Outstanding Aggregation of the Atlantic Brisingid Hymenodiscus coronata (Sars, 1871) (Echinodermata: Asteroidea) in the Strait of Sicily
Abstract
:1. Introduction
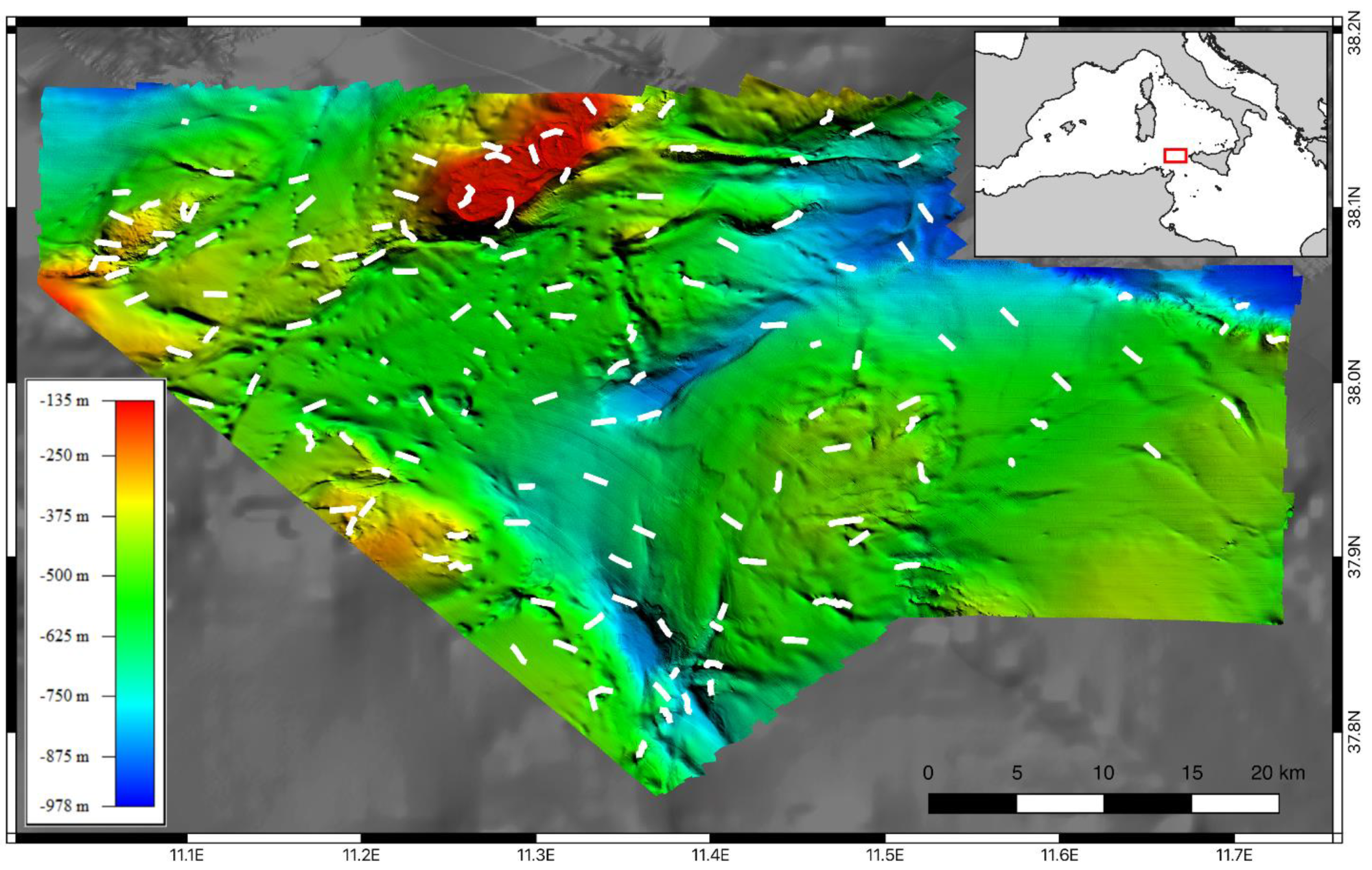
2. Materials and Methods
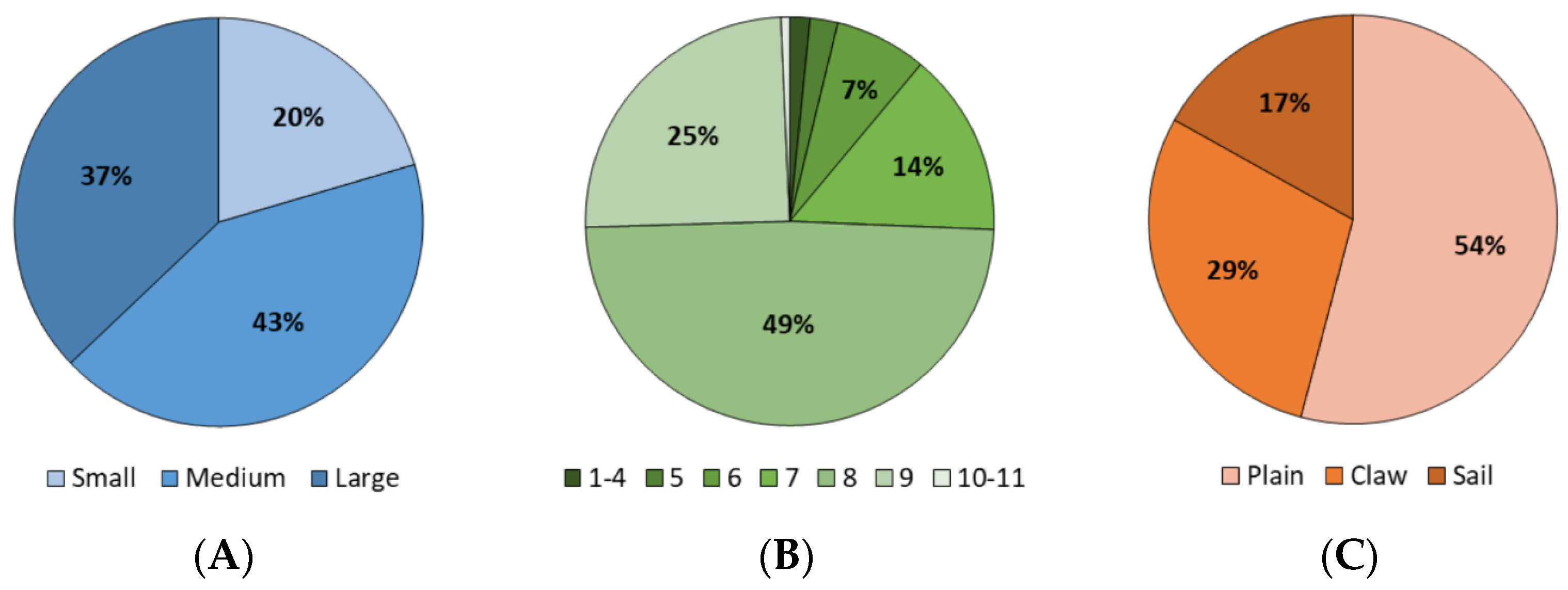
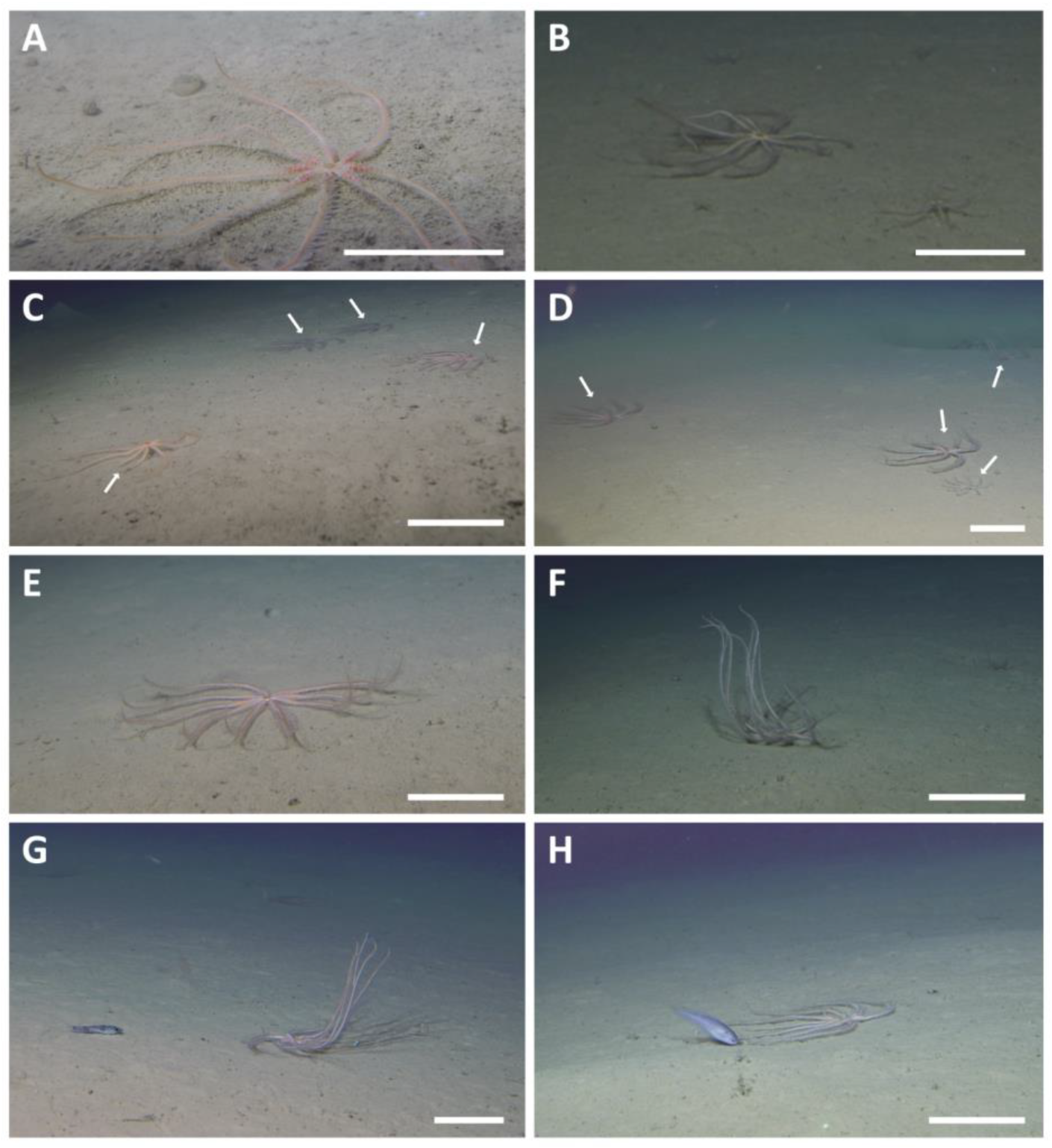
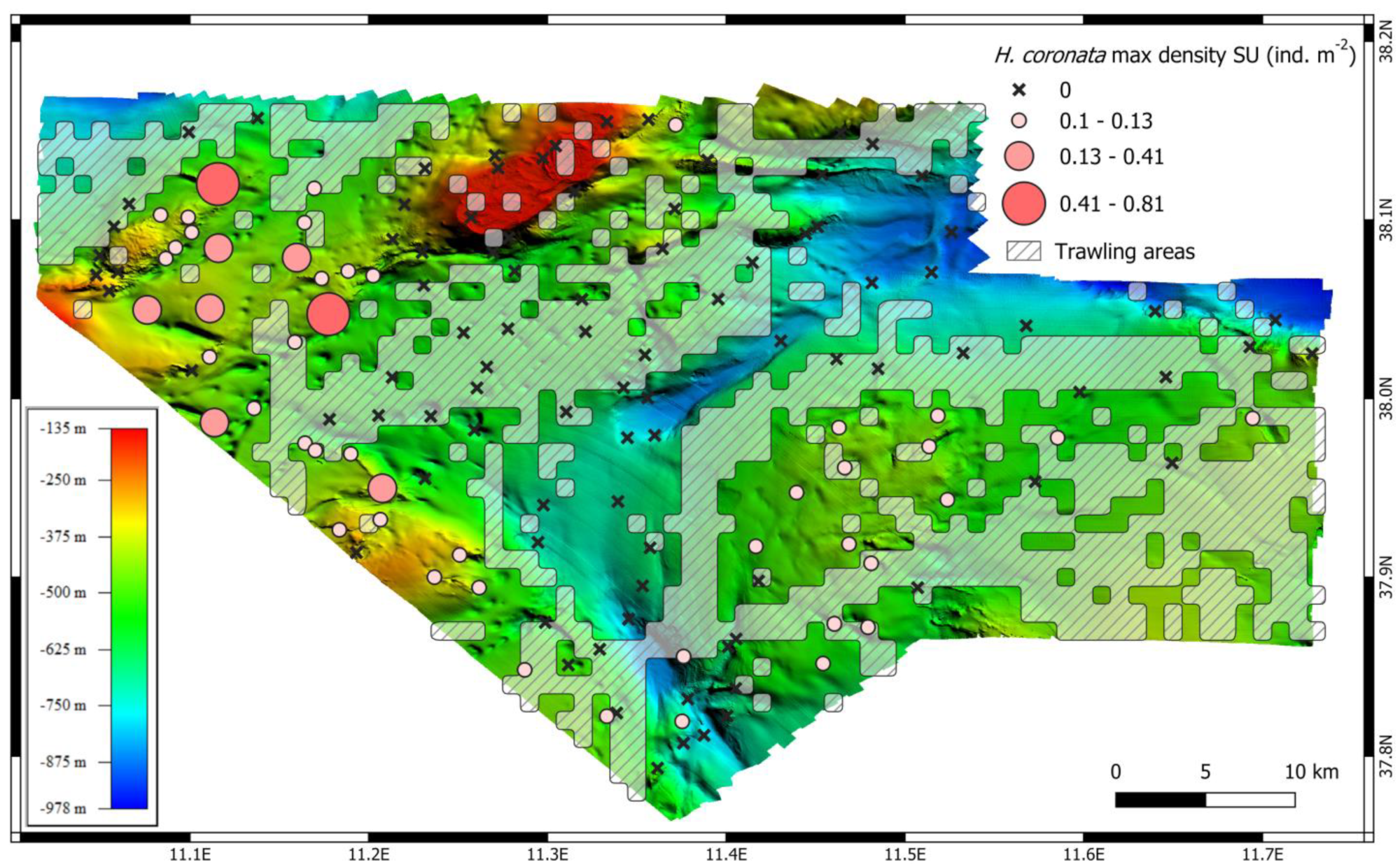
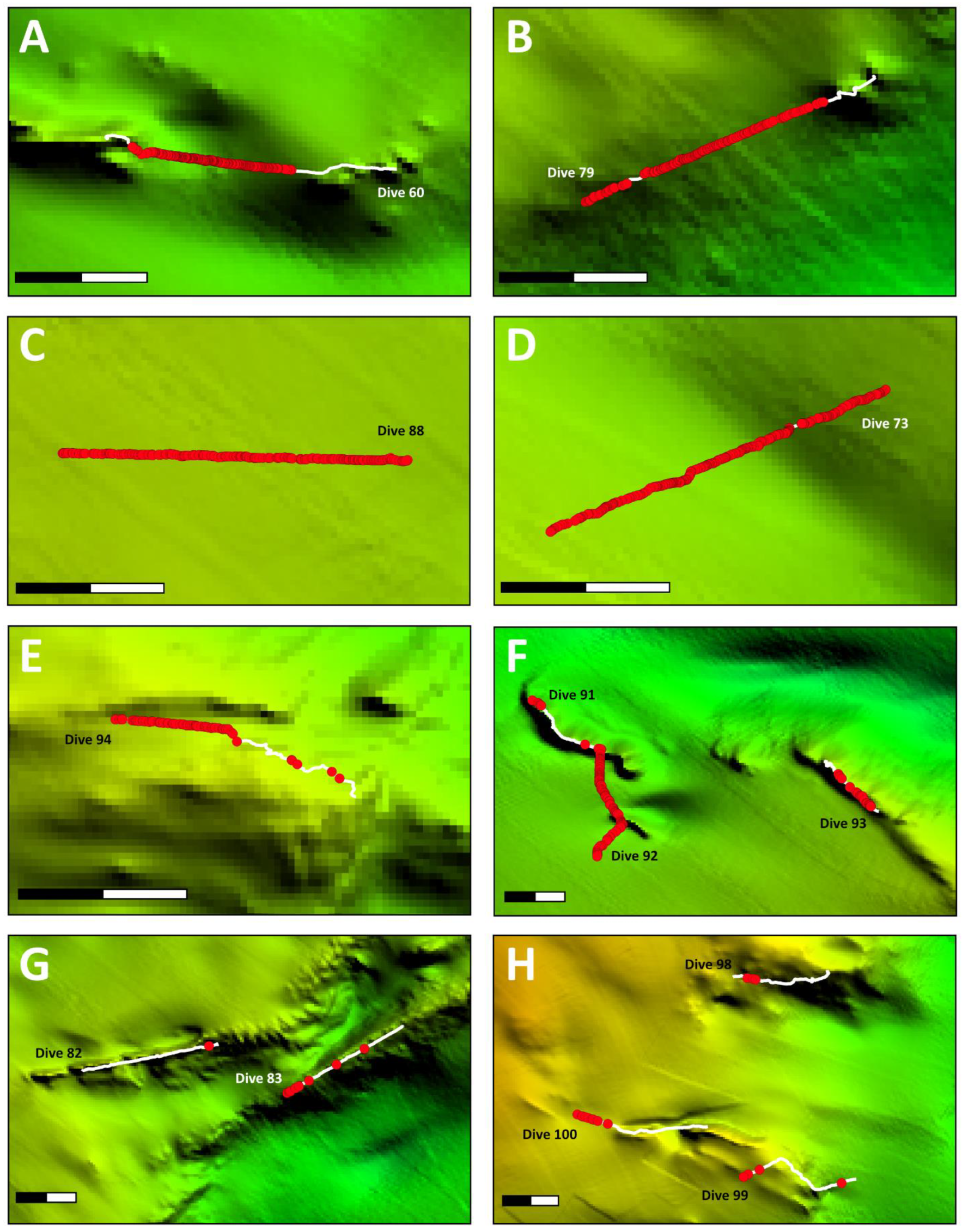
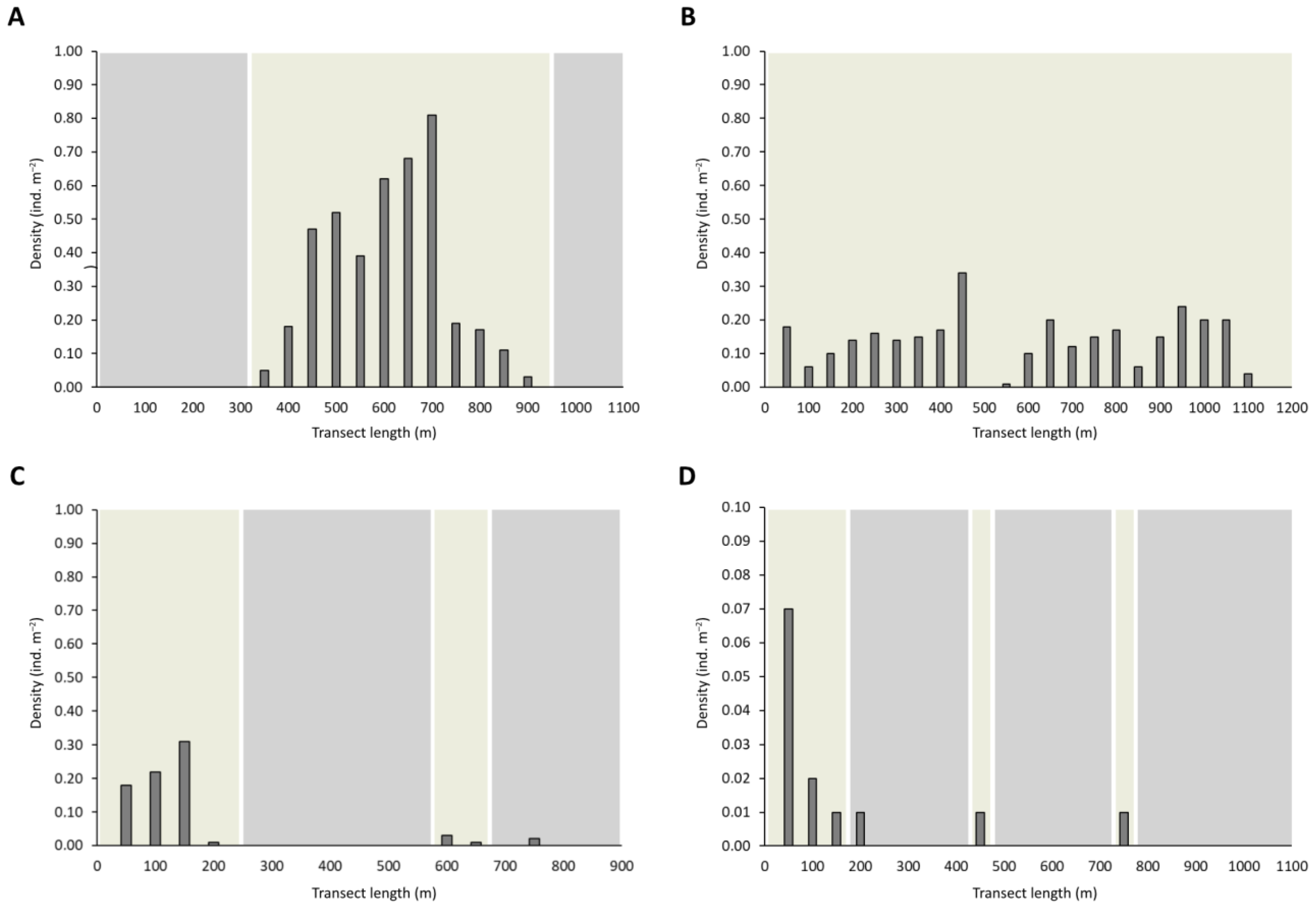
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Mecho, A.; Billett, D.S.M.; Ramírez-Llodra, E.; Aguzzi, J.; Tyler, P.A.; Company, J.B. First Records, Rediscovery and Compilation of Deep-Sea Echinoderms in the Middle and Lower Continental Slope of the Mediterranean Sea. Sci. Mar. 2014, 78, 281–302. [Google Scholar] [CrossRef]
- Leonard, C.; Evans, J.; Knittweis, L.; Aguilar, R.; Alvarez, H.; Borg, J.A.; Garcia, S.; Schembri, P.J. Diversity, Distribution, and Habitat Associations of Deep-Water Echinoderms in the Central Mediterranean. Mar. Biodivers. 2020, 50, 69. [Google Scholar] [CrossRef]
- Gori, A.; Bavestrello, G.; Grinyó, J.; Dominguez-Carrió, C.; Ambroso, S.; Bo, M. Animal Forests in Deep Coastal Bottoms and Continental Shelf of the Mediterranean Sea. In Marine Animal Forests; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Entrambasaguas, L.; Pérez-Ruzafa, Á.; García-Charton, J.A.; Stobart, B.; Bacallado, J.J. Abundance, Spatial Distribution and Habitat Relationships of Echinoderms in the Cabo Verde Archipelago (Eastern Atlantic). Mar. Freshw. Res. 2008, 59, 477. [Google Scholar] [CrossRef]
- Metaxas, A.; Giffin, B. Dense Beds of the Ophiuroid Ophiacantha Abyssicola on the Continental Slope off Nova Scotia, Canada. Deep Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 1307–1317. [Google Scholar] [CrossRef]
- Stevenson, A.; Mitchell, F.J.G.; Davies, J.S. Predation Has No Competition: Factors Influencing Space and Resource Use by Echinoids in Deep-sea Coral Habitats, as Evidenced by Continuous Video Transects. Mar. Ecol. 2015, 36, 1454–1467. [Google Scholar] [CrossRef]
- Iken, K.; Konar, B.; Benedetti-Cecchi, L.; Cruz-Motta, J.J.; Knowlton, A.; Pohle, G.; Mead, A.; Miloslavich, P.; Wong, M.; Trott, T.; et al. Large-Scale Spatial Distribution Patterns of Echinoderms in Nearshore Rocky Habitats. PLoS ONE 2010, 5, e13845. [Google Scholar] [CrossRef]
- Gale, K.S.P.; Gilkinson, K.; Hamel, J.; Mercier, A. Patterns and Drivers of Asteroid Abundances and Assemblages on the Continental Margin of Atlantic Canada. Mar. Ecol. 2015, 36, 734–752. [Google Scholar] [CrossRef]
- Scheibling, R.E.; Lauzon-Guay, J.-S. Feeding Aggregations of Sea Stars (Asterias spp. and Henricia sanguinolenta) Associated with Sea Urchin (Strongylocentrotus droebachiensis) Grazing Fronts in Nova Scotia. Mar. Biol. 2007, 151, 1175–1183. [Google Scholar] [CrossRef]
- Mercier, A.; Hamel, J.-F. Depth-Related Shift in Life History Strategies of a Brooding and Broadcasting Deep-Sea Asteroid. Mar. Biol. 2008, 156, 205–223. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Cobb, J.C.; Talbot-Oliver, T. Density and Dispersion of Luidia clathrata (Echinodermata: Asteroidea) in Old Tampa Bay. Mar. Freshw. Behav. Physiol. 2012, 45, 101–109. [Google Scholar] [CrossRef]
- Mah, C.L. A New Species of Brisingenes from the Hawaii Undersea Military Munitions Assessment Area with an Overview of Hawaiian Brisingid in Situ Video Observations and Functional Morphology of Subambulacral Spines (Forcipulatacea; Asteroidea). Deep Sea Res. Part II 2016, 128, 43–52. [Google Scholar] [CrossRef]
- Zhang, R.; Fau, M.; Mah, C.; Eléaume, M.; Zhang, D.; Zhou, Y.; Lu, B.; Wang, C. Systematics of Deep-Sea Starfish Order Brisingida (Echinodermata: Asteroidea), with a Revised Classification and Assessments of Morphological Characters. Mol. Phylogenet. Evol. 2024, 191, 107993. [Google Scholar] [CrossRef] [PubMed]
- Emson, R.H.; Young, C.M. Feeding Mechanism of the Brisingid Starfish Novodinia antillensis. Mar. Biol. 1994, 118, 433–442. [Google Scholar] [CrossRef]
- Downey, M.E. Revision of the Atlantic Brisingida (Echinodermata: Asteroidea), with Description of a New Genus and Family. Smithson. Contrib. Zool. 1986, 435, 1–57. [Google Scholar] [CrossRef]
- Tanti, C.M.; Schembri, P.J. A Synthesis of the Echinoderm Fauna of the Maltese Islands. J. Mar. Biol. Assoc. UK 2006, 86, 163–165. [Google Scholar] [CrossRef]
- García-Guillén, L.M.; Macías-Ramírez, A.; Ríos, P.; Manjón-Cabeza, M.E. Deep Sea Starfishes (Echinodermata: Asteroidea) from the Avilés Canyon System (Bay of Biscay), Including Two New Records. Estuar. Coast. Shelf Sci. 2022, 277, 107993. [Google Scholar] [CrossRef]
- Sars, G.O. Forhandlinger i Videnskabs-selskabet i Christiania; Brøgger & Christie: Christiania, Norway, 1871; pp. 5–6. [Google Scholar]
- Fisher, W.K. LI.—New Genera and Species of Brisingidæ. Ann. Mag. Nat. Hist. 1917, 20, 418–431. [Google Scholar] [CrossRef]
- Mah, C. Preliminary phylogeny and taxonomic revision of the Brisingida (Asteroidea: Forcipulatacea). In Proceedings of the 9th International Echinoderm Conference, San Francisco, CA, USA, 5–9 August 1996; A.A. Balkema: Rotterdam, The Netherlands, 1998; pp. 273–277. [Google Scholar]
- Clark, A.M.; Downey, M.E. Starfishes of the Atlantic; Chapman Hall: London, UK, 1992; 794p. [Google Scholar]
- Dilman, A.B. Changes in Deep-Sea Asteroid Fauna along the Northern Mid-Atlantic Ridge. Cah. Biol. Mar. 2013, 54, 505–509. [Google Scholar] [CrossRef]
- D’Onghia, G.; Mastrototara, F.; Matarrese, A.; Politou, C.Y.; Mytilineou, C. Biodiversity of the Upper Slope Demersal Community in the Eastern Mediterranean: Preliminary Comparison Between Two Areas With and Without Trawl Fishing. J. Northw. Atl. Fish. Sci. 2003, 31, 263–273. [Google Scholar] [CrossRef]
- Cartes, J.E.; Maynou, F.; Fanelli, E.; Romano, C.; Mamouridis, V.; Papiol, V. The Distribution of Megabenthic, Invertebrate Epifauna in the Balearic Basin (Western Mediterranean) between 400 and 2300 m: Environmental Gradients Influencing Assemblages Composition and Biomass Trends. J. Sea Res. 2009, 61, 244–257. [Google Scholar] [CrossRef]
- Massi, D.; Titone, A.; Micalizzi, R.; Giusto, G.B.; Sinacori, G. Macrozoobenthos Raccolto Nelle Campagne di Pesca a Strascico Condotte Nello Stretto di Sicilia nel Periodo 2003–2010; Technical Report; IAMC-CNR: Mazara del Vallo, Italy, 2011; p. 17. Available online: http://eprints.bice.rm.cnr.it/id/eprint/13012 (accessed on 10 March 2024).
- Öztoprak, B.; Doğan, A.; Dağli, E. Checklist of Echinodermata from the Coasts of Turkey. Turk. J. Zool. 2014, 38, 892–900. [Google Scholar] [CrossRef]
- Terribile, K.; Evans, J.; Knittweis, L.; Schembri, P.J. Maximising MEDITS: Using Data Collected from Trawl Surveys to Characterise the Benthic and Demersal Assemblages of the Circalittoral and Deeper Waters around the Maltese Islands (Central Mediterranean). Reg. Stud. Mar. Sci. 2016, 3, 163–175. [Google Scholar] [CrossRef]
- Gönülal, O.; Dalyan, C. Bathymetric Distribution of Macroinvertebrates in the Northeastern Levantine Sea and the Northeastern Aegean Sea Based on Bottom-Trawl Surveys. Oceanol. Hydrobiol. Stud. 2017, 46, 405–413. [Google Scholar] [CrossRef]
- Buhl-Mortensen, L.; Buhl-Mortensen, P.; Glenner, H.; Båmstedt, U.; Bakkeplass, K. The Inland Deep Sea-Benthic Biotopes in the Sognefjord. In Seafloor Geomorphology as Benthic Habitat; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–372. [Google Scholar] [CrossRef]
- Grinyó, J.; Lo Iacono, C.; Pierdomenico, M.; Conlon, S.; Corbera, G.; Gràcia, E. Evidences of Human Impact on Megabenthic Assemblages of Bathyal Sediments in the Alboran Sea (Western Mediterranean). Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 165, 103369. [Google Scholar] [CrossRef]
- Pérès, J.M.; Picard, J. Nouveau manuel de bionomie benthique de la mer Méditerranée. Rec. Trav. Stat. Mar. End. 1964, 31, 137. [Google Scholar]
- Massi, D.; Titone, A. Composizione Specifica del Megabenthos non Commerciale Della Campagna MEDITS 2018—GSA 16 (Sicilia Meridionale); Technical Report; IRBIM-CNR: Mazara del Vallo, Italy, 2020; p. 13. Available online: http://eprints.bice.rm.cnr.it/id/eprint/20158 (accessed on 10 March 2024).
- Bianchi, C.N.; Morri, C. Biodiversity of the Mediterranean Sea: Situation, Problems and Prospects for Future Research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. Life Mediterr. Sea Look Habitat Changes 2012, 1, 55. [Google Scholar]
- Astraldi, M.; Gasparini, G.P.; Gervasio, L.; Salusti, E. Dense Water Dynamics along the Strait of Sicily (Mediterranean Sea). J. Phys. Oceanogr. 2001, 31, 3457–3475. [Google Scholar] [CrossRef]
- Gasparini, G.P.; Ortona, A.; Budillon, G.; Astraldi, M.; Sansone, E. The Effect of the Eastern Mediterranean Transient on the Hydrographic Characteristics in the Strait of Sicily and in the Tyrrhenian Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2005, 52, 915–935. [Google Scholar] [CrossRef]
- Agostini, V.N.; Bakun, A. ‘Ocean Triads’ in the Mediterranean Sea: Physical Mechanisms Potentially Structuring Reproductive Habitat Suitability (with Example Application to European Anchovy, Engraulis encrasicolus). Fish. Oceanogr. 2002, 11, 129–142. [Google Scholar] [CrossRef]
- Civile, D.; Lodolo, E.; Caffau, M.; Baradello, L.; Ben-Avraham, Z. Anatomy of a Submerged Archipelago in the Sicilian Channel (Central Mediterranean Sea). Geol. Mag. 2016, 153, 160–178. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Sinerchia, M.; Colloca, F. The North Sector of the Strait of Sicily: A Priority Area for Conservation in the Mediterranean Sea. Hydrobiologia 2018, 821, 235–253. [Google Scholar] [CrossRef]
- Van den Beld, I.; Rengstorf, A.; Grehan, A. Development of Standard Operating Procedures for Video Analysis and Classification, GIS Integration, Video Visualization and Image Data Archiving; Final Report INF-08-09-GRE; Earth and Ocean Sciences, NUI: Galway, Ireland, 2007; 73p, Available online: https://maps.marine.ie/infomarData/researchmap/reports/2008/INF-08-09-GRE.pdf (accessed on 10 March 2024).
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Sanchez, R.P. Origen sequencial de los rayos postmetamorficos en Heliaster y Labidiaster (Echinodermata: Asteroidea). Rev. Chil. Hist. Nat. 2000, 73, 573–578. [Google Scholar] [CrossRef]
- Tyler, P.A.; Pain, S.L.; Gage, J.D.; Billett, D.S.M. The Reproductive Biology of Deep-Sea Forcipulate Seastars (Asteroidea: Echinodermata) from the N.E. Atlantic Ocean. J. Mar. Biol. Assoc. UK 1984, 64, 587–601. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Zhou, Y.; Zhang, H. Morphology and Molecular Phylogeny of Two New Species in Genus Freyastera (Asteroidea: Brisingida: Freyellidae), with a Revised Key to Close Species and Ecological Remarks. Deep Sea Res. Part I Oceanogr. Res. Pap. 2019, 154, 103163. [Google Scholar] [CrossRef]
- Sars, G.O.; Sars, M. On Some Remarkable Forms of Animal Life from the Great Deeps Off the Norwegian Coast; Brøgger & Christie: Christiania, Norway, 1872; Volume 1. [Google Scholar]
- Romeu, O.R.; Cartes, J.E.; Solé, M.; Carrassón, M. To What Extent Can Specialized Species Succeed in the Deep Sea? The Biology and Trophic Ecology of Deep-Sea Spiny Eels (Notacanthidae) in the Mediterranean Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 115, 74–90. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Kapiris, K. Feeding ecology of the shortnose greeneye Chlorophthalmus agassizi Bonaparte, 1840 (Pisces: Chlorophthalmidae) in the eastern Ionian Sea (eastern Mediterranean). J. Appl. Ichthyol. 2008, 24, 170–179. [Google Scholar] [CrossRef]
- Consoli, P.; Battaglia, P.; Castriota, L.; Esposito, V.; Romeo, T.; Andaloro, F. Age, growth and feeding habits of the bluemouth rockfish, Helicolenus dactylopterus dactylopterus (Delaroche 1809) in the central Mediterranean (southern Tyrrhenian Sea). J. Appl. Ichthyol. 2010, 26, 583–591. [Google Scholar] [CrossRef]
- Vagenas, G.; Apostolidis, C.; Dogrammatzi, A.; Karachle, P.K. Filling in data gaps on the phaeton dragonet (Synchiropus phaeton (Günther, 1861)): A study on biological characteristics in the eastern Mediterranean (Aegean Sea, Greece). Mar. Biol. Res. 2021, 17, 828–837. [Google Scholar] [CrossRef]
- Scacco, U.; Tiralongo, F.; Mancini, E. Feeding in deep waters: Temporal and size-related plasticity in the diet of the slope predator fish Coelorinchus caelorhincus (Risso, 1810) in the central Tyrrhenian Sea. J. Mar. Sci. Eng. 2022, 10, 1235. [Google Scholar] [CrossRef]
- Azzurro, E.; Ben Souissi, S.J.; Boughedir, W.; Castriota, L.; Deidun, A.; Falautano, M.; Ghanem, R.; Zammit-Mangion, M.; Andaloro, F. The Sicily Strait: A transnational observatory for monitoring the advance of non indigenous species. Biol. Mar. Mediterr. 2014, 21, 105–106. [Google Scholar]
- Spanò, N.; De Domenico, E. Biodiversity in Central Mediterranean Sea. In Mediterranean Identities—Environment, Society, Culture; Fuerst-Bjelis, B., Ed.; Intechopen: London, UK, 2017; Volume 6, pp. 129–148. [Google Scholar] [CrossRef]
- Bo, M.; Al Mabruk, S.A.; Balistreri, P.; Bariche, M.; Batjakas, I.E.; Betti, F.; Bilan, M.; Canese, S.; Cattaneo-Vietti, R.; Corsini-Foka, M.; et al. New Records of Rare Species in the Mediterranean Sea (November 2020). Mediterr. Mar. Sci. 2020, 21, 608–630. [Google Scholar] [CrossRef]
- Smith, C.; Papadopoulou, K.N.; Diliberto, S. Impact of Otter Trawling on an Eastern Mediterranean Commercial Trawl Fishing Ground. ICES J. Mar. Sci. 2000, 57, 1340–1351. [Google Scholar] [CrossRef]
- Colloca, F.; Carpentieri, P.; Balestri, E.; Ardizzone, G.D. A Critical Habitat for Mediterranean Fish Resources: Shelf-Break Areas with Leptometra phalangium (Echinodermata: Crinoidea). Mar. Biol. 2004, 145, 1129–1142. [Google Scholar] [CrossRef]
- Tiralongo, F.; Mancini, E.; Ventura, D.; De Malerbe, S.; Paladini de Mendoza, F.; Sardone, M.; Arciprete, R.; Massi, D.; Marcelli, M.; Fiorentino, F.; et al. Commercial Catches and Discards Composition in the Central Tyrrhenian Sea: A Multispecies Quantitative and Qualitative Analysis from Shallow and Deep Bottom Trawling. Mediterr. Mar. Sci. 2021, 22, 521. [Google Scholar] [CrossRef]
- Fiorentino, F.; Garofalo, G.; Gristina, M.; Gancitano, S.; Norrito, G. Some relevant information on the spatial distribution of demersal resources, benthic biocoenoses and fishing pressure in the Strait of Sicily. In Report of the MedSudMed Expert Consultation on Spatial Distribution of Demersal Resources in the Straits of Sicily and the Influence of Environmental Factors and Fishery Characteristics; MedSudMed Technical Documents No. 2; CNR–IRMA: Trapani, Italy, 2004; pp. 50–66. [Google Scholar]
- Consoli, P.; Esposito, V.; Falautano, M.; Battaglia, P.; Castriota, L.; Romeo, T.; Sinopoli, M.; Vivona, P.; Andaloro, F. The impact of fisheries on vulnerable habitats: The case of trawling on circa-littoral grounds in the Strait of Sicily (central Mediterranean Sea). Mar. Biol. Res. 2017, 13, 1084–1094. [Google Scholar] [CrossRef]
- Di Maio, F.; Geraci, M.L.; Scannella, D.; Russo, T.; Fiorentino, F. Evaluation of the economic performance of coastal trawling off the southern coast of Sicily (Central Mediterranean Sea). Sustainability 2022, 14, 4743. [Google Scholar] [CrossRef]
- Massi, D. Macroinvertebrati Bentonici non Commerciali Della Pesca a Strascico Campagna GRUND 2003—Stretto di Sicilia; ID/WP/DM/1/0704/DRAFT; IAMC-CNR: Mazara del Vallo, Italy, 2004. [Google Scholar]
- Massi, D. Composizione Dello “Sporco” (Macrobenthos Non Commerciale) Della Pesca a Strascico Campagna GRUND 2004—Stretto di Sicilia; ID/WP/DM/2/1005/DRAFT; IAMC-CNR: Mazara del Vallo, Italy, 2005. [Google Scholar]
- Massi, D.; Titone, A. Composizione Dello “Sporco” (Macrobenthos Non Commerciale) Della Pesca a Strascico Campagna MEDITS 2016—Stretto di Sicilia; Technical Report; IAMC-CNR: Mazara del Vallo, Italy, 2017. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, M.; Giova, A.; Bo, M.; Canese, S.; Enrichetti, F.; Romeo, T.; Salvati, E.; Greco, S. Outstanding Aggregation of the Atlantic Brisingid Hymenodiscus coronata (Sars, 1871) (Echinodermata: Asteroidea) in the Strait of Sicily. Diversity 2024, 16, 238. https://doi.org/10.3390/d16040238
Toma M, Giova A, Bo M, Canese S, Enrichetti F, Romeo T, Salvati E, Greco S. Outstanding Aggregation of the Atlantic Brisingid Hymenodiscus coronata (Sars, 1871) (Echinodermata: Asteroidea) in the Strait of Sicily. Diversity. 2024; 16(4):238. https://doi.org/10.3390/d16040238
Chicago/Turabian StyleToma, Margherita, Antonio Giova, Marzia Bo, Simonepietro Canese, Francesco Enrichetti, Teresa Romeo, Eva Salvati, and Silvestro Greco. 2024. "Outstanding Aggregation of the Atlantic Brisingid Hymenodiscus coronata (Sars, 1871) (Echinodermata: Asteroidea) in the Strait of Sicily" Diversity 16, no. 4: 238. https://doi.org/10.3390/d16040238
APA StyleToma, M., Giova, A., Bo, M., Canese, S., Enrichetti, F., Romeo, T., Salvati, E., & Greco, S. (2024). Outstanding Aggregation of the Atlantic Brisingid Hymenodiscus coronata (Sars, 1871) (Echinodermata: Asteroidea) in the Strait of Sicily. Diversity, 16(4), 238. https://doi.org/10.3390/d16040238










