Abstract
Climate change (CC) represents an increasing threat to mangroves worldwide and can amplify impacts caused by local anthropogenic activities. The direct effects of CC on mangrove forests have been extensively discussed, but indirect impacts such as the alteration of ecological processes driven by specific functional groups of the biota are poorly investigated. Ecological roles of key functional groups (FGs) in mangroves from the Atlantic–Caribbean–East Pacific (ACEP) and Indo-West Pacific (IWP) regions are reviewed, and impacts from CC mediated by these FGs are explored. Disruption by CC of ecological processes, driven by key FGs, can reinforce direct effects and amplify the loss of ecological functionality and further degradation of mangrove forests. Biogeochemistry mediator microbiotas of the soil, bioturbators, especially semiterrestrial crabs (Ocypodoids and Grapsoids) and herbivores (crustaceans and Insects), would be the most affected FG in both regions. Effects of climate change can vary regionally in the function of the combination of direct and indirect drivers, further eroding biodiversity and mangrove resilience, and impairing the predictability of ecosystem behaviour. This means that public policies to manage and conserve mangroves, as well as rehabilitation/restoration programs, should take into consideration the pressures of CC in specific regions and the response of key FGs to these pressures.
1. Introduction
Globally, mangroves occupy around 147,359 km2 of sheltered tropical and subtropical coasts, river deltas, and estuaries, inhabiting littoral tidal soils [1]. Mangrove forests are distributed in two main geographic regions, the Atlantic–Caribbean–East Pacific (ACEP) from the West Americas to West Africa and the Indo-West Pacific (IWP) from East Africa to the West Pacific (Figure 1), each containing a specific tree species pool. Forests in the ACEP region support fewer tree species than similar habitats in the IWP region (8 vs. 40, respectively), and though both regions share mangrove flora at the genus level, the IWP has several endemic genera while the ACEP shows only two, Pelliciera and Laguncularia [2].
Notwithstanding, mangrove trees occupy the same total niche (or functional space) in both the ACEP and IWP regions, establishing at the intertidal land–sea interface vegetation fringes of varying width. Diversity, structural complexity, productivity, and biomass are strongly influenced by local abiotic factors, mostly coastal geomorphology, rainfall, tidal amplitude, temperature, salinity, and soil characteristics (nutrients and oxygen content, grain size, humidity) [3,4], as well as biotic factors, like soil micro- and macro-organism communities’ composition, bioturbation, pollination, and herbivory [5,6,7,8].
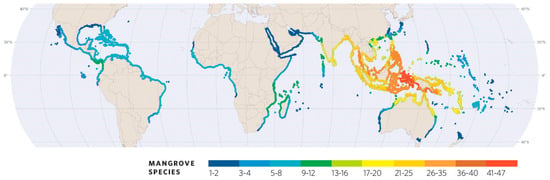
Figure 1.
Specific diversity of worldwide mangroves. Colours indicate the number of catalogued regional species, but considering that local stands have only limited sets of regional species. The Americas–Caribbean and West Africa are the ACEP region, and from East Africa towards the East, this is the IWP region [Adapted from ‘World Atlas of Mangroves’, [9]].
Figure 1.
Specific diversity of worldwide mangroves. Colours indicate the number of catalogued regional species, but considering that local stands have only limited sets of regional species. The Americas–Caribbean and West Africa are the ACEP region, and from East Africa towards the East, this is the IWP region [Adapted from ‘World Atlas of Mangroves’, [9]].
Associated mangrove fauna, both invertebrate (mostly Insects, Polychaetes, Nematodes, Molluscs, and Crustaceans) and vertebrates (fish, birds, and mammals), and likely many other taxa/groups follow the same pattern of higher diversity in the IWP mangroves [2,10,11]. Brachyuran crabs (Crustacea: Decapoda), for example, are far richer in species diversity in IWP forests, where tree and Grapsoid crab richness are positively correlated, totaling over 40 mangrove tree species and around 50 Grapsoids [12,13]. Mangrove community structure and ecological functions are regulated by interactions among these and other species, fitted to the particular physical–chemical conditions that assembled these communities since their rise and dispersion in the early Tertiary [2,14]. Like any other ecosystem, emergent attributes of mangrove community structure and function evolve from interactions among organisms, or, in other words, among their niches. The ecological role of some key groups of organisms, for example, as ‘ecosystem engineers’, like the conspicuous life habits of crab and teredinid populations, or the biochemical cycles mediated by the diverse microbiota, are drivers of structural and compositional attributes of mangrove forests [7,8,15].
Mangrove ecosystems are productive and biodiverse but vulnerable to several threats [16]. Climate change represents an increasing direct threat to mangroves [17], whereas indirectly it can reinforce other natural or anthropogenic impacts [16,18,19]. Impacts of climate change (CC) include sea level rise (SLR), coastal acidification, rising temperatures, changes in precipitation patterns, increased storms and extreme weather events, changes to ocean currents, and rising atmospheric CO2 [20]. Worldwide, mangroves are vulnerable to storms and hurricanes, particularly in the IWP and the Caribbean region in the ACEP. Although East South America and West Africa are still relatively free from the effects of these extreme events, they are predicted to increase in frequency and intensity in these two subregions, as observed recently in southern Brazil [21,22]. These events result in leaf loss, branch breakages, and uprooting and erosion in extreme cases. SLR can increase the drowning and erosion of fringe forests, and burying of basin forests, along with decreasing extension and biodiversity of stands [23,24], even though in some places SLR could favour mangrove expansion by landward migration [25,26]. Extreme rainfall and drought events can also put more pressure on mangrove forests by affecting physiological water balance and salt control mechanisms [27].
However, while the direct effects of CC over mangroves are a current research open avenue [17,20,28], their indirect effects through impacts on the associated biota are not. Indeed, the effects of CC on key organisms such as components of specific functional groups (FGs) can affect forest development and/or structure, potentially leading to functional alterations and forest degradation [29]. Though the concept of ‘functional group’ and ‘guild’ are similar, the sharing by species of a similar resource is the guild (structural) side, and the ecosystem processes these species group influence through resource exploitation, their functional side [30]. Biogeochemistry mediators (which include decomposers), bioturbators, wood borers, and pollinators are examples of FGs in mangroves. For instance, several chemical processes associated with mangrove growth (and so forest structure) depend on the microbiota composition of the sediment [31,32,33]. Crabs and shrimps, and some juvenile and adult fish species, excavate and maintain burrows in soil for refuge [6,34,35,36], influencing sediment microbiota, structure, and physical–chemical conditions (redox potential in particular) [37], which have implications in root and stem development and hence tree growth.
Traditional guild categories such as herbivores and omnivores also play significant roles in the mangrove forest structure through their trophic function, so we consider them as FGs. For instance, some herbivore Brachyuran crabs, through the consumption and burial of specific mangrove propagules, determine tree species that develop and hence forest richness, architecture, and biomass being significant ‘ecosystem engineers’ [7,38,39,40,41]. Omnivores like the neotropical red crab Goniopsis cruentata can develop crucial roles at several trophic levels through predation, herbivory, and detritivory [8,35,42].
An extensive literature search was conducted on major mangrove faunal functional groups involved in the ecological and structural features of mangrove forests in the ACEP and IWP regions summarised in Table 1. It focuses on the FGs of organisms that perform ecological roles that directly ‘shape’ the structural (and hence architectural) features of the forest (tree species, density, size, biomass) since we are interested in structural/physical resistance and the resilience of mangroves to CC. Selected images of these FGs are provided in Figure 2. There are differences in regional/local biotic assemblages inside the main regions (ACEP and IWP), but to capture general attributes of each region to compare them with more confidence, we stressed the FG function. Likewise, when possible, we put groups in the same taxonomic resolution in both regions for better comparison, but the diversity of some of them can vary by one or more orders of magnitude in one region in respect to the other, so further information about more diverse groups can be found in the literature herein.

Figure 2.
Fauna of mangroves. (A) Langurs (Presbytis sp.); (B) Episesarma versicolor; (C) mudskipper (in their burrow); (D) Goniopsis cruentata (eating a Rhizophora propagule); (E) teredinid (Neoteredo sp.) (circle shows the anterior portion of an exposed individual, surrounded by calcified galleries); (F) fiddler crab (Tubuca sp.); (G) Dysphania sp. (herbivore, larva feeds on Kandelia candel). All images from IWP, except (D,E) from ACEP. [Credits: E. Ashton (A,B,F,G); M. Zimmer (C); C.E. Alencar (D); A. Ferreira (E)].
To our knowledge, other groups, like Polychaetes and Nematodes, which, respectively, can represent from 60 to 90% of mangrove soil species, do not influence forest tree composition and structure despite their significant ecological roles in mangrove soils [43,44,45] and were thus not included in this discussion. Likewise, the contribution to ecosystem functioning of diverse types and populations of soil micro- and meso-invertebrates has not been considered due to their extreme diversity and limitations in sampling and/or conservation, which makes their functional role poorly documented [46].

Table 1.
Functional groups (FGs) used in this work to characterise faunal ecological roles, their main faunal components, and their natural effects/interaction on mangrove forests. OM, organic matter; AGB, aboveground biomass; BGB, below-ground biomass; ACBS, anastomosed communal burrow systems; ACEP, Atlantic–Caribbean–East Pacific; (*), East Pacific (ACEP) species; (A), East Atlantic (ACEP) species; IWP, Indo-West Pacific mangroves.
Table 1.
Functional groups (FGs) used in this work to characterise faunal ecological roles, their main faunal components, and their natural effects/interaction on mangrove forests. OM, organic matter; AGB, aboveground biomass; BGB, below-ground biomass; ACBS, anastomosed communal burrow systems; ACEP, Atlantic–Caribbean–East Pacific; (*), East Pacific (ACEP) species; (A), East Atlantic (ACEP) species; IWP, Indo-West Pacific mangroves.
| Functional Group (1) | Functional/ Ecosystemic Role | Major Faunal Group | Effects over Forest (2) | Main Groups and Species Involved | |
|---|---|---|---|---|---|
| ACEP | IWP | ||||
| Biogeochemistry mediators | C, N, P, Fe, and S cycle mediators | Micro-organisms | Effect over ecological processes that cycle nutrients and metals C cycling/N fixation, ammonification, nitrification, denitrification/sulphate reduction Changes in C:N:P ratio of organic matter > changes digestibility and biodegradability of OM Changes trace (essential and non-essential) elements’ availability to plants > regulation plant growth/Hg methylation Control redox conditions > induces iron plaque formation > regulate nutrient uptake by roots > protect trees from toxic substances | Bacteria; Archaea; Cyanobacteria; Fungi (Mycorrhizae); Algae (Diatomacea); Protists; N fixing, ammonifying, nitrifying, and denitrifying bacteria; Phosphate-solubilizing bacteria; Sulphate-reducing bacteria. | Bacteria; Archaea; Cyanobacteria; Fungi (Mycorrhizae); Algae (Diatomacea); Protists; N fixing, ammonifying, nitrifying, and denitrifying bacteria; Phosphate-solubilizing bacteria; Sulphate-reducing bacteria. |
| Bioturbators/burrowers | Ecosystem engineering by sediment disturbance (burrowing, scraping, feeding), sediment oxygenation, particle reworking, sediment architecture, and creation of new habitats | Decapod Crustaceans: Ocypodoidea Xanthoidea Grapsoidea Portunidae Stomatopoda Alpheidae Thalassinidae | Oxygenation > increases redox potential > increase OM mineralisation > dissociate sulphides Decrease sediment OM content > accelerate nutrient and pollutant cycling > maintain productivity Sediment architecture > creation of new habitats > increases micro- and macro-biota diversity Burial/topple of small propagules > biased recruitment > forest structure > AGB/BGB content > carbon content and cycling | Semi-terrestrial Decapods: Fiddler crabs and Ucides cordatus, U. occidentalis (*) (Ocypodoidea) (3) Sesarma rectum, S. curacaoense, S. aequatoriale (*), S. rhizophorae (*), Armases angustipes, A. occidentale (*) (Sesarmidae, (3) Grapsoidea) + other crabs inhabiting ACBS (4) Xanthoidea Callinectes spp. (Portunidae) Pistol shrimp Alpheus spp. (Alpheidae) | Semi-terrestrial Decapods: Fiddler crabs (Ocypodidae) Episesarma versicolor Neosarmatium spp. Perisesarma dussumieri (Sesarmidae) (3) Mud crab Scylla spp. (Portunidae) Xanthoidea Mantis shrimp Squilla spp. (Stomatopoda) Pistol shrimp Alpheus spp.(Caridea) Mud lobters Thalassina spp. (Thalassinidae) |
| Fishes | Burrows making/occupancy > sediment disturbance > oxidation > biota diversification | Cyprinodonts, Gobiids, Fundulids, Rivulins, Poeciliids, Eleotrids | Cyprinodonts, Eleotrids, Gobiids: Periophthalmodon spp., Periophthalmus spp., Scartelaos spp., Boleophthalmus spp. (Mudskippers) | ||
| Herbivores (including Omnivore crabs) | Propagules consumption | Brachyuran and Sesarmid crabs | Propagule consumption > biased recruitment > forest structure/architecture > AGB/BGB content > carbon content and cycling | Goniopsis cruentata, G. pelii (A), G. pulchra (*) (Grapsidae) Ucides cordatus, U. occidentalis (*) (Ucididae) | Neosarmatium smithii, N. meinerti, N. africanum, Metopograpsus latifrons (Sesarmidae) |
| Folivory | Sesarmid crabs | Selective canopy consumption > forest productivity and biomass? | Aratus pisonii, A. pacificus (*) (Sesarmidae, Grapsoidea) | Neosarmatium spp., Perisesarma spp., Parasesarma spp. Episesarma versicolor (Sesarmidae) | |
| Insects | Leaf consumption, defoliation, sap feeding >affect leaf and bud areas > canopy and forest architecture/structure >reproductive and vegetative growth > reproductive output > tree species recruitment | Lepidopterans, Dipterans, Homopterans, Hemipterans, Orthopterans, Coleopterans. | Coleopterans, Lepidopterans, Dipterans, Homopterans, Hemipterans, Orthopterans. | ||
| Litter/detritus consumption | Crabs and gastropod molluscs (5) | Alter soil carbon content and cycling Increase export of carbon and nutrients | Goniopsis cruentata, G. pelii (A), G. pulchra (*) (Grapsidae) Ucidescordatus, U. occidentalis (*) (Ucididae) Sesarmidae (Grapsoidea) Littoraria spp. Thais spp. (*) Cerithidea spp. (*) (Gastropoda) | Parasesarma eumolpe, P. onychophorum, Perisesarma spp. (Sesarmidae) Fiddler crabs (Ocypodidae) Telescopium telescopium, Terebralia palustris Potamiidae Littoraria spp. Cerithidea spp. (Gastropoda) | |
| leaves, flowers, and fruits | Primates | Seed dispersal? | Sapajus libidinosus, S. xanthosternos, S. apella, Alouatta palliata, A. pigra Cebus capucinus (*) | Langurs (Presbytis), proboscis monkey (Nasalis larvatus), Macaca fascicularis, Trachypithecus auratus, T. cristatus, Piliocolobus badius | |
| Wood-borers/ chewers (Xylovores) | Wood-boring Cellulose-lignin processers | Crustaceans: Brachyuran crabs, Isopoda | Consumption of aerial roots meristems > forest architecture > changes in live and dead biomass > carbon stock Promotion of root sprouting (in Rhizophora mangle) > increase in structural complexity of roots > increase in root biomass Effects on structural support and nutrient supply > changes in epifauna and epifloral communities > community-level impacts | Sphaeroma terebrans, S. peruvianum (*) Limnoria lignorum (*) (Isopoda, Crustacea) G. cruentata (Grapsidae) | Sphaeroma terebrans Limnoria spp. (Isopoda) |
| Teredinid and Pholadidae molluscs | Biodegradation > increase in OM lability > accelerate nutrient cycling and outwelling of POM from the tunnelling of deadwood Significant tunnelling of deadwood enhances the benthic structural and niche complexity > provide habitat for many taxa and enhances environmental buffering within deadwood | Teredo spp. (Teredinidae) | Bactronophorus thoracites, Dicyathifer mannii, Lyrodus massa, Spathoteredo obtusa, Teredo spp., Bankia spp. (Teredinidae) | ||
| Insects | Wood consumption > increasing OM biodegradability Effects on canopy architecture, tree growth and reproduction, and internal nutrient cycling | Coccotrypes rhizophorae, Euplatypus sp. (*) (Scolytidae, Coleoptera) Termites (Blattodeans) | Dendroctonus spp. Phaenops spp. (Coleoptera) Cenoloba spp. Zeuzera conferta (Lepidoptera) Termites (Blattodeans) | ||
| Pollinators | Pollination | Insects | Enable tree reproduction > forest structure/architecture > AGB/BGB content > carbon content and cycling | Hymenopteran, Lepidopteran, -Dipteran | Hymenopteran, Lepidopteran, Dipteran |
| Bats | Phyllostomidae | Pteropodidae: Macroglossus minimus, Eonycteris spelaea | |||
(1) We consider a function as significant in the context of our focus on species that have functional roles (i.e., that take part/affect structural processes of mangrove community assemblage); for example, some fishes fits in our category of ‘sediment disturbance’ functional role, and we focus on them, not on fishes that perform other ecological roles in mangroves; this is similar for all species and functional groups/roles addressed here. (2) Symbol ‘>’ means ‘lead to/influences’. (3) To maximise space, only some examples of abundant species of Grapsoid and Ocypodoid crabs of similar ecological function in Atlantic and East Pacific were included in the ACEP column; most Sesarmids are part of more than one FG (bioturbators/burrowers, herbivores, etc.), so appear for the first time listed and posteriorly under ‘Sesarmidae (Grapsoidea)’ (all species of significant genera Ucides, Aratus, Goniopsis, however, were included). Likewise, families and superfamilies of specific genera in IWP column were omitted if the same ACEP, and only some examples of species were included. For more details about local fauna, see References. (4) In some neotropical mangroves, several Brachyuran crabs share burrows with the main owner Ucides cordatus (and probably U. occidentalis in East Pacific), in anastomosed communal burrow systems (ACBS), so we included them in this category [35] (5) Gastropoda are abundant in mangroves and mainly feed on soil litter and detritus, playing roles in shaping the biochemical properties of mangrove sediment and water. However, effect of snails over forest structure is unknown. As with mangroves themselves, the diversity of mangrove associate Gastropoda (and the size of most common species) is highest in the IWP and some East Pacific region mangroves (Cantera et al., 1999 [47]), so their functional role seems more significant than in general ACEP mangroves, despite being reported as seed predators in Florida mangroves (Nagelkerken et al., 2008 [48]).
This paper first revisits the ecological processes mediated by key FGs of fauna and microbes in mangroves, and then how CC could influence such processes and affect the forests. A literature review allowed us to hypothesise that indirect effects of CC drivers on worldwide mangroves, through functional degradation, can be as significant as direct impacts. We offer more insights into how these FGs are related to the resistance and resilience of mangroves to CC, with the aim of contributing to mangrove conservation, rehabilitation, and restoration/rehabilitation (R/R) protocols.
2. Functional Groups, Their Specific Roles, and Effects over Mangroves
2.1. Biogeochemistry Mediators
Micro-organisms are an extremely important group of organisms in mangrove ecology and functionality [31,32,33,49]. The cycling of carbon (C), nutrients, and several metals in mangrove soils involve a wide range of bacterial groups, with some involved in several cycles, for example, nitrogen (N) and also with roles in sulphur (S), iron (Fe), and phosphorus (P) cycles [50,51]. In Australian mangroves, bacteria and fungi can reach around 90% of the total microbial biomass, which also includes algae and protozoa [52,53]. In addition, microphytobenthos (such as Diatomacea and other microbial mats dominated by Cyanobacteria) contribute to significant amounts of buried C and trace elements in mangrove forests and in hypersaline tidal flats (apicum/sabkha) [54,55].
Mangroves are highly dependent on the efficiency of specific microbial communities both in soil and waters to fix N from the atmosphere, and they process, transform (ammonification, nitrification, denitrification), and conserve different forms of this nutrient. Soil and planktonic microbes involved in these pathways, mostly bacteria and archaea, are highly diverse and also include fungi and protists [51]. Gaseous N2 fixation by bacteria is one of the most important processes in mangroves, and biological N2 fixation could supply up to 60% of mangrove requirements of N in mangroves [31,56].
A large proportion of organic P is attached to humic compounds not readily available for plants and microbiota. Mycorrhizal fungi and phosphate-solubilizing bacteria (PSB) associated with roots that make N available to trees constitute an extremely significant association for mangrove nutrition [31,51]. In Avicennia marina forests, phosphatase (enzymes from PSB) activity and conversion to inorganic forms of P are highest in the rhizosphere, together with high densities of PSB, which suggest strong root–soil–phosphobacteria relationships [57].
Biogeochemical cycles of S and Fe are interlinked (and influenced both by roots and bioturbation) and influence N metabolism and P availability [51]. Sulphate-reducing bacteria are the most significant OM decomposers in anaerobic mangrove soils, playing a role in the mineralisation of organic S and availability of soluble Fe and P required by ecosystem biota [31].
2.2. Bioturbators/Burrowers
2.2.1. Crabs and Thalassinids
Several functional roles are performed by semiterrestrial crabs (Crustacea: Brachyura), which are one of the most ecologically significant key groups. This group is dominated mostly by Sesarmid (Grapsoidea) and Ocypodoidea (e.g., fiddler crabs), and in the ACEP also by some Xanthoidea. In the ACEP, the large burrower herbivore Ucides cordatus (Ocypodoidea) [12,35] and other various fossorial crabs can also be found in communal anastomosed long-lasting tunnels between the roots of Rhizophora mangle [35], contributing to oxygenating underground tree roots and adjacent soil. Through burrowing and soil bioturbation, these ‘ecosystem engineers’ can determine microbiota and infaunal diversity, and through changes in sediment and porewater physical chemistry, can influence nutrient availability and therefore tree species growth and productivity [6,8,35,58,59].
Burrowing by some fiddler crabs (Ocypodoidea) in the ACEP can bury small mangrove propagules, like those of Laguncularia racemosa, and lead to the predominance of large propagules of Rhizophora mangle trees, with higher architectural complexity and biomass standing stock, and hence carbon content [8]. Similarly, in IWP mangroves, burrowing and litter grazing by crabs result in a significant reduction in soil organic matter (OM) and its export to adjacent waters, as well as influencing topography and sediment biogeochemistry through changing granulometry, drainage, and redox conditions while maintaining nutrients inside the forest [6,60]. The eventual breakdown of OM, mostly by bacteria, enhances nutrient cycling that supports high levels of mangrove productivity [61]. The removal of crabs from mangroves in Australia resulted in an increase in soil sulphide and ammonium levels and a reduction in cumulative growth and reproductive output of the trees [62].
Mud lobsters, Thalassina spp., were shown to influence the topography and physical–chemical features of the soil and in mangrove succession in SE Asia [63,64]. Whilst burrowing and extracting organic material, built-up mounds of mud can accumulate 1 to 2 m high, creating areas of dry mud suitable for the establishment of mangrove ferns and other burrowing Crustacea such as fiddler crabs and large Episesarma [10]. Thalassinids are also present in Caribbean, East Pacific coast, and other northern South America mangroves [65].
2.2.2. Fish
Fish are conspicuous mangrove inhabitants [34,66,67]. Several juvenile and adult fish (cyprinodonts, gobiids, fundulids, rivulins, poeciliids, and eleotrids) are dependent on structural complexity for refuge, and several species search for mangroves’ structural protection and environmental buffering, like those offered by crab burrows and other crevices in the soil among the roots or within dead wood [34,36,68,69].
Some fish are bioturbators, like the conspicuous burrower mudskipper gobiids in IWP mangroves. Burrows vary in structure between species with a vertical shaft and horizontal side tunnels (sometimes being shared with a commensal pistol shrimp (Alpheus) or crab). Periophthalmodon transports air into their burrows and stores it in a dome-like chamber, while other species, including Periophthalmus, Scartelaos, and Boleophthalmus, have air in their spawning chambers [70]. The presence of these fish also influences sediments through bioturbation processes, with associated effects on soil biogeochemistry, biota diversification, and tree and root development but, unlike crabs, fish do not remove litter from soils to burrows.
2.3. Herbivores
Herbivory in mangroves is predominantly by crustaceans and insects and essentially depends on the chemical characteristics of leaves of different tree species. In general, due to the adaptation to support strong UV radiation and high soil salinity, mangrove leaves have substances that also work as deterrents to herbivores. In a pioneering study on herbivory on the three dominant mangrove species of the ACEP, Lacerda et al. [71] reported that Avicennia schaueriana showed a significantly smaller leaf area eaten and number of leaves attacked than Rhizophora mangle and Laguncularia racemosa, which in turn had similar values. The authors attributed this to the leaf chemical composition of A. schaueriana with higher values of Na, crude fibre, and ash content and lower values of total phenols and soluble carbohydrates compared to the other two species.
A large proportion, up to 74%, of the mangrove tree biomass is stored in trunks [72], heavily influenced by local physical–chemical parameters. Often, significant volumes of mangrove tree trunks and roots fall as large wood debris entering the detrital food chain. Trunks and roots of both live and dead trees are consumed by herbivores. Wood-consuming guilds range from saprophytic (fungi) to mechanical consumption (coleopteran, termites, and teredinids) and form distinct zones according to mangrove shore elevation [15]. Basidiomycetes fungi are found on dead wood above the hightide mark, coleopteran and termites are found in dead wood from above the hightide mark down to the mid-intertidal areas of mangrove forests, and teredinids are found from the mid down to subtidal areas [15]. Basidiomycetes are essential in mangrove forests as they provide resources for many other organisms and enable the regeneration of forests and soil formation [73,74], such as Deuteromycetes and Ascomycetes [55].
2.3.1. Crabs
Leaf-eating crabs are important for energy and carbon flow and provide a food source for predators. Crab faeces are rich in N [75] and, combined with sloppy feeding [76], produce smaller fragments available for deposit feeders. By removing the leaf litter and being stored below-ground in burrows, carbon is retained within the mangrove system. A study of sesarmid crabs in Thai mangroves in the IWP, Thailand, revealed that they removed 87% of the daily leaf litter fall (>30% of the total NPP) by either ingestion or burial [77].
Several crab species feed on mangrove leaves in the canopy, the most conspicuous being Leptarma leptosoma and Parasesarma erythrodactylum in the IWP and Aratus pisonii (A. pacificus in the East Pacific coast [78]) in the ACEP. In mangroves at Tampa Bay, Florida, A. pisonii showed a preference for the red mangrove Rhizophora mangle over those of Laguncularia racemosa and Avicennia germinans, and the percentage of damaged R. mangle leaves was about 20–30 times greater than the other species. R. mangle leaf stomata were 3 to 20 times more abundant in the crab guts compared to the leaf stomata of the other species [79]. In the IWP region, in Australia, Avicennia marina was preferred by P. erythrodactylum over other local abundant mangrove species Bruguieria gymnorhiza and Rhizophora stylosa due to Avicennia’s lower tannin and lignin content [76,80]. However, the proportion of the total leaf area eaten was low (0.50 to 6.17%), both in the IWP and ACEP mangroves [81].
While herbivory by crabs has never been reported to affect canopy characteristics, they can, through differential propagule consumption, determine which tree species establish in a site, and thus the structural/architectural features and biomass of the forest, which can indirectly influence infaunal diversity and nutrient cycling [8,38,59,82]. The ACEP crab Goniopsis cruentata has a significant structural role through the high consumption of propagules of Laguncularia racemosa and Avicennia sp., thus promoting the predominance of R. mangle in the forest, which is architecturally more complex and comparatively stocks more biomass. Yet, this crab promotes increases in prop roots’ complexity and biomass by eating the cortex of R. mangle roots [7,35], which induces their sprouting [83].
Leaves deposited on the soil as litter are mostly consumed by Ucides cordatus (U. occidentalis in the East Pacific coast [78]) in the ACEP region [84] but, in the IWP region, many more crab species share this functional role, mostly sesarmids of various genera, e.g., Clistocoeloma, Episesarma, Neosarmatium, Parasesarma, among others. Parasesarma eumolpe and P. onychophorum, with different consumption preferences on leaf litter and propagules of different species, promote differences in tree species composition depending on the crab population. Litter decomposes for many weeks in sesarmid burrows while being consumed by crabs, leading to a significant decrease in tannin content, and an increase in N content through microbial metabolism, which significantly improves the nutritional value of mangrove leaf litter [71,85]. The experiment of Camilleri [76] showed that under laboratory conditions, regarding the amount of leaf material processed by Parasesarma erythrodactylum, 20% was lost from the mandibles due to “sloppy feeding”, 68% was egested as faeces, and 12% was converted into crab biomass. This balance highlights the significant role played by these crabs in accelerating nutrient and carbon cycling in mangroves. The lower diversity of leaf-eating crabs in the ACEP determines a higher risk of functional disruption in ACEP mangroves by local extinction of these species compared to the high diversity of the leaf-eating crab community in the IWP region.
Several Grapsoids (Brachyura: Grapsoidea) included in this FG are omnivorous, and beyond plant matter (bark, leaves, propagules), they feed on soil detritus, carrion, and small invertebrates, including congenerics and fiddler crabs in the case of Goniopsis cruentata and East Pacific congeneric G. pulchra [78,86], thus exerting both bottom-up and top-down influences on the food chain. Predation of this dominant species over fiddler crabs decreases the burying of Laguncularia racemosa propagules by fiddler crab populations and hence increases their recruitment, with implications on tree dominance and forest structure and thus biomass [8].
2.3.2. Insects
Studies on functional roles of insects in mangroves are scarce [81], limiting our knowledge on their full ecological functions in mangroves. Recent studies have found mangroves to be rich in insect species in IWP mangroves (up to 3000 species in Singapore sites, having few phytophagous and fungivores and a large proportion of predators), around 50% of which are mangrove-specific [87]. Most important phytophagous groups are Coleoptera and Lepidoptera [48,88]. In the ACEP, termites (Blattodea) are particularly abundant [89].
Insect herbivory signs include propagule boring, the erosion of leaf margins, holes in the blade of the leaf, or evidence of leaf miners. In Australia, the proportion of leaf productivity consumed by insect herbivores was only about 2% [90]. The extent of herbivory depends on diverse factors affecting the palatability and nutritional value of leaves that vary with age, season, and between species [10]. In Belize (ACEP), 66 species of leaf-eating insects [91] have been identified, whereas in the Andaman and Nicobar Islands, in the IWP, nearly 200 species were identified [92]. Some insects can cause heavy defoliation events [5], despite being limited by mangroves tannin content [71]. Epidemic proportions of defoliation have been reported; for example, in Indonesia, every tree in an area of 5 to 10 km2 was completely defoliated by caterpillars of the moth Ophiusa [93].
Insect defoliations and loss of apical buds can potentially reduce reproductive and vegetative growth, reducing reproductive output and hence influencing tree recruitment [3,94]. Under specific situations, herbivory can significantly affect leaf area and thus canopy and forest architecture/structure. In a rehabilitated area in semiarid northeast Brazil, severe damage of planted saplings by a Lepidoptera infestation impaired restoration [95]. In nutrient-rich mangroves in Florida, [96] reported higher values of total leaf area eaten (26%) compared to pristine sites. Low levels of folivory observed worldwide under pristine conditions results in nearly intact leaves falling to the litter, suggesting that the grazing pathway may have little influence on nutrient cycling in several mangrove forests. This assumption, however, does not seem to be valid for mangroves impacted by anthropogenic pressures. In an intertidal mangrove island in Belize, herbivory by two specialised, endophytic insects (Ecdytolopha sp., which feed on apical buds, and Marmara sp., which mine stem periderm) also increased in P- and NPK-fertilised trees compared to N-fertilised and control trees [97].
In the Mexican Atlantic coast, mangroves affected by diverse anthropogenic activities, regardless of plant species, show more plants attacked by insects and more leaves and leaf area damaged and removed by herbivores. This higher overall damage to plants is reflected in developmental instability in plants, which, although variable amongst mangrove species, was higher in disturbed compared to pristine forests. This effect is positively correlated with herbivory levels, indicating that herbivores might be a significant source of stress in mangroves impacted by anthropogenic activities [98].
2.3.3. Primates
Some monkey species are exclusively herbivorous, and in SE Asia, langurs (Presbytis) and the proboscis monkey (Nasalis larvatus) eat mangrove leaves, flowers, and fruits. In Sarawak (IWP), Sonneratia species were recorded as the preferred mangrove tree consumed by proboscis monkeys followed by Avicennia [99]. It was estimated that a daily food intake of 12% body weight amounts to 60 kg leaves and fruit km2 day−1 or 21 tonnes annually at the average densities of 500 kg/km2 [100,101], an amount of potential ecological significance [10]. However, more recent studies of semi-wild populations in Sabah, with limited available habitats, showed that N. larvatus mainly forages Rhizophora apiculata and Bruguiera parviflora trees, consisting of 63% young leaves, 5% immature flowers, and 4% young fruits [102]. Overall, there are a wide range of studies concerning the grazing of mangroves by primates in Asia covering a range of mangrove species, e.g., Nypa fruticans, Aegiceras corniculatum, Rhizophora spp., Bruguiera sexangula, B. gymnorrhiza, Sonneratia alba, Avicennia alba, A. officinalis, A. marina, Ceriops tagal, and monkeys, including the Ebony-leaf monkey (Trachypithecus auratus), Silvery-leaf monkey (T. cristatus), Long-tailed macaque (Macaca fascicularis), Red Colobus (Piliocolobus badius), and the aforementioned proboscis monkey.
There are fewer studies published on mangrove-associated monkeys in the ACEP region. Two interesting studies focussing on Howler monkeys (Alouatta pigra) in Mexico and Honduras [103,104] showed that these monkeys focused on flowers during the dry season and leaves during the wet and dry seasons, with no recording of fruit or propagule consumption. Other species have been observed to consume mangrove plant matter in South America, including the Bearded capuchin (Sapajus libidinosus), Yellow-breasted capuchin (S. xanthosternos), Tufted capuchin (S. apella), the Red-handed howler monkey (Alouatta palliata), and Cebus capucinus, consuming tea mangrove Pelliciera rhizophorae propagules [105], but the functional role of monkeys in ACEP mangroves has been suggested to be less important than that in the IWP region [106].
2.4. Wood Borers
2.4.1. Crustaceans and Molluscs
Several marine isopods (Crustacea) like Sphaeroma sp. are wood-borers in mangroves. Sphaeroma terebrans, first recorded in Brazil [107] and recorded up to Florida in the ACEP region, although likely introduced from the IWP [108], can affect the development and even survival of specific mangrove trees, affecting tree diversity and forest architecture [109,110], leading to changes in live and dead biomass and, hence, in aboveground carbon stock. S. terebrans has a non-herbivore relationship with mangroves since the isopod does not ingest root biomass; rather, their excavated burrows serve to protect themselves from exposure and desiccation. Isopods also use their burrows for filter feeding and reproduction. This relationship is quite controversial; authors have suggested it being either beneficial or highly damaging to mangroves. While reducing root production and even promoting root tip atrophy and breaking, S. terebrans may induce mangroves to initiate multiple lateral roots or the replacement of root structure [110,111,112]. This can affect structural support and nutrient supply to the tree and may induce changes in epifauna and epifloral communities, leading to community-level impacts [113,114].
Decomposers of wood span from the high- to low-intertidal regions of the mangrove forest (e.g., basidiomycetes, coleopterans, termites, and teredinids). Molluscs of the families Teredinidae (shipworms) and Pholadidae (piddocks) burrow into mangrove wood. Teredinid mollusks (e.g., Teredo spp.) are particularly important in breaking down dead wood; they have a significant role in biodegradation, and their tunnel density can affect the amount of carbon stored in, and released by, the forest. Yet, when the teredinid tunnels become vacant, they are exploited by many macro-benthic taxa, enhancing trophic and functional resilience [15,69]. The impacts of teredinid bioerosion have been largely noted in the IWP region [15], but they have also been recorded in the ACEP region, impacting Avicennia germinans [115].
2.4.2. Insects
Wood-boring Coleopterans (e.g., cosmopolitan Coccotrypes rhizophorae), bore tunnels in the bark of live trees, cause a loss of branches and plant stress. At the individual tree level, wood-borer insects affect canopy architecture, tree growth and reproduction, and internal nutrient cycling, mostly in nutrient translocation before leaf shading, being able to cause death to branches and trees [48]. They also create new habitats for several other species of arthropods. Scaling up, forest structure, and dynamics of the forest are directly affected by creating light gaps due to wood-borer activity [81]. Effects on ecosystem nutrient cycling are due to changes in the quality and quantity of litter [97]. On small, offshore R. mangle mangrove islands in Belize (ACEP), xylem- and phloem-feeding wood-borers from several insect species, consume the wood by girdling, pruning, and hollowing, and can destroy over 50% of the canopy, a high damage rate compared to less than 6% removed by leaf-feeding herbivores of the canopy [81].
Large proportions of dead wood in high-intertidal areas of mangrove forests will often be consumed by termites, with estimates of pieces containing 70% termite galleries (Personnal observation, IH). Termites build nests above the hightide mark and create enclosed galleries on the branches that allow them to travel long distances in relative safety. Additionally, termites are also found inside dead wood on the forest floor in high- and mid-intertidal areas, with their galleries out of reach from tidal immersion. Often, termite nests are found with other wood-degrading guilds, including coleopteran larvae and basidiomycetes (out of reach from tidal immersion).
2.5. Pollinators
2.5.1. Insects
Several insect species develop pollination function in IWP mangroves [88], where more diverse mangrove flora supports richer insect assemblages. Conversely, this function in ACEP mangroves seems to be performed by some unspecialised Hymenopteran, Lepidopteran, and Dipteran species [116,117,118]. Although sporadic records of flower visitors such as bees and butterflies have been recorded, Rhizophora mangle, the typical and widely distributed representative of the genus in the ACEP, is considered as mainly anemophilous [119], but it can also be pollinated by insects [120].
2.5.2. Bats
Two families of bats are morphologically specialised for nectar feeding, Pteropodidae in the IWP region and Phyllostomidae in the ACEP region [121]. In the IWP region, both fruit bats and flying foxes use the trees as a roost and a source of food. The long-tongued fruit bat (Macroglossus minimus) and the cave fruit bat (Eonycteris spelaea) in Peninsular Malaysia eat Sonneratia nectar and pollen and are major pollinators. In the ACEP region, species from the family Phyllostomidae have been recorded to feed and act as pollinators for Rhizophora sp. However, it should be noted that compared to insects and to a lesser extent birds, bats are less crucial to pollination, and nectar-eating bats are not exclusively dependent on mangroves, although they provide an important link with terrestrial habitats [121]. Fruit bats that feed on nectar and fruit also play a role as herbivores.
3. Specific Responses of Functional Groups to Climate Change and Effects over Mangroves
Predicted and occurring effects of CC over mangrove forests have been extensively addressed; see [17,20,28] for examples of recent reviews. The effects of such changes on FGs need to be studied, since together with direct human-driven stressors, CC impacts on FGs can lead to a degradation of functions and resilience of mangrove forests and, in specific cases, to mangrove mortality. Mangrove structural weakness and/or mortality impair the resistance and protective capacity of coastal ecosystems, reducing protection against CC. Moreover, different CC impacts can affect the same FG, whereas the same impact can affect several FGs simultaneously (Table 2, Figure 3).

Table 2.
Effects of climate changes on ecosystem and functional groups (FGs) and their direct and indirect effects over mangrove forests. Numbers in parentheses characterise effects in Figure 2 (listed impacts are not necessarily in sequence, and include direct impacts driven by FG(s), but also indirect through other impact/FG action, or synergy among various FGs/impacts; see text for details). ACEP, Atlantic–Caribbean–East Pacific; IWP, Indo-West Pacific mangroves.
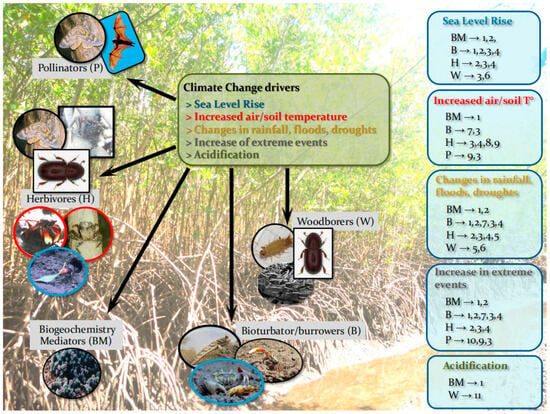
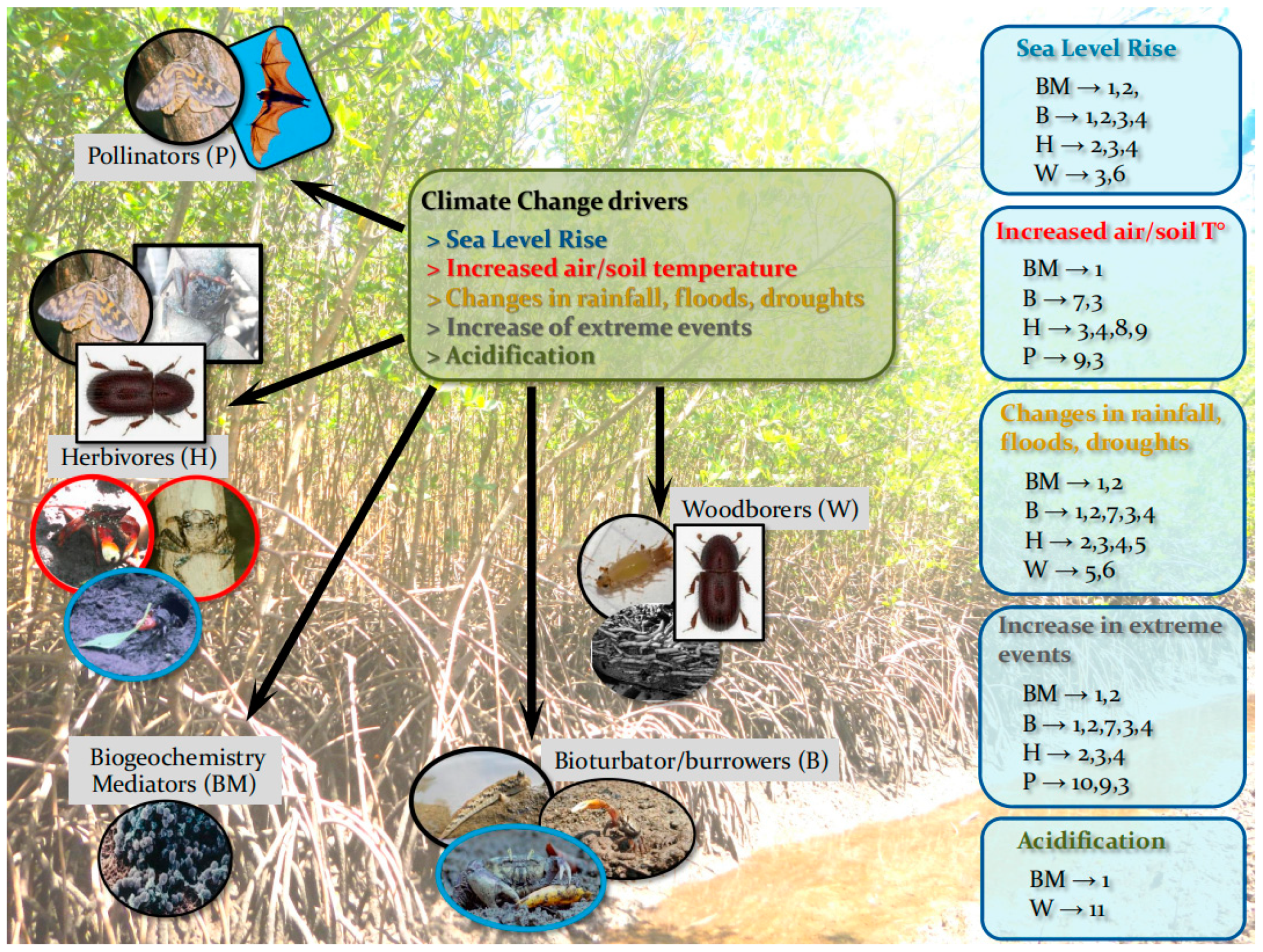
Figure 3.
Effects of climate change (in green box) on FGs and the direct effects of FGs on the forest (blue boxes). The numbers of the effects do not necessarily express event order and are (1) disruption to soil biogeochemical processes; (2) decreased nutrient availability > impact on forest productivity; (3) changing forest structure and biomass/C stock; (4) changes in propagule recruitment patterns; (5) changing existing forest zonation patterns; (6) decrease in forest structural resistance; (7) decrease/increase sediment aeration by sediment reworking; (8) mass defoliation; (9) disruption to tree development; (10) disruption to pollination and reproductive output; (11) decrease in inputs of OM, litter and deadwood processing, and nutrient cycling reduction. [Note: For more (indirect) effects see Table 2. The herbivore FG includes the several mobile Grapsoids (Sesarmids in IWP and G. cruentata (Grapsidae) and a few Sesarmids in ACEP) that live in forest soil and climb trunks and roots, also with omnivore and preying habits (in red circles). Some of these crabs are simultaneously bioturbator/burrowers and major Herbivores (in blue circles)].
3.1. Sea Level Rise (SLR)
An increase in inundation period can affect soil metabolism through biogeochemistry mediators by increasing anoxia periods, shifting for more anaerobic metabolism and decreasing nutrient availability, having an impact on forest productivity. Soil biogeochemical processes can also be affected by disruption in fossorial crab populations.
Inundation can affect Brachyurans and Thalassinid lobsters’ distribution at the burrow and root level, leading to changes in litter and tree propagule consumption mediated by Sesarmids and other Grapsoids and hence tree species recruitment, leading to changes in above- and below-ground biomass content and in forest structure/architecture. This may have implications in forest structural resistance (for example to storminess), determined mostly by zonation patterns and root design, as well as post-storm resprouting, since, unlike Avicennia, Sonneratia, or Aegiceras trees, mature Rhizophora do not have regeneration meristems [122]. A more lasting inundation (in neap and spring tides) and consequent higher OM exportation in a highly productive mangrove forest at the East Pacific coast of the ACEP led to lower populations of litter consumer, Ucides occidentalis, which was hypothesised to lead to a lower transference rate of vegetal OM to higher trophic levels, and hence a further decreased secondary production [123].
SLR can increase the effect of wood-borer isopods, like Sphaeroma sp. An increase in the inundation period can also increase teredinid molluscs’ (e.g., Teredo spp.) biodegradation, which can affect the amount of carbon stored in, and released by, the forest [15]. SLR may also alter the distribution of other biodegrading organisms sensitive to tidal elevation, having a direct impact on mangrove carbon cycles and OM outwelling [15]. SLR can also lead to pollutant remobilisation, an increase in releasing deposited reduced species (e.g., chalcophile metals) by the erosion-driven oxidation of sediments, under soluble, more bioavailable species [55], and thus an increase in its toxicity to mangrove fauna.
3.2. Global and Regional Increases in Air and Soil Temperature
Rising temperatures can disrupt the local and regional distribution of fiddler crabs, with implications in sex ratio and species competition [124,125] and hence bioturbation patterns, affecting sediment bacterial communities that mediate OM composition and nutrient/pollutant availability, and eventually biogeochemical processes [6,126,127], leading to mangrove functional degradation. Decreases in crab bioturbation can be associated with the increased recruitment of architecturally less complex and less carbon content Laguncularia racemosa at Neotropics [7,8], and the release of more greenhouse gases from soil (e.g., nitrous oxide, N2O) [128]. On the other hand, increases in temperature can increase thermohaline stress over gills, and so burrowing for protection is common in some species, potentially altering propagule consumption/recruitment patterns [7], though increasing soil aeration and OM degradation. Effects of increased temperatures can also affect crabs indirectly through disease outbreaks, algal blooms, pollution, or hypoxia [129].
Increases in temperature can potentialize the effect of insects on the function of forests at the regional level. Global and regional increases in temperature can also increase the herbivory of live wood-boring insect assemblages and thus intensify structural damage, mostly of young and restored mangroves or mangroves at their latitudinal limits [130]. Disruption in trees’ reproduction because of disruption in the activity period of pollination agents can also occur but seems restricted to the IWP, where insect pollination is important. The anemophily and mixed-mating systems of mangrove tree species and their less specialised association with pollinators could dampen the risk of CC disruption of this function in ACEP mangroves.
3.3. Altered Precipitation Regimes (Floods and Extended Drought Events)
Effects from floods can be like those described for SLR ones, although episodic. Disruption of biogeochemical processes can result from changes in salinity and flooding of sediments and a decrease in soil nutrient input. An increase in the flooding period can promote an increase in wood-borers’ activity, leading to more structural damage and OM export to adjacent coastal ecosystems [15]. Extended flooding events can increase anoxia in pore waters, affecting iron plaque formation and the nutrient absorption capacity of roots [131].
While mangrove swamp crabs are expected to be good osmoregulators [86], sudden or permanent inputs of low-salinity water could cause mortality by osmotic accommodation failure, particularly if synergically occurring with high temperatures [132]. Flooding and drought can increase erosion/sedimentation rates, leading to changes in regional Thalassinid and crab assemblages, affecting species, respectively, less resistant to lower or higher salinity, and/or thermal stress, changing propagule recruitment patterns, with associated effects on forest structure and biomass distribution. Extended droughts can augment the effects of temperature stress in soil organisms, including crabs, which can decrease litter transformation in detritus.
3.4. Increase in Extreme Weather Events (Storms, Hurricanes)
Damage by extreme storms and tsunamis is mitigated by trees with large stems and roots or similar aboveground heterogeneous complexity such as pneumatophores, decreasing the force of winds and water currents [133,134]. Species of Rhizophora, Bruguiera, and Sonneratia, for example, can reduce the impact of these pressures, minimizing damage landwards. However, mature Rhizophora spp., for instance, lack resprouting meristems, hampering further regeneration, unlike Laguncularia racemosa and species of Avicennia, Sonneratia, and Aegiceras [122,135]. Thus, the composition and structure of the forest mediated by major propagule consumers, for example, can influence post-storm regeneration capacity, which depends in part on the manner of FG reestablishment.
Extreme weather events like intense El Niño, which are predicted to increase in strength [136], can lead to mangrove mortality, mainly by the disruption of soil features from sea level changes and thermohaline stress [137,138,139]. Soil disruption can kill micro- and macro-biota that influence biogeochemical cycles, leading to mangrove dieback and the release of CO2 and nitrous oxide to the atmosphere.
Storms and changes in inundation can modify the population dynamics of Brachyuran crabs, leading to food chain and community assemblage disruptions, for example, the top-down influence exerted by carnivores, and in propagule recruitment mediated by herbivores. The decrease or destruction of canopy cover, soil disruption, and branch removal by storms can affect crab richness and abundance, changing the taxonomic and functional composition [59,140], with similar effects of SLR over mangrove stand’s structural resistance. Analogous effects are expected on Thalassinids.
Increased storminess and related root and soil disruption, such as extended hypoxia, can affect the distribution of mangrove burrow-dwelling fishes, mainly the small species, altering biota related to roots, burrows, and even sediment biogeochemistry, potentially increasing the effect on stressed mangroves. Evidence suggests that root complexity increases the density and diversity of fish larvae, since higher fish larval abundance and a higher number of taxa occur in Rhizophora species [141]. Increased storminess may also disrupt the pollination of mangrove stands in the IWP, but likely less so in the ACEP, thus decreasing the reproductive output and primary productivity of mangroves, influencing forest structure post-storm.
Increased storminess may also disrupt the pollination of mangrove stands in the IWP and thus decrease the reproductive output and primary productivity of mangroves, influencing forest structure post-storm, but less likely in the ACEP, where despite changes in pollinator assemblages after hurricanes, the anemophily of Rhizophora and the mixed-mating system of Laguncularia racemosa, for example, allow for after-event pollination [142].
3.5. Water Acidification
Mangroves can help buffer seawater acidification, but a decrease in water pH can impair mangrove development through changes in nutrient–plant relationships and several other ecological processes mediated by microbiota in mangrove sediments [48,143], decreasing mangrove resistance to other stressors.
Decreases in pH may inhibit the ability of teredinids to process wood. Teredinids are bivalve molluscs, and their calcareous shells (valves) are denticulated with teeth-like projections which permit them to create tunnels by rasping through the wood, processing the fixed carbon as they do [15]. A decrease in pH may impact the development of teredinid valves (like on all molluscs and crustaceans) at both the juvenile and adult stages, thus reducing their ability to process large volumes of wood in mid- to low-intertidal areas of mangrove forests. This would have an impact of the amount of mangrove dead biomass being broken down and made available to other animals, habitats, and ecosystems, e.g., in the form of macro-benthic cryptic fauna exploiting vacant teredinid tunnels [69], dissolved and particulate OM outwelling, and connectivity to other adjacent habitats through carbon export [144]. This would have an impact on the trophic structure and resilience with mangrove forests and connectivity between adjacent ecosystems.
4. Discussion
Human activities and climate changes represent threats to mangroves, particularly when they act synergistically. Beyond the direct effects on the diversity and structure of forests, indirect effects of CC through organisms that are directly associated with mangrove functioning (or functionality) can alter the ecological processes of the forest and emergent properties of the community, impairing recovery or leading to further mangrove degradation and loss/decrease in resilience and/or mangrove dieback. Despite different levels of biodiversity between ACEP and IWP mangroves, bioturbator/burrowers, especially semiterrestrial crabs (Ocypodoids and Grapsoids in the ACEP and mostly Sesarmid Grapsoids in the IWP), herbivores (crustaceans and insects), and biogeochemistry mediators, mostly bacteria, would likely be the most affected FGs in both regions. Thus, the outcome of specific climate impacts is determined by local forest composition (in terms of tree species and FG) since, for example, the physical/biological resistance of different trees determines the level of impact of the climate driver and the post-event recovering capacity. On the other hand, the effects of the disruption of FGs on mangroves can delay or impair recovery, decreasing resistance to further climatic disturbances.
Species that are part of more than one FG have several key roles and occupy ample niche dimensions of the habitat. In ACEP mangroves, Goniopsis cruentata (and probably congeneric G. pulchra) is an omnivore crab with herbivorous and raptorial habits, and a controller of propagule recruitment (influencing forest development, structure, and biomass) and also of populations of ecosystem engineers like fiddler crabs. This crab is a key species occupying several spatial and trophic niche dimensions within the soil–tree interface occupied by several Sesarmid species in the IWP [35,145]. Similarly, Ucides cordatus (U. occidentalis in East Pacific coast) performs several functional roles and is the main neotropical litter and Rhizophora propagule consumer and a major bioturbator/burrower and soil microbiota influencer. A loss of one of these dominant species of crabs can strongly affect a large functional portion of an ACEP stand. Dominant (often also called key) species can occupy broadest niches and so pervade the ecosystem exerting control on the occurrence of other species [146]. Studies have shown that interspecific interactions among selected dominant species can be a main determinant of community and ecosystem functioning [147,148,149].
Recent studies show that mangrove invertebrate macrofauna (at least crustaceans and molluscs) have extreme low functional redundancy (i.e., every species performs ecological function(s) in part or entirely different to the ecological function(s) of others) relative to intertidal position, diet, and behaviour [13,145,150]. Models predict that species-poor systems have low functional redundancy and are more prone to experiencing functional loss with species extinction [13,151]. This latter study found that despite peaks of taxonomic distinctness and functional richness (i.e., the volume of functional space occupied by all species in the community) of mangrove macrobenthos (crustaceans and molluscs) in Southeast Asian (Indonesia and Hong Kong) and (unexpectedly) in two South American locations (Colombia and North Brazil), the extreme low functional redundancy of ecological functions performed by only one species is common at 60% of the 16 worldwide mangrove locations studied, showing the vulnerability of these forests to species loss.
However, there is evidence that a high number of species, or species within each FG (or a high functional redundancy), should lead to greater ecosystem stability in facing stressors [152,153], which could favour the higher resilience of IWP mangroves to CC (except in extreme events) relative to the ACEP. Hence, evidence suggests that the risk of functional collapse in the ACEP region (and in semiarid/arid realms) mangrove stands is higher compared to the most part of IWP mangroves in S-SE Asia and Australia [29,150]. This highlights the necessity to prepare increasingly threatened Neotropical mangroves for CC, protecting their biodiversity and planning effective conservation and rehabilitation measures to face anthropic pressures and environmental changes in the Anthropocene [24].
5. Conclusions
The extension, magnitude, and temporal span of the effects of CC depend on a specific set of factors at regional and local levels, and the biological and climate systems’ behaviour can be unpredictable. Indeed, since CC can affect the phenology and distribution of species, and hence their interactions, it is difficult to fully predict the impact of these drivers over species and ecosystem functioning and resilience. CC can alter the balance between the abundance and richness of biota and open niche spaces previously unoccupied by species but close others. Species can respond independently to CC and with community dynamics linked by feedback mechanisms, making it difficult to predict responses. It is possible, however, to detect early stress factors (e.g., the extinction of key species, wood-borer damage, functional degradation) through regular monitoring, and to manage mangroves to increase their resistance and resilience through their conservation and rehabilitation/restoration.
While the impacts of key biotic groups can be restricted to specific mangrove stands, the synergistic effects of CC, their effect via FGs, and human degradation drivers can affect forest ecological processes and contribute to their degradation, loss of resiliency, and mortality at a regional level. Where multiple stressors occur, like pollution, human disturbance, the overexploitation of resources, altered hydrology, and hydrodynamics, the consequent reduction in the abundance and diversity of species will cause a decrease in functional diversity, which in turn can reduce ecosystem service provision and ecological resilience to CC. The predictions presented here will require verification through dedicated research in global mangrove ecosystems.
Sacco et al.’s [154] ten golden rules for reforestation also apply to mangrove ecosystems to maximise biodiversity recovery. These are to, firstly, protect existing pristine forests so there is conservation of functional diversity. Involve all stakeholders, maximise biodiversity recovery to meet multiple goals, including CC mitigation, and select appropriate areas for restoration and species to maximise mangrove biodiversity and function. To fully apply these rules, however, further research is required on key FGs and their multitrophic biotic interactions in mangrove ecosystems to understand responses to CC and the wider implications on sustainable livelihoods and food security services that mangroves provide. The rehabilitation/restoration of mangrove forests regarding their functional processes is of paramount importance, concentrating efforts to first restore/recruit key functional groups that recover several important ecological processes for the community with enhanced species richness and functionality to effectively face CC challenges.
Author Contributions
Conceptualisation, A.C.F. and L.D.L.; methodology, all authors; formal analysis, A.C.F., L.D.L. and E.C.A.; investigation, all authors; writing—original draft, all authors; writing—review and editing, all authors; supervision, A.C.F.; funding acquisition, L.D.L. and A.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the INCT-TMCOcean—Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq grant number 405.765/2022-3 and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico—FUNCAP grant number 00159-001-0009/2019-1.
Data Availability Statement
Data sharing is not applicable to this article since no datasets were generated or analysed during the current study.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Bunting, P.; Rosenqvist, A.; Hilarides, L.; Lucas, R.M.; Thomas, N.; Tadono, T.; Worthington, T.A.; Spalding, M.; Murray, N.J.; Rebelo, L.-M. Global Mangrove Extent Change 1996–2020: Global Mangrove Watch Version 3.0. Remote Sens. 2022, 14, 3657. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Latham, R.E. Global patterns of diversity in mangrove floras. In Species Diversity in Ecological Communities: Historical and Geographical Perspectives; Ricklefs, R.E., Schluter, D., Eds.; University of Chicago Press: Chicago, IL, USA, 1993; pp. 215–229. [Google Scholar]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; López-Hofman, L.; Ewe, S.M.L.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Ferreira, A.C.; Borges, R.; Ward, R. Mangroves of Brazil. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Thammineni, P., Ashton, E.C., Eds.; Springer: Singapore, 2022; pp. 521–563. [Google Scholar] [CrossRef]
- Cannicci, S.; Burrows, B.; Fratini, S.; Smith, T.J., III; Ofenberg, J.; Dahdouh-Guebas, F. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008, 89, 186–200. [Google Scholar] [CrossRef]
- Kristensen, E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 2008, 59, 30–43. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ganade, G.; Attayde, J.L. Restoration versus natural regeneration in a neotropical mangrove: Effects on plant biomass and crab communities. Ocean Coast. Manag. 2015, 110, 38–45. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Bezerra, L.E.A.; Mathews-Cascon, H. Aboveground stock in a restored Neotropical mangrove: Influence of management and brachyuran crab assemblage. Wetlands Ecol. Manag. 2019, 27, 223–242. [Google Scholar] [CrossRef]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves; ITTO-ISME-FAO: Okinawa, Japan, 2011. [Google Scholar] [CrossRef]
- Hogarth, P.J. The Biology of Mangroves; Oxford University Press: London, UK, 1999. [Google Scholar]
- Lee, S.Y. Mangrove macrobenthos: Assemblages, services, and linkages. J. Sea Res. 2008, 59, 16–29. [Google Scholar] [CrossRef]
- Lee, S.Y. Ecological role of grapsid crabs in mangrove ecosystems: A review. Mar. Freshw. Res. 1998, 49, 335–343. [Google Scholar] [CrossRef]
- Cannicci, S.; Lee, S.Y.; Bravo, H.; Cantera-Kintz, J.R.; Dahdouh-Guebas, F.; Fratini, S.; Fusi, M.; Jimenez, P.J.; Nordhaus, I.; Porri, F.; et al. A functional analysis reveals extremely low redundancy in global mangrove invertebrate fauna. Proc. Natl. Acad. Sci. USA 2021, 118, e2016913118. [Google Scholar] [CrossRef]
- Duke, N.C. Mangrove Floristics and Biogeography Revisited: Further Deductions from Biodiversity Hot Spots, Ancestral Discontinuities, and Common Evolutionary Processes. In Mangrove Ecosystems: A Global Biogeographic Perspective; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Teilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 17–54. [Google Scholar]
- Hendy, I.W.; Shipway, J.R.; Tupper, M.; Etxabe, A.G.; Ward, R.D.; Cragg, S.M. Biodegraders of large woody debris across a tidal gradient in an Indonesian mangrove ecosystem. Front. Glob. Chang. 2022, 5, 852217. [Google Scholar] [CrossRef]
- Ashton, E.C. Threats to Mangroves and Conservation Strategies. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Thammineni, P., Ashton, E.C., Eds.; Springer: Singapore, 2022; pp. 217–230. [Google Scholar] [CrossRef]
- Alongi, D.M. Climate Change and Mangroves. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Thammineni, P., Ashton, E.C., Eds.; Springer: Singapore, 2022; pp. 175–198. [Google Scholar] [CrossRef]
- Gilman, E.; Ellison, J.; Duke, N.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Moomaw, W.R.; Chmura, G.L.; Davies, G.T.; Finlayson, C.M.; Middleton, B.A.; Natali, S.M.; Perry, J.E.; Roulet, N.; Sutton-Grier, A.E. Wetlands in a changing climate: Science, policy and management. Wetlands 2018, 38, 183–205. [Google Scholar] [CrossRef]
- Ward, R.; Friess, D.; Day, R.; Mackenzie, R. Impacts of climate change on global mangrove ecosystems: A region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef]
- I.P.C.C. Climate Change 2013: The Physical Science Basis; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- da Rocha Júnior, R.L.; dos Santos Silva, F.D.; Pinto, D.D.C.; Costa, R.L.; Gomes, H.B.; Herdies, D.L.; Guidson Farias de Freitas, I.; Silva Vila Nova, T. Analysis of temperature extremes in the South of Brazil. Rev. Bras. Climatol. 2022, 30, 445–460. [Google Scholar] [CrossRef]
- Godoy, M.D.P.; Meireles, A.J.A.; Lacerda, L.D. Mangrove response to land use change in estuaries along the semiarid coast of Ceará, Brazil. J. Coast. Res. 2018, 34, 524–533. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Borges, R.; Ferreira, A.C. Neotropical mangroves: Conservation and sustainable use in a scenario of global climate change. Aquatic Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1347–1364. [Google Scholar] [CrossRef]
- Godoy, M.D.P.; Lacerda, L.D. Mangroves response to climate change: A review of recent findings on mangrove extension and distribution. An. Acad. Bras. Cien. 2015, 87, 651–667. [Google Scholar] [CrossRef]
- Ward, R.D.; Lacerda, L.D.; Cerqueira, A.S.; Silva, V.H.M.C.; Hernandez, O.C. Vertical accretion rates of mangroves in northeast Brazil: Implications for future responses and management. Estuar. Coast. Shelf. Sci. 2023, 289, 108382. [Google Scholar] [CrossRef]
- Mafi-Gholami, D.; Zenner, E.K.; Jaafari, A.; Ward, R.D. Modeling multi-decadal mangrove leaf area index in response to drought along the semi-arid southern coasts of Iran. Sci. Tot. Env. 2019, 656, 1326–1336. [Google Scholar] [CrossRef]
- Ward, R.; Lacerda, L.D. Responses of mangrove ecosystems to sea level change. In Dynamic Sedimentary Environment of Mangrove Coasts; Friess, D., Sidik, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–253. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Lacerda, L.D.; Rodrigues, J.V.M.; Bezerra, L.E.A. New contributions to mangrove rehabilitation/restoration protocols and practices. Wetlands Ecol. Manag. 2023, 31, 89–114. [Google Scholar] [CrossRef]
- Blondel, J. Guilds or functional groups: Does it matter? Oikos 2003, 100, 223–231. [Google Scholar] [CrossRef]
- Holguín, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Sham, A.; Elbadawi, A.A.; Hassan, A.H.; Alhosani, B.K.K.; El-Esawi, M.A.; AlKhajeh, A.S.; AbuQamar, S.F. A Consortium of Rhizosphere-Competent Actinobacteria Exhibiting Multiple Plant Growth-Promoting Traits Improves the Growth of Avicennia marina in the United Arab Emirates. Front. Mar. Sci. 2021, 8, 715123. [Google Scholar] [CrossRef]
- Farrer, E.C.; Van Bael, S.A.; Clay, K.; Smith, M.K.H. Plant microbial symbioses in coastal systems: Their ecological importance and role in coastal restoration. Estuar. Coasts 2022, 45, 1805–1822. [Google Scholar] [CrossRef]
- Barletta, M.; Saint-Paul, U.; Barletta-Bergan, A.; Ekau, W.; Schories, D. Spatial and temporal distribution of Myrophis punctatus (Ophichtidae) and associated fish fauna, in a north Brazilian intertidal mangrove forest. Hydrobiologia 2000, 426, 65–74. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Alencar, C.E.R.D.; Bezerra, L.E.A. Interrelationships among ecological factors of brachyuran crabs, trees and soil in mangrove community assemblage in Northeast Brazil. Community Ecol. 2019, 20, 277–290. [Google Scholar] [CrossRef]
- Lira, M.G.S.; Berbel-Filho, W.M.; Espírito-Santo, H.M.V.; Tatarenkov, A.; Avise, J.C.; Leaniz, C.G.; Consuegra, S.; Lima, S.M.Q. Filling the gaps: Phylogeography of the self-fertilizing Kryptolebias species (Cyprinodontiformes: Rivulidae) along South American mangroves. J. Fish Biol. 2021, 99, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.D.; Carvalho, C.E.V.; Tanizaki, K.F.; Ovalle, A.R.; Rezende, C.E. The biogeochemistry and trace metals distribution of mangrove rhizospheres. Biotropica 1993, 25, 252–257. [Google Scholar] [CrossRef]
- Smith, T.J., III; Chan, H.T.; McIvor, C.C.; Robblee, M.B. Comparisons of seed predation in tropical tidal forests from three continents. Ecology 1989, 70, 146–151. [Google Scholar] [CrossRef]
- McKee, K.L. Mangrove species distribution and propagule predation in Belize: An exception to the dominance-predation hypothesis. Biotropica 1995, 27, 334–345. [Google Scholar] [CrossRef]
- Bosire, J.O.; Kairo, J.G.; Kazungu, J.; Koedam, N.; Dahdouh-Guebas, F. Predation on propagules regulates regeneration in a high-density reforested mangrove plantation. Mar. Ecol. Prog. Ser. 2005, 299, 149–155. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ganade, G.; Freire, F.A.M.; Attayde, J.L. Propagule predation in a Neotropical mangrove: The role of the Grapsid crab Goniopsis cruentata. Hydrobiologia 2013, 707, 135–146. [Google Scholar] [CrossRef]
- Lima-Gomes, R.C.; Cobo, V.J.; Fransozo, A. Feeding behaviour and ecosystem role of the red mangrove crab Goniopsis cruentata (Latreille, 1803) (Decapoda, Grapsoidea) in a subtropical estuary on the brazilian coast. Crustaceana 2011, 84, 735–747. [Google Scholar] [CrossRef]
- Cutrim, A.S.T.; Sousa, L.K.S.; Ribeiro, R.P.; Oliveira, V.M.; Almeida, Z.S. Structure of a Polychaete community in a mangrove in the northern coast of Brazil. Acta Biol. Col. 2018, 23, 286–294. [Google Scholar] [CrossRef]
- Murugesan, P.; Sarathy, P.P.; Muthuvelu, S.; Mahadevan, G. Diversity and distribution of Polychaetes in Mangroves of East Coast of India. In Mangrove Ecosystem Ecology and Function; Sharma, S., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Spedicato, A.; Zeppilli, D.; Thouzeau, G.; Michaud, E. Nematode diversity patterns in mangroves: A review of environmental drivers at different spatial scales. Biodivers. Conserv. 2023, 32, 1451–1471. [Google Scholar] [CrossRef]
- Chew, L.L.; Chong, V.C. Copepod community structure and abundance in a tropical mangrove estuary, with comparisons to coastal waters. Hydrobiologia 2011, 666, 127–143. [Google Scholar] [CrossRef]
- Jaime, R.; Cantera, K.; Thomassin, B.A.; Arnaud, P.M. Faunal zonation and assemblages in the Pacific Colombian mangroves. Hydrobiologia 1999, 413, 17–33. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Blaber, S.J.M.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.G.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.M.; Sasekumar, A.; et al. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Q.; Li, J.; Jian, S.; Ren, H. Mangrove succession enriches the sediment microbial community in South China. Sci. Rep. 2016, 6, 27468. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguín, G. Plant growth-promoting bacteria: A potential tool for arid mangrove reforestation. Trees 2002, 16, 159–166. [Google Scholar] [CrossRef]
- Alongi, D.M. Macro- and Micronutrient Cycling and Crucial Linkages to Geochemical Processes in Mangrove Ecosystems. J. Mar. Sci. Eng. 2021, 9, 456. [Google Scholar] [CrossRef]
- Alongi, D.M. Bacterial productivity and microbial biomass in tropical mangrove sediments. Micro. Ecol. 1988, 15, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Bano, N.; Nisa, M.; Khan, N.; Saleem, M.; Harrison, P.J.; Ahmed, S.I.; Azam, F. Significance of bacteria in the flux of organic matter in the tidal creeks of the mangrove ecosystem of the Indus River delta, Pakistan. Mar. Ecol. Prog. Ser. 1997, 157, 1–12. Available online: https://www.int-res.com/articles/meps/157/m157p001.pdf (accessed on 1 May 2024). [CrossRef]
- Brown, D.R.; Marotta, H.; Peixoto, R.B.; Enrich-Prast, A.; Barroso, G.C.; Soares, M.L.G.; Machado, W.; Pérez, A.; Smoak, J.M.; Sanders, L.M.; et al. Hypersaline tidal flats as important “blue carbon” systems: A case study from three ecosystems. Biogeosciences 2021, 18, 2527–2538. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Ward, R.D.; Borges, R.; Ferreira, A.C. Mangrove trace-metal Biogeochemistry response to global climate change. Front. For. Glob. Chang. 2022, 5, 817992. [Google Scholar] [CrossRef]
- Zuberer, D.A.; Silver, W.S. Biological dinitrogen fixation (Acetylene reduction) associated with Florida mangroves. Appl. Environ. Microbiol. 1978, 35, 567–575. [Google Scholar] [CrossRef]
- Abhijith, R.; Vennila, A.; Purushothaman, C.S.; Padua, S.; Shilta, M.T.; Mahesh, V. Influence of sediment chemistry on mangrove-phosphobacterial relationship. Int. J. Chem. Stud. 2018, 6, 1677–1686. Available online: https://core.ac.uk/download/pdf/158619563.pdf (accessed on 1 May 2024).
- Warren, J.H.; Underwood, A.J. Effects of burrowing crabs on the topography of mangrove swamps in New South Wales. J. Exp. Mar. Biol. Ecol. 1986, 102, 223–235. [Google Scholar] [CrossRef]
- Barbanera, A.; Markesteijn, L.; Kairo, J.; Juma, J.A.; Karythis, S.; Skov, M.W. Functional responses of mangrove fauna to forest degradation. Mar. Freshw. Res. 2022, 73, 762–773. [Google Scholar] [CrossRef]
- Nozarpour, R.; Shojaei, M.G.; Naderloo, R.; Nasi, F. Crustaceans functional diversity in mangroves and adjacent mudflats of the Persian Gulf and Gulf of Oman. Marine Environ. Res. 2023, 186, 105919. [Google Scholar] [CrossRef]
- Macintosh, D.J. Ecology and productivity of Malaysian mangrove crab populations (Decapoda: Brachyura). In Proceedings of the Asian Symposium on Mangrove Environment: Research and Management, Kuala Lumpur, Malaysia, 25–29 August 1980; Soepadmo, E., Rao, A.N., Macintosh, D.J., Eds.; University of Malaya Press: Kuala Lumpur, Malaysia, 1984; pp. 354–377. [Google Scholar]
- Smith, T.J., III; Boto, K.G.; Frusher, D.D.; Giddins, R.L. Keystone species and mangrove forest dynamics: The influence of burrowing by crabs on soil nutrient status and forest productivity. Estuar. Coast. Shelf. Sci. 1991, 33, 19–32. [Google Scholar] [CrossRef]
- Havanond, S. Effects of mud lobster (Thalassina anomala Herbst) on plant succession in mangrove forests. Bull. Mar. Sci. 1987, 41, 635–636. [Google Scholar]
- Hossain, M.S.; Bujang, J.S.; Kamal, A.H.M.; Zakaria, M.H.; Muslim, A.M.; Nadzri, M.I. Effects of burrowing mud lobsters (Thalassina anomala Herbst 1804) on soil macro- and micronutrients in a Malaysian mangrove. Estuar. Coast. Shelf Sci. 2019, 228, 106358. [Google Scholar] [CrossRef]
- Felder, D.L. Diversity and ecological significance of deep-burrowing macrocrustaceans in coastal tropical waters of the Americas (Decapoda: Thalassinidea). Interciencia 2001, 26, 440–449. [Google Scholar]
- Arceo-Carranza, D.; Gamboa, E.; Teutli-Hernández, C.; Badillo-Alemán, M.; Herrera-Silveira, J.A. Fish as an indicator of ecological restoration of mangroves on the north coast of Yucatán. Rev. Mex. Biodivers. 2016, 87, 489–496. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5548498 (accessed on 1 May 2024). [CrossRef]
- Lewis, R.R.; Gilmore, R.G. Important considerations to achieve successful mangrove forest restoration with optimum fish habitat. Bull. Mar. Sci. 2007, 80, 823–837. [Google Scholar]
- Hernández-Mendoza, L.C.; Escalera-Vázquez, L.; Arceo-Carranza, D. Estuarine fish feeding changes as indicator to mangrove restoration success in seasonal karstic wetlands. Front. Glob. Chang. 2022, 4, 743232. [Google Scholar] [CrossRef]
- Hendy, I.; Michie, L.; Taylor, B.W. Habitat creation and biodiversity maintenance in mangrove forests: Teredinid bivalves as ecosystem engineers. PeerJ 2014, 2, e591. [Google Scholar] [CrossRef] [PubMed]
- Ishimatsu, A.; Khoo, K.H.; Takita, T. Deposition of air in burrows of tropical mudskippers as an adaptation to the hypoxic mudflat environment. Science 1998, 81, 289–297. Available online: https://www.jstor.org/stable/i40135492 (accessed on 1 May 2024).
- Lacerda, L.D.; José, D.V.; Rezende, C.E.; Francisco, M.C.F.; Wasserman, J.C.; Martins, J.C. Leaf chemical characteristics affecting herbivory in a New World mangrove forest. Biotropica 1986, 18, 350–355. [Google Scholar] [CrossRef]
- Ong, J.E.; Gong, W.K.; Wong, C.H. Allometry and partitioning of the mangrove, Rhizophora apiculata. For. Ecol. Manag. 2004, 188, 395–408. [Google Scholar] [CrossRef]
- Ananda, K.; Sridhar, K.R. Diversity of filamentous fungi on decomposing leaf and woody litter of mangrove forests in the southwest coast of India. Curr. Sci. 2004, 87, 1431–1437. Available online: https://www.jstor.org/stable/24109484 (accessed on 1 May 2024).
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur. J. For. Res. 2008, 127, 1–22. [Google Scholar] [CrossRef]
- Lee, S.Y. Herbivory as an ecological process in a Kandelia candel (Rhizophoraceae) mangal in Hong Kong. J. Trop. Ecol. 1997, 7, 337–348. Available online: https://www.jstor.org/stable/2559631 (accessed on 1 May 2024). [CrossRef]
- Camilleri, J. Leaf choice by crustaceans in a mangrove forest in Queensland. Mar. Biol. 1989, 102, 453–459. [Google Scholar] [CrossRef]
- Thongtham, N.; Kristensen, E.; Purangprasan, S.Y. Leaf removal by sesarmid crabs in Bangrong Mangrove forest, Phuket, Thailand: With emphasis on the feeding ecology of Neoepisesarma versicolor. Estuar. Coast. Shelf. Sci. 2008, 80, 573–580. [Google Scholar] [CrossRef]
- Medina-Contreras, D.; Arenas-González, F.; Cantera-Kintz, J.; Sánchez-González, A.; Giraldo, A. Food web structure and isotopic niche in a fringe macro-tidal mangrove system, Tropical Eastern Pacific. Hydrobiologia 2020, 847, 3185–3199. [Google Scholar] [CrossRef]
- Erickson, A.A.; Saltis, M.; Bell, S.S.; Dawes, C.J. Herbivore feeding preferences as measured by leaf damage and stomatal ingestion: A mangrove crab example. J. Exp. Mar. Biol. Ecol. 2003, 289, 123–138. [Google Scholar] [CrossRef]
- Rani, V.; Sreelakshmi, C.; Nandan, S.B.; Santu, K.S.; Preethy, C.M. Feeding ecology of Parasesarma plicatum and its relation to carbon structuring in mangrove ecosystem. Hydrobiologia 2023, 850, 911–927. [Google Scholar] [CrossRef]
- Feller, I.C. The role of herbivory by wood-boring insects in mangrove ecosystems in Belize. Oikos 2002, 97, 153–176. [Google Scholar] [CrossRef]
- Alongi, D.M.; Christoffersen, P. Benthic infauna and organism-sediment relations in a shallow, tropical coastal area—Influence of outwelled mangrove detritus and physical disturbance. Mar. Ecol. Prog. Ser. 1992, 81, 229–245. Available online: https://www.jstor.org/stable/24827336 (accessed on 1 May 2024). [CrossRef]
- Calderon, D.G.; Echeverri, B.R. Obtaining Rhizophora mangle seedlings by stimulation of adventitious roots using an air-layering technique. In Mangrove Ecosystem Studies in Latin America and Africa; Kjerfve, B., Ed.; UNESCO: Paris, France, 1997; pp. 98–107. [Google Scholar]
- Schories, D.; Barletta-Bergan, A.; Barletta, M.; Krumme, U.; Mehlig, U.; Rademaker, V. The keystone role of leaf-removing crabs in mangrove forests of North Brazil. Wetlands Ecol. Manag. 2003, 11, 243–255. [Google Scholar] [CrossRef]
- Ashton, E.C. Mangrove sesarmid crab feeding experiments in Peninsular Malaysia. J. Exp. Mar. Biol. Ecol. 2002, 273, 97–119. [Google Scholar] [CrossRef]
- Burggren, W.; McMahon, B. Biology of the Land Crabs; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar] [CrossRef]
- Yeo, D.; Srivathsan, A.; Puniamoorthy, J.; Maosheng, F.; Grootaert, P.; Chan, L.; Guènard, B.; Damken, C.; Wahab, R.A.; Yuchen, A.; et al. Mangroves are an overlooked hotspot of insect diversity despite low plant diversity. BMC Biol. 2021, 19, 202. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.H. The natural history of insect herbivory on mangrove trees in and near Singapore. Raffles Bull. Zool. 1990, 38, 119–203. [Google Scholar]
- Feller, I.C.; Mathis, W.N. Primary herbivory by wood-boring insects along an architectural Gradient of Rhizophora mangle. Biotropica 1997, 29, 440–451. [Google Scholar] [CrossRef]
- Robertson, A.I.; Duke, N.C. Insect herbivory on mangrove leaves in North Queensland. Aust. J. Ecol. 1987, 12, 1–7. [Google Scholar] [CrossRef]
- Farnsworth, E.J.; Ellison, A.M. Patterns of Herbivory in Belizean Mangrove Swamps. Biotropica 1991, 23, 555–567. [Google Scholar] [CrossRef]
- Veenakumari, K.; Mohanraj, P.; Bandyopadbyay, A.K. Insect herbivores and their natural enemies in the mangals of the Andaman and Nicobar Islands. J. Nat. Hist. 1997, 31, 1105–1126. [Google Scholar] [CrossRef]
- Whitten, A.J.; Damanik, S.J. Mass defoliation of mangroves in Sumatra, Indonesia. Biotropica 1986, 18, 176. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, J.; Cui, X.; Xu, F.; Lin, G.; Lin, G. Insect outbreaks have transient effects on carbon fluxes and vegetative growth but longer-term impacts on reproductive growth in a mangrove forest. Agric. For. Meteorol. 2019, 279, 107747. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Freire, F.A.M.; Rodrigues, J.V.M.; Bezerra, L.E.A. Mangrove recovery in semiarid coast shows increase in ecological processes from biotic and abiotic drivers in response to hydrological restoration. Wetlands 2022, 42, 80. [Google Scholar] [CrossRef]
- Onuf, C.P.; Teal, J.M.; Valiela, I. Interactions of nutrients, plant growth and herbivory in a mangrove ecosystem. Ecology 1977, 58, 514–526. [Google Scholar] [CrossRef]
- Feller, I.C. The effects of nutrient enrichment on growth and herbivory in dwarf red mangrove (Rhizophora mangle L.). Ecol. Monogr. 1995, 65, 477–505. [Google Scholar] [CrossRef]
- Maldonado-López, Y.; Vaca-Sánchez, M.S.; Canché-Delgado, A.; García-Jaín, S.E.; González-Rodríguez, A.; Cornelissen, T.; Cuevas-Reyes, P. Leaf herbivory and fluctuating asymmetry as indicators of mangrove stress. Wetlands Ecol. Manag. 2019, 27, 571–580. [Google Scholar] [CrossRef]
- Salter, R.E.; Mackenzie, N.A.; Nightingale, N.; Aken, K.M.; Chai, P.P.K. Habitat use, ranging behaviour and food habits of the Proboscis monkey, Nasalis larvatus (van Wurmb), in Sarawak. Primates 1985, 26, 436–451. [Google Scholar] [CrossRef]
- Yeager, C.P. Feeding ecology of the Proboscis monkey (Nasalis larvatus). Int. J. Primatol. 1989, 10, 497–530. [Google Scholar] [CrossRef]
- Dierenfeld, E.S.; Koontz, E.W.; Goldstein, R.S. Feed intake, digestion and passage of the Proboscis monkey (Nasalis larvatus) in captivity. Primates 1992, 33, 399–405. [Google Scholar] [CrossRef]
- Tangah, J.; Ashton, E.C.; Chan, H.T.; Baba, S. Mangroves of Malaysia. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Thammineni, P., Ashton, E.C., Eds.; Springer: Singapore, 2022; pp. 373–395. [Google Scholar] [CrossRef]
- Snarr, K.A. Seismic activity response as observed in mantled howlers (Alouatta palliata), Cuero y Salado Wildlife Refuge, Honduras. Primates 2005, 46, 281–285. [Google Scholar] [CrossRef]
- Luecke-Bridgeman, L. Diet of the black howler monkey (Alouatta pigra) in mangrove forests and the phytochemistry of mangrove plants. Am. J. Phys. Anthropol. 2012, 147, 197. [Google Scholar] [CrossRef]
- Mendez-Carvajal, P. Population Study of Coiba Howler Monkeys (Alouatta coibensis coibensis) and Coiba Capuchin Monkeys (Cebus capucinus imitator), Coiba Island National Park, Republic of Panama. J. Primatol. 2012, 1, 104. [Google Scholar] [CrossRef]
- Santos, R.R.; Bridgeman, L.L. Mangrove-living primates in the Neotropics: An ecological review. In Primates in Flooded Habitats: Ecology and Conservation; Nowak, K., Barnett, A.A., Matsuda, I., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 54–58. [Google Scholar] [CrossRef]
- Cuddington, K.; Byers, J.E.; Wilson, W.G.; Hastings, A. Ecosystem Engineers: Plants to Protists; Academic Press: Cambridge, MA, USA, 2007; ISBN 978-0123738578. [Google Scholar]
- Davidson, T.; de Rivera, C.E.; Hsieh, H.-L. Damage and alteration of mangroves inhabited by a marine wood-borer. Mar. Ecol. Prog. Ser. 2014, 516, 177–185. [Google Scholar] [CrossRef]
- Perry, D.M.; Brusca, R.C. Effect of the root-boring isopod Sphaeroma peruvianum on red mangrove forests. Mar. Ecol. Prog. Ser. 1989, 57, 287–292. [Google Scholar] [CrossRef]
- Svavarsson, J.; Melckzedeck, K.W.; Osore, E.O. Does the wood-borer Sphaeroma terebrans (Crustacea) shape the distribution of the mangrove Rhizophora mucronata? Ambio 2002, 31, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Brown, B.J.; Lowrie, S. Isopod and insect root borers may benefit Florida mangroves. Science 1978, 201, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Ribi, G. Does the wood boring isopod Sphaeroma terebrans benefit red mangroves (Rhizophora mangle)? Bull. Mar. Sci. 1981, 31, 925–928. [Google Scholar]
- Olafsson, E. Are wood-boring isopods a real threat to the well-being of mangrove forests? Ambio 1998, 27, 760–761. [Google Scholar]
- Brooks, R.A. Discovery of Sphaeroma terebrans, a wood-boring isopod, in the red mangrove, Rhizophora mangle, habitat of Northern Florida Bay. Ambio 2004, 33, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.B.; Angelini, C. Wood traits and tidal exposure mediate shipworm infestation and biofouling in southeastern U.S. estuaries. Ecol. Eng. 2019, 132, 1–12. [Google Scholar] [CrossRef]
- Nadia, T.C.L. Fenologia, Ecologia da Polinização e Reprodução de Espécies de Manguezal, no Município de Goiana—PE. Ph.D. Dissertation, Universidade Federal de Pernambuco, Recife, Brazil, 2009. [Google Scholar]
- Diniz, U.M.; Nadia, T.L.; Mello, M.A.R.; Machado, I.C. Few plants and one dominant fly shape a unique pollination network in a neotropical mangrove. Aquat. Bot. 2022, 180, 103526. [Google Scholar] [CrossRef]
- Landry, C.L. Pollinator-mediated competition between two coflowering Neotropical mangrovespecies, Avicennia germinans (Avicenniaceae) and Laguncularia racemosa (Combretaceae). Ann. Bot. 2013, 111, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nadia, T.L.; Machado, I.C. Wind pollination and propagule formation in Rhizophora mangle L. (Rhizophoraceae): Resource or pollination limitation? An. Acad. Bras. Cien. 2014, 86, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Núnez, D.A.; Mancera-Pineda, J.E. Pollination and fruit set in the main neotropical mangrove species from the Southwestern Caribbean. Aquat. Bot. 2012, 103, 60–65. [Google Scholar] [CrossRef]
- Fleming, T.H.; Geiselman, C.; Kress, W.J. The evolution of bat pollination: A phylogenetic perspective. Ann. Bot. 2009, 104, 1117–1143. [Google Scholar] [CrossRef] [PubMed]
- Villamayor, B.M.R.; Rollon, R.N.; Samson, M.S.; Albano, G.M.G.; Primavera, J.H. Impact of Haiyan on Philippine mangroves: Implications to the fate of the widespread monospecifc Rhizophora plantations against strong typhoons. Ocean Coast. Manag. 2016, 132, 1–14. [Google Scholar] [CrossRef]
- Castellanos-Galindo, G.A.; Cantera, J.; Valencia, N.; Giraldo, S.; Peña, E.; Kluger, L.C.; Wolff, M. Modeling trophic flows in the wettest mangroves of the world: The case of Bahía Málaga in the Colombian Pacific coast. Hydrobiologia 2017, 803, 13–27. [Google Scholar] [CrossRef]
- Arakaki, J.; Grande, F.R.; Arvigo, A.L.; Pardo, J.C.F.; Fogo, B.R.; Sanches, F.H.C.; Miyai, C.A.; Marochi, M.Z.; Costa, T.M. Battle of the borders: Is a range-extending fiddler crab affecting the spatial niche of a congener species? J. Exp. Mar. Biol. Ecol. 2020, 532, 151445. [Google Scholar] [CrossRef]
- Jimenez, P.J.; Vorsatz, L.D.; Costa, T.M.; Cannicci, S. Temperature Extremes and Sex-Related Physiology, Not Environmental Variability, Are Key in Explaining Thermal Sensitivity of Bimodal-Breathing Intertidal Crabs. Front. Mar. Sci. 2022, 9, 858280. [Google Scholar] [CrossRef]
- Booth, J.M.; Fusi, M.; Marasco, R.; Mbobo, T.; Daffonchio, D. Fiddler crab bioturbation determines consistent changes in bacterial communities across contrasting environmental conditions. Sci. Rep. 2019, 9, 3749. [Google Scholar] [CrossRef]
- Fusi, M.; Booth, J.M.; Marasco, R.; Merlino, G.; Garcias-Bonet, N.; Barozzi, A.; Garuglieri, E.; Mbobo, T.; Diele, K.; Duarte, C.M.; et al. Bioturbation Intensity Modifies the Sediment Microbiome and Biochemistry and Supports Plant Growth in an Arid Mangrove System. Microbiol. Spectr. 2022, 10, e0111722. [Google Scholar] [CrossRef]
- Otero, X.L.; Araújo Jr, J.M.C.; Barcellos, D.; Queiroz, H.M.; Romero, D.J.; Nóbrega, G.N.; Siqueira Neto, M.; Ferreira, T.O. Crab Bioturbation and Seasonality Control Nitrous Oxide Emissions in Semiarid Mangrove Forests (Ceará, Brazil). App. Sci. 2020, 10, 2215. [Google Scholar] [CrossRef]
- Thirukanthan, C.S.; Azra, M.N.; Seman, N.J.A.; Agos, S.M.; Arifin, H.; Aouissi, H.A.; Lananan, F.; Gao, H. A scientometric review of climate change and research on crabs. J. Sea Res. 2023, 193, 102386. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest insects and climate change. Curr. For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef]
- Kumar, A.; Ramanathan, A. Speciation of selected trace metals (Fe, Mn, Cu and Zn) with depth in the sediments of Sundarban mangroves: India and Bangladesh. J. Soils Sediments 2015, 15, 2476–2486. [Google Scholar] [CrossRef]
- Nurdiani, R.; Zeng, C. Effects of temperature and salinity on the survival and development of mud crab, Scylla serrata (Forsskål), larvae. Aquacult. Res. 2007, 38, 1529–1538. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Jayatissa, L.P.; Di Nitto, D.; Bosire, J.O.; Seen, D.L.; Koedam, N. How effective were mangroves as a defence against the recent tsunami? Curr. Biol. 2005, 15, R443–R447. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, K.; Rajendran, N. Coastal mangrove forests mitigated tsunami. Estuar. Coast. Shelf Sci. 2005, 65, 601–606. [Google Scholar] [CrossRef]
- Baldwin, A.; Egnotovich, M.; Ford, M.; Platt, W. Regeneration in Fringe Mangrove Forests Damaged by Hurricane Andrew. Plant Ecol. 2001, 157, 151–164. [Google Scholar] [CrossRef]
- Cai, W.; Ng, B.; Geng, T.; Jia, F.; Wu, L.; Wang, G.; Liu, Y.; Gan, B.; Yang, K.; Santoso, A.; et al. Anthropogenic impacts on twentieth-century ENSO variability changes. Nat. Rev. Earth Environ. 2023, 4, 407–418. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C.; Reef, R.; Hickey, S.; Ball, M.C. Mangrove dieback during fluctuating sea levels. Sci. Rep. 2017, 7, 1680. [Google Scholar] [CrossRef]
- Servino, R.N.; Gomes, L.E.O.; Bernardino, A.F. Extreme weather impacts on tropical mangrove forests in the Eastern Brazil Marine Ecoregion. Sci. Total Environ. 2018, 628–629, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.O.; Otero, X.L.; Nóbrega, G.N.; Queiroz, H.M.; Barcellos, D.; Vidal-Torrado, P. Mangroves Along the Brazilian Coast. In The Soils of Brazil; Schaefer, C.E.G.R., Ed.; World Soils Book Series; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Diele, K.; Tran Ngoc, D.M.; Geist, S.J.; Meyer, F.W.; Pham, Q.H.; Saint-Paul, U.; Tran, T.; Berger, U. Impact of typhoon disturbance on the diversity of key ecosystem engineers in a monoculture mangrove forest plantation, Can Gio Biosphere Reserve, Vietnam. Global Planet. Chang. 2013, 110, 236–248. [Google Scholar] [CrossRef]
- Muzaki, F.K.; Giffari, A.; Saptarini, D. Community structure of fish larvae in mangroves with different root types in Labuhan coastal area, Sepulu–Madura. AIP Conf. Proc. 2017, 1854, 020025. [Google Scholar] [CrossRef]
- Landry, C.L. Changes in pollinator assemblages following hurricanes affect the mating system of Laguncularia racemosa (Combretaceae) in Florida, USA. J. Trop. Ecol. 2013, 29, 209–216. [Google Scholar] [CrossRef]
- Lovelock, C.E. Soil Respiration and Belowground Carbon Allocation in Mangrove Forests. Ecosystems 2008, 11, 342–354. [Google Scholar] [CrossRef]
- Saavedra-Hortua, D.A.; Friess, D.A.; Zimmer, M.; Gillis, L.G. Sources of particulate organic matter across mangrove forests and adjacent ecosystems in different geomorphic settings. Wetlands 2020, 40, 1047–1059. [Google Scholar] [CrossRef]
- Ashton, E.C.; Macintosh, D.J.; Hogarth, P.J. A baseline study of the diversity and community ecology of crab and molluscan macrofauna in the Sematan mangrove forest, Sarawak, Malaysia. J. Trop. Ecol. 2003, 19, 127–142. [Google Scholar] [CrossRef]
- McNaughton, S.J.; Wolf, L.L. Dominance and the Niche in Ecological Systems. Science 1970, 167, 131–139. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Lohbeck, M.; Bonger, F.; Martinez-Ramos, M.; Poorter, L. The importance of biodiversity and dominance for multiple ecosystem functions in a human-modified tropical landscape. Ecology 2016, 97, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Delfan, N.; Shojaei, M.J.; Naderloo, R. Patterns of structural and functional diversity of macrofaunal communities in a subtropical mangrove ecosystem. Est. Coast. Shelf. Sci. 2021, 252, 107288. [Google Scholar] [CrossRef]
- Henderson, C.J.; Gilby, B.L.; Schlacher, T.A.; Connolly, R.M.; Sheaves, M.; Maxwell, P.S.; Flint, N.; Borland, H.P.; Martin, T.S.H.; Olds, A.D. Low redundancy and complementarity shape ecosystem functioning in a low-diversity ecosystem. J. Anim. Ecol. 2020, 89, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Walker, B. Conserving biological diversity through ecosystem resilience. Conserv. Biol. 1995, 9, 747–752. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge and needs for future research. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Sacco, A.D.; Hardwick, K.A.; Blakesley, D.; Brancalion, P.H.S.; Breman, E.; Rebola, L.C.; Chomba, S.; Dixon, K.; Elliott, S.; Ruyonga, G.; et al. Ten golden rules for reforestation to optimize carbon sequestration, biodiversity recovery and livelihood benefits. Glob. Chang. Biol. 2021, 27, 328–1348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).