Abstract
Surveillance of endoparasites at the host community level is rarely reported for ungulates. Yet, changes in the composition and abundance of species in ungulate assemblages, coupled with environmental and climate change, bring into focus the need for baseline data on endoparasite occurrence in host species at the community level. We investigated the prevalence and intensity of eggs of endoparasites in feces of a dynamic boreal ungulate community in Yukon, Canada, that included reintroduced bison (Bison bison), as well as introduced elk (Cervus canadensis), naturally colonizing mule deer (Odocoileus hemionus), and resident populations of caribou (Rangifer tarandus), moose (Alces americanus), and thinhorn sheep (Ovis dalli). We also examined the change in endoparasite prevalence and intensity in bison fecal samples collected eight years apart. The prevalence of eggs detected in feces differed across species for most endoparasite groups. We also provide new records of several endoparasites in novel hosts or new geographic records. We detected a substantially greater prevalence and intensity of trichostrongyle-type eggs in bison feces between samples collected eight years apart. Our data emphasize the need for targeted pathogen surveillance programs to monitor the movement of various ungulate and associated endoparasites. This is particularly pertinent since our data potentially supports evidence for the continued northward expansion and host switching of protostrongylid species, which may have health implications for animals at a new interface.
Keywords:
bison; caribou; cestodes; community ecology; fecal egg counts; moose; Moniezia; protostrongylid; sheep; trichostrongyle 1. Introduction
Ungulates are often culturally and economically important to local people and key constituents in local food webs. Changes in endoparasite interactions have the potential to influence host species at both individual and population levels. The impacts of endoparasites on ungulate hosts range from inconsequential to severe morbidity and mortality [1], and these impacts can vary among host species. Endoparasitic infections can present as a component of multifactorial interactions that impact population health and survival in ungulate populations [2,3,4]. As such, monitoring the prevalence and intensity of endoparasites in ungulates is of importance, particularly when the host species composition and abiotic environment is subject to rapid climate and landscape changes.
Our knowledge of the prevalence and intensity of endoparasites in ungulates is often based on investigations of a single host species. Many endoparasites have specific host species required to complete their life cycle (host-specific). However, host species do not often occur in isolation, as they are typically part of an assemblage of species occupying similar habitats or utilizing similar food resources, which may facilitate the transmission of generalist parasites across species. Thus, transmission between species may impact whole communities [5]. Therefore, the composition and history of a complex ungulate host community can impact the prevalence and intensity of endoparasites among community members. Ungulate communities can be dynamic in their species composition, with changes in species resulting from natural or human activities that add or remove wild or domestic species to local assemblages [6,7].
The ungulate community in southwestern Yukon, Canada, has been particularly dynamic over the last 35–70 years [6,7]. Resident species, such as caribou (Rangifer tarandus), moose (Alces americanus), and thinhorn sheep (Ovis dalli), share a landscape with relatively “new” species, including bison (Bison bison), elk (Cervus canadensis), and mule deer (Odocoileus hemionus). Bison were reintroduced to the region in the late 1980s as part of a national recovery program for an endangered species [8,9], elk were introduced in the 1950s to increase ungulate species available to local hunters [10], and mule deer naturally colonized the region from the Rocky Mountains, beginning in the 1950s [11]. Wildlife managers and local people that use these species lack information on how endoparasite prevalence or intensity differs among ungulate hosts in this dynamic ungulate community.
Moreover, climate change is expected to have a range of effects on the endoparasites that exist in northern ecosystems, including, but not limited to, range expansion of more southerly endoparasite species [12,13,14,15,16], increases in endoparasite diversity [13,16], enhanced environmental parasite survival, and prolonged infective seasons. These changes have the potential to result in heavier endoparasite burdens, and potentially pathogenic effects, in host species [12,13,17]. For many northern ungulates, climate change may also be significantly changing host–protostrongylid species interactions [12], as has been seen with Umingmakstrongylus pallikuukensis, the nematode lungworm of muskox (Ovibos moschatus) [16,18]. Both the northward expansion of hosts carrying novel endoparasites and the anthropogenic translocation of non-native species are particularly concerning when considering aberrant hosts—hosts in which parasites are not adapted with potential for increased impacts on host health [19]. Evaluating and establishing the prevalence and intensity of endoparasites is important for comparing against future changes in the ranges of host species, endoparasite emergence, and host-switching events.
Considerable effort has been expended in recent decades on reintroducing bison to their native range. However, there has been concern regarding the reintroduction of bison (as well as that of elk and deer) impacting access to food and habitat resources as well as the health of resident ungulates, such as moose, caribou, and sheep [6,7,8,9]. This concern, coupled with that of climate change impacts, suggests that the surveillance of endoparasites across species within the ungulate community is of interest. Thus, the aim of our study was to approximate the prevalence and intensity of fecally-shed endoparasites among hosts in an ungulate community that included reintroduced bison. An ancillary objective of our study was to compare short-term changes in the prevalence and intensity of fecal endoparasites in bison specifically over eight years.
2. Materials and Methods
Our study area was located within the distributional range of the Aishihik bison population, a reintroduced population located in southwestern Yukon, Canada [7,8,20]. Our 8000 km2 study area was within the Boreal Cordillera Ecozone [21], which was semi-arid and characterized by short, cool summers and long, cold winters. Vegetative cover primarily consisted of alpine tundra and, at lower elevations, boreal forest dominated by white spruce (Picea glauca) or trembling aspen (Populus tremulodies). Bison, caribou, moose, and thinhorn sheep were common and widely distributed across our study area. Elk, mule deer, and semi-feral horses (Equus ferus) also occurred in our study area, but these species were localized and occurred at low densities [6,7]. Mountain goats (Oreamnos americanus) occurred within 25km of our study area, but none had been observed within the bison range during >20 years of survey effort [7].
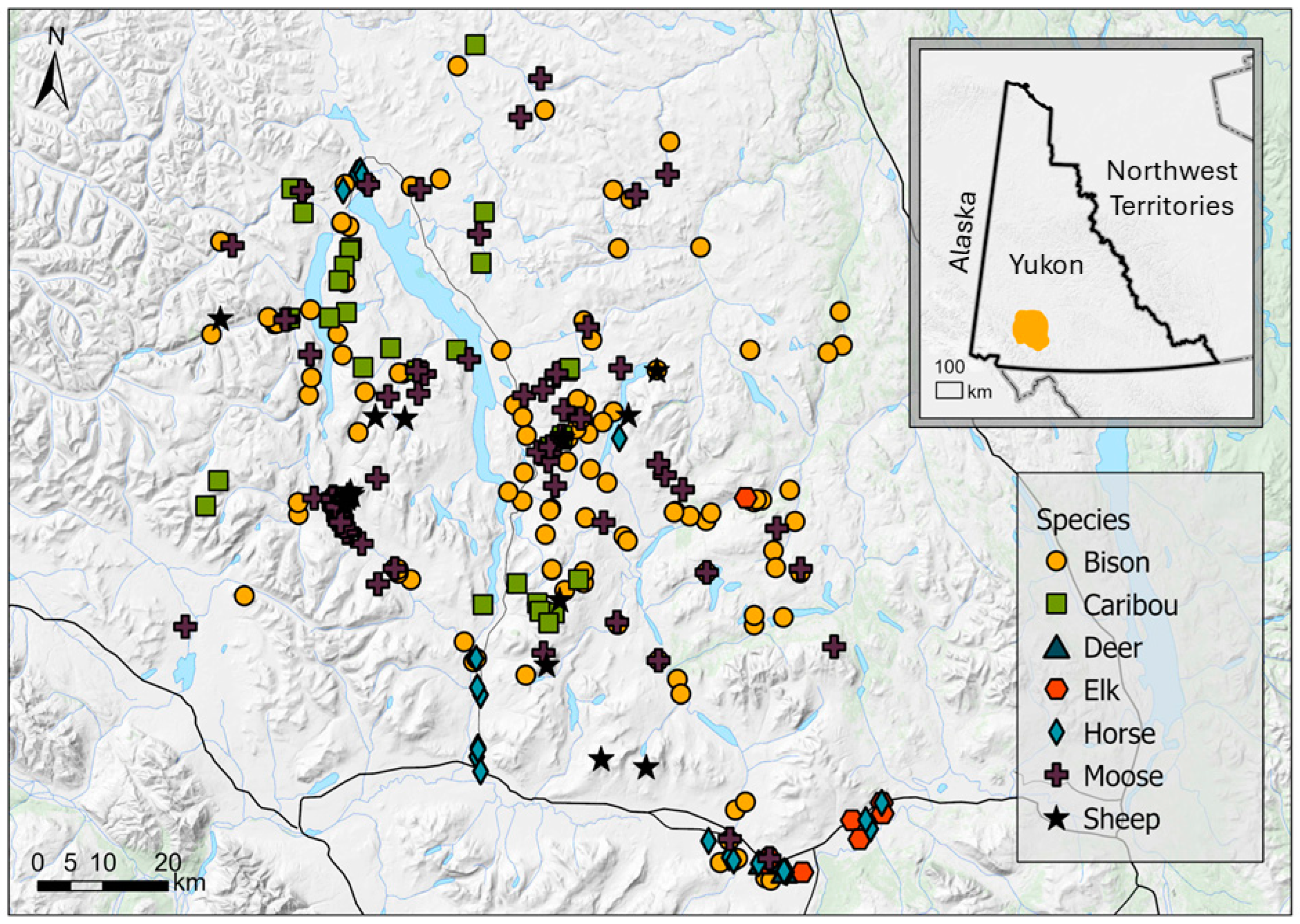
We collected fecal samples (>100 g each) from the ground from six ungulate species (Table 1) within our study area, including bison (n = 83 fecal samples), caribou (n = 38), moose (n = 139), thinhorn sheep (n = 35), elk (n = 9), and mule deer (n = 4; Figure 1). Sample sizes were imbalanced and reflected, in part, the relative abundance of these species in our study area and the difficulty of finding fecal samples in a remote landscape with limited access. During late February and March 2010, we used a helicopter to locate fresh tracks in the snow of the target species and follow them to feeding or bedding areas. We easily identified species that deposited the feces based on the size and shape of the feces [22], as well as the tracks and other signs (e.g., bedding and feeding sites) left in the snow [7]. In the case of sheep and deer, habitat was also used to identify the species that left the feces, with sheep pellets found in high alpine habitats and deer pellets found in low-elevation forests. We collected up to six samples from each site. At each site, we walked a loose transect through which a group of animals had spent time feeding, resting, and defecating. To attempt to obtain samples from different individuals, we collected a single sample every 10–15m to avoid the chance of sampling the same animal twice. Sampling occurred broadly across our large study area, except for elk, whose population was localized. We did not know the sex or age class of animals that deposited the samples. Fecal collections were non-invasive so permits from animal care committees or the government wildlife management agency were not required.
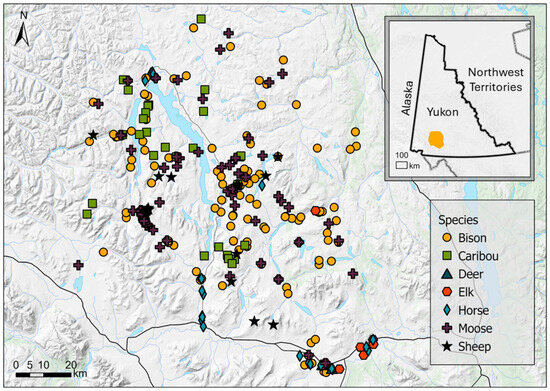
Figure 1.
Locations where ungulate fecal samples were collected by species in Yukon, Canada.
Samples were frozen when collected in the field and stored frozen at −20°C until processed in the lab. The collection and storage of frozen fecal samples is a routine practice for studies examining the endoparasites of northern ungulates. Many wild ungulates are only sampled by management agencies for various pathogens in winter, so our results may be compared to those of other studies on other northern ungulates where fecal samples were collected similarly. For instance, frozen fecal samples collected in winter on top of the snow have been used in numerous studies of northern ungulates, including muskox, sheep, caribou, and moose [12,18,23,24,25,26]. Additionally, protostrongylid nematodes of Arctic ungulates, such as caribou and muskox, have been shown to have high freezing survival [27,28]. It is important to note that our aim was not a precise identification and quantification of the intensity of endoparasites in our study species, which may be affected by using frozen versus fresh species or the timing of sample collection [28]. Rather, we aimed to provide a robust comparison of the relative prevalence and intensity across our study species using samples collected at the same time of year and subject to the same collection and storage protocols; thus, eliminating bias in our set of samples.
In 2019, fecal samples were analyzed for gastrointestinal nematode eggs and coccidian oocysts using a modified Wisconsin double centrifuge technique as previously described [29]. Nematode (generic trichostrongyle, nematodirid, Moniezia sp., Trichuris sp.) eggs were identified to family or genus level based on morphology and then counted to a maximum of 1000. After this number, the total number of eggs per slide was estimated based on the extent of the slide covered; for example, 1000 eggs at 50% of the slide was estimated to be equal to 2000 eggs across the entire slide. Fecal egg counts were calculated by dividing the total number of eggs per sample by the grams of feces. Coccidian oocysts (Eimeria spp.) were identified by morphology to genus and categorized with the following classification system: 0 = 0 per slide; 1+ = 1–50; 2+ = 51–250; 3+ = 251–1000; 4+ = >1000. Additionally, all sheep, caribou, mule deer, and elk fecal samples were analyzed for first stage (L1) of lung or muscle-dwelling protostrongylid nematodes (spike-tailed larvae of Protostrongylus or Orthostrongylus spp. and dorsal-spined larvae of Parelaphostrongylus or Varestrongylus spp.) using a quantitative beaker Baermann technique [30]. In samples where > 1000 larvae were estimated upon visualization of the sample in a petri dish, three aliquots of 50 microliters were removed with a micropipette after swirling the petri dish. The number of larvae from each aliquot was counted and averaged across the three aliquots, and then larvae per gram of feces was calculated based on the post-centrifugation sample volume and the number of grams of feces. All elk samples were analyzed for trematode eggs using a Flukefinder® modified trematode sedimentation procedure (Flukefinder®, Soda Springs, ID, USA). The fecal intensity for each sample was calculated by dividing the number of eggs or larvae by the number of grams of feces, usually 5 g, to calculate the eggs or larvae per gram of feces (EPG or LPG). Prevalence was calculated as the percentage of samples that were positive. Both the median and mean fecal intensity were calculated only for positive samples (i.e., we did not include 0 values).
To examine changes in the prevalence or intensity of endoparasites in bison specifically, we collected additional bison fecal samples in late winter 2018 using the same field sampling strategy as that of late winter 2010. Laboratory analyses of the 2018 bison samples (n = 87) occurred in 2019, at the same time as for the 2010 bison samples (n = 83).
3. Results
Overall, we found differences in the prevalence among all ungulate species for most endoparasite groups (Table 1). For instance, dorsal-spined protostrongylid larvae were found in only one caribou sample (Table 1). Elk samples were negative for trematode eggs, and all deer samples were negative for all endoparasite species (Table 1). We found a higher prevalence of cestodes (i.e., Moniezia sp.) in bison (27 of 83 samples; 33%) than in any other ungulate species.

Table 1.
Percent (and number) of individuals from different ungulate species in the boreal forest of northwestern Canada that were positive for various endoparasites in fecal samples collected in late winter 2010.
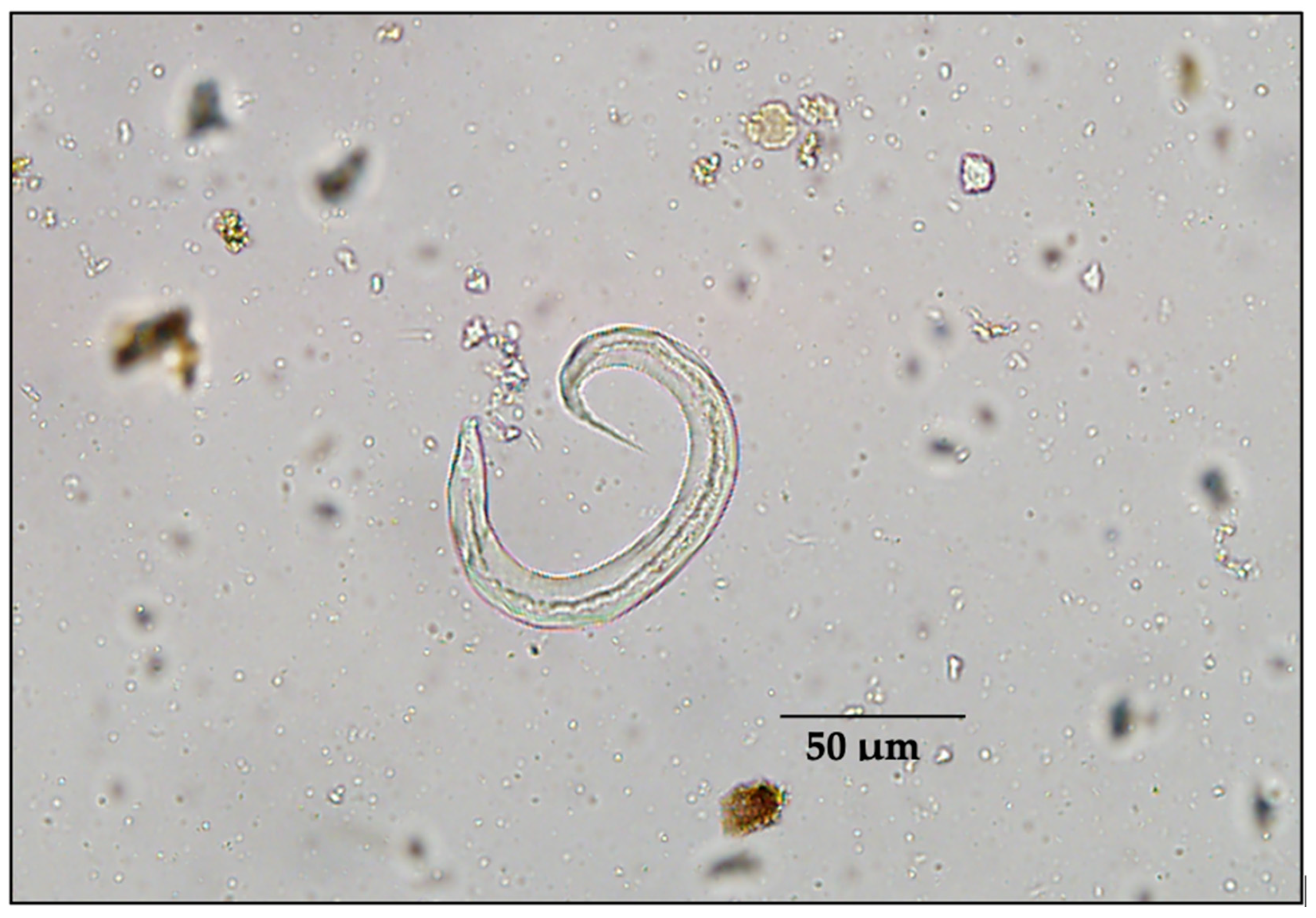
The intensity of fecal endoparasites varied between species (Table 2). Most striking was the intensity of spike-tailed larvae (most likely Protostrongylus stilesi) in sheep feces (Figure 2), which was almost 10 times that in any other species. Cestodes were substantially greater in moose than in any other species of ungulates in our study area. The mean intensity of trichostrongyles, nematodirids, and Trichuris sp. was low among all ungulate species (Table 2). Protostrongylid larvae/gram ranged from 0.6 to 1675.6 in thinhorn sheep, and cestodes eggs per gram of feces ranged from 0.2 to 250 in moose (Table 2). Coccidian oocysts (Eimeria spp.) were observed in 2/38 caribou samples (5.3%), both at the lowest intensity category (1+). A similar prevalence (4/83 samples [4.8%]) of Eimeria spp. oocysts was found in our 2010 bison samples, with an intensity category for three of these samples being 1+ and the other being 2+.

Table 2.
Median, mean ± SD, and range of eggs per gram of feces of different ungulate species in the boreal forest of southwestern, Yukon, Canada, in late winter 2010.
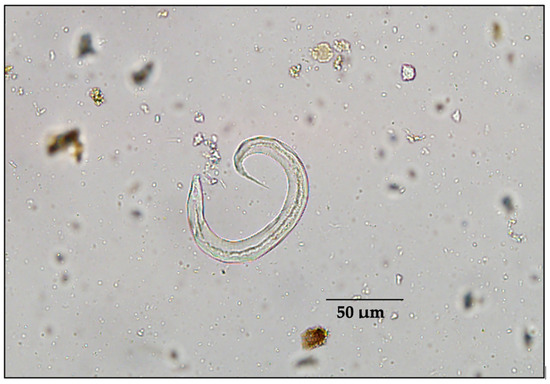
Figure 2.
Example of spike-tailed protostrongylid first-stage larvae (L1), most likely Protostrongylus stilesi in thinhorn sheep (Ovis dalli) or Orthostrongylus macrotis in cervids.
Our results from bison fecal samples collected in 2010 compared to 2018 were generally similar, with the notable exception of trichostrongyles, where prevalence increased from 1% to 93% over the 8-year period (Table 3). The intensity of trichostrongyles in bison fecal samples also increased substantially over 8 years (Table 1). We observed a trivial change (<5%) in the prevalence of Trichuris sp. and Eimeria sp. While there was no change in the prevalence of cestodes in bison fecal samples over the two years, we observed a 32% increase in the intensity of infection between 2010 and 2018 (Table 3).

Table 3.
Comparison of prevalence (%; [n]) and median (mean ± SD [range]) intensity of endoparasites from bison (Bison bison) fecal samples collected in 2010 (n = 83) and 2018 (n = 87) in southwestern Yukon, Canada.
4. Discussion
We provide data on the prevalence and intensity of endoparasites observed in fecal samples from a dynamic ungulate community in northern Canada, several decades after the reintroduction of bison and elk to the region and following the natural range expansion of mule deer into the region. Studies of endoparasite infections in ungulates at the community level are rare. Our key finding was that the prevalence and intensity of endoparasites differed among members of this ungulate community. Our snapshot of endoparasite occurrence among species in this assemblage may serve as a baseline and can be used to examine differences between species and assess changes in the future.
With respect to comparisons within this dynamic community, prevalence differed significantly across species for all endoparasite groups. Notably, caribou had the highest prevalence of trichostrongyle (most likely Teladorsagia spp.), moose and thinhorn sheep had a relatively high prevalence of nematodirids (most likely Nematodirus spp.), and sheep had a relatively high prevalence of Trichuris spp. (whipworm). Species of Nematodirus are pathogenic in domestic sheep, but their impact on thinhorn sheep is not known. Dorsal-spined larvae (most likely Varestrongylus eleguneniensis or Parelaphostrongylus andersoni) were found only in caribou feces. Molecular identification would be needed to determine if generalist species of endoparasites were universally found across host species in our study area (e.g., trichostrongyles, nematodirids, and cestodes). However, our study suggests that some species of parasites are more host-specific within this region (e.g., Trichuris sp., Eimeria sp., protostrongylid larvae, and dorsal-spined larvae).
Another key finding of our study is that we also provide new host and geographic records for particular endoparasites in several ungulate species in the Yukon, including Moniezia sp., particularly commonly found in our bison samples. Anoplocephalid tapeworms such as Moniezia sp. are generally widespread across southern North America, but further species-level molecular classification is necessary to understand their impacts on natural and emerging hosts in northern ecosystems [16]. Moniezia sp. can cause diarrhea and reduced weight gain in young domestic sheep [30], and heavy burdens appear to have been the cause of death in some muskox calves [31]. Thus, better understanding the extent of infection of this and other tapeworms may be valuable in understanding population level health impacts in bison and other ungulates that share habitat with them.
The spike-tailed protostrongylid larvae that we identified in caribou and elk are likely Orthostrongylus macrotis, which has not to our knowledge been reported this far north in Canada (but see [32]). Adults of O. macrotis inhabit airways and produce spike-tailed protostrongylid larvae and are usually reported from mule deer and pronghorn (Antilocapra americana) [33,34,35,36]. Less commonly, they have been documented in moose in Alberta [37] and elk in Wyoming [38]. Colonization of new hosts by similar multi-host lungworms has been documented and attributed to climate change [16,39,40,41,42]. At this time, very little is understood about the ecology and pathological impacts of O. macrotis on its hosts, particularly those that are incidental or aberrant hosts of this normally trachea- and bronchi-dwelling lungworm [16,32].
We propose two potential hypotheses for the presence of spike-tailed protostrongylid larvae in feces of elk and caribou in the Yukon. First, the movement of mule deer northward or deliberate introduction of elk could have brought O. macrotis into contact with caribou [31]. Elk introduced to the Yukon were not tested for endoparasites prior to their translocation, underscoring the need to test for pathogens, including endoparasites, prior to the translocation of ungulates to prevent “pathogen pollution” [43]. Second, though less likely based on known host predilections, it is possible that these are larvae of Protostrongylus stilesi from thinhorn sheep. This parasite has spilled into muskox from thinhorn sheep in Alaska, secondary to muskox reintroduction in the Brooks Range [44]. Without genetic analysis, we cannot know definitively which protostrongylid species has colonized elk and caribou in the Yukon and if this colonization has been persistent.
Interestingly, while we found Protostrongylus spp. spike-tailed larvae in our thinhorn sheep samples, we did not identify DSL of Parelaphostrongylus odocoilei. Prevalences of DSL of P. odocoilei were 91% and 100% in thinhorn sheep from Faro and Kluane National Park, Yukon, although not present in Ivaavik National Park, much further north in Yukon [45]. It is possible that DSL of P. odocoilei had decreased survival when stored at −20°C compared to those of Protostrongylus spp.; however, we did recover DSL from other hosts in our study. Prevalences for Protostrogylus spp. approaching 100% are also not uncommon across the northern range of thinhorn sheep and in the Yukon range from 56% to 100% [45]. Protostrongylus rushi was also found in a single, preserved thinhorn sheep specimen [46]. Larvae of both Protostrongylus and Parelaphostrongylus migrate through the lungs, where they can cause hemorrhage and inflammation, and have been implicated in multifactorial pneumonia in wild sheep. Fecal examination for lungworm larvae is not sufficient for predicting the extent of associated disease in thinhorn sheep, as larval counts poorly reflect the extent of lung involvement of these parasites [47]. Overall, the population-level impacts of infections with lungworm and co-infections with lung- and muscle-worm on thinhorn sheep are unknown.
Our caribou samples had a lower prevalence of DSL (3%) compared to previous studies. This may, again, reflect both prolonged freezing at −20°C, the time of year of sampling (protostrongylids often peak in spring and fall, not late winter), or a genuinely low prevalence in this caribou population. DSL have been reported in other caribou populations in the Yukon, with prevalences of 47% and 29%, respectively [5]. The DSL species identified previously in the Yukon were Parelaphostrongylus andersoni and Varestrongylus sp. [5]. Caribou in the Northwest Territories (NWT) had a DSL prevalence of 5.7% [48]. Parelaphelostrongylus odocoilei has also been identified in caribou in the NWT [45]. Our results are consistent with a low (zero) prevalence of DSL in moose and elk [16,38,49]. We did not conduct a Baermann examination for larvae in bison as protostrongylids have not been reported in bison, and any Dictyocaulus spp. lungworm larvae (more commonly found in moose) would not have survived freezing.
A final key finding from our study is that, with the exception of trichostrongyles, we found only minor changes in the prevalence or intensity of endoparasites observed in bison feces between samples collected in 2010 and 2018. The marked increase seen in the prevalence and intensity of trichostrongyles in bison over the 8-year period may be a result of either a true increase in prevalence or a result of samples being stored for an extended duration at −20°C. Previous studies have demonstrated that freezing decreases the recovery rates for strongylid nematode eggs in particular [28], which may explain our low trichostrongyle egg counts in some host species. However, prevalence is generally more conserved with prolonged freezing than intensity [28].
Bison are susceptible to a diverse fauna of trichostrongyles, which can have significant impacts on their health [50]. Given the high prevalence (93%) found in our 2018 samples, it would be valuable to conduct further fecal surveys that include molecular analyses (e.g., metabarcoding) that identify the proportions of trichostrongyle species present [51,52]. Further work on the species identification and impact of anoplocephalid tapeworms (likely Moniezia sp.) in bison is also suggested.
There is mounting evidence that climate change will have several significant impacts on host–parasite ecosystems in northern biomes. For example, protostrongylid survival rates are expected to increase, and transmission periods may also lengthen, which may lead to a range expansion of protostrongylids [14,17,53,54]. This may be particularly concerning in caribou, as many populations are experiencing declines with multifactorial causes [55], and wild sheep, where protostrongylid prevalence and intensity are already high in some populations. Additionally, healthy bison populations are important for recovery efforts [20]. Anthropogenic impacts associated with wildlife management decisions may influence host–endoparasite dynamics by changing primary, intermediate, and aberrant host densities and distributions and the extent to which host species interact with each other. Finally, multi-parasitism (infection with multiple parasites) may have greater impacts on species, both at individual and population levels [56]. Further studies that incorporate analysis at the individual level will be necessary to ascertain the effect of infection with multiple parasites within a given study population. Fecal studies based on the morphology of eggs, larvae, and oocysts such as this one are limited by our inability to identify parasites to species level but are often more achievable in remote environments. Nonetheless, our data provide baseline information against which to compare with future endoparasite surveys. Molecular (genetic) analyses to definitively identify endoparasite species diversity [27,51,52] should be pursued in the future to help guide ungulate conservation in the face of climate change and anthropogenic perturbation.
In summary, despite inherent limitations imposed by using frozen samples collected during the winter season only, our study has provided a rare and valuable comparison of the relative prevalence and intensity of endoparasites among several ungulate species in the same region at the same time. We also provided new records of particular endoparasites in the region or in specific species, which contribute important information on these parasites. Lastly, we have provided information on the short-term apparent change in prevalence and intensity of endoparasites found shed in bison feces.
Author Contributions
Conceptualization, C.L.A., E.J.J. and T.S.J.; data curation, C.L.A. and T.S.J.; formal analysis, C.L.A. and T.S.J.; funding acquisition, E.J.J. and T.S.J.; investigation, C.L.A., B.W. and E.J.J.; methodology, C.L.A., B.W., E.J.J. and T.S.J.; project administration, E.J.J. and T.S.J.; resources, B.W., N.J.H. and T.S.J.; supervision, B.W., N.J.H., E.J.J. and T.S.J.; validation, B.W.; visualization, C.L.A. and T.S.J.; writing—original draft, C.L.A. and T.S.J.; writing—review and editing, B.W., N.J.H. and E.J.J. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by Environment and Climate Change Canada (Habitat Stewardship Program), Yukon Department of Environment, the National Science and Engineering Research Council of Canada (NSERC), and scholarships from the Western College of Veterinary Medicine’s Department of Microbiology and Research Office.
Institutional Review Board Statement
Field sampling was approved by the Government of Yukon. No animals were harassed, harmed, or handled during the collection of samples.
Data Availability Statement
Data can be obtained at reasonable request from the corresponding author. Locations of these species are sensitive due to their conservation status and because some are hunted.
Acknowledgments
We thank the staff and pilots that helped collect samples in the field or store them prior to analysis. H.A. Miller is thanked for making the map.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoberg, E.P.; Kocan, A.A.; Rickard, L.G. Gastrointestinal strongyles in wild ruminants. In Parasitic Diseases of Wild Mammals; Samuel, W.M., Pybus, M.J., Kocan, A.A., Eds.; Wiley: New York, NY, USA, 2001; pp. 193–227. [Google Scholar]
- Gulland, F.M.D. The role of nematode parasites in soay sheep (Ovis aries L.) mortality during a population crash. Parasitology 1992, 105, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Lankester, M.W. Extrapulmonary lungworms of cervids. In Parasitic Diseases of Wild Mammals; Samuel, W.M., Pybus, M.J., Kocan, A.A., Eds.; Wiley: New York, NY, USA, 2001; pp. 228–278. [Google Scholar]
- Eljaki, A.A.; Al Kappany, Y.M.; Grosz, D.D.; Smart, A.J.; Hildreth, M.B. Molecular survey of trichostrongyle nematodes in a Bison bison herd experiencing clinical parasitism, and effects of avermectin treatment. Vet. Parasitol. 2016, 227, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kutz, S.J.; Asmundsson, I.; Hoberg, E.P.; Appleyard, G.D.; Jenkins, E.J.; Beckmen, K.; Branigan, M.; Butler, L.; Chilton, N.B.; Cooley, D.; et al. Serendipitous discovery of a novel protostrongylid (Nematoda: Metastrongyloidea) in caribou, muskoxen, and moose from high latitudes of North America based on DNA sequence comparisons. Can. J. Zool. 2007, 85, 1143–1156. [Google Scholar] [CrossRef]
- Jung, T.S.; Stotyn, S.A.; Czetwertynski, S.M. Dietary overlap and potential competition in a dynamic ungulate community in Northwestern Canada. J. Wildl. Manag. 2015, 79, 1277–1285. [Google Scholar] [CrossRef]
- Jung, T.S.; Hegel, T.M.; Stotyn, S.A.; Czetwertynski, S.M. Co-occurrence of reintroduced and resident ungulates on a shared winter range in northwestern Canada. Écoscience 2015, 22, 7–16. [Google Scholar]
- Jung, T.S. Investigating local concerns regarding large mammal restoration: Group size in a growing population of reintroduced bison (Bison bison). Glob. Ecol. Conserv. 2020, 24, e01303. [Google Scholar] [CrossRef]
- Jung, T.S.; Czetwertynski, S.M.; Schmiegelow, F.K.A. Boreal forest titans do not clash: Low overlap in winter habitat selection by moose (Alces americanus) and reintroduced bison (Bison bison). Eur. J. Wildl. Res. 2018, 64, 25. [Google Scholar] [CrossRef]
- Strong, W.L.; Chambers, J.H.S.; Jung, T.S. Range constraints for introduced elk in southwest Yukon, Canada. Arctic 2013, 66, 470–482. [Google Scholar] [CrossRef]
- Hoefs, M. Mule, Odocoileus hemionus, and white-tailed, O. virginianus, deer in the Yukon. Can. Field-Nat. 2001, 115, 296–300. [Google Scholar] [CrossRef]
- Jenkins, E.J.; Veitch, A.M.; Kutz, S.J.; Hoberg, E.P.; Polley, L. Climate change and the epidemiology of protostrongylid nematodes in northern ecosystems: Parelaphostrongylus odocoilei and Protostrongylus stilesi in Dall’s sheep (Ovis d. dalli). Parasitology 2006, 132, 387–401. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Brooks, D.R. A macroevolutionary mosaic: Episodic host-switching, geographical colonization and diversification in complex host-parasite systems. J. Biogeogr. 2008, 35, 1533–1550. [Google Scholar] [CrossRef]
- Kutz, S.J.; Jenkins, E.J.; Veitch, A.M.; Ducrocq, J.; Polley, L.; Elkin, B.; Lair, S. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host–parasite interactions. Vet. Parasitol. 2009, 163, 217–228. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn, N.P. Gastrointestinal Nematodes of Western Canadian Cervids: Molecular Diagnostics, Faunal Baselines and Management Considerations. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2010. [Google Scholar]
- Kutz, S.J.; Ducrocq, J.; Verocai, G.G.; Hoar, B.M.; Colwell, D.D.; Beckmen, K.B.; Polley, L.; Elkin, B.T.; Hoberg, E.P. Parasites in ungulates of arctic North America and Greenland. Adv. Parasitol. 2012, 79, 99–252. [Google Scholar] [PubMed]
- Kutz, S.J.; Hoberg, E.P.; Polley, L.; Jenkins, E.J. Global warming is changing the dynamics of Arctic host–parasite systems. Proc. R. Soc. B. 2005, 272, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Kafle, P.; Peller, P.; Massolo, A.; Hoberg, E.; Leclerc, L.-M.; Tomaselli, M.; Kutz, S.J. Range expansion of muskox lungworms track rapid arctic warming: Implications for geographic colonization under climate forcing. Sci. Rep. 2020, 10, 17323. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J.; Peters-Kennedy, J.; Stokol, T.; Gerhold, R.W.; Beckstead, R.B.; Divers, T.J. Diagnosis of Parelaphostrongylus spp. infection as a cause of meningomyelitis in calves. J. Vet. Diagn. Investig. 2011, 23, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Harms, N.J.; Jung, T.S.; Andrew, C.L.; Surujballi, O.P.; VanderKop, M.; Savic, M.; Powell, T. Health status of reintroduced wood bison (Bison bison athabascae): Assessing the conservation value of an isolated population in northwestern Canada. J. Wildl. Dis. 2019, 55, 44–53. [Google Scholar] [PubMed]
- Ecological Stratification Working Group. A National Ecological Framework for Canada; Agriculture and Agri-Food Canada and Environment Canada: Ottawa, ON, Canada, 1995.
- Jung, T.S.; Kukka, P.M. Influence of habitat type on decay and disappearance of elk (Cervus canadensis) pellets in boreal forest of northwestern Canada. Wildl. Biol. 2016, 22, 160–166. [Google Scholar] [CrossRef]
- Verocai, G.G.; Kutz, S.J.; Simard, M.; Hoberg, E.P. Varestrongylus eleguneniensis sp. n. (Nematoda: Protostrongylidae): A widespread, multi-host lungworm of wild North American ungulates, with an emended diagnosis for the genus and explorations of biogeography. Parasit. Vectors 2014, 7, 556. [Google Scholar] [CrossRef] [PubMed]
- Filip-Hutsch, K.; Czopowicz, M.; Świsłocka, M.; Ratkiewicz, M.; Borkowska, A.; Kowalczyk, R.; Demiaszkiewicz, A.W. Patterns of parasite eggs, oocysts and larvae shedding by moose in the Biebrza marshland (NE Poland). Int. J. Parasitol. Parasites Wildl. 2020, 11, 191–197. [Google Scholar] [CrossRef]
- Świsłocka, M.; Borkowska, A.; Matosiuk, M.; Czajkowska, M.; Duda, N.; Kowalczyk, R.; Ratkiewicz, M. Sex-biased polyparasitism in moose (Alces alces) based on molecular analysis of faecal samples. Int. J. Parasitol. Parasites Wildl. 2020, 13, 171–177. [Google Scholar] [CrossRef]
- Idland, L.; Juul, A.M.; Solevåg, E.K.; Tysnes, K.R.; Robertson, L.J.; Utaaker, K.S. Occurrence of faecal endoparasites in reindeer (Rangifer tarandus) in two grazing areas in northern Norway. Acta Vet. Scand. 2021, 63, 13. [Google Scholar] [CrossRef] [PubMed]
- Kafle, P.; Peacock, S.J.; Grond, S.; Orsel, K.; Kutz, S. Temperature-dependent development and freezing survival of protostrongylid nematodes of Arctic ungulates: Implications for transmission. Parasit. Vectors 2018, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Schurer, J.; Davenport, L.; Wagner, B.; Jenkins, E. Effects of sub-zero storage temperatures on endoparasites in canine and equine feces. Vet. Parasitol. 2014, 204, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.D.; Todd, A.C. Survey of gastrointestinal parasitism in Wisconsin dairy cattle. J. Am. Vet. Med. Assoc. 1962, 141, 706–709. [Google Scholar]
- Narsapur, V.S. Pathogenesis and biology of anoplocephaline cestodes of domestic animals. Ann. Rech. Vet. 1988, 19, 1–17. [Google Scholar] [PubMed]
- Samuel, W.M.; Gray, D.R. Parasitic infection in muskoxen. J. Wildl. Manag. 1974, 38, 775–782. [Google Scholar] [CrossRef]
- Verocai, G.G.; Kafle, P.; Sulliotti, V.; Lejeune, M.; Hoberg, E.P.; Kutz, S.J. Morphometry of first-stage larvae of Orthostrongylus macrotis (Nematoda: Protostrongylidae), lungworm of wild ungulates from western North America. J. Parasitol. 2022, 108, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Boddicker, M.L.; Hugghins, E.J. Helminths of big game mammals in South Dakota. J. Parasitol. 1969, 55, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Greiner, E.C.; Worley, D.E.; O’Gara, B.W. Protostrongylus macrotis (Nematoda: Metastrongyloidea) in pronghorn antelope from Montana and Wyoming. J. Wildl. Dis. 1974, 10, 70–73. [Google Scholar] [CrossRef]
- Pybus, M.J. Survey of hepatic and pulmonary helminths of wild cervids in Alberta, Canada. J. Wildl. Dis. 1990, 26, 453–459. [Google Scholar] [CrossRef]
- Belem, A.M.G.; Couvillion, C.E.; Siefker, C.; Griffin, R.N. Evidence for arrested development of abomasal nematodes in white-tailed deer. J. Wildl. Dis. 1993, 29, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Samuel, W.M.; Barrett, M.W.; Lynch, G.M. Helminths in moose of Alberta. Can. J. Zool. 1976, 54, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Thorne, T. Diseases of Wildlife in Wyoming, 2nd ed.; Wyoming Game and Fish Department: Cheyenne, WY, USA, 1982.
- deVos, J.C., Jr.; McKinney, T. Potential impacts of global climate change on abundance and distribution of elk and mule deer in western North America. Final. Rep. West. Assoc. Fish Wildl. Agencies 2007, 32, 1369–1378. [Google Scholar]
- Kutz, S.J.; Hoberg, E.P.; Molnár, P.K.; Dobson, A.; Verocai, G.G. A walk on the tundra: Host–parasite interactions in an extreme environment. Int. J. Parasitol. Parasites Wildl. 2014, 3, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Hoberg, E.P.; Brooks, D.R. Evolution in action: Climate change, biodiversity dynamics and emerging infectious disease. Phil. Trans. R. Soc. B 2015, 370, 20130553. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.R.; Hoberg, E.P.; Boeger, W.A. The Stockholm Paradigm: Climate Change and Emerging Disease; University of Chicago Press: Chicago, IL, USA, 2019. [Google Scholar]
- Cunningham, A.; Daszak, P.; Rodríguez, J. Pathogen pollution: Defining a parasitological threat to biodiversity conservation. J. Parasitol. 2003, 89, S78–S83. [Google Scholar]
- Hoberg, E.P.; Kutz, S.J.; Nagy, J.; Jenkins, E.J.; Elkin, B.; Branigan, M.; Cooley, D. Protostrongylus stilesi (Nematoda: Protostrongylidae): Ecological isolation and putative host-switching between Dall’s sheep and muskoxen in a contact zone. Comp. Parasitol. 2002, 69, 1–9. [Google Scholar] [CrossRef]
- Jenkins, E.J.; Appleyard, G.D.; Hoberg, E.P.; Rosenthal, B.M.; Kutz, S.J.; Veitch, A.M.; Schwantje, H.M.; Elkin, B.T.; Polley, L. Geographic distribution of the muscle-dwelling nematode Parelaphostrongylus odocoilei in North America, using molecular identification of first-stage larvae. J. Parasitol. 2005, 91, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Kutz, S.J.; Veitch, A.M.; Hoberg, E.P.; Elkin, B.T.; Jenkins, E.J.; Polley, L. New host and geographic records for two protostrongylids in Dall’s sheep. J. Wildl. Dis. 2001, 37, 761–774. [Google Scholar] [CrossRef]
- Jenkins, E.J.; Veitch, A.M.; Kutz, S.J.; Bollinger, T.K.; Chirino-Trejo, J.M.; Elkin, B.T.; West, K.H.; Hoberg, E.P.; Polley, L. Protostrongylid parasites and pneumonia in captive and wild thinhorn sheep (Ovis dalli). J. Wildl. Dis. 2007, 43, 189–205. [Google Scholar] [CrossRef]
- Johnson, D.; Harms, N.J.; Larter, N.C.; Elkin, B.T.; Tabel, H.; Wei, G. Serum biochemistry, serology, and parasitology of boreal caribou (Rangifer tarandus caribou) in the Northwest Territories, Canada. J. Wildl. Dis. 2010, 46, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Barone, C.D.; Wit, J.; Hoberg, E.P.; Gilleard, J.S.; Zarlenga, D.S. Wild ruminants as reservoirs of domestic livestock gastrointestinal nematodes. Vet. Parasitol. 2020, 279, 109041. [Google Scholar] [CrossRef] [PubMed]
- Tessaro, S.V. Review of the diseases, parasites and miscellaneous pathological conditions of North American bison. Can. Vet. J. 1989, 30, 416–422. [Google Scholar] [PubMed]
- Avramenko, R.W.; Bras, A.; Redman, E.M.; Woodbury, M.R.; Wagner, B.; Shury, T.; Liccioli, S.; Windeyer, M.C.; Gilleard, J.S. High species diversity of trichostrongyle parasite communities within and between western Canadian commercial and conservation bison herds revealed by nemabiome metabarcoding. Parasit. Vectors 2018, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Beaumelle, C.; Redman, E.M.; de Rijke, J.; Wit, J.; Benabed, S.; Debias, F.; Duhayer, J.; Pardonnet, S.; Poirel, M.T.; Capron, G.; et al. Metabarcoding in two isolated populations of wild roe deer (Capreolus capreolus) reveals variation in gastrointestinal nematode community composition between regions and among age classes. Parasit. Vectors 2021, 14, 594. [Google Scholar] [CrossRef] [PubMed]
- Hoberg, E.P.; Polley, L.; Jenkins, E.J.; Kutz, S.J. Pathogens of domestic and free-ranging ungulates: Global climate change in temperate to boreal latitudes across North America. Rev. Sci. Tech. 2008, 27, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Hoberg, E.P.; Polley, L.; Jenkins, E.J.; Kutz, S.J.; Veitch, A.M.; Elkin, B.T. Integrated approaches and empirical models for investigation of parasitic diseases in northern Wildlife. Emerg. Infect. Dis. 2008, 14, 10–17. [Google Scholar] [CrossRef]
- Festa-Bianchet, M.; Ray, J.C.; Boutin, S.; Côté, S.D.; Gunn, A. Conservation of caribou (Rangifer tarandus) in Canada: An uncertain future. Can. J. Zool. 2011, 89, 419–434. [Google Scholar] [CrossRef]
- van Beest, F.M.; Petersen, H.H.; Krogh, A.K.H.; Frederiksen, M.L.; Schmidt, N.M.; Hansson, S.V. Estimating parasite-condition relationships and potential health effects for fallow deer (Dama dama) and red deer (Cervus elaphus) in Denmark. Int. J. Parasitol. Parasites Wildl. 2023, 21, 143–152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).