Assessment of Sessile Benthic Communities in Jeju Island, Republic of Korea, Using Autonomous Reef Monitoring Structures (ARMS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. ARMS Deployment and Recovery
2.3. Image Analysis
2.4. DNA Extraction and Metabarcoding
2.5. Statistical Analyses
3. Results
3.1. Sessile Benthic Community Composition
3.2. Composition of Sessile Benthic Community Using DNA Metabarcoding
3.3. Comparison of Image Analysis and DNA Metabarcoding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, N.; Simon-Blecher, N.; Ben-Ezra, S.; Yuval, M.; Doniger, T.; Leray, M.; Karako-Lampert, S.; Tarazi, E.; Levy, O. Evaluating biodiversity for coral reef reformation and monitoring on complex 3D structures using environmental DNA (eDNA) metabarcoding. Sci. Total Environ. 2023, 856, 159051. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Chapin, F.S.; Cornelissen, J.H.C.; Díaz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Chang. Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef]
- Higgins, E.; Scheibling, R.E.; Desilets, K.M.; Metaxas, A. Benthic community succession on artificial and natural coral reefs in the northern Gulf of Aqaba, Red Sea. PLoS ONE 2019, 14, e0212842. [Google Scholar] [CrossRef] [PubMed]
- Mermillod-Blondin, F.; François-Carcaillet, F.; Rosenberg, R. Biodiversity of benthic invertebrates and organic matter processing in shallow marine sediments: An experimental study. J. Exp. Mar. Biol. Ecol. 2005, 315, 187–209. [Google Scholar] [CrossRef]
- Brown, E.A.; Chain, F.J.J.; Zhan, A.; MacIsaac, H.J.; Cristescu, M.E. Early detection of aquatic invaders using metabarcoding reveals a high number of non-indigenous species in Canadian ports. Divers. Distrib. 2016, 22, 1045–1059. [Google Scholar] [CrossRef]
- Danovaro, R.; Carugati, L.; Berzano, M.; Cahill, A.E.; Carvalho, S.; Chenuil, A.; Corinaldesi, C.; Cristina, S.; David, R.; Dell’Anno, A.; et al. Implementing and innovating marine monitoring approaches for assessing marine environmental status. Front. Mar. Sci. 2016, 3, 213. [Google Scholar] [CrossRef]
- David, R.; Uyarra, M.C.; Carvalho, S.; Anlauf, H.; Borja, A.; Cahill, A.E.; Carugati, L.; Danovaro, R.; De Jode, A.; Feral, J.P.; et al. Lessons from photo analyses of Autonomous Reef Monitoring Structures as tools to detect (bio-)geographical, spatial, and environmental effects. Mar. Pollut. Bull. 2019, 141, 420–429. [Google Scholar] [CrossRef] [PubMed]
- van der Heyde, M.; Bunce, M.; Wardell-Johnson, G.; Fernandes, K.; White, N.E.; Nevill, P. Testing multiple substrates for terrestrial biodiversity monitoring using environmental DNA metabarcoding. Mol. Ecol. Resour. 2020, 20, 732–745. [Google Scholar] [CrossRef]
- Bae, S.; Kim, P.; Yi, C.H. Biodiversity and spatial distribution of ascidian using environmental DNA metabarcoding. Mar. Environ. Res. 2023, 185, 105893. [Google Scholar] [CrossRef]
- Ribas-Deulofeu, L.; Loubeyres, M.; Denis, V.; De Palmas, S.; Hwang, S.-J.; Woo, S.; Song, J.-I.; Chen, C.A. Jeju Island: A sentinel for tracking ocean warming impacts on high-latitude benthic communities. Coral Reefs 2023, 42, 1097–1112. [Google Scholar] [CrossRef]
- Chung, H.; Cho, K.W.; Chung, K.H.; Kim, J.H.; Shin, J.; Seo, Y.; Kang, J.S.; Lee, I.K. Ecological characteristics of algal whitening in coastal zone of Seogwipo area, Cheju Island. Algae 1998, 13, 361–374. [Google Scholar]
- Denis, V.; Ribas-Deulofeu, L.; Loubeyres, M.; De Palmas, S.; Hwang, S.-J.; Woo, S.; Song, J.-I.; Chen, C.A. Recruitment of the subtropical coral Alveopora japonica in the temperate waters of Jeju Island, South Korea. Bull. Mar. Sci. 2014, 91, 85–96. [Google Scholar] [CrossRef]
- Lee, K.T.; Lee, H.M.; Subramaniam, T.; Yang, H.S.; Park, S.R.; Kang, C.K.; Keshavmurthy, S.; Choi, K.S. Dominance of the scleractinian coral Alveopora japonica in the barren subtidal hard bottom of high-latitude Jeju Island off the south coast of Korea assessed by high-resolution underwater images. PLoS ONE 2022, 17, e0275244. [Google Scholar] [CrossRef]
- Hong, H.-K.; Keshavmurthy, S.; Kang, C.-K.; Hwang, K.; Park, S.R.; Cho, S.-H.; Choi, K.-S. Alveopora japonica repopulation of a bare substrate off Jeju Island, Korea. Bull. Mar. Sci. 2015, 91, 477–478. [Google Scholar] [CrossRef]
- Kim, T.; Kang, D.-H. An encrusting hard coral enclosing soft coral in the high-latitude Asia–Pacific marginal distribution zone. Diversity 2022, 14, 856. [Google Scholar] [CrossRef]
- Yang, H.-S.; Cho, Y.-G.; Kim, T.; Heo, S.-J. First report with molecular confirmation of the colonial sphenopid Palythoa mutuki (Cnidaria: Anthozoa: Zoantharia: Sphenopidae) forming massive colonies in Southern Jeju Island, Korea. J. Mar. Sci. Eng. 2023, 11, 574. [Google Scholar] [CrossRef]
- Kim, T.; Kim, T.; Yang, H.-S.; Choi, S.K.; Son, Y.B.; Kang, D.-H. Alveopora japonica conquering temperate reefs despite massive coral bleaching. Diversity 2022, 14, 86. [Google Scholar] [CrossRef]
- Kennedy, E.V.; Ordoñez, A.; Lewis, B.E.; Diaz-Pulido, G. Comparison of recruitment tile materials for monitoring coralline algae responses to a changing climate. Mar. Ecol. Prog. Ser. 2017, 569, 129–144. [Google Scholar] [CrossRef]
- Yang, H.-S.; Kim, T.; Lee, K.-T.; Kim, T.; Baker, D.M.; Kang, D.-H. Use of autonomous reef monitoring structures to monitor changes in the marine environment in Jeju, South Korea: A brief review. Ocean Sci. J. 2023, 58, 17. [Google Scholar] [CrossRef]
- Leray, M.; Knowlton, N. DNA bar coding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Pearman, J.K.; Anlauf, H.; Irigoien, X.; Carvalho, S. Please mind the gap—Visual census and cryptic biodiversity assessment at central Red Sea coral reefs. Mar. Environ. Res. 2016, 118, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, L.; Caley, M.J.; Brainard, R.E.; Knowlton, N. The diversity of coral reefs: What are we missing? PLoS ONE 2011, 6, e25026. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-T.; Perrois, G.; Yang, H.-S.; Kim, T.; Choi, S.K.; Kang, D.-H.; Kim, T. Impact of super typhoon ‘Hinnamnor’ on density of kelp forest and associated benthic communities in Jeju Island, Republic of Korea. J. Mar. Sci. Eng. 2023, 11, 1035. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.I.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. Planetary boundaries: Exploring the safe operating space for humanity. Ecol. Soc. 2009, 14, 32. [Google Scholar] [CrossRef]
- Richardson, K.; Steffen, W.; Lucht, W.; Bendtsen, J.; Cornell, S.E.; Donges, J.F.; Drüke, M.; Fetzer, I.; Bala, G.; von Bloh, W.; et al. Earth beyond six of nine planetary boundaries. Sci. Adv. 2023, 9, eadh2458. [Google Scholar] [CrossRef] [PubMed]
- Borja, A.; Elliott, M.; Andersen, J.H.; Berg, T.; Carstensen, J.; Halpern, B.S.; Heiskanen, A.-S.; Korpinen, S.; Lowndes, J.S.S.; Martin, G.; et al. Overview of integrative assessment of marine systems: The ecosystem approach in practice. Front. Mar. Sci. 2016, 3, 20. [Google Scholar] [CrossRef]
- Steyaert, M.; Lindhart, M.; Khrizman, A.; Dunbar, R.B.; Bonsall, M.B.; Mucciarone, D.A.; Ransome, E.; Santodomingo, N.; Winslade, P.; Head, C.E.I. Remote reef cryptobenthic diversity: Integrating autonomous reef monitoring structures and in situ environmental parameters. Front. Mar. Sci. 2022, 9, 932375. [Google Scholar] [CrossRef]
- Carter, A.; Prekel, S. Benthic colonization and ecological successional patterns on a planned nearshore artificial reef system in Broward County, SE Florida. In Proceedings of the 11th International Coral Reef Symposium, Lauderdale, FL, USA, 7–11 July 2008; pp. 1209–1213. [Google Scholar]
- Fredericq, S.; Krayesky-Self, S.; Sauvage, T.; Richards, J.; Kittle, R.; Arakaki, N.; Hickerson, E.; Schmidt, W.E. The critical importance of rhodoliths in the life cycle completion of both macro- and microalgae, and as holobionts for the establishment and maintenance of marine biodiversity. Front. Mar. Sci. 2019, 5, 502. [Google Scholar] [CrossRef]
- Carugati, L.; Corinaldesi, C.; Dell’Anno, A.; Danovaro, R. Metagenetic tools for the census of marine meiofaunal biodiversity: An overview. Mar. Genom. 2015, 24, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Rshaidat, M.M.; Snider, A.; Rosebraugh, S.; Devine, A.M.; Devine, T.D.; Plaisance, L.; Knowlton, N.; Leray, M. Deep COI sequencing of standardized benthic samples unveils overlooked diversity of Jordanian coral reefs in the northern Red Sea. Genome 2016, 59, 724–737. [Google Scholar] [CrossRef]
- West, K.M.; Adam, A.A.S.; White, N.; Robbins, W.D.; Barrow, D.; Lane, A.; Richards, T. The applicability of eDNA metabarcoding approaches for sessile benthic surveying in the Kimberley region, north-western Australia. Environ. DNA 2022, 4, 34–49. [Google Scholar] [CrossRef]
- Sugihara, K.; Yamano, H.; Choi, K.-S.; Hyeong, K. Zooxanthellate scleractinian corals of Jeju Island, Republic of Korea. In Integrative Observations and Assessments; Springer: Berlin/Heidelberg, Germany, 2014; pp. 111–130. [Google Scholar] [CrossRef]
- Vieira, C.; Keshavmurthy, S.; Ju, S.-J.; Hyeong, K.; Seo, I.; Kang, C.-K.; Hong, H.-K.; Chen, C.A.; Choi, K.-S. Population dynamics of a high-latitude coral Alveopora japonica Eguchi from Jeju Island, off the southern coast of Korea. Mar. Freshw. Res. 2016, 67, 594–604. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, J.E.; Kim, J.H.; Kim, S.; Keshavmurthy, S.; Agostini, S.; Reimer, J.D.; Chen, C.A.; Choi, K.-S.; Park, S.R.; et al. The origin of the subtropical coral Alveopora japonica (Scleractinia: Acroporidae) in high-latitude environments. Front. Ecol. Evol. 2020, 8, 12. [Google Scholar] [CrossRef]
- McCoy, S.J.; Kamenos, N.A. Coralline algae (Rhodophyta) in a changing world: Integrating ecological, physiological, and geochemical responses to global change. J. Phycol. 2015, 51, 6–24. [Google Scholar] [CrossRef]
- Schils, T. Branching Lithophyllum coralline algae: Dominant reef builders on herbivory-depressed tropical reefs after high coral mortality. Diversity 2023, 15, 1025. [Google Scholar] [CrossRef]
- Gómez-Lemos, L.A.; Doropoulos, C.; Bayraktarov, E.; Diaz-Pulido, G. Coralline algal metabolites induce settlement and mediate the inductive effect of epiphytic microbes on coral larvae. Sci. Rep. 2018, 8, 17557. [Google Scholar] [CrossRef]
- Abdul Wahab, M.A.; Ferguson, S.; Snekkevik, V.K.; McCutchan, G.; Jeong, S.; Severati, A.; Randall, C.J.; Negri, A.P.; Diaz-Pulido, G. Hierarchical settlement behaviours of coral larvae to common coralline algae. Sci. Rep. 2023, 13, 5795. [Google Scholar] [CrossRef] [PubMed]
- Leite, B.R.; Duarte, S.; Troncoso, J.S.; Costa, F.O. Artificial seaweed substrates complement ARMS in DNA metabarcoding-based monitoring of temperate coastal macrozoobenthos. Diversity 2023, 15, 657. [Google Scholar] [CrossRef]
- Palomino-Alvarez, L.A.; Vital, X.G.; Castillo-Cupul, R.E.; Suárez-Mozo, N.Y.; Ugalde, D.; Cervantes-Campero, G.; Muciño-Reyes, M.R.; Homá-Canché, P.; Hernández-Díaz, Y.Q.; Sotelo-Casas, R.; et al. Evaluation of the use of Autonomous Reef Monitoring Structures (ARMS) for describing the species diversity of two coral reefs in the Yucatan Peninsula, Mexico. Diversity 2021, 13, 579. [Google Scholar] [CrossRef]
- Edmunds, P.J. Patterns in the distribution of juvenile corals and coral reef community structure in St. John, US Virgin Islands. Mar. Ecol. Prog. Ser. 2000, 202, 113–124. [Google Scholar] [CrossRef]
- Obst, M.; Exter, K.; Allcock, A.L.; Arvanitidis, C.; Axberg, A.; Bustamante, M.; Cancio, I.; Carreira-Flores, D.; Chatzinikolaou, E.; Chatzigeorgiou, G.; et al. A marine biodiversity observation network for genetic monitoring of hard-bottom communities (ARMS-MBON). Front. Mar. Sci. 2020, 7, 572680. [Google Scholar] [CrossRef]
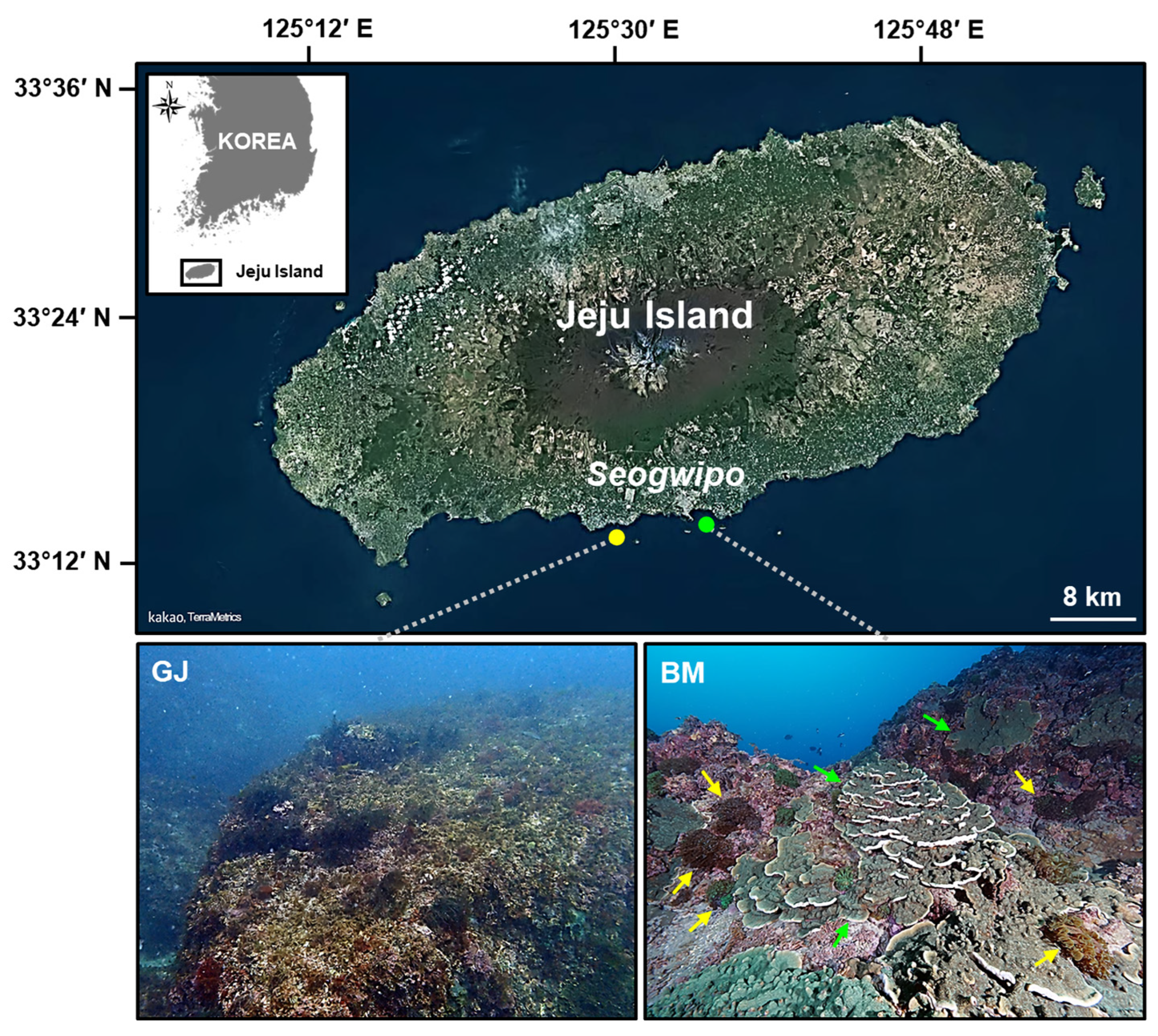
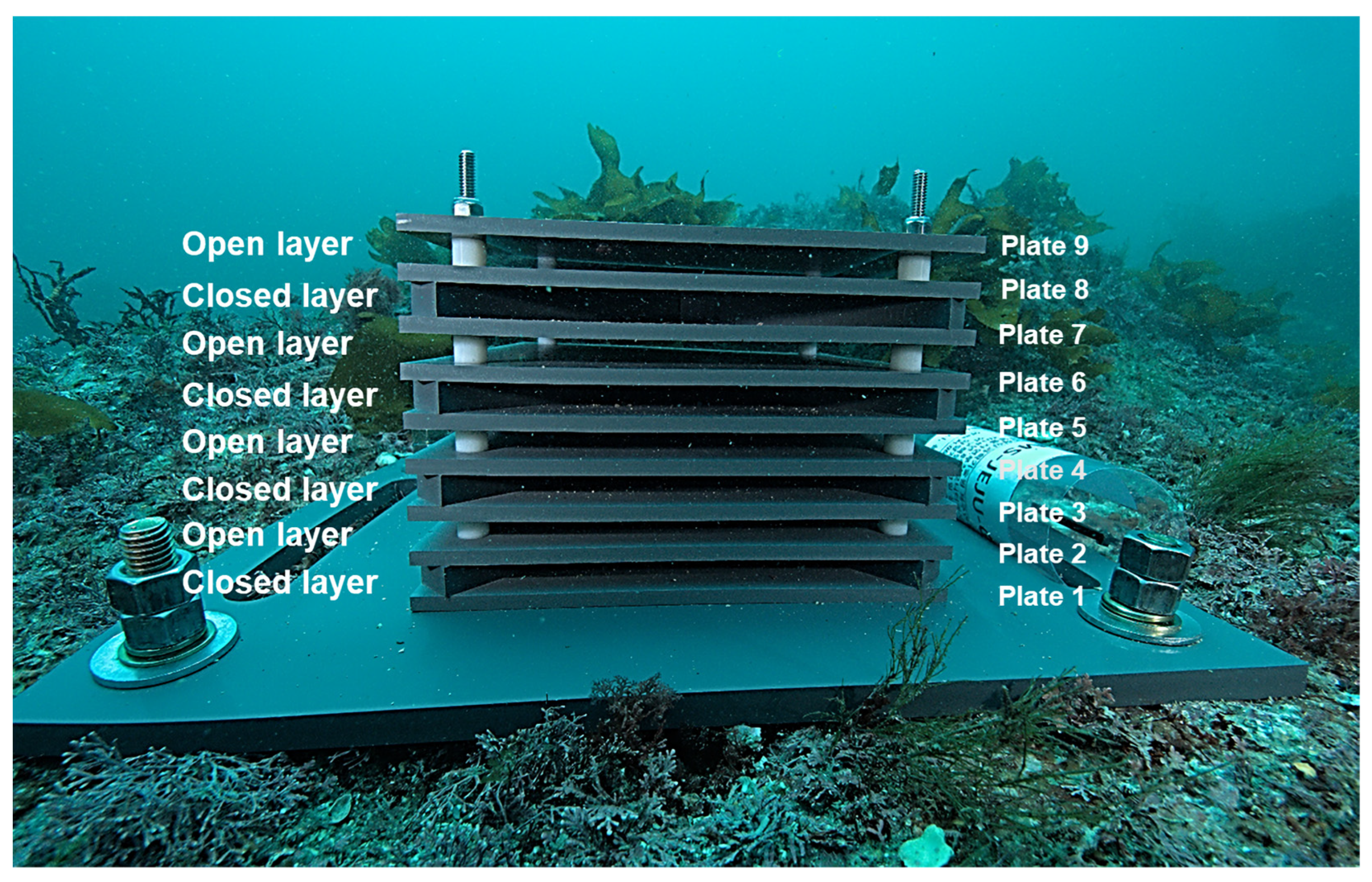
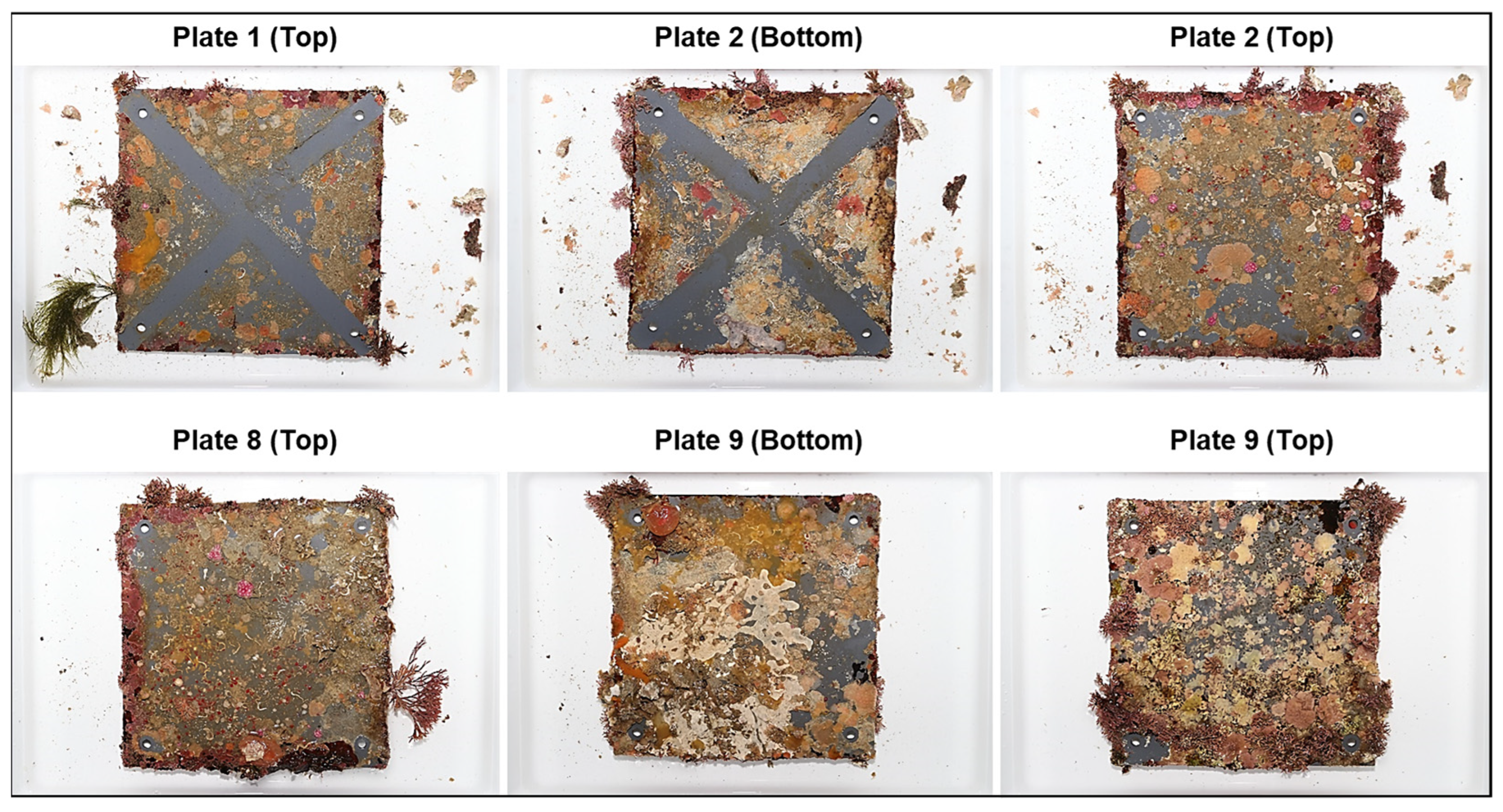
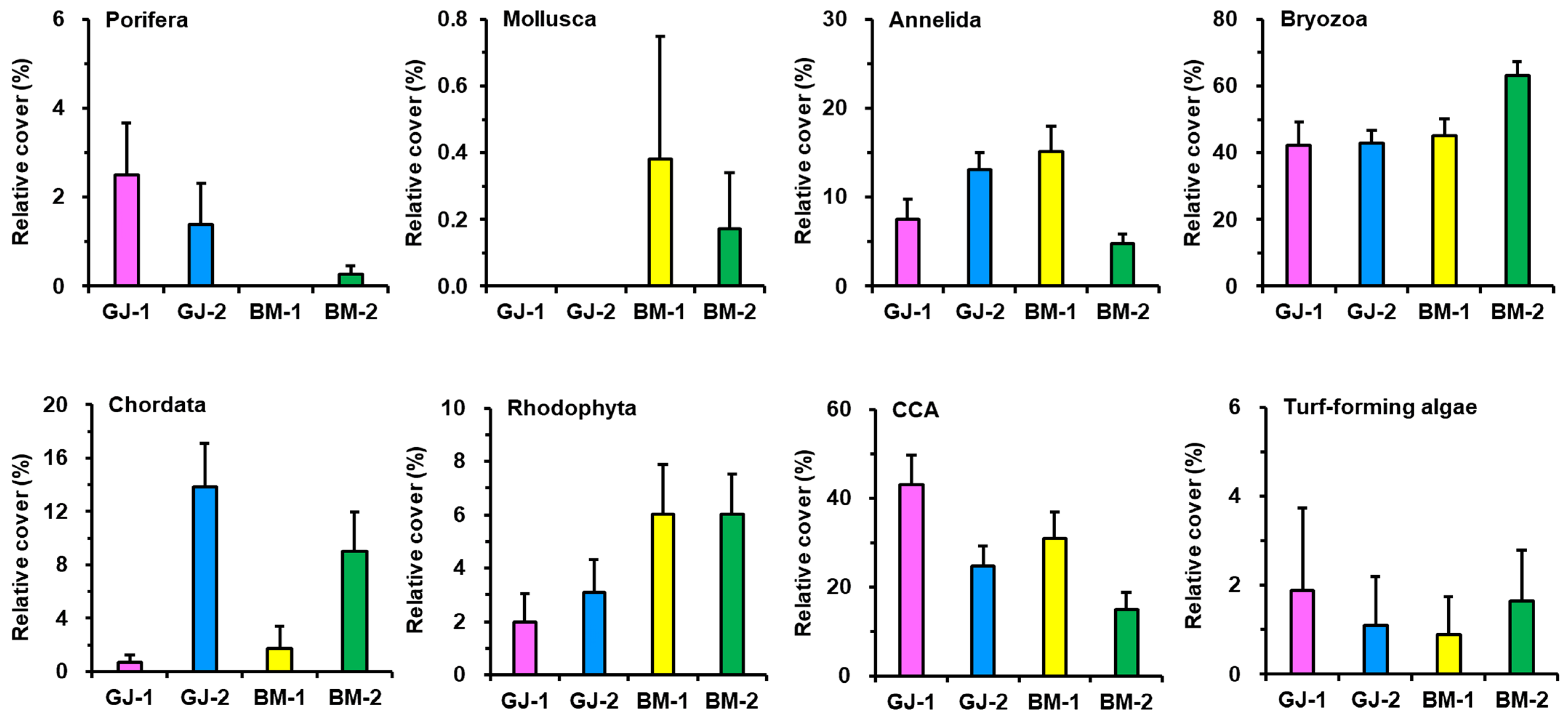
| Site | Deployment Date | Recovery Date | Installation Period | Site ID | Latitude | Longitude | Depth (m) |
|---|---|---|---|---|---|---|---|
| Gangjeong | 18 August | 19 August | 1 year (12 months) | GJ-1 | 33°13′21.86″ N | 126°28′41.07″ E | 13 |
| 18 August | 21 June | 3 years (34 months) | GJ-2 | 33°13′22.08″ N | 126°28′44.25″ E | 13 | |
| Bomok | 18 August | 19 August | 1 year (12 months) | BM-1 | 33°14′19.48″ N | 126°35′11.86″ E | 12 |
| 18 August | 21 June | 3 years (34 months) | BM-2 | 33°14′16.88″ N | 126°35′10.87″ E | 12 |
| Global Test | R Statistic | Significance Level (p) |
|---|---|---|
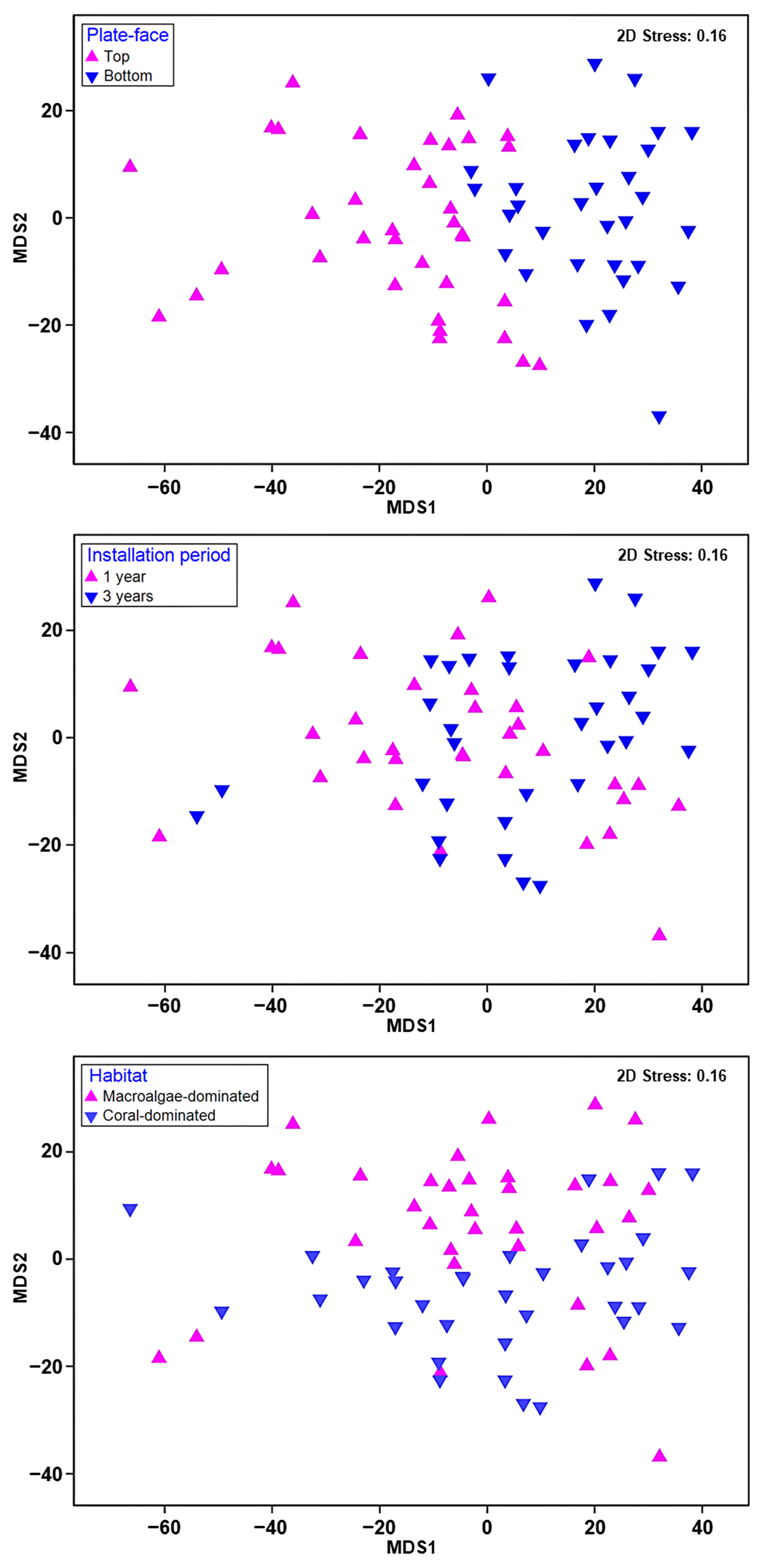
| Plate face | 0.437 | 0.001 |
| Installation period | 0.178 | 0.001 |
| Habitat | 0.064 | 0.011 |
| Layer | 0.018 | 0.164 |
| Groups: Top and bottom (plate face) Average dissimilarity = 42.26 | ||||
| Sessile Benthic Category | Average Abundance | Contribution (%) | Cumulative (%) | |
| Top | Bottom | |||
| CCA | 6.47 | 2.70 | 27.19 | 27.19 |
| Bryozoa | 5.68 | 7.70 | 17.43 | 44.62 |
| Annelida | 1.61 | 3.77 | 17.17 | 61.79 |
| Chordata | 0.84 | 2.27 | 14.98 | 76.77 |
| Rhodophyta | 2.22 | 0.36 | 13.86 | 90.63 |
| Turf-forming algae | 0.56 | 0.10 | 4.37 | 94.99 |
| Porifera | 0.34 | 0.39 | 4.25 | 99.24 |
| Mollusca | 0.07 | 0.05 | 0.76 | 100.0 |
| Groups: 1 year and 3 years (installation period) Average dissimilarity = 38.13 | ||||
| Sessile Benthic Category | Average Abundance | Contribution (%) | Cumulative (%) | |
| 1 year | 3 years | |||
| CCA | 5.49 | 3.91 | 23.59 | 23.59 |
| Chordata | 0.29 | 2.73 | 20.09 | 43.68 |
| Bryozoa | 6.19 | 7.08 | 17.66 | 61.34 |
| Annelida | 2.71 | 2.54 | 15.65 | 76.98 |
| Rhodophyta | 1.17 | 1.51 | 12.65 | 89.63 |
| Turf-forming algae | 0.28 | 0.4 | 4.88 | 94.50 |
| Porifera | 0.41 | 0.32 | 4.66 | 99.16 |
| Mollusca | 0.07 | 0.05 | 0.84 | 100.0 |
| Groups: Macroalgae-dominated and coral-dominated (habitat) Average dissimilarity = 36.60 | ||||
| Sessile Benthic Category | Average Abundance | Contribution (%) | Cumulative (%) | |
| Macroalgae-Dominated | Coral-Dominated | |||
| CCA | 5.28 | 4.11 | 23.99 | 23.99 |
| Bryozoa | 6.18 | 7.08 | 18.79 | 42.78 |
| Annelida | 2.71 | 2.54 | 16.26 | 59.04 |
| Chordata | 1.72 | 1.30 | 15.85 | 74.89 |
| Rhodophyta | 0.83 | 1.85 | 13.96 | 88.85 |
| Porifera | 0.64 | 0.09 | 5.18 | 94.03 |
| Turf-forming algae | 0.29 | 0.39 | 5.07 | 99.10 |
| Mollusca | 0.00 | 0.13 | 0.90 | 100.0 |
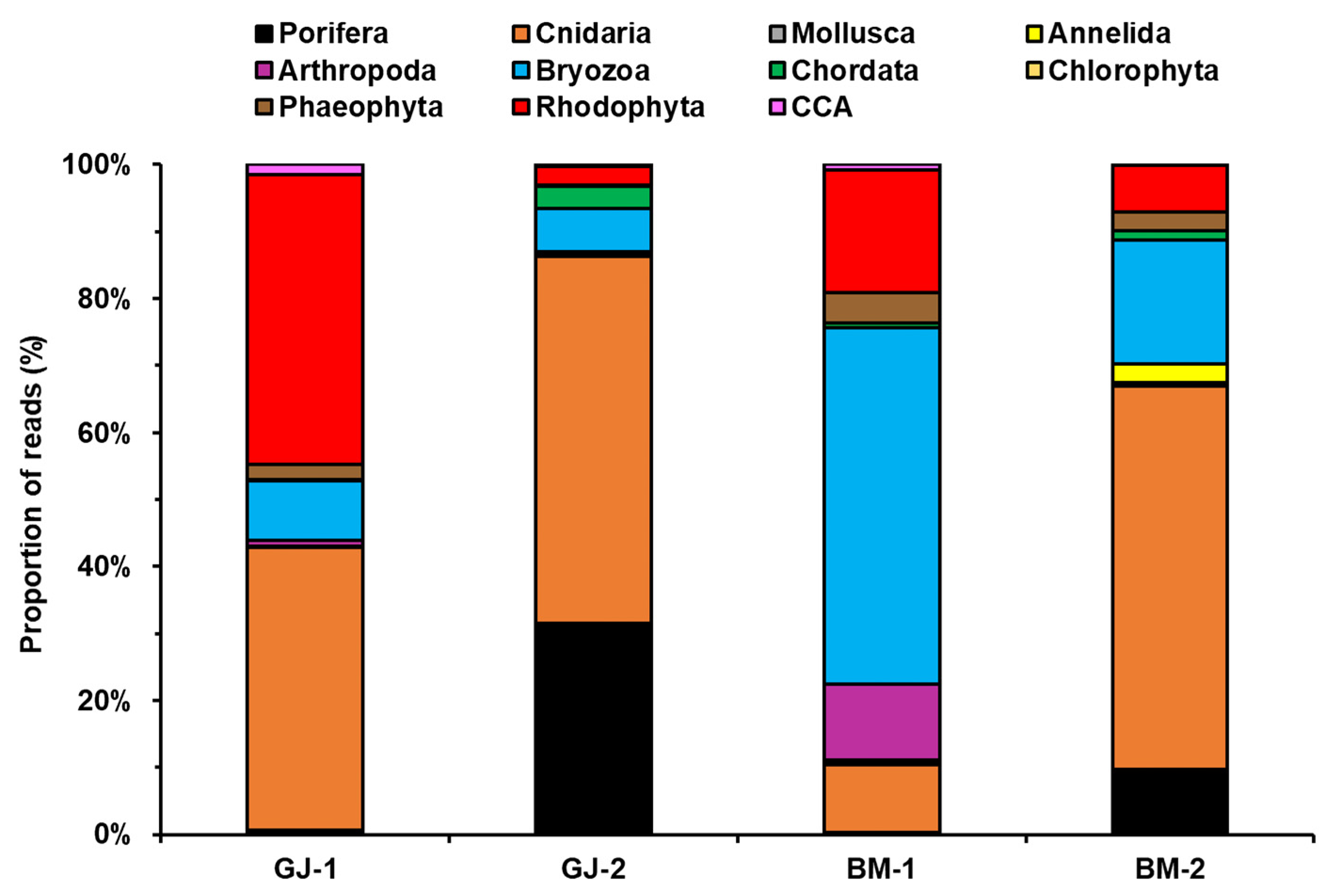
| Site ID | Total Reads | Target Reads * | Target Reads (%) |
|---|---|---|---|
| GJ-1 | 213,664 | 67,731 | 31.7 |
| GJ-2 | 266,440 | 73,537 | 27.6 |
| BM-1 | 233,258 | 34,522 | 14.8 |
| BM-2 | 204,740 | 12,080 | 5.9 |
| Total | 918,102 | 187,870 | 20.5 |
| Sessile Benthic Categories | GJ | BM | All | ||||
|---|---|---|---|---|---|---|---|
| GJ-1 | GJ-2 | Total | BM-1 | BM-2 | Total | ||
| Porifera | 6 | 19 | 21 | 5 | 9 | 12 | 26 |
| Cnidaria | 15 | 27 | 32 | 11 | 12 | 19 | 35 |
| Mollusca | 1 | 1 | 2 | 2 | |||
| Annelida | 1 | 1 | 2 | 1 | 3 | 3 | 3 |
| Arthropoda | 2 | 1 | 2 | 1 | 1 | 2 | |
| Bryozoa | 15 | 11 | 21 | 11 | 12 | 19 | 28 |
| Chordata | 1 | 1 | 1 | 2 | 2 | 3 | 3 |
| Chlorophyta | 1 | 1 | 1 | ||||
| Phaeophyta | 9 | 4 | 12 | 7 | 5 | 9 | 15 |
| Rhodophyta | 34 | 24 | 43 | 16 | 13 | 23 | 49 |
| CCA | 8 | 4 | 9 | 1 | 1 | 2 | 9 |
| Total number of species | 92 | 92 | 144 | 56 | 58 | 93 | 173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-T.; Kim, T.; Park, G.-H.; Oh, C.; Park, H.-S.; Kang, D.-H.; Kang, H.-S.; Yang, H.-S. Assessment of Sessile Benthic Communities in Jeju Island, Republic of Korea, Using Autonomous Reef Monitoring Structures (ARMS). Diversity 2024, 16, 83. https://doi.org/10.3390/d16020083
Lee K-T, Kim T, Park G-H, Oh C, Park H-S, Kang D-H, Kang H-S, Yang H-S. Assessment of Sessile Benthic Communities in Jeju Island, Republic of Korea, Using Autonomous Reef Monitoring Structures (ARMS). Diversity. 2024; 16(2):83. https://doi.org/10.3390/d16020083
Chicago/Turabian StyleLee, Kyeong-Tae, Taihun Kim, Gun-Hoo Park, Chulhong Oh, Heung-Sik Park, Do-Hyung Kang, Hyun-Sil Kang, and Hyun-Sung Yang. 2024. "Assessment of Sessile Benthic Communities in Jeju Island, Republic of Korea, Using Autonomous Reef Monitoring Structures (ARMS)" Diversity 16, no. 2: 83. https://doi.org/10.3390/d16020083
APA StyleLee, K.-T., Kim, T., Park, G.-H., Oh, C., Park, H.-S., Kang, D.-H., Kang, H.-S., & Yang, H.-S. (2024). Assessment of Sessile Benthic Communities in Jeju Island, Republic of Korea, Using Autonomous Reef Monitoring Structures (ARMS). Diversity, 16(2), 83. https://doi.org/10.3390/d16020083








