Analyzing Species Diversity in Rocky Intertidal Communities over Multiple Spatial Scales among Understudied Eastern Pacific Ecoregions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sample-Based Incidence and Abundance Scoring
2.3. Spatial Scales and Measures of Diversity
3. Results
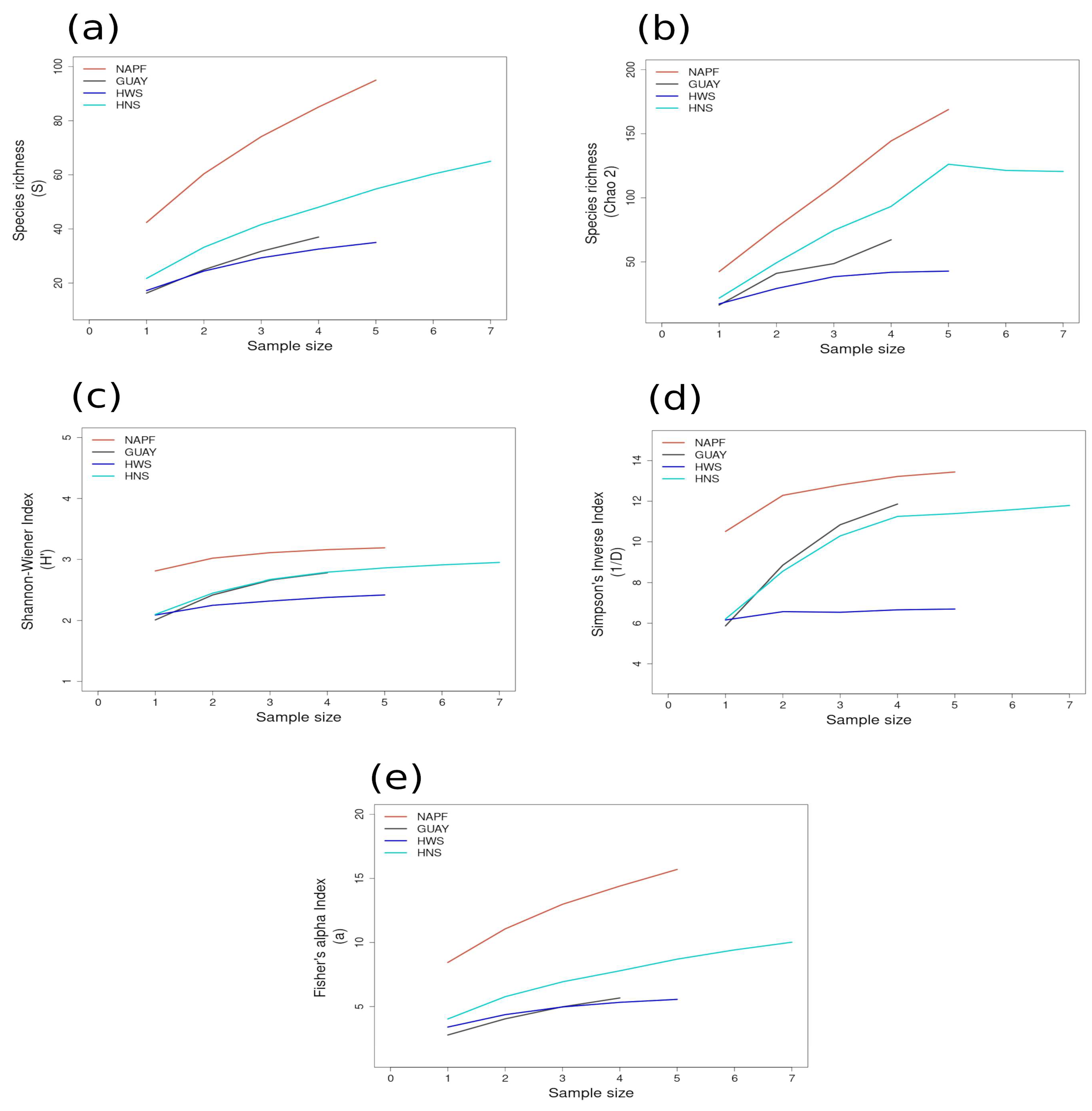
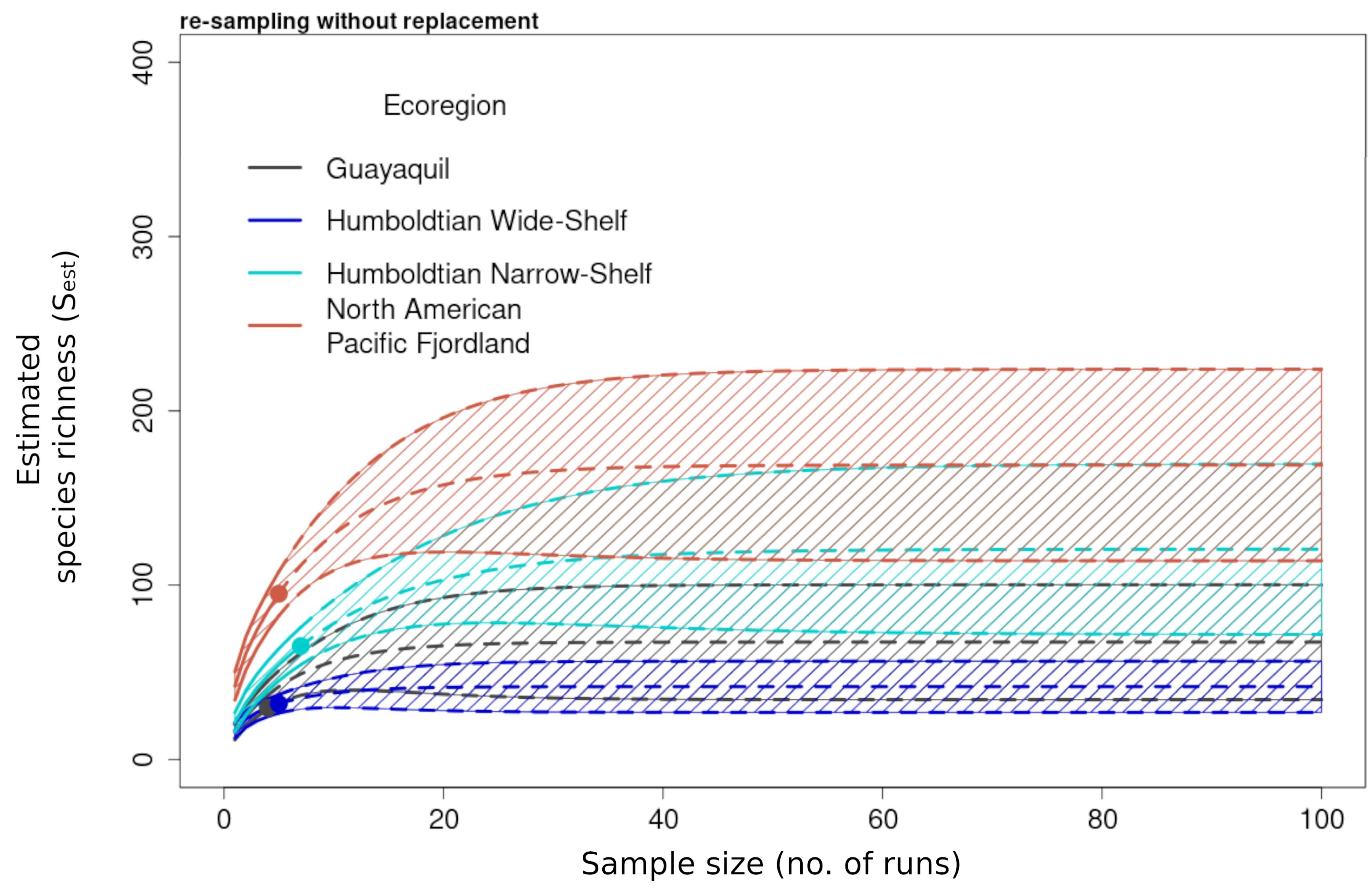
3.1. Summary of Species Inventory
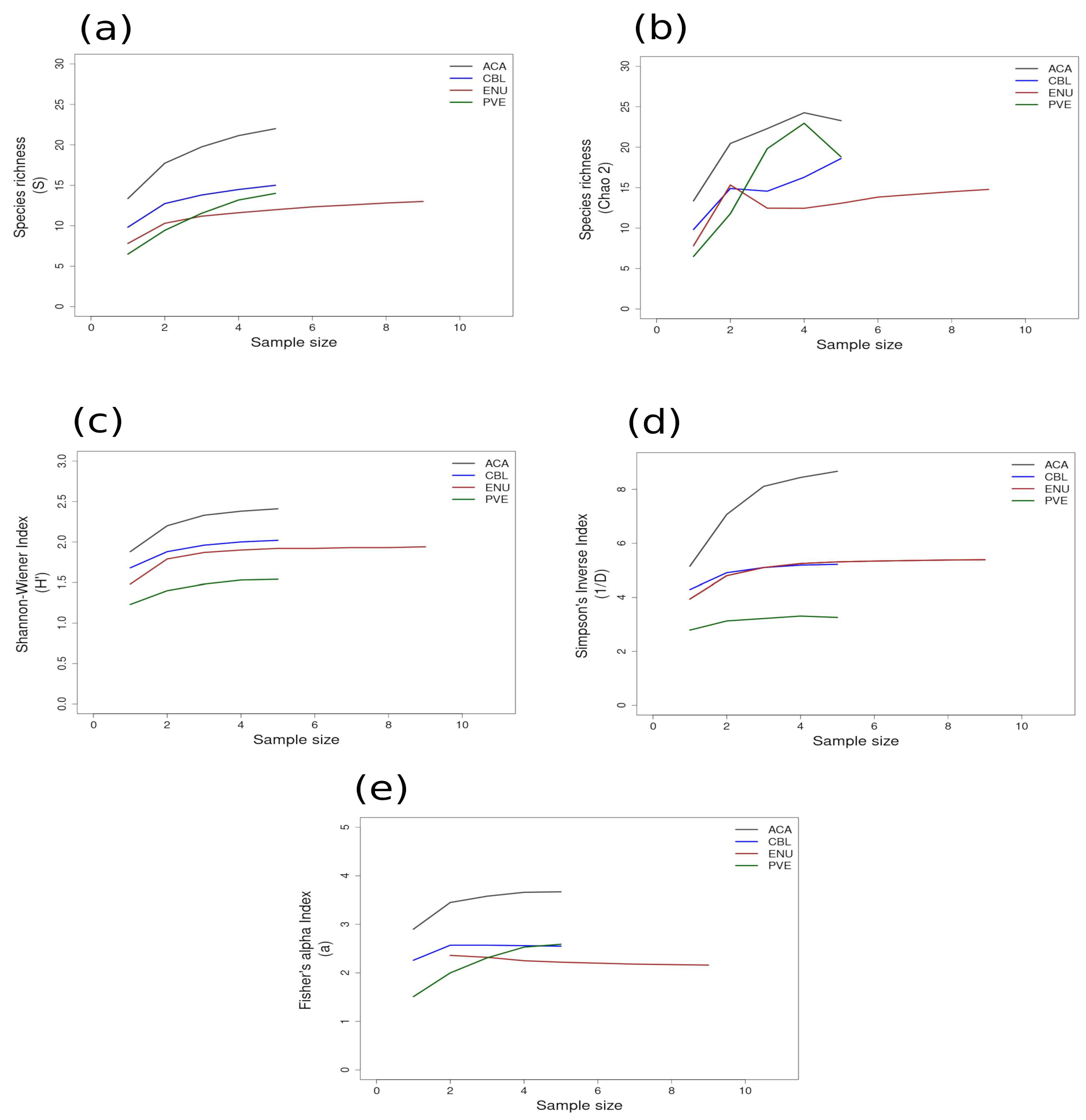
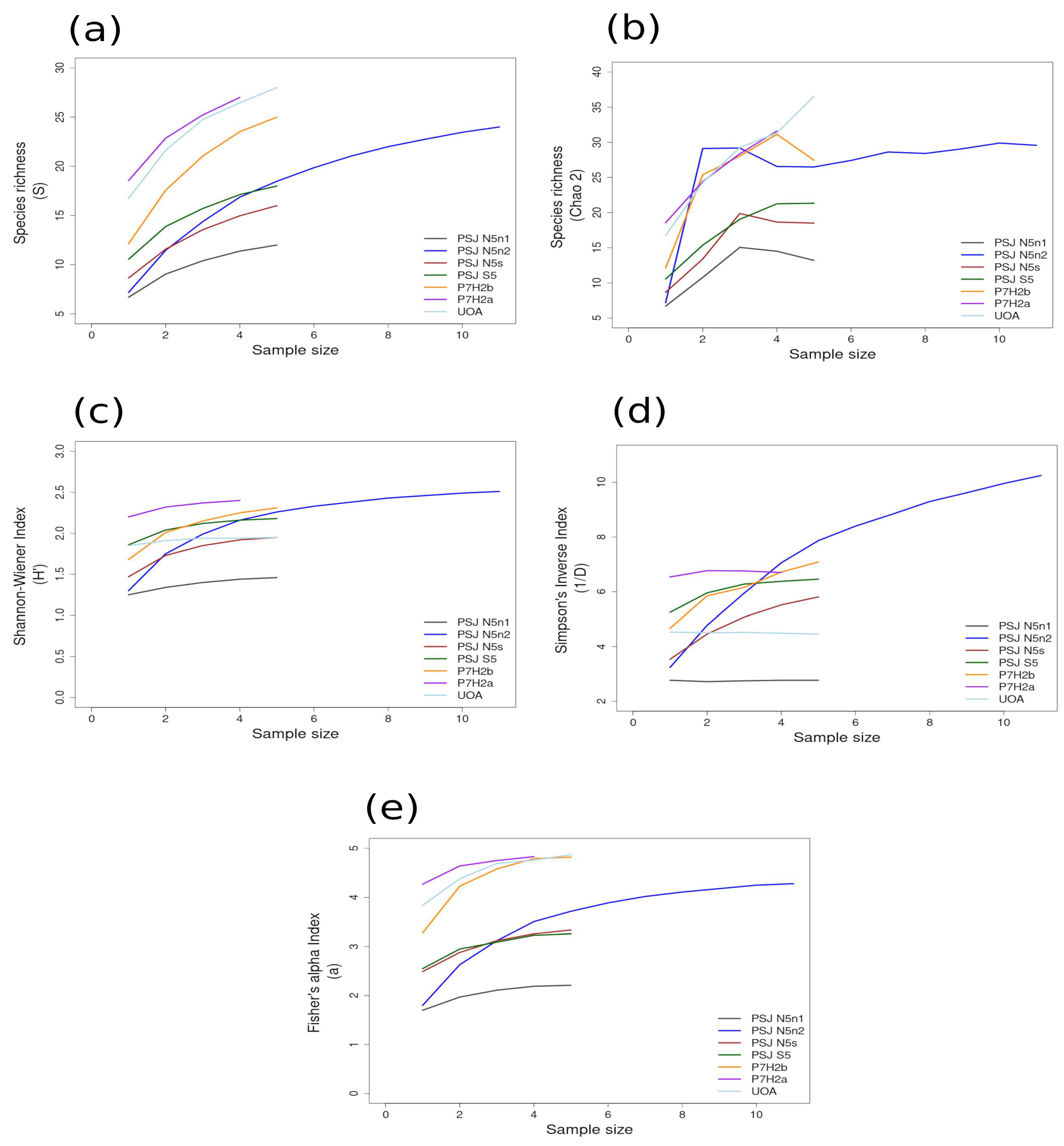
3.2. Alpha Diversity
3.2.1. Richness and Diversity Curves
3.2.2. Hutcheson’s t-Test of the Shannon–Wiener Indices
3.3. Beta Diversity
3.3.1. Patterns of Zonation: β1 Scale
3.3.2. Patterns of Similarity: β2 Scale
3.3.3. Patterns of Similarity: β3 Scale
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibanez-Erquiaga, B.; Pacheco, A.S.; Rivadeneira, M.M.; Tejada, C.L. Biogeographical Zonation of Rocky Intertidal Communities along the Coast of Peru (3.5–13.5° S Southeast Pacific). PLoS ONE 2018, 13, e0208244. [Google Scholar] [CrossRef]
- Valqui, J.; Ibañez-Erquiaga, B.; Pacheco, A.S.; Wilbur, L.; Ochoa, D.; Cardich, J.; Pérez-Huaranga, M.; Salas-Gismondi, R.; Pérez, A.; Indacochea, A.; et al. Changes in Rocky Intertidal Communities after the 2015 and 2017 El Niño Events along the Peruvian Coast. Estuar. Coast. Shelf Sci. 2021, 250, 107142. [Google Scholar] [CrossRef]
- Bosman, A.L.; Hockey, P.A.R.; Siegfried, W.R. The Influence of Coastal Upwelling on the Functional Structure of Rocky Intertidal Communities. Oecologia 1987, 72, 226–232. [Google Scholar] [CrossRef]
- Bustamente, R.H.; Branch, G.M.; Eekhout, S.; Robertson, B.; Zoutendyk, P.; Schleyer, M.; Dye, A.; Hanekom, N.; Jurd, M.; McQuaid, C.; et al. Gradients of Intertidal Primary Productivitiy around the Coast of South Africa and Their Relationships with Consumer Biomass. Oecologia 1994, 102, 189–201. [Google Scholar] [CrossRef]
- Konar, B.; Iken, K.; Edwards, M. Depth-Stratified Community Zonation Patterns on Gulf of Alaska Rocky Shores. Mar. Ecol. 2008, 30, 63–73. [Google Scholar] [CrossRef]
- Okuda, T.; Noda, T.; Yamamoto, T.; Ito, N.; Nakaoka, M. Latitudinal Gradient of Species Diversity: Multi-Scale Variability in Rocky Intertidal Sessile Assemblages along the Northwestern Pacific Coast. Popul. Ecol. 2004, 46, 159–170. [Google Scholar] [CrossRef]
- Menge, B.A. Predation Intensity in a Rocky Intertidal Community: Relation between Predator Foraging Activity and Environmental Harshness. Oecologia 1978, 34, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Menge, B. Top-down and Bottom-up Community Regulation in Marine Rocky Intertidal Habitats. J. Exp. Mar. Biol. Ecol. 2000, 250, 257–289. [Google Scholar] [CrossRef] [PubMed]
- Steneck, R.S.; Watling, L. Feeding Capabilities and Limitation of Herbivorous Molluscs: A Functional Group Approach. Mar. Biol. 1982, 68, 299–319. [Google Scholar] [CrossRef]
- Engle, J.M. Unified Monitoring Protocols for the Multi-Agency Rocky Intertidal Network; U.S. Department of the Interior Minerals Management Service Pacific OCS Region: Camarillo, CA, USA, 2008; Volume 1, p. 84.
- Bell, E.C. Environmental and Morphological Influences on Thallus Temperature and Dessication of the Intertidal Alga Mastocarpus Papillatus. J. Exp. Mar. Biol. Ecol. 1995, 191, 29–55. [Google Scholar] [CrossRef]
- Harley, C.G.D.; Helmuth, B. Local and Regional Scale Effects of Wave Exposure, Thermal Stress, and Absolute versus Effective Shore Level on Patterns of Intertidal Zonation. Limnol. Oceanogr. 2003, 48, 1498–1508. [Google Scholar] [CrossRef]
- Helmuth, B.; Miezkowska, N.; Moore, P.; Hawkins, S.J. Living on the Edge of Two Changing Worlds: Forecasting the Responses of Rocky Intertidal Ecosystems to Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 373–404. [Google Scholar] [CrossRef]
- Helmuth, B.; Broitman, B.R.; Blanchette, C.A.; Gilman, S.; Halpin, P.; Harley, C.D.G.; O’Donnell, M.J.; Hofmann, G.E.; Menge, B.; Strickland, D. Mosaic Patterns of Thermal Stress in the Rocky Intertidal Zone: Implications for Climate Change. Ecol. Monogr. 2006, 76, 461–479. [Google Scholar] [CrossRef]
- DeVogelaere, A.P.; Foster, M.S. Damage and Recovery in Intertidal Fucus Gardneri Assemblages Following the “Exxon Valdez” Oil Spill. Mar. Ecol. Prog. Ser. 1994, 106, 263–271. [Google Scholar] [CrossRef]
- Wilbur, L.; Küpper, F.C.; Louca, V. Algal Cover as a Driver of Diversity in Communities Associated with Mussel Assemblages across Eastern Pacific Ecoregions. Mar. Ecol. 2023, 45, e12785. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Wilbur, L.; Louca, V.; Ibanez-Erquiaga, B.; Küpper, F.C. A Case for Trans-Regional Intertidal Research in Unstudied Areas in the Northeast and Southeast Pacific: Filling the Gaps. Coasts 2024, 4, 323–346. [Google Scholar] [CrossRef]
- Arakaki, N.; Carbajal, P.; Gamarra, A.; Gil-Kodaka, P.; Ramírez, M. Macroalgas de la Costa Central del Perú; Universidad Nacional Agraria La Molina: Lima, Peru, 2019; p. 128. ISBN 978-612-4387-19-7. [Google Scholar]
- Dawson, Y.E.; Acleto, C.; Foldvik, N. The Seaweeds of Peru; Weinheim Verlag Von J. Cramer: Stuttgart, Germany, 1964; p. 111. ISBN 978-3-7682-5413-7. [Google Scholar]
- Howe, M.A. Marine Algae of Peru; Memoirs of the Torrey botanical club; Press of the New era Print. Co.: New York, NY USA, 1914; p. 285. [Google Scholar]
- Instituto del Mar del Perú. Catálogo Digital de La Biodiversidad Acuática Del Perú. Available online: https://biodiversidadacuatica.imarpe.gob.pe/ (accessed on 20 May 2024).
- Abbott, I.A.; Hollengerg, G.J. Marine Algae of California; Stanford University Press: Redwood City, CA, USA, 1976; p. 844. [Google Scholar]
- Carlton, J.T. Intertidal Invertebrates from Central California to Oregon the Light and Smith Manual, 4th ed.; University of California Press: Redwood City, CA, USA, 2007; p. 1001. [Google Scholar]
- Kozloff, E. Marine Invertebrates of the Pacific Northwest with Additions and Corrections; University of Washington Press: Seattle, WA, USA, 1996; p. 539. ISBN 978-0-295-97562-7. [Google Scholar]
- AlgaeBase:: Listing the World’s Algae. Available online: https://www.algaebase.org/ (accessed on 12 July 2024).
- Steneck, R.S. Herbivory on Coral Reefs: A Synthesis. In Proceedings of the 6th International Coral Reef Symposium, Townsville, Australia, 8–12 August 1988; Volume 1, pp. 37–49. [Google Scholar]
- Steneck, R.S.; Dethier, M.N. A Functional Group Approach to the Structure of Algal-Dominated Communities. Oikos 1994, 69, 476–498. [Google Scholar] [CrossRef]
- Steneck, R.S.; Vavrinec, J.; Leland, A.V. Accelerating Trophic-Level Dysfunction in Kelp Forest Ecosystems of the Western North Atlantic. Ecosystems 2004, 7, 323–332. [Google Scholar] [CrossRef]
- Ricotta, C.; Pavoine, S.; Bacaro, G.; Acosta, A.T.R. Functional Rarefaction for Species Abundance Data. Methods Ecol. Evol. 2012, 3, 519–525. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.-Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and Estimators Linking Individual-Based and Sample-Based Rarefaction, Extrapolation and Comparison of Assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Salinas, H.; Ramirez-Delgado, D. ecolTest:Community Ecology. Available online: https://cran.r-project.org/web/packages/ecolTest/index.html (accessed on 10 November 2022).
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Chao, A. Estimating the Population Size for Capture-Recapture Data with Unequal Catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Lande, R. Statistics and Partitioning of Species Diversity, and Similarity among Multiple Communities. Oikos 1996, 76, 5–13. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Chao, A. Measuring and Estimating Species Richness, Species Diversity, and Biotic Similarity from t-teSampling Data. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Cambridge, UK, 2013; pp. 195–211. ISBN 978-0-12-384720-1. [Google Scholar]
- Colwell, R.K.; Elsensohn, J.E. EstimateS Turns 20: Statistical Estimation of Species Richness and Shared Species from Samples, with Non-Parametric Extrapolation. Ecography 2014, 37, 609–613. [Google Scholar] [CrossRef]
- Jost, L. Entropy and Diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Jost, L. Partitioning Diversity into Independent Alpha and Beta Components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Wiley Blackwell: Hoboken, NJ, USA, 2004; p. 272. [Google Scholar]
- Beck, J.; Schwanghart, W. Comparing Measures of Species Diversity from Incomplete Inventories: An Update. Methods Ecol. Evol. 2010, 1, 38–44. [Google Scholar] [CrossRef]
- Edden, A.C. A Measure of Species Diversity Related to the Lognormal Distribution of Individuals among Species. J. Exp. Mar. Biol. Ecol. 1971, 6, 199–209. [Google Scholar] [CrossRef]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The Relation Between the Number of Species and the Number of Individuals in a Random Sample of an Animal Population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Colwell, R.; Coddington, J.; Colwell, R.K.; Coddington, J.A. Estimating Terrestrial Biodiversity through Extrapolation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [CrossRef]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, Extrapolating, and Comparing Incidence-Based Species Accumulation Curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Warton, D.I.; Wright, S.T.; Wang, Y. Distance-Based Multivariate Analyses Confound Location and Dispersion Effects. Methods Ecol. Evol. 2012, 3, 89–101. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the Amount of Ecologic Association Between Species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; o’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 10 November 2022).
- R Core Team R: A Language and Environment for Statistical Computing: Package: Vienna, Austria 2020. Available online: https://wwwR-project.org/ (accessed on 8 September 2022).
- Anderson, M.; Gorley, R.N.; Clarke, K. PERMANOVA+ for Primer: Guide to Software and Statistical Methods; Primer-E Limited: Auckland, New Zealand, 2008; p. 214. [Google Scholar]
- Barahona Padilla, S.P. Patrones Filogeográficos de dos Moluscos Intermareales a lo Largo de un Gradiente Biogeográfico en la Costa Norte del Perú. Available online: https://repositorio.upch.edu.pe/handle/20.500.12866/892 (accessed on 12 July 2024).
- Schipper, S.R.; Shivak, J.P.; Hind, K.R.; Miller, K.A.; Hughey, J.R.; Gabrielson, P.W.; Martone, P.T. Reinstatement of Corallina Chilensis (Corallinaceae, Rhodophyta) Based on DNA Sequencing of the Type Material Collected by Darwin. Phycologia 2023, 62, 203–216. [Google Scholar] [CrossRef]
- Huber, S. What Is Corallina Officinalis Var. Chilensis? An Examination of Nomenclature, Biogeography, Phylogeny, and Morphology. Master’s Thesis, University of British Columbia, Vancouver, BC, USA, 2020; p. 125. [Google Scholar]
- Hind, K.R.; Gabrielson, P.W.; Lindstrom, S.C.; Martone, P.T. Misleading Morphologies and the Importance of Sequencing Type Specimens for Resolving Coralline Taxonomy (Corallinales, Rhodophyta): Pachyarthron Cretaceum Is Corallina Officinalis. J. Phycol. 2014, 50, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H. Strength, Slope and Variability of Marine Latitudinal Gradients. Mar. Ecol. Prog. Ser. 2004, 273, 251–267. [Google Scholar] [CrossRef]
- McCoy, S.J.; Kamenos, N.A. Coralline Algae (Rhodophyta) in a Changing World: Integrating Ecological, Physiological, and Geochemical Responses to Global Change. J. Phycol. 2015, 51, 6–24. [Google Scholar] [CrossRef]
- Halfar, J.; Steneck, R.S.; Joachimski, M.; Kronz, A.; Wanamaker, A.D., Jr. Coralline Red Algae as High-Resolution Climate Recorders. Geology 2008, 36, 463–466. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean Acidification Causes Bleaching and Productivity Loss in Coral Reef Builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef] [PubMed]
- Ries, J.B. Skeletal Mineralogy in a High-CO2 World. J. Exp. Mar. Biol. Ecol. 2011, 403, 54–64. [Google Scholar] [CrossRef]
- Chaudhary, C.; Saeedi, H.; Costello, M.J. Bimodality of Latitudinal Gradients in Marine Species Richness. Trends Ecol. Evol. 2016, 31, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, H.; Costello, M.J.; Warren, D.; Brandt, A. Latitudinal and Bathymetrical Species Richness Patterns in the NW Pacific and Adjacent Arctic Ocean. Sci. Rep. 2019, 9, 10. [Google Scholar] [CrossRef]
- Bhadja, P.; Poriya, P.; Kundu, R. Community Structure and Distribution Pattern of Intertidal Invertebrate Macrofauna at Some Anthropogenically Influenced Coasts of Kathiawar Peninsula (India). Adv. Ecol. 2014, 2014, 547395. [Google Scholar] [CrossRef]
- Datta, S.N.; Chakraborty, S.K.; Jaiswar, A.K.; Ziauddin, G. A Comparative Study on Intertidal Faunal Biodiversity of Selected Beaches of Mumbai Coast. J. Environ. Biol. 2010, 31, 981–986. [Google Scholar] [PubMed]
- Switzer, P.V. Site Fidelity in Predictable and Unpredictable Habitats. Evol. Ecol. 1993, 7, 533–555. [Google Scholar] [CrossRef]
- Wichard, T.; Charrier, B.; Mineur, F.; Bothwell, J.H.; Clerck, O.D.; Coates, J.C. The Green Seaweed Ulva: A Model System to Study Morphogenesis. Front. Plant Sci. 2015, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.T. Quaternary Uplift of the Peruvian Coast Related to the Subduction of the Nazca Ridge: 13.5 to 15.6 Degrees South Latitude. Quat. Int. 1992, 15–16, 87–97. [Google Scholar] [CrossRef]
- Jaramillo, E.; Dugan, J.E.; Hubbard, D.M.; Melnick, D.; Manzano, M.; Duarte, C.; Campos, C.; Sanchez, R. Ecological Implications of Extreme Events: Footprints of the 2010 Earthquake along the Chilean Coast. PLoS ONE 2012, 7, e35348. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.G.; Fisher, J.L.; Mace, A.J. Larval Recruitment in a Region of Strong, Persistent Upwelling and Recruitment Limitation. Mar. Ecol. Prog. Ser. 2009, 394, 79–99. [Google Scholar] [CrossRef]
- Pfaff, M.C.; Branch, G.M.; Wieters, E.A.; Branch, R.A.; Broitman, B.R. Upwelling Intensity and Wave Exposure Determine Recruitment of Intertidal Mussels and Barnacles in the Southern Benguela Upwelling Region. Mar. Ecol. Prog. Ser. 2011, 425, 141–152. [Google Scholar] [CrossRef]
- Shanks, A.L.; Largier, J.; Brink, L.; Brubaker, J.; Hooff, R. Demonstration of the Onshore Transport of Larval Invertebrates by the Shoreward Movement of an Upwelling Front. Limnol. Oceanogr. 2000, 45, 230–236. [Google Scholar] [CrossRef]
- Vasilis, L.; Wilbur, L.; Kupper, F.C. Upwelling, productivity, and changes in intertidal communities at a Pacific coastal marine protected area following a Coastal El Niño event. Estuar. Coast. Shelf Sci. 2024; 323–346, in review. [Google Scholar]
- Larsen, C.F.; Motyka, R.J.; Freymueller, J.T.; Ivins, E.R. Rapid Viscoelastic Uplift in Southeast Alaska Caused by Post-Little Ice Age Glacial Retreat. Earth Planet. Sci. Lett. 2005, 237, 548–560. [Google Scholar] [CrossRef]
- Larsen, C.F.; Motyka, R.J.; Freymueller, J.T.; Echelmeyer, K.A.; Ivins, E.R. Rapid Uplift of Southern Alaska Caused by Recent Ice Loss. Geophys. J. Int. 2004, 158, 1118–1133. [Google Scholar] [CrossRef]
- Praetorius, S.; Mix, A.; Jensen, B.; Froese, D.; Milne, G.; Wolhowe, M.; Addison, J.; Prahl, F. Interaction between Climate, Volcanism, and Isostatic Rebound in Southeast Alaska during the Last Deglaciation. Earth Planet. Sci. Lett. 2016, 452, 79–89. [Google Scholar] [CrossRef]
- McNeil, C.; O’Neel, S.; Loso, M.; Pelto, M.; Sass, L.; Baker, E.H.; Campbell, S. Explaining Mass Balance and Retreat Dichotomies at Taku and Lemon Creek Glaciers, Alaska. J. Glaciol. 2020, 66, 530–542. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; McFiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.-M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodide Accumulation Provides Kelp with an Inorganic Antioxidant Impacting Atmospheric Chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954–6958. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Adler, R.F. Interdecadal Variability/Long-Term Changes in Global Precipitation Patterns during the Past Three Decades: Global Warming and/or Pacific Decadal Variability? Clim. Dyn. 2013, 40, 3009–3022. [Google Scholar] [CrossRef]
- Peterson, W.; Robert, M.; Bond, N. The Warm Blob-Conditions in the Northeastern Pacific Ocean; PICES Press: Sidney, Austria, 2015; Volume 23, pp. 36–38. [Google Scholar]
- Cavole, L.M.; Demko, A.M.; Diner, R.E.; Giddings, A.; Koester, I.; Pagniello, C.M.L.S.; Paulsen, M.-L.; Ramirez-Valdez, A.; Schwenck, S.M.; Yen, N.K.; et al. Biological Impacts of the 2013–2015 Warm-Water Anomaly in the Northeast Pacific: Winners, Losers, and the Future. Oceanography 2016, 29, 273–285. [Google Scholar] [CrossRef]
- Schneider, N.; Miller, A.J.; Pierce, D.W. Anatomy of North Pacific Decadal Variability. J. Clim. 2002, 15, 586–605. [Google Scholar] [CrossRef]
- Cai, W.; Borlace, S.; Lengaigne, M.; van Rensch, P.; Collins, M.; Vecchi, G.; Timmermann, A.; Santoso, A.; McPhaden, M.J.; Wu, L.; et al. Increasing Frequency of Extreme El Niño Events Due to Greenhouse Warming. Nat. Clim. Change 2014, 4, 111–116. [Google Scholar] [CrossRef]
- Rodríguez-Morata, C.; Díaz, H.F.; Ballesteros-Canovas, J.A.; Rohrer, M.; Stoffel, M. The Anomalous 2017 Coastal El Niño Event in Peru. Clim. Dyn. 2019, 52, 5605–5622. [Google Scholar] [CrossRef]
- Lopez, H.; Lee, S.-K.; Kim, D.; Wittenberg, A.T.; Yeh, S.-W. Projections of Faster Onset and Slower Decay of El Niño in the 21st Century. Nat. Commun. 2022, 13, 1915. [Google Scholar] [CrossRef]
- Szathmary, L.P.; Helmuth, B.; Wethey, D.S. Climate Change in the Rocky Intertidal Zone: Predicting and Measuring the Body Temperature of a Keystone Predator. Mar. Ecol. Prog. Ser. 2009, 374, 46–56. [Google Scholar] [CrossRef]
- Hewson, I.; Button, J.B.; Gudenkauf, B.M.; Miner, B.; Newton, A.L.; Gaydos, J.K.; Wynne, J.; Groves, C.L.; Hendler, G.; Murray, M.; et al. Densovirus Associated with Sea-Star Wasting Disease and Mass Mortality. Proc. Natl. Acad. Sci. USA 2014, 111, 17278–17283. [Google Scholar] [CrossRef]
- Garrity, S.D. Some Adaptations of Gastropods to Physical Stress on a Tropical Rocky Shore. Ecology 1984, 65, 559–574. [Google Scholar] [CrossRef]
- Eschweiler, N.; Molis, M.; Buschbaum, C. Habitat-Specific Size Structure Variations in Periwinkle Populations (Littorina littorea) Caused by Biotic Factors. Helgol. Mar. Res. 2009, 63, 119–127. [Google Scholar] [CrossRef]
- Stickle, W.B.; Carrington, E.; Hayford, H. Seasonal Changes in the Thermal Regime and Gastropod Tolerance to Temperature and Desiccation Stress in the Rocky Intertidal Zone. J. Exp. Mar. Biol. Ecol. 2017, 488, 83–91. [Google Scholar] [CrossRef]
- Wells, H.W.; Gray, I.E. The Seasonal Occurrence of Mytilus Edulis on the Carolina Coast as a Result of Transport around Cape Hatteras. Biol. Bull. 1960, 119, 550–559. [Google Scholar] [CrossRef]
- Jones, S.J.; Mieszkowska, N.; Wethey, D.S. Linking Thermal Tolerances and Biogeography: Mytilus edulis (L.) at Its Southern Limit on the East Coast of the United States. Biol. Bull. 2009, 217, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Seuront, L.; Nicastro, K.R.; Zardi, G.I.; Goberville, E. Decreased Thermal Tolerance under Recurrent Heat Stress Conditions Explains Summer Mass Mortality of the Blue Mussel Mytilus Edulis. Sci. Rep. 2019, 9, 17498. [Google Scholar] [CrossRef]
- Helmuth, B.A. Intertidal Mussel Microclimates: Predicting the Body Temperature of a Sessile Invertebrate. Ecol. Monogr. 1998, 68, 51–74. [Google Scholar] [CrossRef]
- Ricketts, E.F.; Calvin, J. Between Pacific Tides, 5th ed.; Stanford University Press: Stanford, CA, USA, 1985; p. 680. ISBN 978-0-8047-2068-7. [Google Scholar]
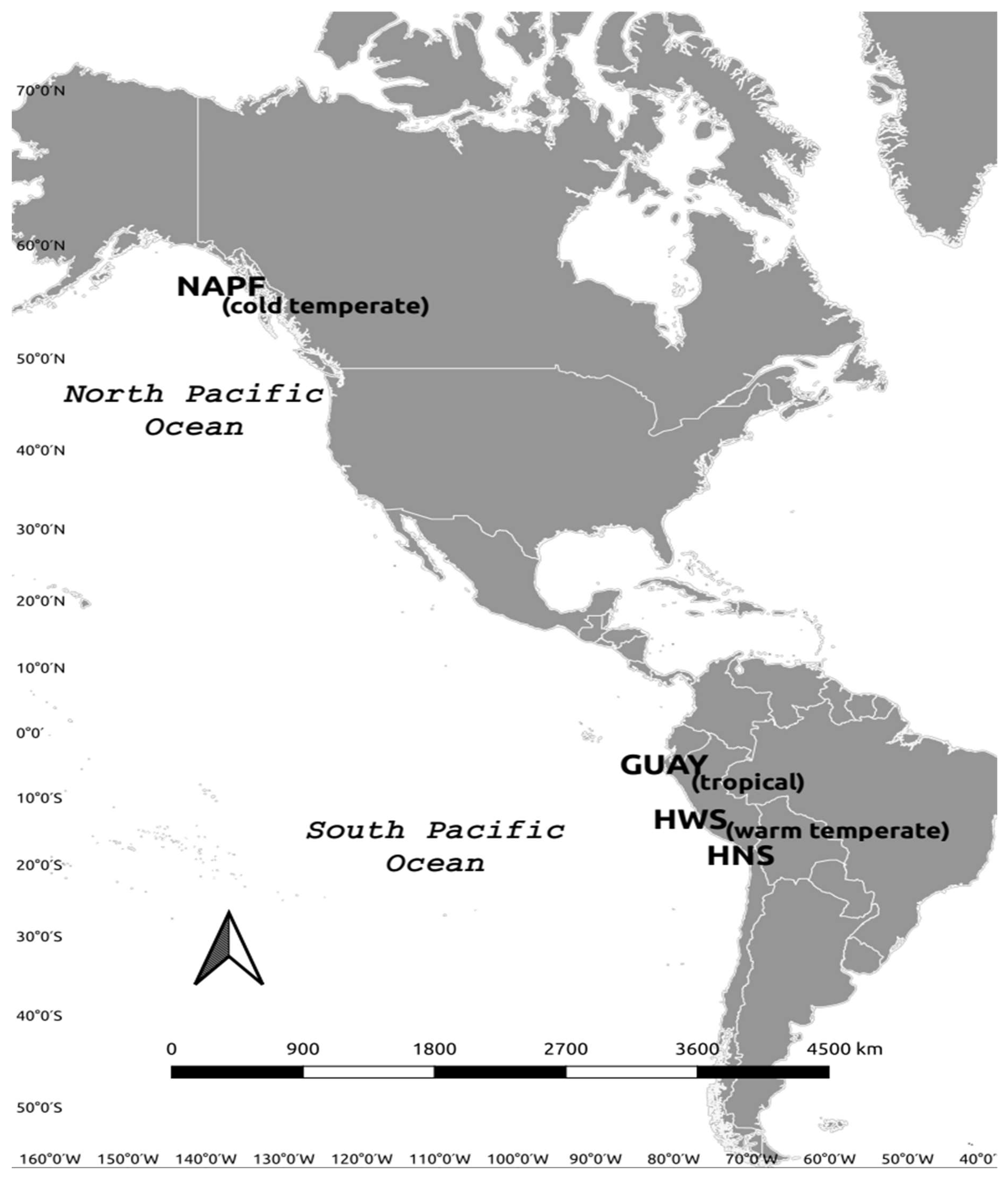






| Ecoregions/Sites | Latitude | Longitude |
|---|---|---|
| Guayaquil (GUAY) | ~0 ° to 6° S | |
| Playa Acapulco (ACA) | 3°44′28.31 S | 80°46′32.04 W |
| Playa Veleros (PVE) | 4°10′38.99 S | 81°08′33.82 W |
| El Nuro (ENU) | 4°13′04.07 S | 81°11′12.72 W |
| Cabo Blanco (CBL) | 4°15′00.22 S | 81°13′56.28 W |
| Humboldtian Wide-Shelf (HWS) | ~12° S to ~13° S | |
| Leon Dormido (LDO) | 12°38′03.69 S | 76°40′19.08 W |
| Playa Ensenada (PEN) | 12°38′51.29 S | 76°40′12.03 W |
| Playa Farallones (PFA1, PFA2) | 12°44′23.20 S, 12°44′23.24 S | 76°38′10.16 W |
| Playa Gallardo (PGA) | 12°57′53.73 S | 76°30′39.44 W |
| Humboldtian Narrow-Shelf (HNS) | ~15° S to ~26° S | |
| Reserva Punta San Juan (PSJ) N5n1-Nn2, N5s, S4, S5 | 15°21′45, 15°21′47.20 S, 15°22′02.76 S, 15°22′03.93 S | 78°11′30.07 W, 75°11′29.79 W, 75°11′16.30 W, 75°11′19.37 W |
| Playa Siete Huecos (P7H) (P7H2a, P7H2b) | 15°24′20.03 S, 15°24′20.05 S | 75°07′52.74 W |
| Universidad de Antofagasta (UOA) | 23°42′02.57 S | 70°25′27.56 W |
| North American Pacific Fjordland (NAPF) | ~50° N to ~59° N | |
| Kresta Point (KPO) | 57°08′27.58 N | 135°30′28.45 W |
| Kayak Island (KIS) | 57°02′21.88 N | 135°21′15.67 W |
| Pirate’s Cove (PCO1, PCO2) | 56°59′12.33 N | 135°22′46.95 W |
| Ecoregion | Shannon–Wiener | Simpson’s Inverse | Fisher’s Alpha | Shannon Exponential | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean (α3) | min (α2) | max (α2) | mean (α3) | min (α2) | max (α2) | mean (α3) | min (α2) | max (α2) | mean (α3) | |
| GUAY (n = 24) | 2.66 ± 0.17 | 1.53 ± 0.12 (PVE) | 2.38 ± 0.05 (ACA) | 10.84 ± 2.21 | 3.31 ± 0.37 (PVE) | 8.44 ± 0.77 (ACA) | 5.67 ± 0.36 | 2.17 ± 0.25 (ENU) | 3.66 ± 0.33 (ACA) | 16.15 |
| HWS (n = 28) | 2.38 ± 0.08 | 1.62 ± 0.01 (LDO) | 2.31 ± 0.02 (PGA) | 6.66 ± 0.91 | 3.48 ± 0.04 (LDO) | 7.49 ± 0.18 (PGA) | 5.56 ± 0.37 | 2.49 ± 0.26 (LDO) | 3.96 ± 0.46 (PFA1) | 11.27 |
| HNS (n = 39) | 2.91 ± 0.09 | 1.44 ± 0.08 (N5n1) | 2.49 ± 0.04 (N5n2) | 11.62 ± 1.51 | 2.77 ± 0.28 (N5n1) | 9.96 ± 0.36 (N5n2) | 9.42 ± 0.47 | 2.21 ± 0.28 (N5n1) | 4.87 ± 0.38 (UOA) | 19.19 |
| NAPF (n = 24) | 3.16 ± 0.07 | 2.61 ± 0.03 | 3.03 ± 0.04 (PCO1) | 13.22 ± 1.06 | 9.27 ± 0.97 (KIS) | 13.64 ± 0.24 (PCO2) | 15.7 ± 0.65 | 7.3 ± 0.53 (KIS) | 10.7 ± 0.67 (PCO1) | 26.82 |
| Hutcheson’s Two-Tailed t-Test Statistic, Bonferroni p-Value < 0.001 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GUAY | ACA | CBL | PVE | |||||||
| ACA | -- | 10.73, <0.001 | 17.31, <0.001 | |||||||
| CBL | 10.73, <0.001 | -- | 8.23, <0.001 | |||||||
| PVE | 17.31, <0.001 | 8.23, <0.001 | -- | |||||||
| ENU | 13.66, <0.001 | 1.48, 0.14 | 7.44, <0.001 | |||||||
| Hutcheson’s two-tailed t-test statistic, Bonferroni p-value < 0.005 | ||||||||||
| HWS | LDO | PEN | PFA1 | PFA2 | ||||||
| LDO | -- | −12.43, <0.001 | −9.22, <0.001 | −9.65, <0.001 | ||||||
| PEN | −12.43, <0.001 | -- | 0.89, 0.37 | 2.62, 0.01 | ||||||
| PFA1 | −9.22, <0.001 | 0.89, 0.37 | -- | −1.26, 0.21 | ||||||
| PFA2 | −9.65, <0.001 | 2.62, 0.01 | −1.26, 0.21 | -- | ||||||
| PGA | −11.11, <0.001 | −0.28, 0.78 | −1.05, 0.29 | −2.57, 0.01 | ||||||
| Hutcheson’s two-tailed t-test statistic, Bonferroni p-value < 0.002 | ||||||||||
| HNS | N5n1 | N5n2 | N5s | S5 | P7H2a | P7H2b | ||||
| N5n1 | -- | −18.48, <0.001 | −7.36, <0.001 | −11.75, <0.001 | −15.42, <0.001 | −13.47, <0.001 | ||||
| N5n2 | −18.48, <0.001 | -- | 11.35, <0.001 | 8.11, <0.001 | 2.93, 0.003 | 4.74, <0.001 | ||||
| N5s | −7.36, <0.001 | 11.35, <0.001 | −4.19, <0.001 | −4.19, <0.001 | −8.26, <0.001 | −6.32, <0.001 | ||||
| S5 | −11.75, <0.001 | 8.11, <0.001 | −4.19, <0.001 | -- | −4.67, <0.001 | 2.58, 0.01 | ||||
| P7H2a | 15.42, <0.001 | 2.93, 0.003 | −8.26, <0.001 | −4.67, <0.001 | -- | 1.83, 0.07 | ||||
| P7H2b | −13.47, <0.001 | 4.74, <0.001 | −6.32, <0.001 | −2.58, 0.01 | 1.83, 0.07 | -- | ||||
| UOA | −7.96, <0.001 | 14.02, <0.001 | 0.14, 0.89 | 5.05, <0.001 | 9.87, <0.001 | 7.44, <0.001 | ||||
| Hutcheson’s two-tailed t-test statistic, Bonferroni p-value < 0.005 | ||||||||||
| NAPF | KIS | KPO | PCO1 | PCO2 | ||||||
| KIS | -- | −2.82, 0.005 | −7.10, <0.001 | −6.17, <0.001 | ||||||
| KPO | −2.82, 0.005 | -- | −4.74, <0.001 | −3.68, <0.001 | ||||||
| PCO1 | −7.10, <0.001 | −4.74, <0.001 | -- | 1.16, 0.25 | ||||||
| PCO2 | −6.17, <0.001 | −3.68, <0.001 | 1.16, 0.25 | -- | ||||||
| WPA | −5.90, <0.001 | −3.47, 0.005 | 1.15, 0.15 | 0.04, 0.97 | ||||||
| Hutcheson’s Two-Tailed t-Test Statistic, Bonferroni p-Value < 0.008 | ||||
|---|---|---|---|---|
| Ecoregion | GUAY | HWS | HNS | NAPF |
| GUAY | -- | −18.34, <0.001 | 32.38, <0.001 | −6.74, <0.001 |
| HWS | −18.34, <0.001 | -- | 20.21, <0.001 | −22.94, <0.001 |
| HNS | 32.38, <0.001 | 20.21, <0.001 | -- | −4.83, <0.001 |
| NAPF | −6.74, <0.001 | −22.94, <0.001 | −4.83, <0.001 | -- |
| Ecoregion | HNS | HWS | GUAY | NAPF |
|---|---|---|---|---|
| HNS | -- | 0.65 | 0.09 | 0.02 |
| HWS | 0.65 | -- | 0.2 | 0.08 |
| GUAY | 0.09 | 0.2 | -- | 0.05 |
| NAPF | 0.02 | 0.08 | 0.05 | -- |
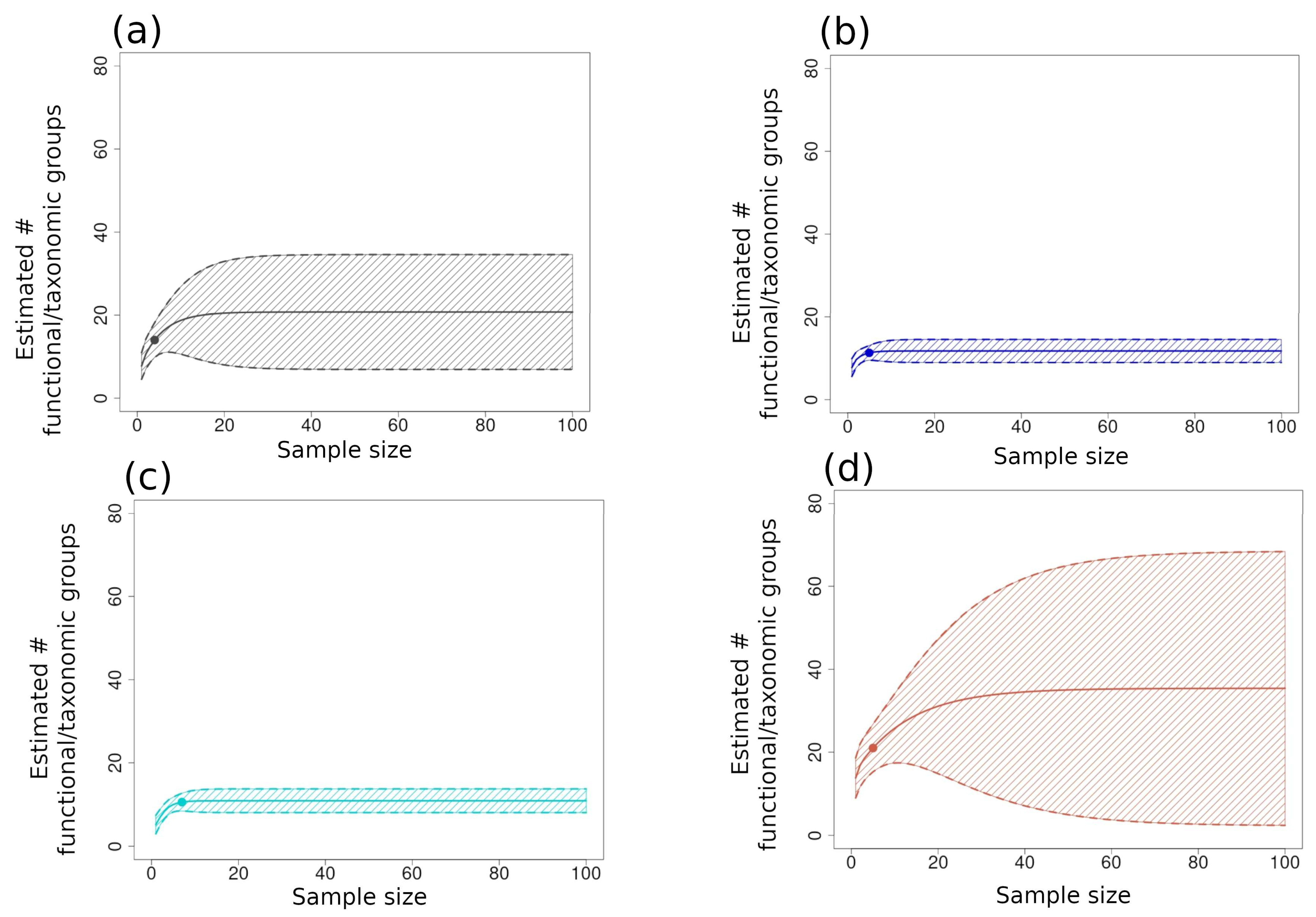
| Fx/Taxa Group Abbreviation | Description | r2 | Pr (>r) |
|---|---|---|---|
| fx. grp 2.5 (NAPF) | red filamentous algae | 0.41 | 0.007 |
| fx grp. 3.5 (NAPF) | corticated foliose algae | 0.43 | 0.008 |
| fx grp. 5 (NAPF) | leathery macro algae | 0.63 | 0.001 |
| Eumalocostraca (NAPF) | hermit crabs | 0.40 | 0.01 |
| Heterobranchia (GUAY) | limpet Siphonaria | 0.41 | 0.01 |
| Patellogastropoda (NAPF) | limpets | 0.57 | 0.001 |
| Theocostraca (NAPF) | barnacles | 0.60 | 0.001 |
| Zoantharia (NAPF) | small colonial actinids | 0.40 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilbur, L.; Küpper, F.C.; Louca, V. Analyzing Species Diversity in Rocky Intertidal Communities over Multiple Spatial Scales among Understudied Eastern Pacific Ecoregions. Diversity 2024, 16, 498. https://doi.org/10.3390/d16080498
Wilbur L, Küpper FC, Louca V. Analyzing Species Diversity in Rocky Intertidal Communities over Multiple Spatial Scales among Understudied Eastern Pacific Ecoregions. Diversity. 2024; 16(8):498. https://doi.org/10.3390/d16080498
Chicago/Turabian StyleWilbur, Lynn, Frithjof C. Küpper, and Vasilis Louca. 2024. "Analyzing Species Diversity in Rocky Intertidal Communities over Multiple Spatial Scales among Understudied Eastern Pacific Ecoregions" Diversity 16, no. 8: 498. https://doi.org/10.3390/d16080498
APA StyleWilbur, L., Küpper, F. C., & Louca, V. (2024). Analyzing Species Diversity in Rocky Intertidal Communities over Multiple Spatial Scales among Understudied Eastern Pacific Ecoregions. Diversity, 16(8), 498. https://doi.org/10.3390/d16080498






