014, 18, 283–294. [Google Scholar] [CrossRef]
- Ojianwuna, C.C.; Enwemiwe, V.N. Spatial distribution of butterflies in different macrohabitat in a university campus in Southern-Nigeria. Int. J. Trop. Insect Sci. 2021, 41, 2657–2668. [Google Scholar] [CrossRef]
- Nagase, A.; Kurashina, M.; Nomura, M.; MacIvor, J.S. Patterns in urban butterflies and spontaneous plants across a University campus in Japan. Pan-Pac. Entomol. 2019, 94, 195–215. [Google Scholar] [CrossRef]
- Prudic, K.L.; Oliver, J.C.; Brown, B.V.; Long, E.C. Comparisons of Citizen Science Data-Gathering Approaches to Evaluate Urban Butterfly Diversity. Insects 2018, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Arlettaz, R.; Schaub, M.; Fournier, J.; Reichlin, T.; Sierro, A.; Watson, J.; Braunisch, V. From Publications to Public Actions: When Conservation Biologists Bridge the Gap between Research and Implementation. BioScience 2010, 60, 835–842. [Google Scholar] [CrossRef]
- McKinley, D.C.; Miller-Rushing, A.J.; Ballard, H.L.; Bonney, R.; Brown, H.; Cook-Patton, S.C.; Evans, D.M.; French, R.A.; Parrish, J.K.; Phillips, T.B.; et al. Citizen science can improve conservation science, natural resource management, and environmental protection. Biol. Conserv. 2017, 208, 15–28. [Google Scholar] [CrossRef]
- Deguines, N.; Princé, K.; Prevot, A.-C.; Fontaine, B. Assessing the emergence of pro-biodiversity practices in citizen scientists of a backyard butterfly survey. Sci. Total Environ. 2020, 716, 136842. [Google Scholar] [CrossRef]
- Miller-Rushing, A.; Primack, R.; Bonney, R. The history of public participation in ecological research. Front. Ecol. Environ. 2012, 10, 285–290. [Google Scholar] [CrossRef]
- Toomey, A.H.; Knight, A.T.; Barlow, J. Navigating the Space between Research and Implementation in Conservation. Conserv. Lett. 2017, 10, 619–625. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Chen, B.; Du, Y.; Huang, X.; Pan, X.; Zhang, Q. Citizen science: Integrating scientific research, ecological conservation and public participation. Biodivers. Sci. 2013, 21, 738–749. [Google Scholar]
- Yang, J.; Xing, D.; Luo, X. Assessing the performance of a citizen science project for monitoring urban woody plant species diversity in China. Urban For. Urban Green. 2021, 59, 127001. [Google Scholar] [CrossRef]
- Zeng, Q.; Wei, Q.; Lei, G. Contribution of citizen science towards cryptic species census: “many eyes” define wintering range of the Scaly-sided Merganser in mainland China. Avian Res. 2018, 9, 6. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Cheng, C.; Liu, Y.; Jennerjahn, T.C. Citizen science to support coastal research and management: Insights from a seagrass monitoring case study in Hainan, China. Ocean. Coast. Manag. 2023, 231, 106403. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Luo, X.; Qin, K.; Merz, R.; Zhou, S. Habitat monitoring of an endangered Asian butterfly, Teinopalpus aureus (Lepidoptera: Papilionidae) and change in local residents’ conservation awareness. J. Insect Conserv. 2018, 22, 721–729. [Google Scholar] [CrossRef]
- Ma, F.Z.; Xu, H.G.; Chen, M.M.; Tong, W.J.; Wang, C.B.; Cai, L. Progress in construction of China butterfly diversity observation network (China BON-Butterflies). J. Ecol. Rural. Environ. 2018, 34, 27–36. [Google Scholar] [CrossRef]
- National Geographic. Citizen Science. Available online: https://education.nationalgeographic.org/resource/citizen-science-article (accessed on 15 August 2023).
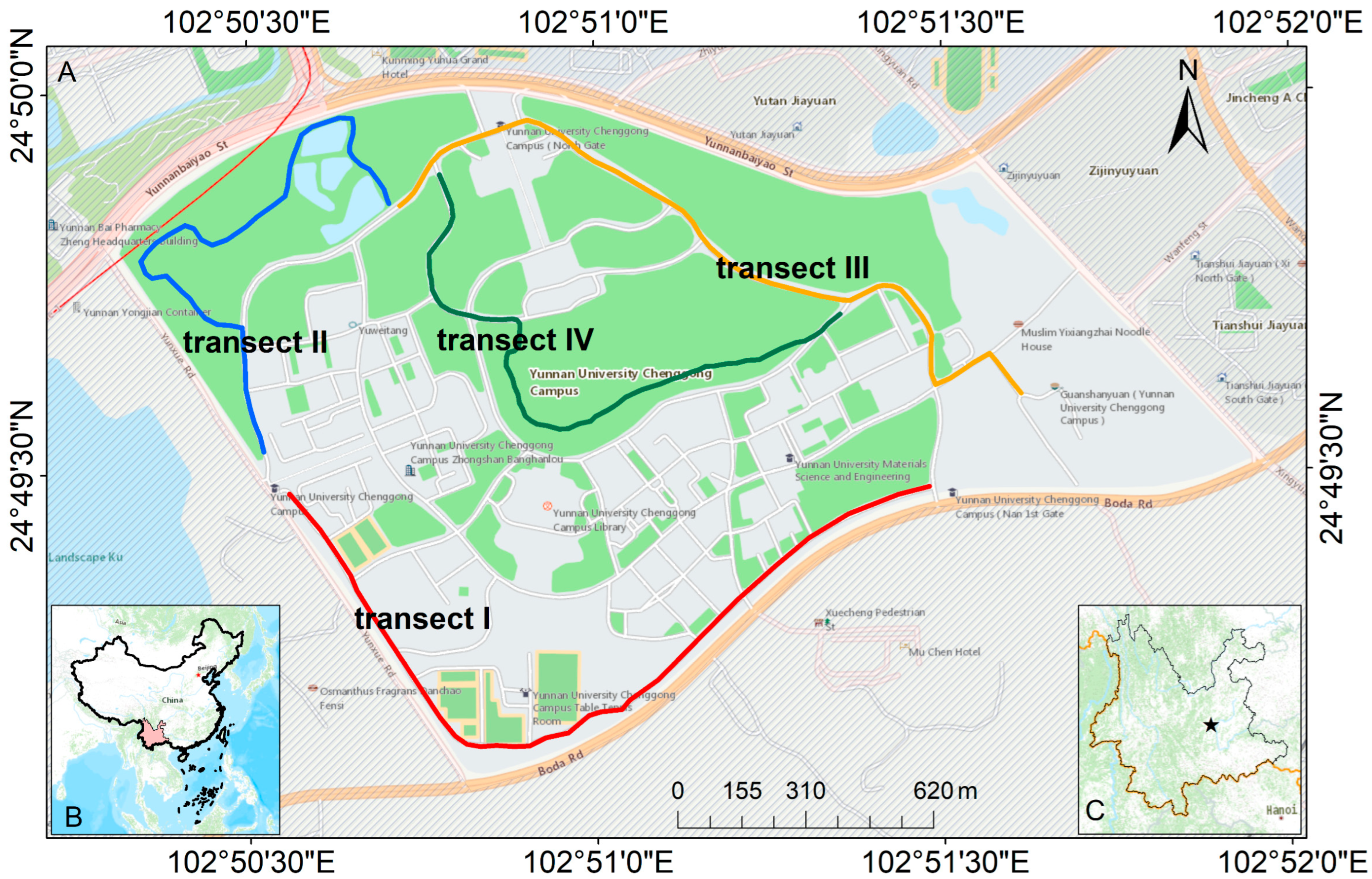








| Transect | Start and End Coordinates | Transect Length/m | Elevation Range/m | Habitat Type | Type of Disturbance |
|---|---|---|---|---|---|
| I | 102.856644, 24.827453 | 2152 | 1952–1993 | Campus road habitat | Human life disturbances: daily travel, physical activity, vehicular traffic, etc. |
| 102.841152, 24.827718 | |||||
| II | 102.840729, 24.828334 | 1735 | 1954–1955 | Campus scenic habitat | Green pruning activities, pedestrian viewing, unscheduled building construction, etc. |
| 102.843720, 24.833994 | |||||
| III | 102.843896, 24.833945 | 2050 | 1955–1994 | Campus road habitat | Human life disturbances: daily travel, vehicular traffic, etc. |
| 102.859606, 24.828714 | |||||
| IV | 102.854640, 24.831672 | 1745 | 1955–2001 | Campus scenic habitat | Green pruning activities, pedestrian viewing, unscheduled building construction, etc. |
| 102.844871, 24.834721 |
| Family | No. of Genera | Percentage | No. of Species | Percentage | No. of Individuals | Percentage |
|---|---|---|---|---|---|---|
| Hesperiidae | 3 | 8.6 | 3 | 6.0 | 10 | 0.3 |
| Papilionidae | 3 | 8.6 | 8 | 16.0 | 110 | 3.0 |
| Pieridae | 10 | 28.6 | 16 | 32.0 | 2469 | 68.1 |
| Nymphalidae | 14 | 40.0 | 17 | 34.0 | 575 | 15.9 |
| Riodinidae | 1 | 2.9 | 2 | 4.0 | 6 | 0.2 |
| Lycaenidae | 4 | 11.4 | 4 | 8.0 | 455 | 12.6 |
| Total | 35 | 100 | 50 | 100 | 3625 | 100 |
| Diversity Index | Species Richness | Shannon Diversity | Simpson Diversity |
|---|---|---|---|
| Hesperiidae | 3.0 ± 0.5 | 2.6 ± 0.5 | 2.4 ± 0.6 |
| Papilionidae | 8.0 ± 1.4 | 3.4 ± 0.4 | 2.2 ± 0.2 |
| Pieridae | 16.0 ± 0.4 | 5.4 ± 0.1 | 3.2 ± 0.1 |
| Nymphalidae | 17.0 ± 5.4 | 4.6 ± 0.3 | 2.5 ± 0.2 |
| Riodinidae | 2.0 ± 0.2 | 1.9 ± 0.3 | 1.8 ± 0.4 |
| Lycaenidae | 4.0 ± 0.5 | 2.4 ± 0.1 | 2.0 ± 0.1 |
| Family | Species | IUCN Global | China Species Red List |
|---|---|---|---|
| Papilionidae | Graphium sarpedon | LC | LC |
| Byasa hedistus | LC | VU | |
| Pieridae | Prioneris thestylis | NE | NT |
| Eurema hecabe | LC | LC | |
| Nymphalidae | Dilipa morgiana | NE | NT |
| Junonia orithya | LC | LC | |
| Danaus chrysippus | LC | LC | |
| Vanessa cardui | LC | LC | |
| Lycaenidae | Lampides boeticus | LC | LC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.-Q.; Li, Y.-P.; Pan, Y.; Wang, C.-Y.; Peng, M.-C.; Hu, S.-J. Butterfly Diversity in a Rapidly Developing Urban Area: A Case Study on a University Campus. Diversity 2024, 16, 4. https://doi.org/10.3390/d16010004
Fang S-Q, Li Y-P, Pan Y, Wang C-Y, Peng M-C, Hu S-J. Butterfly Diversity in a Rapidly Developing Urban Area: A Case Study on a University Campus. Diversity. 2024; 16(1):4. https://doi.org/10.3390/d16010004
Chicago/Turabian StyleFang, Sheng-Quan, Yong-Ping Li, Yue Pan, Chong-Yun Wang, Ming-Chun Peng, and Shao-Ji Hu. 2024. "Butterfly Diversity in a Rapidly Developing Urban Area: A Case Study on a University Campus" Diversity 16, no. 1: 4. https://doi.org/10.3390/d16010004
APA StyleFang, S.-Q., Li, Y.-P., Pan, Y., Wang, C.-Y., Peng, M.-C., & Hu, S.-J. (2024). Butterfly Diversity in a Rapidly Developing Urban Area: A Case Study on a University Campus. Diversity, 16(1), 4. https://doi.org/10.3390/d16010004






