Abstract
The Croatian Natural History Museum (CNHM) houses rich fossil collections from the Neogene deposits of Northern Croatia, comprising numerous scallops (Bivalvia: Pectinidae). During the Middle Miocene (Badenian = Langhian and early Serravallian), this region was located at the southwestern margin of the Central Paratethys. The value of the CNHM’s historical collections has been presented through taxonomic revisions and biostratigraphic, paleoenvironmental and paleogeographic study. Methods included the cross-checking of specimens from museum boxes with all available published data, systematic revision of scallops, recording the abundance of each taxon, defining the preservation state of the specimens, measuring the morphometric elements and taking photographs of each specimen. After the conducted revision of 624 specimens, the number of registered taxa in the collections was reduced from 52 to 33, and their stratigraphic distribution has been updated. The species Lissochlamys excisa (Bronn, 1831) has been recorded for the first time at the southwestern part of the Central Paratethys. The southernmost Badenian record of Delectopecten vitreus (Gmelin, 1791) known so far has been described. Six types of pectinid habitats have been distinguished, based on the provided paleontological and lithological data, also estimating the scallops’ abundance in each of them. Bioerosion and encrustation traces on scallops’ shells represent an additional contribution to paleoecological studies.
1. Introduction
Fossils housed at the museum collections represent a data set used for various research, e.g., taxonomic revisions, and biostratigraphic, paleobiogeographic and paleoecological analyses, giving the opportunity to “open the windows of the past”. The value of the museum collections has been described in many papers (e.g., [,,] and the references therein). Historical museum collections usually represent the first step of the study, serving also as comparative material. Fossil collections with incomplete archive data do not have biostratigraphic value. On the contrary, the collections comprising the detailed notes on locality, stratigraphy and systematics, when combined with the previously published data, provide valuable information for further research and observations.
In this paper, we present the significance of historical museum collections via the example of the Middle Miocene (Langhian and lower Serravallian/Badenian) scallops (Bivalvia: Pectinidae) housed at the Croatian Natural History Museum (CNHM). Specimens were collected by professional geologists from various localities in Northern Croatia. For a major part of the collections published data are available. Most of the specimens are kept in the collection of the academician Vanda Kochansky-Devidé [,]. This collection was donated to the CNHM by the University of Zagreb, Faculty of Science, in 2019. It is significant because it represents the first piece of biostratigraphic research conducted on the Miocene marine deposits of Medvednica Mt., near Zagreb [] (Figure 1). Other pectinids from this area are housed in the CNHM collection Marine Miocene II and were collected by D. Gorjanović-Kramberger during his field research. Those results remained unpublished, as noted in [] (p. 172). Nevertheless, part of the data from D. Gorjanović-Kramberger research is available in []. Kochansky [] (and the references therein) united and combined all the records of the Miocene marine macrofossil invertebrates of Medvednica Mt. known at that time. Scallops from other localities in Northern Croatia (Figure 1) were collected during the field research by Gj. Pilar in Glinsko Pokuplje [,], F. Šuklje in the Samobor area [] and J. Poljak. Collections also have a special historical value based on the handwritten labels containing information about no-longer-available outcrops, and which describe sediments in which some of the scallops were found.
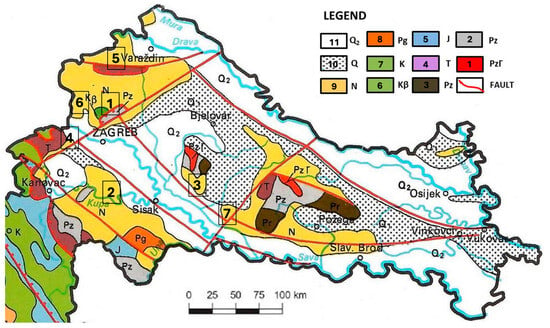
Figure 1.
Middle Miocene (Badenian) localities in Northern Croatia comprising scallops from the CNHM collections (geological map modified after [,]). Neogene deposits are marked with yellow color. Legend: 1. Precambrian metamorphic rocks; 2. Paleozoic granites; 3. Paleozoic sedimentary rocks; 4. Triassic carbonates, sporadically clastites; 5. Jurassic carbonates with scarce volcanoclastites; 6. Cretaceous basalts; 7. Cretaceous dominantly carbonate rocks; 8. Paleogene limestones; 9. Neogene clastic and carbonate rocks; 10. Pleistocene, dominantly unconsolidated clastites; 11. Holocene unconsolidated clastites. Localities: 1. Medvednica Mt. (collection a and e in Figure 2), 2. Glinsko Pokuplje and Zrinjsko-Dvorska kotlina valley area (collection b in Figure 2), 3. Moslavina (collection d in Figure 2), 4. Zaprešić Brijeg hill (collection c in Figure 2), 5. Ivanščica Mt. (collection f in Figure 2), 6. Marijagorička Brda hills (collection a in Figure 2), 7. Psunj Mt. (collection f in Figure 2).
A recent revision of the CNHM pectinids started with M. Bošnjak’s PhD research [], focusing on the Vanda Kochansky-Devidé collection. In this paper, we continue the taxonomic revision, including all the inventoried scallops from the CNHM, altogether 624 specimens from six collections (Figure 2, Supplement S1). Presented data are available in Supplements S1 and S2. We have revised all the available data on the museum specimens and conducted their taxonomic and taphonomic analysis. Furthermore, paleoenvironments which these scallops inhabited are defined, and an updated biostratigraphy of the revised species is provided.
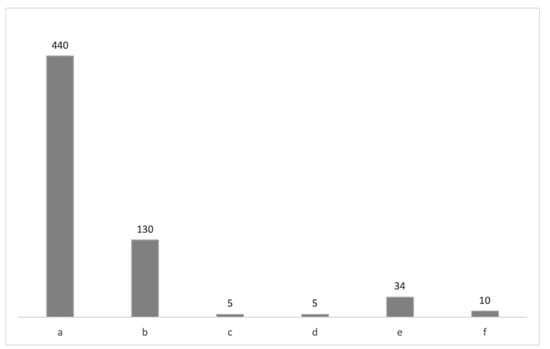
Figure 2.
The number of the analyzed Pectinidae specimens housed in the CNHM collections: (a) the Vanda Kochansky-Devidé collection [,]; (b) Pliocene and Miocene fauna of the Glinsko Pokuplje area and Zrinjsko-Dvorska kotlina valley [,]; (c) Miocene fauna of the Zaprešić Breg locality []; (d) Miocene malacological fauna of the Virovitica, Novska and Moslavina areas; (e) Marine Miocene II; (f) Miocene bivalves of Croatia and Slavonia. All data are presented in Supplements S1 and S2.
Scallops represent a large and diverse group of molluscs living mostly as vagile epifauna in the marine environments, except for some species which are byssally attached or cemented to the bottom. Species living free on the bottom are capable of swimming (e.g., [] (N348), []). Pectinids are common in the fossil record due to their calcite shell with high preservation potential. They are also important as a biostratigraphic tool, and a paleoecological and paleomigrational indicator of the possible open marine corridors. Since the second half of the 20th century, there have been several proposals for the European Miocene biozonation based on scallops (e.g., [,,,,]). Bohn-Havas et al. [] described pectinid assemblage zones in Hungary by proposing five zones and four subzones ranging from uppermost Egerian to upper Badenian. Two different types of fauna have been recognized: nearshore “larger pectinids” and “Chlamys” forms, and deeper water environment indicators with the genus “Amussium” (i.e., Lentipecten, Propeamussium, Palliolum, etc.). Studencka [] (and the references therein) analyzed Badenian pectinid assemblages from Poland, concluding that Badenian subdivision based on scallops is possible only within individual basins due to the isochronous appearance of pectinids throughout the Paratethys Sea. Mandic [] proposed pectinid bivalve zonation of the Central Paratethys, with 12 biozones from the lower Oligocene “Solenovian” crisis to the Mid-Miocene Sarmatian restriction event.
For the Miocene marine deposits of Croatia, scallops are recorded in various localities in Northern Croatia (Figure 1). Here, the aim of the presented systematic revision of the Middle Miocene (Langhian and lower Serravallian/Badenian) Pectinidae from the CNHM collections is to enhance the current knowledge on scallops’ distribution during the Middle Miocene in the Central Paratethys, to update the biostratigraphy of the Badenian deposits in Northern Croatia, and to further insight into the Middle Miocene paleoecology in the southwestern part of the Central Paratethys.
2. Geological Setting
During the Middle Miocene, the Northern Croatian Basin (NCB) paleogeographically belonged to the epicontinental Paratethys Sea. The NCB was part of the Central Paratethys, located at the southwestern part of the Pannonian Basin System (e.g., [,,,] and the references therein). The evolutionary history of the basin is complex and was controlled by tectonics, climate change, volcanic activity, eustatic sea-level changes and other factors, as well as the changing marine connections between the Central Paratethys and the neighboring marine realms which affected deposition and biota (e.g., [,,,,]).
Sediments comprising here presented scallops were deposited during the Badenian, corresponding to the Langhian and lower Serravallian of the standard Neogene time scale []. In papers dealing with the Middle Miocene of the Central Paratethys, various timings of the Badenian stage have been used (e.g., [,] and the references therein). There are disagreements regarding the lower boundary of the Middle Miocene, i.e., Badenian stage, and the subdivisions of the Badenian. According to, e.g., [], the Badenian stage has a three-fold subdivision, which is frequently used in papers referring to the North Croatian Basin (e.g., []). However, e.g., [,] and the references therein consider a two-fold subdivision of the Badenian stage.
The Badenian stage of the Central Paratethys is defined by three third-order sequences, the transgressive–regressive cycles TB 2.3, 2.4 and 2.5 (e.g., [,,,]). In the NCB, the timing of the oldest cycle, TB 2.3, corresponds to the lower Badenian hydrologically open lake (see [] and the references therein) or a corresponding lake system []. The following two cycles mark the fully marine depositional environment, TB 2.4 in the middle Badenian (upper Langhian), and TB 2.5 in the upper Badenian (lower Serravallian; see [] and the references therein). These cycles, marking the timing of the open marine gateways, controlled the migration and distribution of the marine biota in the ancient sea (e.g., [,] and the references therein). The Badenian transgression belonging to the transgressive–regressive cycle TB 2.4 was gradual and is recorded in the younger Badenian deposits in the southeastern parts of the Pannonian Basin System (e.g., []).
Scallops stored at the CNHM are collected from the Middle Miocene (Badenian) deposits shown in Figure 1. The geology of these localities is described in the published explanatory notes of several sheets of the Basic Geological Map of Croatia 1:100,000, including Medvednica Mt. [,], Glinsko Pokuplje and Zrinjsko-Dvorska kotlina valley [], the Zaprešić Brijeg hill [], Ivanščica Mt. [], the Marijagorička Brda hills [], and Psunj Mt. [].
3. Materials and Methods
A complete list of the Badenian Pectinidae from the CNHM is available in Supplement S1. Some outcrops are no longer available for sampling due to urbanization, e.g., the Zaprešić Brijeg hill and part of the research areas on Medvednica Mt. (Figure 1).
Every specimen from the CNHM collections is stored in a box with a museum label, each showing an inventory or temporary inventory number, species name, locality and stratigraphic age (Figure 3). A remark on the sediment type from which the specimen was found is also given for part of the collections, mostly for the specimens from the Vanda Kochansky-Devidé collection (Supplement S1), as noted in the Systematic Part.

Figure 3.
Examples of the museum labels from the CNHM collections showing an inventory or temporary inventory number, species name, locality and stratigraphic age (in Croatian). A remark on the sediment type from which the specimen was found is also given for part of the collections.
The study method included the following steps: (1) cross-checking the content of the museum box with the museum label notes, and afterwards with the museum inventory books and the description of the specimens in the available published papers ([,,,,,]); (2) recording all the data from the museum labels and the additional notes from the available published papers; (3) a systematic revision of specimens according to the literature (listed in the Systematic Part); (4) a cross-examination of the revised taxa with other published data, recorded localities and stratigraphic distributions; (5) recording and counting the abundance of each taxa; (6) recording the preservation state of the specimens; (7) counting completely preserved pectinid shells, specifically the articulated and fragmented ones; (8) measuring the morphometric elements of the specimens; (9) taking a photograph of each specimen at the CNHM using the Canon EOS 6D camera made by Canon, Japan, with the lenses Sigma 105 1:2.8 DG MACRO HSM and Canon 24–105 mm 1:3.5–5.6 IS STM.
The morphometric elements of the preserved scallops were measured using the electronic digital caliper. Measured elements encompassed the following dimensions (following [,,,]) (Figure 4):
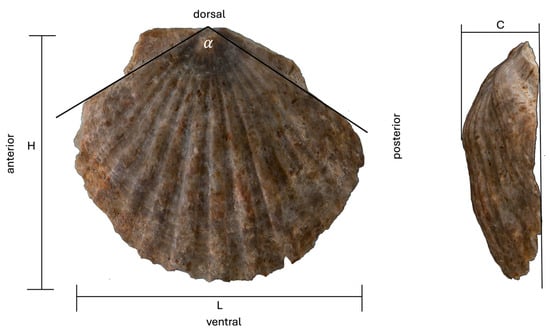
Figure 4.
Measured valve elements. Legend: L, valve length; H, valve height; α, umbonal angle; C, valve convexity.
- -
- (Maximum) valve length, L: the maximum dimension from the anterior to posterior margin of the valve;
- -
- (Maximum) valve height, H: perpendicular to the length; the maximum dimension from the dorsal to the ventral margin of the valve;
- -
- Umbonal angle, α: an angle with sides tangential to the anterior and posterior margin of the valve;
- -
- Valve convexity, C: the maximum inflation of the valve(s).
4. Systematic Part (following [,])
- Order Pectinida Gray, 1854
- Superfamily Pectinoidea Rafinesque, 1815
- Family Pectinidae Rafinesque, 1815
Pectinidae gen. et sp. indet.
- vp. 1944 Aequipecten scabrellus Lmk.—Kochansky, p. 239.
- Materials: one specimen from Medvednica Mt. found in marls (Figure 1, Supplement S1).
- Remarks: Due to the preservation state not possible to determine the specimen.
Pectinidae gen. et sp. indet., juv.
- vp. 1944 Chlamys sp.—Kochansky, p. 239.
- Materials: two juvenile specimens found in corrallinacean calcarenites on Medvednica Mt. (Figure 1, Supplement S1).
- Remarks: Due to the growth stage of the specimens, it is not possible to give a more detailed determination.
- Subfamily Pectininae Rafinesque, 1815
- Tribe Pectinini Rafinesque, 1815
Genus Gigantopecten Rovereto, 1899
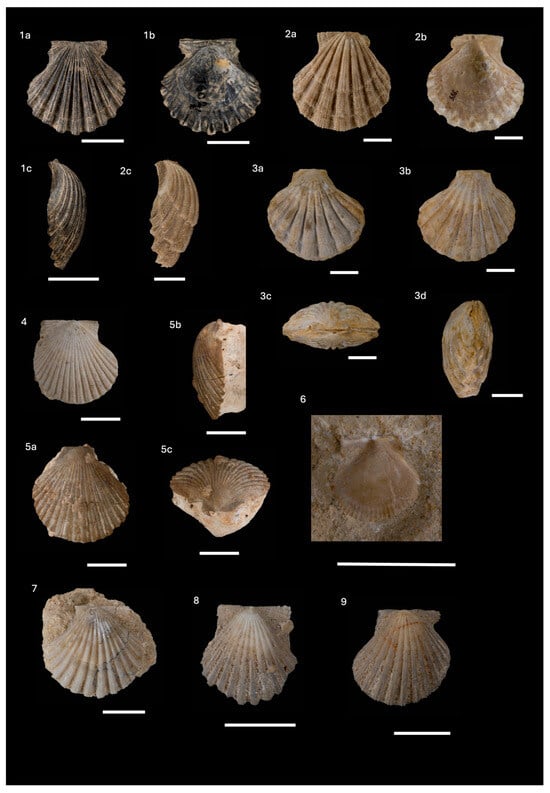
Gigantopecten nodosiformis (de Serres in Pusch, 1837)
| (Figure 5(1–3)) |
- *1837 Pecten nodosiformis M. de Serres—Pusch, p. 42, pl. 5, fig. 9a–c.
- 1867 Pecten latissimus Brocc.—Hörnes, p. 395, pl. 56, figs. 1–4, pl. 57, figs. 1–4.
- v1873 Pecten latissimus Brocc.—Pilar, p. 92.
- 1897 Macrochlamys latissima Br.—Sacco, p. 32, pl. 9, fig. 5, pl. 10, figs. 1–5.
- 1939 Chlamys latissima Brocchi—Roger, p. 37, pl. 18, fig. 1, pl. 19, fig. 1, pl. 28, fig. 1.
- v1944 Macrochlamys (Nodipecten) latissimus Brocc.—Kochansky, p. 240.
- v1944 Lyropecten melii Ugol.—Kochansky, p. 240.
- v1957 Chlamys latissima nodosiformis (Pusch)—Kochansky-Devidé, p. 43.
- 1960 Chlamys latissimi (Brocchi)—Csepreghy-Meznerics, p. 33, pl. 30, pl. 31.
- 1960 Chlamys latissima nodosiformis (de Serres)—Csepreghy-Meznerics, p. 33, pl. 26, figs.
- 1–5, pl. 27, figs. 1–2, pl. 28, figs. 1–2, pl. 29, figs. 1–2, pl. 32, figs. 1–2.
- 1977 Chlamys latissima nodosiformis (De Serres)—Nicorici, p. 132, pl. 13, pl. 14, fig. 1, pl. 15, figs. 1–4.
- 1978 Chlamys (Macrochlamys) latissima nodosiformis (De Serres, 1829)—Steininger et al., p. 345, pl. 10, figs. 2,3.
- 1985 Chlamys (Macrochlamys) latissimus nodosiformis (Serres in Pusch, 1837)—Atanacković, p. 38, pl. 5, fig. 3.
- 1988 Chlamys (Macrochlamis) Iatissima nodosiformis (de Serres in Pusch, 1837)—Studencka
- & Studencki, p. 26, text-pl. 4, pl. 5, fig. 1a–b.
- 2001 Gigantopecten nodosiformis (Pusch, 1837)—Schultz, p. 249, pl. 36, fig. 3, pl. 37, figs. 1, 2, pl. 38, fig. 2, pl. 39.
- 2004 Macrochlamis nodosiformis (de Serres in Pusch, 1837)—Mandic, p. 141, fig. 6.1,2.
- v2017 Gigantopecten nodosiformis (de Serres in Pusch, 1837)—Bošnjak, p. 91, pl. 4, fig. 4.
- 2019 Gigantopecten nodosiformis (Pusch, 1837)—Studencka, p. 11, textfigs. 2–4.
- Material: six shells, 11 valves and 25 valve fragments from Medvednica Mt. (part of them found in the “Litavac limestone” and Lithothamnion bioclastic limestone), Glinsko Pokuplje (one specimen found in the Lithothamnion bioclastic limestone), Ivanščica Mt., Psunj Mt. and Moslavina (Figure 1, Supplements S1 and S2).
- Remarks: Specimens are consistent with the descriptions referred to in the synonymy. This species is in the CNHM collections, represented under a few names (Supplement S1): Pecten latissimus, Lyropecten melii, Lyropecten nodosiformis, Chlamys latissima nodosiformis. Species G. nodosiformis is in the Badenian deposits of Northern Croatia, mostly recorded in the Lithothamnion limestone and the “Litavac limestone” (e.g., [,]).
- Distribution: Early Miocene (Karpatian)?, Middle Miocene (Badenian) of the Central Paratethys, common in the Badenian ([,] and the references therein). Present in the Mediterranean to the NE Atlantic; occurred in the Early Miocene (Burdigalian), and present there in the Middle and Late Miocene ([,] and the references therein).
Gigantopecten tournali (de Serres, 1829)
| (Figure 5(4,5)) |
- *1829 Pecten Tournali Nobis.—Serres, p. 263–264, pl. 6, fig. 1.
- 1867 Pecten tournali Serres—Hörnes, p. 398, pl. 58, figs. 1–6.
- v1944 Inaequipecten tournali Serr.—Kochansky, p. 242.
- 1939 Chlamys tournali De Serres—Roger, p. 19, pl. 2, fig. 4, pl. 3, fig. 1, pl. 4, fig. 1, pl. 8, fig.
- 1, pl. 9, fig. 1, pl. 10, figs.1–2.
- 1960 Chlamys tournali (de Serres)—Csepreghy-Meznerics, p. 32, pl.23, figs. 3–5, pl. 24, fig. 2, pl. 25, figs. 1–2.
- 1977 Chlamys tournali (De Serres)—Nicorici, p. 131, pl. 12, fig. 4.
- 1978 Chlamys (Macrochlamys) tournali—Steininger et al., p. 345, pl. 9, fig. 1.
- 2001 Gigantopecten tournali (de Serres, 1829)—Schultz, p. 254, pl. 41, figs. 1, 2.
- 2004 Macrochlamis tournali (de Serres, 1829)—Mandic, p. 142, fig. 6.3,4.
- v2017 Gigantopecten tournali (de Serres, 1829)—Bošnjak, p. 92, pl. 4, fig. 5.
- vp.2017 Flabellipecten sp.—Bošnjak, p. 89, pl. 4, fig. 2.
- Material: Two left and one right valve found in the “Litavac limestone” on Medvednica Mt. (Figure 1, Supplements S1 and S2).
- Remarks: CNHM specimens are consistent with the descriptions referred to in the synonymy. Specimen Inv. No. 6.458./1606. is in [] erroneously described as Flabellipecten sp.
- Distribution: “Late Ottnangian” (Karpatian following []) to Badenian of the Central Paratethys following [] (p. 142); in the Western Paratethys in the Upper Marine Molasse; in the Mediterranean and NE Atlantic from the Burdigalian to ? Tortonian [].
Genus Oopecten Sacco, 1897
Oopecten solarium (Lamarck, 1819)
| (Figure 6(1–7)) |
- *1819 Pecten solarium—Lamarck, p. 179, Nr. 1.
- 1867 Pecten besseri Andrz.—Hörnes, p. 404, pl. 62, figs. 1,2 (?: pl. 63, figs. 1−5).
- v1873 Pecten besseri Andrz.—Pilar, p. 93.
- v1873 Pecten rollei Hörnes—Pilar, p. 93.
- vp.1873 Pecten flabelliformis Defrance—Pilar, p. 93.
- vp.1944 Aequipecten opercularis L.—Kochansky, p. 239.
- v1944 Flabellipecten incrassatus Partsch—Kochansky, p. 242.
- v1944 Flabellipecten besseri Andrz.—Kochansky, p. 242.
- vp.1944 Flabellipecten besseri Andrzejowski– Kochansky, p. 242.
- 1957 Pecten pseudobeudanti Depéret & Roman—Kochansky-Devidé, p. 39.
- 1960 Flabellipecten solarium (Lamarck)—Csepreghy-Meznerics, p. 15, pl. 7, figs. 3–7, pl. 8, figs. 1–3.
- 1960 Chlamys (Oopecten) solarium (Lamarck, 1819)—Kojumdgieva, p. 67, pl. 23, fig. 1.
- 1977 Chlamys solarium (Lamarck)—Nicorici, p. 131, pl. 11, fig. 1, pl. 12, figs. 1–3.
- 1978 Pecten (Flabellipecten) solarium—Steininger et al., p. 346, pl. 10, figs. 3–7, pl. 11, fig. 1.
- 2001 Flabellipecten solarium (Lamarck, 1819)—Schultz, p. 236 (pars).
- 2003 Flabellipecten solarium (Lamarck, 1819)—Bongrain, p. 671, figs. 3, 4A–C.
- 2004 Oopecten solarium (Lamarck, 1819)—Mandic, p. 140, fig. 5.8.
- v2017 Oopecten solarium (Lamarck, 1819)—Bošnjak, p. 90, pl. 4, fig. 3.
- vp.2017 Aequipecten opercularis (Linnaeus, 1758)—Bošnjak, p. 81.
- vp.2017 Flabellipecten besseri (Andrzejowski, 1830)—Bošnjak, p. 88.
- vp.2017 Flabellipecten sp.—Bošnjak, p. 89.
- Material: four shells, 12 valves and 16 fragments from Glinsko Pokuplje (one specimen found in the “Litavac limestone”), Medvednica Mt. (from the “Litavac limestone” and corrallinacean calcarenites) and Moslavačka gora Mt. (Figure 1, Supplements S1 and S2).
- Remarks: Classification in this paper follows the opinion of [], where the author described characteristics of the species Oopecten solarium and discussed its classification in the literature. Some of the specimens are erroneously described in [] as Aequipecten opercularis (Temp. Inv. No. VKD-120-6-2), Flabellipecten besseri (Inv. No. 6.459./1607., Temp. Inv. No. VKD-130-3) and Flabellipecten sp. (Inv. No. 6.457./1605., Temp. Inv. Nos. VKD-131-2-2, VKD-131-4) (Supplement S1). The right valve of the specimen with Temp. Inv. No. VKD-667 is determined as Oopecten aff. solarium (Figure 6(5); Supplements S1 and S2) since it shows differences in the number of ribs, height and in ear morphology compared to the descriptions in the synonymy (e.g., []). This CNHM specimen was originally described as Pecten pseudobeudanti Depéret & Roman [] (p. 39), and in [] (p. 38–39) as Flabellipecten solarium (Lamarck). One complete (Inv. No. 1096.1; [] p. 93) and two incomplete (Temp. Inv. Nos. VKD-131-1-1 and VKD-131-3) valves (Figure 6(6,7); Supplements S1 and S2) are determined as Oopecten cf. solarium based on the visible diagnostic elements mostly corresponding to the species solarium. The latter two incomplete specimens were erroneously described as Flabellipecten besseri and Flabellipecten sp. in [].
- Distribution: The species is typical for the Badenian deposits of the Central Paratethys [,]. In the Mediterranean to E Atlantic region from Burdigalian to Messinian ([] and the references therein).
Genus Flabellipecten Sacco, 1897
Flabellipecten besseri (Andrzejowski, 1830)
| (Figure 6(8–10)) |
- *1830 Pecten Besseri Nobis—Andrzejowski, p. 103–104, Nr. 12, pl. 6, fig. 1.
- 1867 Pecten besseri Andrz.—Hörnes, p. 404, pl. 62, figs. 1,2 (?: pl. 63, figs. 1−5).
- vp.1873 Pecten flabelliformis Defrance—Pilar, p. 93.
- vp.1944 Flabellipecten besseri Andrz.—Kochansky, p. 242.
- 1960 Flabellipecten besseri (Andrzejowsky)—Csepreghy-Meznerics, p. 14, pl. 5, fig. 7, pl. 6, figs. 1,2.
- 1960 Pecten (Flabellipecten) besseri Andrzejowsky, 1830—Kojumdgieva, p. 66, pl. 22, figs. 3–5.
- 1977 Flabellipecten besseri (Andrzejowsky)—Nicorici, p. 128, pl. 4, figs. 1,2, pl. 5, figs. 1–4, pl. 6, fig. 1.
- 1978 Pecten (Flabellipecten) besseri Andrzejowsky, 1830—Steininger et al., p. 345, pl. 10, figs. 4,5; pl. 11, figs. 2,3.
- 1985 Pecten (Flabellipecten) besseri Andrzejowsky, 1830—Atanacković, p. 42, pl. 6, figs. 6–8.
- 2001 Flabellipecten besseri (Andrzejowski, 1830)—Schultz, p. 227, pl. 21, figs. 2, 3.
- vp.2017 Flabellipecten besseri (Andrzejowski, 1830)—Bošnjak, p. 88.
- Material: two valves and one fragment found in the “Litavac limestone” in Glinsko Pokuplje and Medvednica Mt. (Figure 1, Supplements S1 and S2).
- Remarks: Part of the specimens determined as Pecten besseri in the CNHM documentation are here revised as Oopecten solarium (see Supplement S1). Kochansky [] (p. 242) described the presence of Flabellipecten besseri in the same layers as Oopecten solarium (revised in this paper, originally Flabellipecten incrassatus), and noted a more abundant presence of the latter species in the Badenian deposits in the investigated area. One valve fragment (Inv. No. 5.947./1096.2; []) is determined as Flabellipecten cf. besseri (Figure 6(10); Supplement S1) based on the similarity of the ribs to the species besseri, but the dorsal part of the valve has not been preserved.
- Distribution: Middle Miocene, Badenian in the Central Paratethys [].
Genus Oppenheimopecten Teppner, 1922
Oppenheimopecten revolutus (Michelotti, 1847)
| (Figure 7(1–3)) |
- *1847 Pecten revolutus mihi—Michelotti, p. 87, Nr.4.
- 1897 Pecten revolutus Micht.—Sacco, p. 63, pl. 20, figs. 10–15.
- v1944 Pecten revolutus Micht.—Kochansky, p. 243, pl. 13, figs. 3,4.
- 1960 Pecten revolutus Michelotti—Csepreghy-Meznerics, p. 12, pl. 4, figs. 8,9, pl. 5, figs. 1–3.
- 1977 Pecten revolutus Michelotti—Nicorici, p. 126, pl. 3, figs. 1–4.
- 1978 Pecten (O.) revolutus Mich.—Steininger et al., p. 341, pl. 12, fig. 1.
- 2001 Oppenheimopecten revolutus (Michelotti, 1847)—Schultz, p. 261, pl. 40, fig. 2a + b.
- v2017 Pecten revolutus Michelotti, 1847—Bošnjak, p. 94, pl. 5, fig. 1.
- Material: one shell, 16 valves and two fragments from Medvednica Mt. (part of them found in the “Litavac limestone” and corrallinacean calcarenites) and Moslavina (Figure 1, Supplements S1 and S2).
- Remarks: Specimens described in detail in [] and consistent with the description referred to in the synonymy.
- Distribution: Karpatian and Badenian in the Central Paratethys [,,].
Oppenheimopecten aduncus (Eichwald, 1830)
| (Figure 7(4,5)) |
- *1830 Pecten aduncus, m.—Eichwald, p. 213, Fussnote 96.
- v1944 Pecten cf. benedictus Lmk.—Kochansky, p. 243.
- 1960 Pecten aduncus Eichwald—Csepreghy-Meznerics, p. 9, pl. 1, figs. 1–7, pl. 2, figs. 1–3.
- 1960 Pecten (Pecten) aduncus Eichwald, 1853—Kojumdgieva, p. 65, pl. 22, figs. 1, 2.
- 1977 Pecten aduncus Eichwald—Nicorici, p. 125, pl. 1, figs. 1–5, pl. 2, figs. 1–3.
- 1978 Pecten (Oppenheimopecten) aduncus—Steininger et al., p. 346, pl. 12, figs. 2–5.
- 1985 Pecten (Pecten) aduncus Eichwald, 1830—Atanacković, p. 42, pl. VI, figs. 3,4,5.
- 2001 Oppenheimopecten aduncus (Eichwald, 1830)—Schultz, p. 257, pl. 43, figs. 1a + b, 2a + b.
- 2013 Pecten aduncus Eichwald, 1830—Harzhauser et al., p. 368, pl. 3, figs. 1–2.
- v2017 Pecten aduncus Eichwald, 1830—Bošnjak, p. 94, pl. 4, fig. 6.
- v2017 Pecten cf. benedictus Lamarck, 1819—Bošnjak, p. 96, pl. 5, fig. 3.
- Material: one complete shell and one left valve from Medvednica Mt. (valve found in the “Litavac limestone”) (Figure 1, Supplements S1 and S2).
- Remarks: Description of CNHM specimens as referred to in synonymy. Specimen under Temp. Inv. No. VKD-134 (Figure 7(5)) was erroneously described as Pecten cf. benedictus Lamarck in [,].
- Distribution: Badenian in the Paratethys, Middle to Upper Miocene of the Mediterranean and NE Atlantic [].
Genus Pecten O. F. Müller, 1776
Pecten subarcuatus styriacus Hilber, 1879
| (Figure 8(1–3)) |
- *1879 Pecten Styriacus Hilb.—Hilber, p. 455, 456, 464, pl. 6, figs. 13–15.
- vp.1873 Pecten flabelliformis Defrance—Pilar, p. 93.
- vp.1944 Aequipecten opercularis L.—Kochansky, p. 239.
- v1944 Pecten fuchsi Font.—Kochansky, p. 243.
- 2001 Pecten subarcuatus styriacus Hilber, 1879—Schultz, p. 271, pl. 42, figs. 2, 3.
- 2004 Pecten subarcuatus styriacus Hilber, 1879—Mandic, p. 142, fig. 7.
- vp.2017 Aequipecten opercularis (Linnaeus, 1758)—Bošnjak, p. 81.
- v2017 ? Pecten fuchsi Tournouer, 1874—Bošnjak, p. 95, pl. 5, fig. 2.
- Material: four valves and seven valve fragments from Glinsko Pokuplje and Medvednica Mt. (found in the “Litavac limestone” and marls) (Figure 1, Supplements S1 and S2).
- Remarks: The classification of the specimens follows the opinion of []. Specimens under the Temp. Inv. Nos. VKD-133 and VKD-120-2 (Figure 8(1–3), Supplement S1) erroneously described as presumably Pecten fuchsi and Aequipecten opercularis in [] following the opinion of [].
- Distribution: According to [] and the references therein, Pecten subarcuatus styriacus inhabited the Central Paratethys from the late Eggenburgian to late Badenian.
Genus Costellamussiopecten Bongrain, Cahuzac & Freneix, 1994
Costellamussiopecten cristatus badense (Fontannes, 1882)
| (Figure 8(4–6)) |
- *1882 Pleuronectia Badensis—Fontannes, p. 199.
- p.1867 Pecten cristatus Bronn.—Hörnes, p. 419, pl. 66, fig. 1.
- v1873 Pecten (Pleuronectes) cristatus Bronn—Pilar, p. 93.
- 1902 Amussium cristatum Bronn.—Depéret & Roman, p. 171, pl. 26, figs. 1–4, pl. 27, fig. 1.
- 1902 Amussium cristatum mutation badense Fontannes—Depéret & Roman, p. 174, pl. 25, figs. 1–6.
- 1929. Pecten sp.?—Šuklje, p. 6.
- v1944 Amussium cristatum Bronn.—Kochansky, p. 241.
- v1957 Amussium cristatum badense (Fontannes)—Kochansky-Devidé, p. 41.
- 1960 Amussium cristatum badense Fontannes—Csepreghy-Meznerics, p. 18, pl. 34, figs. 7–11.
- 1963 Amussium (Amussium) cristatum badense Fontannes—Atanacković, p. 62, pl. 6, fig. 6, 6a.
- 1977 Amussium cristatum badense (Fontannes)—Nicorici, p. 130, pl. 9, figs. 1–5, pl. 10, figs. 1–4.
- 1978 Amussium cristatum badense (Fontannes, 1879)—Steininger et al., p. 342, pl. 6, figs. 1–4.
- 1985 Amussium (Amussium) cristatum badensis (Fontanes, 1880)—Atanacković, p. 36, pl. 2, figs. 13,14.
- 2001 Amussium cristatum badense (Fontannes, 1882)—Schultz, p. 157, pl. 15, fig. 13.
- 2004 Costellamussiopecten cristatus badense (Fontannes, 1882)—Mandic, p. 140, figs. 5.5–7.
- v2017 Costellamussiopecten cristatus badense (Fontannes, 1882)—Bošnjak, p. 83, pl. 3, fig. 2.
- Material: 18 valves and 11 valve fragments from Glinsko Pokuplje, Zaprešić Brijeg hill and Medvednica Mt. (majority of them found in clayey marls with sand intercalations, and one specimen in marls) (Figure 1, Supplements S1 and S2).
- Remarks: Classification with Costellamussiopecten follows [], given that Cristatopecten Bongrain & Cahuzac, 2004 [] is its junior subjective synonym. One specimen (Inv. No. 6.224./1372.; []) originally described as Pecten sp. is here determined as Costellamussiopecten cf. cristatus badense (Fontannes, 1882) due to its similarity of exterior valve characteristics with this subspecies (Figure 8(6)).
- Distribution: The Karpatian to Badenian of the Central Paratethys, and the Burdigalian to Messinian of the Mediterranean to NE Atlantic (from [], p. 140).
- Tribe Aequipectinini von Teppner, 1922
Genus Aequipecten Fischer, 1886
Aequipecten elegans (Andrzejowski, 1830)
| (Figure 9(1–3)) |
- *1830 Pecten elegans—Andrzejowski, p. 102–103, pl. 5, fig. 5a,b.
- 1867 Pecten elegans Andrz.—Hörnes, p. 416, pl. 64, fig. 6.
- vp.1873 Pecten scabrellus Lamarck—Pilar, p. 93.
- vp.1929 Pecten scabrellus Lamarck—Šuklje, p. 6.
- 1939 Chlamys elegans Andrzejowski—Roger, p. 111, pl. 14, figs. 4–6.
- v1944 Pecten (Chlamys) elegans Andrz.—Kochansky, p. 239.
- vp.1944 Aequipecten scabrellus Lmk.—Kochansky, p. 239.
- 1960 Chlamys elegans (Andrzejowsky)—Csepreghy-Meznerics, p. 19, pl. 11, figs. 9−16, pl. 12, fig. 1.
- 1977 Chlamys elegans elegans Andrzejowsky—Nicorici, p. 137, pl. 24, figs. 1–3, pl. 25, figs. 1–6, pl. 26, figs. 1–5, pl. 27, figs. 1–6.
- 1978 Chlamys (Aequipecten) elegans (Andrzejowsky, 1830)—Steininger et al., p. 343, pl. 7, figs. 4–7.
- 1985 Chlamys (Aequipecten) elegans (Andrzejowsky, 1830)—Atanacković, p. 39, pl. 4, figs. 1−3.
- 2001 Aequipecten elegans (Andrzejowski, 1830)—Schultz, p. 188, pl. 17, fig. 13.
- vp.2017 Aequipecten scabrella (Lamarck, 1819)—Bošnjak, p. 83.
- v2017 Aequipecten elegans (Andrzejowski, 1830)—Bošnjak, p. 80, pl. 2, fig. 4.
- Material: shells, valves and one fragment from Glinsko Pokuplje, Zaprešić Brijeg hill and Medvednica Mt. (part of them found in the “Litavac limestone”, Lithothamnion bioclastic limestone and marls) (Figure 1, Supplements S1 and S2).
- Description: rounded valves with 9 to 10 prominent radial ribs; secondary riblets (3–4) can be seen at ribs as well as in ribs’ interspaces; on the exterior surface prominent commarginal lines present; auricles ornamented with ribs.
- Remarks: The description of the CNHM specimens is consistent with the descriptions referred to in the synonymy. Aequipecten elegans resembles A. scabrellus, with the difference in the smaller number of ribs and wider interspaces in A. elegans (e.g., [,]).
- Distribution: In the Central Paratethys, the species is recorded in the Badenian deposits [,].
Aequipecten macrotis (Sowerby in Smith, 1847)
| (Figure 9(4,5)) |
- *1847 Pecten macrotis—Sowerby in Smith, p. 420, pl. 17, fig. 15.
- vp.1929 Pecten scabrellus Lamarck—Šuklje, p. 6.
- v1944 Chlamys sp.—Kochansky, p. 239.
- v1944 Aequipecten opercularis L.—Kochansky, p. 239.
- vp.1944 Aequipecten scabrellus Lmk.—Kochansky, p. 239.
- 2001 Aequipecten macrotis macrotis (Sowerby, 1847)—Schultz, p. 192, pl. 18, fig. 2a + b.
- 2004 Aequipecten macrotis (Sowerby in Smith, 1847)—Mandic, p. 138, fig. 5.1,2.
- vp.2017 Chlamys sp.—Bošnjak, p. 76.
- vp.2017 Aequipecten opercularis (Linneaus, 1758)—Bošnjak, p. 81.
- vp.2017 Aequipecten scabrella (Lamarck, 1819)—Bošnjak, p. 83.
- v2017 Aequipecten sp.—Bošnjak, p. 84.
- 2020 Aequipecten macrotis (Sowerby in Smith, 1847)—Mandic et al., p. 68, fig. 6I.
- Material: valves and fragments from Zaprešić Brijeg hill, Moslavina and Medvednica Mt. (part of them found in the “Litavac limestone”, corrallinacean calcarenites and marls; all specimens of Aequipecten cf. macrotis found in the “Litavac limestone”) (Figure 1, Supplements S1 and S2).
- Remarks: This species has 18–24 primary ribs. The CNHM specimens’ descriptions are consistent with the descriptions referred to in the synonymy. Fragments under the Temp. Inv. No. VKD-122 (Supplement S1) are determined as Aequipecten cf. macrotis (Sowerby in Smith, 1847).
- Distribution: Eggenburgian to Badenian of the Central Paratethys; Miocene of the Mediterranean and NE Atlantic [,,].
Aequipecten malvinae (Du Bois de Montpéreux, 1831)
| (Figure 9(6)) |
- *1831 Pecten malvinae—Du Bois de Montpéreux, p. 71, 72, pl. 8, figs. 2,3.
- vp.1944 Aequipecten opercularis L.—Kochansky, p. 239.
- vp.1944 Chlamys sp.—Kochansky, p. 238.
- 1978 Chlamys (Aequipecten) malvinae (Dubois, 1851)—Steininger et al., p. 342, pl. 7, figs. 2,3.
- 1986 Chlamys (Aequipecten) malvinae (du Bois de Montpéreux, 1831)—Studencka, p. 35, pl. 4, figs. 4,7,10
- 2001 Aequipecten malvinae (Dubois de Montpéreux, 1831)—Schultz, p. 197, pl. 18, figs. 4,5
- 2004 Aequipecten malvinae (Dubois, 1831)—Mandic, p. 138, fig. 5.3,4.
- vp. 2017 Aequipecten opercularis (Linneaus, 1758)—Bošnjak, p. 81.
- vp.2017 Chlamys sp.—Bošnjak, p. 74.
- Material: fragments and one valve from Medvednica Mt. found in the “Litavac limestone” and corrallinacean calcarenites; a specimen of Aequipecten cf. malvinae found in clayey marls with sand intercalations (Figure 1, Supplements S1 and S2).
- Remarks: This species has 27–30 ribs, with 2–3 lateral ones []. Descriptions of the CNHM specimens are consistent with the descriptions referred to in the synonymy. One specimen (Temp. Inv. No. VKD-117-1-1; Figure 9(6), Supplement S1) is determined as Aequipecten cf. malvinae (Du Bois de Montpéreux, 1831).
- Distribution: Badenian of the Central Paratethys; Tarkhanian and Konkian of the Eastern Paratethys; Burdigalian to Tortonian of the Mediterranean and NE Atlantic (from []).
Aequipecten scabrellus (Lamarck, 1819)
| (Figure 9(7–9)) |
- *1819 Pecten scabrellus—Lamarck, p. 183.
- vp.1873 Pecten scabrellus Lamarck—Pilar, p. 93.
- vp.1944 Chlamys sp.—Kochansky, p. 239.
- vp.1944 Aequipecten scabrellus Lmk.—Kochansky, p. 239.
- 1960 Chlamys scabrella (Lamarck)—Csepreghy-Meznerics, p. 20, pl. 12, figs. 2−20.
- 1985 Chlamys (Aequipecten) seniensis (Lamarck, 1819)—Atanacković, p. 39, pl. 3, figs. 6,7.
- 1986 Chlamys (Aequipecten) scabrella (Lamarck, 1819)—Studencka, p. 38, pl. 3, fig. 9, pl. 4, figs. 1−4, 6.
- 2001 Aequipecten scabrellus (Lamarck, 1819)—Schultz, p. 202, pl. 18, figs. 6, 8−10.
- vp.2017 Chlamys sp.—Bošnjak, p. 76.
- vp.2017 Aequipecten scabrella (Lamarck, 1819)—Bošnjak, p. 83.
- Material: valves and fragments from Glinsko Pokuplje and Medvednica Mt. (part of them found in the “Litavac limestone”, corrallinacean calcarenites and marls) (Figure 1, Supplements S1 and S2).
- Remarks: This species has 15 to 21 ribs, mostly 18 to 19 []. Descriptions of the CNHM specimens are consistent with the descriptions referred to in the synonymy.
- Distribution: Early and Middle Miocene of the Central Paratethys; Tarkhanian of the Eastern Paratethys; Lower Miocene of the Western Paratethys; Miocene and Pliocene of the Mediterranean and NE Atlantic [].
Aequipecten sp. indet.
- vp.1929 Pecten scabrellus Lamarck—Šuklje, p. 6.
- vp.1944 Aequipecten opercularis L.—Kochansky, p. 239.
- vp.2017 Aequipecten opercularis (Linneaus, 1758)—Bošnjak, p. 81.
- Materials: fragments from Medvednica Mt. (found in the “Litavac limestone” and clayey marls with sand intercalations) and the Zaprešić Brijeg hill (Figure 1, Supplements S1 and S2).
- Remarks: Preserved valve fragments with rib morphology indicate the genus Aequipecten, due to the preservation state lacking the other diagnostic elements determined on the genus level.
- Tribe indet.
Pectininae gen. et sp. indet.
| (Figure 10(1,2)) |
- Material: two valves from the Zaprešić Brijeg hill and Medvednica Mt. (Figure 1, Supplements S1 and S2).
- Description: rounded valves, pectinoid shape; slightly convex; one specimen (Inv. No. 6.223./1371.a; Figure 10(1)) on the anterior and posterior side fragmented with both auricles preserved, and the other one (Inv. No. 6.592./1738.; Figure 10(2)) anteriorly fragmented with one preserved auricle; exterior of the valve partially covered by radial ribs.
- Remarks: Specimen Inv. No. 6.592./1738. (Figure 10(2)) was originally described in the CNHM documentation as Pecten laythajanus Partsch, and in [] (p. 89) as Flabellipecten laythajanus. Due to the incomplete preservation state, specimens are here determined on the subfamily level.
- Subfamily Camptonectinae Habe, 1977
Genus Delectopecten R. B. Stewart, 1930
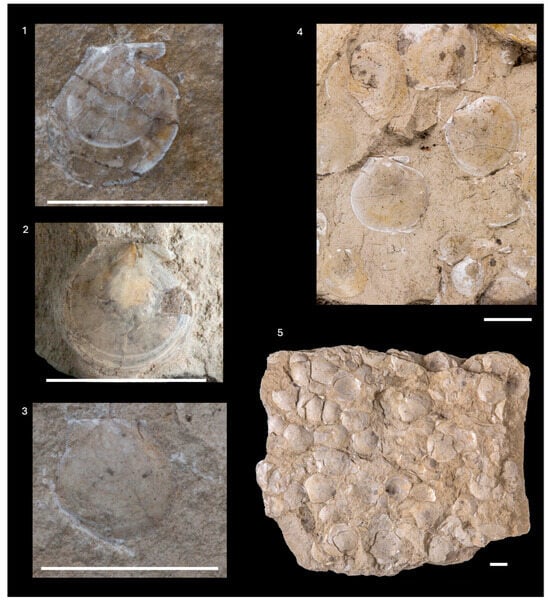
Delectopecten vitreus (Gmelin, 1791)
| (Figure 11) |
- *1791 Ostrea vitrea—Gmelin, 3328.
- v1944 Pseudamussium (Lissochlamys) sp.—Kochansky, p. 241.
- v1957 Chlamys auensis zollikoferi (Bittner)—Kochansky-Devidé, p. 41–43, pl. 1, figs. 1–3, pl. 2, figs. 1–7.
- 1978 Palliolum (Palliolum) zoellikoferi (Bittner, 1884)—Steininger et al., p. 342, pl. 6, figs. 5–8.
- 1986 Palliolum (Delectopecten) vitreum (Gmelin, 1791)—Studencka, p. 34–35.
- 2001 Palliolum (Palliolum) auense auense (Kittl, 1882)—Schultz, p. 166, pl. 15, fig. 5.
- 2015 Delectopecten vitreus (Gmelin, 1791)—Studencka, p. 311, text-fig. 3.
- v2017 Palliolum (Palliolum) auensis zollikoferi—Bošnjak, p. 77.
- Material: Specimens preserved as mold and imprint accumulations on marly plates. They are common in the Badenian clayey marls with sand intercalations on Medvednica Mt. and in the platy calcareous marls/limestones with high calcareous content from the Marijagorička Brda hills. Originally described as Valbersdorf [i.e., Walbersdorf] facies [,] (Figure 1, Supplements S1 and S2).
- Dimensions: Part of the specimens were first measured by []. New measurements are shown in Supplement S2. Maximum height—20.6 mm, minimum height—5.8 mm; maximum length—22 mm, minimum length—5.8 mm; maximum umbonal angle—134°, minimum umbonal angle—82°.
- Description: Valves subcircular to oval, exterior smooth, only few specimens have commarginal lines. Auricles are small and prominent. Ears on left valve not separated from the valve, ending perpendicularly. Anterior auricle of right valve has well-developed byssus notch and is pointed off valve. Clearly visible anterior ear ornamentation bears radial riblets. Hinge line straight. Specimens are described in detail in [,].
- Remarks: The characteristics of the revised specimens correspond to the species Delectopecten vitreus described in, e.g., [,,,]. Kochansky-Devidé [] revised previously collected specimens described in [], as well as new collected specimens. She determined the subspecies Chlamys auensis zoelikoferi based on valve shape, dimensions (length/height ratio value of around 1) and umbonal angle values (more than 110°). New measurements provided here (Supplement S2) show variability in dimensions, with the criteria of [] not strong enough to determine the specimens as a subspecies. Diagenetic processes have also affected the shape of the shell. The shape and external ornamentation variability of Delectopecten vitreus is described in, e.g., [,] (after []). Bošnjak [] fulfills the discussion on these specimens, mentioning the distribution of the Badenian horizon with “Chlamys auensis zollikoferi” in Northern Croatia.
- Distribution: In the Paratethys recorded in the upper Oligocene of the North Alpine Foreland Basin [], and the Badenian deposits in Poland and Slovakia ([] and the references therein), and Croatia ([,]; this paper). In the Mediterranean and the North Sea Basin dated to the Pliocene ([,] and the references therein).
- Subfamily Palliolinae Korobkov in Eberzin, 1960
- Tribe Pseudentoliini Waller, 2006
Genus Korobkovia Glibert & van de Poel, 1965
Korobkovia denudata (Reuss, 1867)
| (Figure 12(1–6)) |
- *1867 Pecten denudatus Rss.—Reuss, p. 139, pl. 7, fig. 1.
- 1875 Pecten denudatus Reuss—Hörnes, p. 383, pl. 14, figs. 21–22.
- 1897 Pseudamussium corneum var. denudata Sacco—Sacco, p. 51, pl. 14, figs. 30–39.
- 1928 Amussium denudatum Reuss—Depéret & Roman, p. 187, textfig. 8, pl. 28, figs. 4, 4a, 5, 6, 6a, 7, 8.
- vp.1944 Amussium cristatum Bronn.—Kochansky, p. 241.
- v1944 Amussium corneum Sow. var. denudata Rss.—Kochansky, p. 242.
- v1957 Amussium denudatum (Reuss)—Kochansky-Devidé, p. 40, pl. 2, fig. 8.
- 1960 Amussium denudatum (Reuss)—Csepreghy-Meznerics, p. 18, pl. 34, figs. 13–14.
- 1960 Pseudamussium denudatum (Reuss, 1867)—Kojumdgieva, p. 85, pl. 1, figs. 12–13, pl. 2, figs. 1–2.
- 1985 Pseudamussium denudatum (Reuss, 1867) –Atanacković, p. 41, pl. 6, figs. 1–2.
- 2001 Lentipecten (Lentipecten) corneus denudatus (Reuss, 1867)—Schultz, p. 153, pl. 15, fig. 2.
- 2014 Lentipecten denudatus (Reuss 1867)—Mikuž & Gašparič, p. 157–158, pl. 1, fig. 3.
- 2015 Lentipecten corneus denudatus (Reuss, 1867)—Studencka, p. 103, fig. 4A-D.
- vp.2017 Costellamussiopecten cristatus badense (Fontannes, 1882)—Bošnjak, p. 85.
- v2017 Lentipecten denudatus (Reuss, 1867)—Bošnjak, p. 86, pl. 3, fig. 3.
- v2017 Lentipecten (Lentipecten) corneus denudatus (Reuss, 1867)—Bošnjak, p. 86, pl. 3, fig. 4.
- 2020 Korobkovia denudata (Reuss, 1867)—Mandic et al., p. 63–64, textfig. 5L.
- Material: twelve valve molds and imprints and five fragments from clayey marls with sand intercalations on Medvednica Mt. (Figure 1, Supplements S1 and S2).
- Remarks: Classification following the opinion of [], where the authors revised the genus Korobkovia based on the ornamentation of the inner side of the left valve and remarked on the different opinion of []. According to the shell characteristics described in [,], weak radial ribs in the internal part of the left valve can be seen in several CNHM specimens. Specimens VKD-129 (Figure 12(1–6)) and VKD-130 were classified as Lentipecten (Lentipecten) corneus denudatus and Lentipecten denudatus in [], as well as specimen VKD-128-4-1, which was classified as Costellamussiopecten cristatus badense, are here revised as Korobkovia denudata.
- Distribution: Ottnangian to Badenian of the Central Paratethys, Early to Middle Miocene of the Eastern Paratethys and Early to Late Miocene of the Mediterranean ([] and the references therein).
- Tribe Palliolini Waller, 1991
Genus Lissochlamys Sacco, 1897
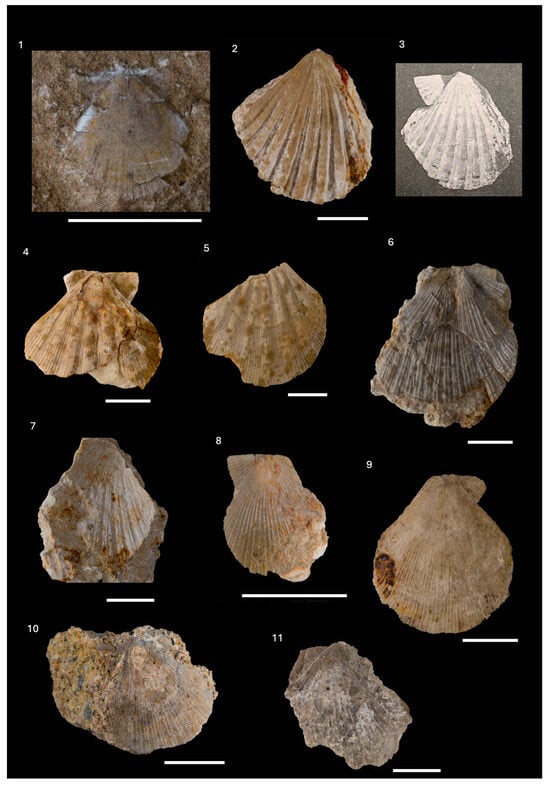
Lissochlamys excisa (Bronn, 1831)
| (Figure 12(7)) |
- *1831 Pecten excisus n.—Bronn, p. 117.
- v1873 Pecten multicostulatus, sp.—Pilar, p. 93.
- v1874 Pecten multicostulatus, n. sp.—Pilar, p. 215, pl. 1, fig. 1.
- 1897 Pecten perlaevis Almera et Bofill—Almera & Bofill, p. 406, 407, pl. 7, fig. 1.
- 1939 Chlamys excisa Bronn—Roger, p. 88, pl. 12, figs. 4–7, pl. 21, fig. 1a–c.
- 1979 Palliolum (Lissochlamys) excisum (Bronn, 1831)—de Porta, p. 371.
- 2001 ? Palliolum (Lissochlamis) excisum (Bronn, 1831)—Schultz, p. 169.
- Material: one left valve from Glinsko Pokuplje (Figure 1, Supplements S1 and S2).
- Description: orbicular left valve outline; convex; disc exterior with numerous vaguely visible thin radial ribs; ears of similar length.
- Remarks: The description of the specimen is consistent with the descriptions referred to in the synonymy. There are rare data on this species in the literature regarding the Neogene deposits of the Paratethys. A CNHM specimen was originally described by [,] as a new species named Pecten multicostatus and collected from the Middle Miocene marls in Banovina. Its only known Middle Miocene counterpart outside Central Paratethys, originally named as Pecten perlaevis, is present in the Langhian deposits of San Vicente in Catalonia (Tarragona, NE Spain) [,]. According to [] and the references therein, this species is mostly recorded in fine-grained sandstones of the Pliocene age.
- Distribution: Badenian of the Central Paratethys—Romania [] and Croatia (this paper), Middle Miocene to Pliocene of the Mediterranean Province ([,] and the references therein, this study); Pliocene of the Atlantic Province ([,] and the references therein).
- Subfamily Pedinae Bronn, 1862
Pedinae gen. et sp. indet.
| (Figure 13(1)) |
- vp. Chlamys sp.—Kochansky, p. 238.
- vp. Chlamys sp.—Bošnjak, p. 76.
- Material: one valve from clayey marls with sand intercalations on Medvednica Mt. (Figure 1, Supplement S1).
- Description: A shell of chlamydoid shape; in part of the ventral margin of the valve visible numerous radial ribs, which are not clear in the valve dorsal margin. Ears partially preserved, not clear.
- Remarks: Specimen in [,] is classified as Chlamys sp. Due to the incomplete preservation state of the specimen, classification is here kept on the subfamily level.
- Tribe Chlamydini von Teppner, 1922
Genus Manupecten Monterosato, 1889
Manupecten puymoriae (Mayer-Eymar, 1857)
| (Figure 13(2,3)) |
- *1857 Pecten puymoriae Mayer—Mayer-Eymar, p. 377–378.
- 1920 Pecten (Manupecten) puymoriae May.—Dollfus & Dautzenberg, p. 422, pl. 42, figs. 5–13.
- 1939 Chlamys puymoriae Mayer-Eymar—Roger, p. 181, pl. 26, figs. 3–6, 8–12.
- v1944 Pecten (Manupecten) puymoriae Mayer—Kochansky, p. 240, pl. 13, fig. 2.
- v2017 ? Manupecten var. fasciculatus (Millet, 1854)—Bošnjak, p. 79, pl. 2, fig. 2.
- Material: one right valve found in the “Litavac limestone” from Medvednica Mt. (Figure 1, Supplements S1 and S2).
- Remarks: Due to the slightly eroded shell or poor preservation, not all fine characteristics stated in [,] are visible. Yet, a fine shell surface network is visible in some parts of the exterior side of the valve. This species is highly variable (e.g., []). Kochansky [] gave a figure of the specimen housed in the CNHM collection (Table XIII, fig. 2; fig. 3c in []), but the same sample from the collection is missing the ear visible in the original paper (Figure 13(2,3)).
- Distribution: According to [], this species has been recorded from Burdigalian up to Langhian, and presumably in the Pliocene. It is distributed in the late Early Miocene deposits of France, Switzerland and Germany (after []).
Manupecten linguafelis (Almera & Bofill, 1897)
| (Figure 13(4,5)) |
- 1867 Pecten reussi Hörnes—Hörnes, p. 407, pl. 64, fig. 1. [non d’Orbigny, 1850]
- *1897 Pecten linguafelis Almera et Bofill.—Almera & Bofill, p. 403, pl. 4, fig. 11.
- 1897 Manupecten pesfelis L.—Sacco, p. 36, pl. 12, fig. 1.
- 1939 Chlamys fasciculata Millet—Roger, p. 180, pl. 26, figs. 7, 13–17, text-fig. 91.
- v1944 Flexopecten pes-felis L.—Kochansky, p. 240.
- 1960 Chlamys fasciculata (Millet)—Csepreghy-Meznerics, p. 33, pl. 32, figs. 3–7.
- 1977 Chlamys fasciculata (Millet)—Nicorici, p. 145, pl. 35, fig. 2a–d, text-fig. 6.
- 1985 Chlamys (Manupecten) fasciculata (Millet)—Atanacković, p. 41, pl. 5, fig. 2.
- 2001 Manupecten fasciculatus (Millet, 1854)—Schultz, p. 219, pl. 19, fig. 11.
- v2017 Manupecten pesfelis (Linnaeus, 1758)—Bošnjak, p. 79, pl. 2, fig. 2.
- 2020 Manupecten linguafelis (Amlera & Bofill, 1897)—Mandic et al., p. 65, fig. 6A.
- Material: two valves found in corrallinacean calcarenites on Medvednica Mt. (Figure 1); one partially preserved with lower half of valve (Supplements S1 and S2).
- Remarks: Sacco [] noted the variability of the species. Roger [] differentiated the very similar species Manupecten pesfelis and M. fasciculata by the smaller number of ribs, shell outline and other characteristics. Mandic et al. [] gave remarks on the validity of the name Manupecten linguafelis.
- Distribution: Ottnangian to Badenian of the Central Paratethys; Middle to Late Miocene of the Mediterranean and NE Atlantic (after [] and the references therein).
Genus Talochlamys Iredale, 1929
Talochlamys costai (Fontannes, 1884)
| (Figure 13(6,7)) |
- *1884 Pecten costai—Fontannes, p. 20–21, pl. 8, figs. 9,10.
- 1897 Chlamys gloriamaris Dub.—Sacco, p. 5, pl. 1, figs. 8–11.
- 1939 Chlamys costai (Font.)—Roger, p. 160, pl. 22, fig. 8; pl. 23, figs. 9–10.
- v1944 Chlamys aff. gloriamaris Dub.—Kochansky, p. 238.
- vp.1944 Chlamys tauroperstriata Sacco—Kochansky, p. 237.
- 1960 Chlamys costai (Fontannes)—Csepreghy-Meznerics, p. 25, pl. 16, fig. 3.
- 1977 Chlamys jakloweciana Kittl—Nicorici, p. 143–144, pl. 31, fig. 2a-c, pl. 42, fig. 2a-e.
- 2001 Chlamys costai (Fontannes, 1884)—Schultz, p. 173, pl. 16, fig. 7 and 8.
- v2017 Chlamys aff. gloriamaris (Dubois, 1831)—Bošnjak, p. 74, pl. 1, fig. 1.
- vp. 2017 Talochlamys multistriata (Poli, 1795)—Bošnjak, p. 76.
- Material: one right valve found in the Lithothamnion bioclastic limestone from Medvednica Mt. (Figure 1, Supplements S1 and S2).
- Remarks: Description of the CNHM specimens consistent with the detailed description in []. Visible shagreen microsculpture, differing this species from Talochlamys multistriata, as noted by []. One fragmented specimen (Temp. Inv. No. VKD-114-2, Supplement S1) is determined as Talochlamys cf. costai (Fontannes, 1884) (Figure 13(7)). The same specimen was originally determined as Chlamys tauroperstriata in [], and later as Talochlamys multistriata in [].
- Distribution: Eggenburgian to Badenian of the Central Paratethys; Aquitanian to Pliocene in the Mediterranean and E Atlantic ([,], this study).
Talochlamys multistriata (Poli, 1795)
| (Figure 13(8–11)) |
- *1795 Ostrea multistriata—Poli, p. 164, pl. 28, fig. 14.
- vp.1873 Pecten scabrellus Lamarck—Pilar, p. 93.
- 1897 Chlamys multistriata (Poli)—Sacco, p. 6–7, pl. 1, figs. 12–14.
- 1939 Chlamys multistriata Poli—Roger, p. 165, pl. 22, figs. 5–7, 11, 15, pl. 23, fig. 5, pl. 24, figs. 8–9.
- v1944 Chlamys multistriata Poli—Kochansky, p. 237.
- vp.1944 Chlamys tauroperstriata Sacco—Kochansky, p. 237.
- vp.1944 Chlamys tauroperstriata Sacco var.—Kochansky, p. 238, pl. 13, fig. 1.
- v1944 Chlamys sp. aff. varia L.—Kochansky, p. 237.
- 1960 Chlamys multistriata (Poli)—Csepreghy-Meznerics, p. 23, pl. 14, figs. 22–25, pl. 15, fig. 1.
- 1977 Chlamys multistriata (Poli)—Nicorici, p. 144, pl. 36, figs. 1–4, pl. 37, figs. 1–7.
- 1985 Chlamys (Chlamys) multistriata (Poli, 1795)—Atanacković, p. 37, pl. 3, figs. 2,3.
- 1993 Crassadoma multistriata (Poli, 1795)—Waller, p. 212, Figs. 5a,d,g; 6c-j.
- 2001 Crassadoma ? multistriata s. l. (Poli, 1795)—Schultz, p. 176, pl. 16, figs. 6, 9–12, pl. 17, figs. 1–4 (cum syn.).
- 2004 Crassadoma multistriata (Poli, 1795)—Mandic, p. 138, fig. 4.8,9.
- v2017 Chlamys sp. aff. varia Linnaeus, 1758—Bošnjak, p. 75, pl. 1, fig. 2.
- vp.2017 Talochlamys multistriata (Poli, 1795)—Bošnjak, p. 76, pl. 1, figs. 5,6.
- 2020 Talochlamys multistriata (Poli, 1795)—Mandic et al., p. 68, fig. 6H.
- Material: valves and fragments from Glinsko Pokuplje and Medvednica Mt. (found in the Lithothamnion bioclastic limestone, and Talochlamys cf. multistriata in corrallinacean calcarenites) (Figure 1, Supplements S1 and S2).
- Remarks: The description of the CNHM specimens is consistent with the detailed description in [] and other descriptions referred to in the synonymy. The species has a variable morphology (e.g., [,]). Mandic [] described the resemblance of this species with the species Chlamys costai and Chlamys justiniana and the difference by the absence of the shagreen microsculpture. Two CNHM specimens (Temp. Inv. Nos. VKD-112 and VKD-114-1, Supplement S1; Figure 13(10,11)) are not very well preserved and are determined as Talochlamys cf. multistriata.
- Distribution: Eggenburgian to Badenian of the Central Paratethys; Sakaraulian to Konkian of the Eastern Paratethys; Burdigalian to Recent in the Mediterranean, E Atlantic and W Indian Ocean (from [] and the references therein).
Talochlamys cf. brussonii, juv. (de Serres, 1829)
- *1829 Hinnites brussonii—de Serres, p. 134, pl. 5, figs. 1,2.
- 1867 Hinnites defrancei Michelotti—Hörnes, p. 423, pl. 67, fig. 3 [excl. 1–2, 4 = H. crispus]
- 1897 Hinnites brussoni v. taurinensis Sacco—Sacco, p. 11, pl. 2, figs. 6–17.
- 1910 Hinnites brussonii De Serr. var. taurinensis—Schaffer, p. 32, pl. 15, fig. 6.
- 1939 Chlamys brussoni De Serres—Roger, p. 175, pl. 23, figs. 7–8, pl. 24, figs. 1–3.
- vp.1944 Hinnites brussoni De Serr. var. taurinensis Sacco—Kochansky, p. 244.
- 1960 Chlamys brussoni taurocostata (Sacco)—Csepreghy-Meznerics, p. 36, pl. 34, fig. 1.
- 1977 Chlamys brussoni defrancei Michelotti—Nicorici, p. 152, pl. 42, fig. 1a-d, pl. 43, fig. 1a–c.
- 1977 Chlamys brussoni leufroyi De Serres—Nicorici, p. 151, pl. 43, figs. 1–3.
- 2001 Hinnites brussoni taurinensis Sacco, 1897—Schultz, p. 222, pl. 20, fig. 1.
- 2004 Hinnites crispus (Brocchi, 1814)—Mandic, p. 136, fig. 4.6,7.
- vp.2017 Hinnites brussoni De Serr. var. taurinensis Sacco, 1897—Bošnjak, p. 78, pl. 2, fig. 1.
- 2020 Talochlamys brussonii (Serres, 1829)—Mandic et al., p. 67, fig. 6B-G.
- Material: juvenile specimens found in tuff from Medvednica Mt. (Figure 1, Supplement S1).
- Remarks: The description of the CNHM specimens is consistent with the detailed description in [] and with determinations referred to in the synonymy. The species Talochlamys brussonii is similar to Hinnites crispus, from which it differs by the shagreen microsculpture of the shell which is absent in H. crispus (e.g., [,,] and the references therein). Here, a shagreen microsculpture has not been detected on the presented specimens, possibly due to the preservation state.
Talochlamys sp. indet.
- vp.1944 Hinnites brussoni De Serr. var. taurinensis Sacco—Kochansky, p. 244.
- vp.2017 Hinnites brussoni De Serr. var. taurinensis Sacco, 1897—Bošnjak, p. 78, pl. 2, fig. 1.
- Material: five specimens found in corrallinacean calcarenites from Medvednica Mt. (Figure 1, Supplement S1).
- Remarks: Specimens are in the juvenile stage and are therefore determined on the genus level.
5. Results
5.1. Taxonomy
The systematic revision of the Middle Miocene (Badenian) scallops from the museum’s collections encompassed 624 specimens. The number of present taxa, after the revision, is reduced from 52 (in the original collections) to 33 (Table 1, Supplement S1).

Table 1.
Revised list of the Pectinidae from the CNHM collections ordered according to the Systematic Part.
The revised list (Table 1) also presents the original, now outdated, determinations from the collections’ documentation. Some species names in the museum documentation had an erroneous (e.g., Pecten flabelliformis) or incomplete authorship (e.g., Pecten malvinae). However, the list is here revised and corrected (Table 1). Various names were available for one species, particularly for the taxa Gigantopecten nodosiformis, Oopecten solarium, Talochlamys multistriata, etc. (Table 1), which are now consolidated. The most tangled were the specimens from the genus Aequipecten, originally archived as the specimens from the genera Pecten, Chlamys and Aequipecten. After the examination, they were grouped into four species, with part of the specimens determined in open nomenclature, as described in the Systematic Part.
5.2. Taphonomy
The preservation state of pectinid specimens is shown in Figure 14. The majority of the specimens are preserved as a single valve. Well-preserved specimens enabled the study of diagnostic elements for the purpose of their revision. The accumulations of Delectopecten vitreus (Gmelin, 1791) are partly counted as a “valve” preservation state (Figure 14), since their valve dimensions could be measured (Supplement S2).
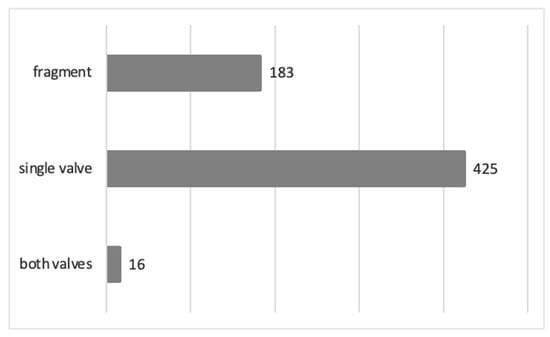
Figure 14.
The preservation state of the revised specimens (Supplement S1).
On some scallops’ valves, bioerosion and encrusters have been detected, as shown in Figure 15.

Figure 15.
Bioerosion and encrustations on scallops from the CNHM collection. Bioerosion: (1–3); encrustations: (4–6). (1)—drill-holes on (a) Gigantopecten nodosiformis (de Serres in Pusch, 1837) (Inv. No. 6.621./1767.) and (b) Pecten subarcuatus styriacus Hilber, 1879 (Temp. Inv. No. VKD-120-2); (2)—Entobia traces on Gigantopecten nodosiformis (de Serres in Pusch, 1837) (Inv. No. 6.621./1767.); (3)—polychaetes traces on Gigantopecten nodosiformis (de Serres in Pusch, 1837) (Inv. No. 6.627./1773.); (4)—spirorbid polychaetes on Gigantopecten nodosiformis (de Serres in Pusch, 1837) (Inv. No. 6.618./1764.); (5)—red algae on Gigantopecten nodosiformis (de Serres in Pusch, 1837) (Inv. No. 6.618./1764.); (6)—bryozoans on Gigantopecten nodosiformis (de Serres in Pusch, 1837) (Inv. No. 6.621./1767.). Scale bar: 1 cm.
Bioerosions consist of drill-holes (Figure 15(1)) possibly made by predator gastropods or octopuses, which are visible on the valves of Gigantopecten nodosiformis (Figure 5(2) and Figure 15(1a)), Pecten subarcuatus styriacus (Figure 8(1) and Figure 15(1b)), Oopecten solarium (Figure 6(4b)) and Flabellipecten besseri (Figure 6(8)). Also, Entobia borings of clionid sponges can be seen on a G. nodosiformis valve (Figure 5(2) and Figure 15(2)), as well as traces of polychaetes (Figure 5(3) and Figure 15(3)).
Encrusters are represented by spirorbid polychaetes detected on the valve of Gigantopecten nodosiformis (Figure 5(1) and Figure 15(4)), red algae on the shell of G. nodosiformis (Figure 5(2) and Figure 15(5)) and mostly by bryzoans, as seen on valves of G. nodosiformis (Figure 5(1) and Figure 15(6)), Gigantopecten tournali (Figure 5(5)) and Oppenheimopecten aduncus (Figure 6(4b)).
Detailed analysis of the taphonomy of scallops is planned for future research.
5.3. Lithology
The original notes stored with the CNHM specimens gave us a clue about the sediments in which some of the CNHM scallops were found. This mostly refers to the Vanda Kochansky-Devidé collection. Table 2 shows the abundance of revised scallops according to lithological records from the museum documentation. For part of the specimens there are no data on the sediment from which scallops were collected.

Table 2.
Scallops’ abundance according to the partly recorded lithology (Supplement S1, Systematic Part). Taxon order according to the Systematic Part. Legend: (1) Pectinidae gen. et sp. indet., (2) Gigantopecten nodosiformis, (3) Gigantopecten tournali, (4) Oopecten solarium, (5) Oopecten aff. solarium, (6) Oopecten cf. solarium, (7) Flabellipecten besseri, (8) Oppenheimopecten revolutus, (9) Oppenheimopecten aduncus, (10) Pecten subarcuatus styriacus, (11) Costellamussiopecten cristatus badense, (12) Aequipecten elegans, (13) Aequipecten macrotis, (14) Aequipecten cf. macrotis, (15) Aequipecten malvinae, (16) Aequipecten cf. malvinae, (17) Aequipecten scabrellus, (18) Aequipecten sp. indet., (19) Delectopecten vitreus, (20) Korobkovia denudata, (21) Pedinae gen. et sp. indet., (22) Manupecten puymoriae, (23) Manupecten linguafelis, (24) Talochlamys costai, (25) Talochlamys cf. costai, (26) Talochlamys multistriata, (27) Talochlamys cf. multistriata, (28) Talochlamys cf. brussonii, juv., (29) Talochlamys sp. indet.
6. Discussion
The revision of the CNHM Pectinidae (Supplement S1, Table 1) provided new insights into the paleogeographic distribution of certain species (described in the Systematic Part) during the Badenian (=Langhian and early Serravallian).
6.1. Biostratigraphy and Paleogeography
The significance of scallops as a biostratigraphic tool is evident in their range, as seen in Figure 16. All revised specimens correspond to the Middle Miocene—Badenian/Langhian and lower Serravallian , which is in accordance with previous records from Northern Croatia. There are seven species occurring only during the Badenian in the Central Paratethys (Figure 16): Gigantopecten nodosiformis, Oopecten solarium, Flabellipecten besseri, Oppenheimopecten aduncus, Aequipecten elegans, Aequipecten malvinae, and Lissochlamys excisa. Among them, Flabellipecten besseri and Aequipecten elegans are known only from the deposits of the Central Paratethys. Two species from the revised list, also known only from the Central Paratethys, are Oppenheimopecten revolutus and Pecten subarcuatus styriacus. Their stratigraphic range also encompasses the Early Miocene (Figure 16).
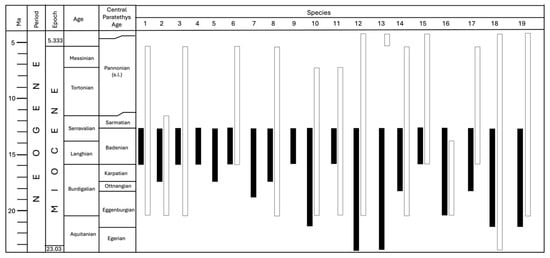
Figure 16.
The stratigraphic range of the revised Middle Miocene scallops in the Paratethys (black columns) and the Mediterranean and northeastern Atlantic (white columns) as described in the Systematic Part. The stratigraphy follows []. Species legend: 1. Gigantopecten nodosiformis (de Serres in Pusch, 1837), 2. Gigantopecten tournali (de Serres, 1829), 3. Oopecten solarium (Lamarck, 1819), 4. Flabellipecten besseri (Andrzejowski, 1830), 5. Oppenheimopecten revolutus (Michelotti, 1847), 6. Oppenheimopecten aduncus (Eichwald, 1830), 7. Pecten subarcuatus styriacus Hilber, 1879, 8. Costellamussiopecten cristatus badense (Fontannes, 1882), 9. Aequipecten elegans (Andrzejowski, 1830), 10. Aequipecten macrotis (Sowerby in Smith, 1847), 11. Aequipecten malvinae (Du Bois de Montpéreux, 1831), 12. Aequipecten scabrellus (Lamarck, 1819), 13. Delectopecten vitreus (Gmelin, 1791), 14. Korobkovia denudata (Reuss, 1867), 15. Lissochlamys excisa (Bronn, 1831), 16. Manupecten puymoriae (Mayer-Eymar, 1857), 17. Manupecten linguafelis (Almera & Bofill, 1897), 18. Talochlamys costai (Fontannes, 1884), 19. Talochlamys multistriata (Poli, 1795).
One newly determined specimen of Lissochlamys excisa (Bronn, 1831) (Figure 12(7)) represents the first record of this species in the Badenian deposits of the southwestern margin of the Central Paratethys, so far known in this area only from Romania [].
The revised species Delectopecten vitreus (Figure 11), an indicator of deeper marine environments, marks its southernmost distribution in the Central Paratethys known so far, further than it was previously reported (e.g., [] and the references therein).
Deposits with Delectopecten vitreus (Figure 11) and Korobkovia denudata (Figure 12(1–6)), another indicator of deeper marine environments, are mostly distributed in the central part of Medvednica Mt. (Figure 1), where the Badenian sediments were deposited in a deep trough forming an over 500 m-thick succession []. These deposits comprise the planktic and specialized chemosymbiotic benthic fauna (e.g., [,,,]).
A horizon with Delectopecten vitreus (formerly “Chlamys auensis zollikoferi”) is present in Croatia only sporadically, and the determination of this species has been a matter of debate for a long time (e.g., [,]). A high abundance of this scallop is recorded in the Badenian deposits of Medvednica Mt. and the Marijagorička Brda hills near Zagreb [,] (Figure 1). Its presence was also noted in other areas of Northern Croatia (e.g., [,]). The species Delectopecten vitreus today lives from the sublittoral to abyssal depths [,], and has a long planktotrophic development enabling its distant dispersal []. This ontogenic characteristic of Delectopecten vitreus should be considered in the study of possible open marine corridors, since its records correspond to the Badenian transgressive cycles in Northern Croatia (e.g., []).
6.2. Depositional Environment
Comparing the original notes stored with the specimens with the newly published papers, we can distinguish several types of environments, associated with the specific scallop communities (Figure 16, Figure 17 and Figure 18, Table 2, Supplement S1):
- The marginal marine scallop-bearing environment is defined by the sandy dolomitic breccia, i.e., “Litavac limestone”. It marks the marine transgression, mostly on the dolomitic base-rock. Partly or completely dissolved clasts leave the cavities in the rock. This is one of the widely distributed Badenian deposit types in Northern Croatia, with the most diversified pectinid fauna composed of the taxa Gigantopecten nodosiformis, Gigantopecten tournali, Oopecten solarium, Flabellipecten besseri, Oppenheimopecten revolutus, Pecten subarcuatus styriacus, Manupecten puymoriae, Aequipecten elegans, A. macrotis, A. scabrellus and A. malvinae (Figure 16 and Figure 17, Table 2, Supplement S1). In the CNHM collections, we have recorded 111 specimens from the “Litavac limestone”, marking it as one of the most fossiliferous deposits (Figure 17).
- “Litavac limestone” is often synonymized with the Lithothamnion bioclastic limestone (e.g., []). However, Lithothamnion limestone is made predominantly of the coralline algae and often of bryzoans, usually representing the packstone to grainstone type of the carbonate rock. Therefore, “Litavac limestone” represents the marginal marine environment (deposited in the desiccation vadose zone), while Lithothamnion bioclastic limestone was deposited slightly deeper on the shelf, usually not deeper than 60 m (e.g., []) (Figure 18). In some cases, it can be compared to the coralligenous constructions, i.e., the hard substrate formations of biogenic origin formed mainly by the deposition of the incrustated algae (e.g., []). A well-known example of the Lithothamnion bioclastic limestone is the Badenian “Leitha limestone” from the Vienna and Styrian Basins, which comprises even an richer pectinid assemblage than Croatian limestone and was widely distributed in the Central Paratethys (e.g., []). Scallops’ abundance in the Lithothamnion bioclastic limestone is low (17 specimens), and some of the taxa from the Lithothamnion limestone also occur in the “Litavac limestone” (Figure 17, Table 2, Supplement S1).
- Corrallinacean calcarenites are less firmly bound than bioclastic limestones. They comprise red algae detritus and other dominantly carbonate clasts and characterize environments with rapid deposition (Figure 18). These deposits also represent a common fossiliferous facies on Medvednica Mt. (Figure 17, Table 2, Supplement S1) comprising several species of scallops, i.e., Oppenheimopecten revolutus, Manupecten linguafelis, Oopecten solarium, and the genera Talochlamys and Aequipecten. However, their abundance is not so high (41 specimen; Figure 17, Table 2) compared to the “Litavac limestone” and deeper environments.
- Marls were in most cases deposited in the lagoons or sheltered bays (Figure 18). These deposits are less common in the studied area, which bear mainly Aequipecten and are represented by 29 specimens (Figure 17, Table 2, Supplement S1).
- Clayey marls with sand intercalations represent the facies with the most abundant scallops (193 specimens; Figure 17 and Figure 18, Table 2, Supplement S1). Kochansky [] compared them with the Walbersdorf facies, i.e., facies equivalent to the “schlier” of the Vienna Basin. This facies marks deeper marine realms, with various depths recorded on Medvednica Mt. (Figure 1) [,]. This is also seen by the recorded scallops, which mostly include Costellamussiopecten cristatus badense, Delectopecten vitreus, and Korobkovia denudata (Table 2, Supplement S1).
Part of the specimens did not have a remark on the sediment type (Figure 17). As today these outcrops are no longer available for research due to urbanization, or their location is not fully known, this lack of information represents one of the limiting factors from the study of the historical collections.
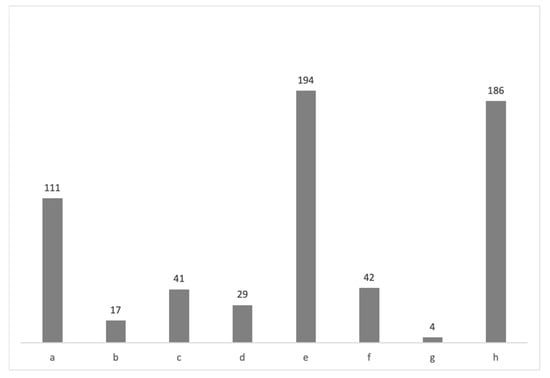
Figure 17.
The abundance of the CNHM scallops in the described environments (Figure 18, Table 2, Supplement S1). Legend: (a) “Litavac limestone”; (b) Lithothamnion bioclastic limestone; (c) Corrallinacean calcarenites; (d) marls; (e) clayey marls with sand intercalations; (f) platy calcareous marls/limestones with a high calcareous content; (g) other (tuff); (h) unknown (no data).
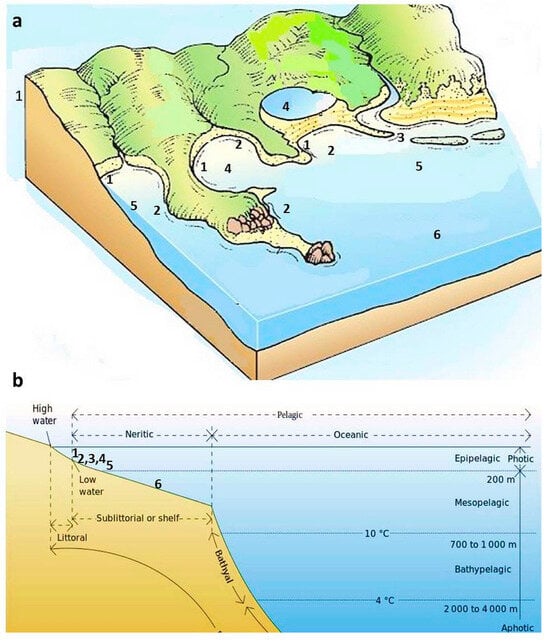
Figure 18.
Environments associated with the specific scallop communities based on the CNHM specimens (Supplement S1). (a) Distribution of the various scallop-bearing deposits in the coastal and deeper marine areas (modified following []); (b) a schematic profile of ocean zones with the probable position of various types of deposits comprising the pectinids (modified following []). Legend: 1—“Litavac limestone”; 2—Lithothamnion bioclastic limestone; 3—corrallinacean calcarenites; 4—marls; 5—clayey marls with sand intercalations; 6—platy calcareous marls/limestones.
6.3. Paleoecology
Large pectinid shells represent a firm base for the attachment of various encrusting organisms, mostly inhabiting live specimens (attached to the outer side of the shell). At some of the Middle Miocene localities comprising the inner shelf coralline algal deposits, encrustation occurs at one third of the studied large echinoids and bivalves (ostreids and pectinids, e.g., []). Among the most common encrusters on large bivalve shells, balanoid barnacles, bryozoans and spirorbid polychaetes occur (e.g., [,]). Bryozoan encrustations are visible on specimens of Gigantopecten nodosiformis, Gigantopecten tournali and Oppenheimopecten aduncus from the CNHM collection (Figure 15(6)). Pectinids from the coastal and inner shelf deposits (“Litavac limestone” and Lithothamnion bioclastic limestone) are also commonly encrusted with red algae (see: Gigantopecten nodosiformis in Figure 15(5)).
Mollusks living in marginal marine Miocene habitats were commonly exposed to mechanical and chemical bioerosion of boring sponges and marine predators (e.g., []). Predators are detected by the characteristics of the drill-hole, e.g., [,,,,]. Possible scallop predators here might have been octopuses, e.g., [,,,], or gastropods, e.g., [,,]. Such borings can be seen on some scallop shells of, e.g., Gigantopecten nodosiformis (Figure 15(1a)), Pecten subarcuatuas styriacus (Figure 15(1b)), Oopecten solarium and Flabellipecten besseri, although pectinids developed advanced strategies against the predators, including their swimming ability and the mechanical strength of the shell [].
Most of the bioerosion and encrustations on the CNHM specimens are seen on valves of Gigantopecten nodosiformis (Figure 15). Among the papers dealing with the Miocene molluscs’ taphonomy (e.g., [,,,,,]), data from the Central Paratethys are mostly based on bioerosion (e.g., [,,,,,,,]) and rarely on encrustations (e.g., []). This topic requires further study, and observations made here contribute to the understanding of the marine paleoecology from the southwestern margin of the Central Paratethys.
7. Conclusions
CNHM Pectinidae are very well preserved, with visible diagnostic characteristics enabling their further study and revision, and providing the updated list of the Middle Miocene (Badenian/Langhian and lower Serravallian) scallops from Northern Croatia.
The revision encompassed 624 specimens stored in six collections and collected from various localities of the Badenian (Langhian and lower Serravallian) age in Northern Croatia. The results, after the consolidation of various names representing the same species, showed a decreased number of scallop taxa (from 52 to 33) present in the collections. One of the most complicated cases were the specimens belonging to the genus Aequipecten because of their outdated miscellaneous determinations assigned to three genera.
An updated distribution of the typical Badenian scallop fauna is comparable with other contemporaneous localities. Here, the recorded species occurring only during the Badenian in the Central Paratethys are Gigantopecten nodosiformis, Oopecten solarium, Flabellipecten besseri, Oppenheimopecten aduncus, Aequipecten elegans, Aequipecten malvinae and Lissochlamys excisa. The species Flabellipecten besseri and Aequipecten elegans are known exclusively from the Badenian of the Central Paratethys.
The results of the presented revision indicated new distribution ranges of the species Lissochlamys excisa (Bronn, 1831) and Delectopecten vitreus (Gmelin, 1791), marking their southernmost occurrences in the Central Paratethys during the Badenian.
Comparing the original notes stored in the collection documentation with the newly published papers, we distinguished six types of depositional environments associated with the specific scallop communities and grouped them from the shallower to the deeper marine environments: (1) “Litavac limestone”; (2) Lithothamnion bioclastic limestone; (3) corrallinacean calcarenites; (4) marls; (5) clayey marls with sand intercalations and (6) platy calcareous marls/limestones with a high calcareous content. The scallops’ abundance in each environment is also noted. The most fossiliferous environments are clayey marls with sand intercalations and the “Litavac limestone”, while the Lithothamnion bioclastic limestone has the lowest scallop abundance. However, this grouping only refers to one part of the specimens, while the other part of the collections does not contain available notes on the sediment type.
The paleoecologic analysis was complemented by defining the traces of bioerosion and encrustations on scallops’ valves. Predation marks in the shape of drill-holes have been recorded on several valves, as well as encrustations made by clionid sponges, red algae and bryzoans. Most of these traces are recorded on valves of Gigantopecten nodosiformis from marginal marine environments.
The value and importance of historical collections as a paleontological database (“dark data”, e.g., []) are shown in all the information extracted from the specimens and the collections documentation here presented. Especially valuable are the collections with thoroughly described localities and a biostratigraphy of the deposits (e.g., [,]). Additionally, due to the changes in, e.g., stratigraphy and toponymy, all these collection notes help users to better understand previous papers and descriptions when revising them from today’s point of view.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d16080508/s1, Supplement S1: Middle Miocene scallops (Bivalvia: Pectinidae) from the CNHM collections; Supplement S2: measurements of the Middle Miocene scallops (Bivalvia: Pectinidae) from the CNHM collections. Measured specimens include completely preserved valves, and valves with sufficient diagnostic elements.
Author Contributions
Conceptualization, M.B., O.M. and J.S.; methodology, M.B., O.M. and J.S.; validation, O.M.; investigation, M.B., O.M. and J.S.; formal analysis, M.B. and O.M.; data curation, M.B. and O.M.; writing—original draft preparation, M.B.; writing—review and editing, M.B., O.M. and J.S.; visualization, M.B. and J.S.; supervision, O.M. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Authors are grateful to Nives Borčić, the senior museum technician of the Croatian Natural History Museum, for the photographs of the specimens. Authors would like to thank the reviewers and editors on their valuable comments and suggestions which improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| CNHM | Croatian Natural History Museum |
| Inv. No. | Inventory number |
| Temp. Inv. No. | temporary inventory number |
References
- Winston, J.E. Archives of a small planet: The significance of museum collections and museum- based research in invertebrate taxonomy. In Linnaeus Tercentenary: Progress in Invertebrate Taxonomy; Zhang, Z.-Q., Shear, W.A., Eds.; Zootaxa, Magnolia Press: Auckland, New Zealand, 2007; Volume 1668, pp. 47–54. [Google Scholar] [CrossRef]
- Allmon, W.D.; Dietl, G.P.; Hendricks, J.R.; Ross, R.M. Bridging the two fossil records: Paleontology’s “big data” future resides in museum collections. In Museums at the Forefront of the History and Philosophy of Geology: History Made, History in the Making: Geological Society of America Special Paper; Rosenberg, G.D., Clary, R.M., Eds.; The Geological Society of America: Boulder, CO, USA, 2018; Volume 535, pp. 1–10. [Google Scholar]
- Dominici, S.; Forli, M.; Bogi, C.; Guerrini, A.; Benvenuti, M. Paleobiology from museum collections: Comparing historical and novel data on Upper Miocene molluscs of the Livorno Hills. Riv. It. Paleontol. Strat. 2020, 126, 65–109. [Google Scholar]
- Kochansky, V. Miozäne marine Fauna des südlichen Abhanges der Medvednica, Zagreber Gebirge [Fauna marinskog miocena južnog pobočja Medvednice (Zagrebačke gore)]. Geol. Vjesn. Hrv. Drž Geol. Zav. Hrv. Drž. Geol. Muz. 1944, 2, 171–280, (In Croatian with German summary). [Google Scholar]
- Kochansky-Devidé, V. Ueber die Fauna des marinen Miozäns und über den Tortonischen „Schlier“ von Medvednica (Zagreber Gebirge) [O fauni marinskog miocena i o tortonskom „šliru“ Medvednice (Zagrebačke gore)]. Geol. Vjesn. 1957, 10, 39–50, (In Croatian with German summary). [Google Scholar]
- Gorjanović-Kramberger, D. Geologische Übersichtskarte des Königreiches Kroatien-Slavonien. Erläuterungen zur geolo-gischen Karte von Agram, Zone 22, Col XIV. Nakl. Kralj. Zemalj. Vlade Odjel Za Unut. Poslove Zagreb, 1908; 1–75, (In Croatian and German). [Google Scholar]
- Pilar, G. Trećegorje i podloga mu u Glinskom Pokuplju (Geognostička istraživanja od godine 1871.). Rad Jugosl. Akad. Znan. Umjet. 1873, 25, 53–179. (In Croatian) [Google Scholar]
- Pilar, G. Njekoje važnije okamine iz Pokupskoga trećogorja. (Dodatak razpravi naštampanoj u knjigi 25. “Radu”). Rad Jugosl. Akad. Znan. Umjet. 1874, 26, 214–216. [Google Scholar]
- Šuklje, F. Mediterranean fauna in Zaprešić Brijeg locality in the Samoborska Gora Mt. [Mediteranska fauna Zaprešić-brihega u Samoborskoj gori]. Vijesti Geološkog Zavoda 1929, 3, 1–52. [Google Scholar]
- Sremac, J.; Bošnjak, M.; Velić, J.; Malvić, T.; Bakrač, K. Nearshore Pelagic Influence at the SW Margin of the Paratethys Sea—Examples from the Miocene of Croatia. Geosicences 2022, 12, 120. [Google Scholar] [CrossRef]
- Velić, I.; Velić, J. Geological Map of Croatia. In Proceedings of the First Croatian Geological Congress, Opatija, Croatia, 18–21 October 1995. [Google Scholar]
- Bošnjak, M. Paleoekologija i Biostratigrafija Badenskih (Srednjomiocenskih) Naslaga Medvednice na Temelju Mekušaca i Pratećih Fosilnih Organizama [Paleoecology and Biostratigraphy of the Middle Miocene (Badenian) Deposits of the Medvednica Mt. Based on Mollusks and Associated Fossil Organisms]. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2017; pp. 1–214, (in Croatian with English abstract). [Google Scholar]
- Cox, L.R.; Newell, N.D.; Boyd, D.W.; Branson, C.C.; Casey, R.; Chavan, A.; Coogan, A.H.; Dechaseaux, C.; Fleming, C.A.; Haas, F.; et al. Part N, Mollusca 6, Bivalvia. In Treatise on Invertebrate Paleontology; Moore, R.C., Ed.; The Geological Society of America and The University of Kansas: Lawrence, Kansas, 1969; Volume 1, N1–N489+i-xxxviii. [Google Scholar]
- Carpenter, K.E.; de Angelis, N. The Living Marine Resources of the Eeastern Central Atlantic. Volume 2, Bivalves, Gastropods, Hagfishes, Sharks, Batoid Fishes, and Chimaeras; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; pp. 665–1509. [Google Scholar]
- Báldi, T. Proposal for biozonation of the European Oligocene and Miocene on the basis of molluscs. In Report on Activity of the R.C.M.N.S. Working Group; 1975; pp. 41–47. [Google Scholar]
- Demarcq, G. Essai de synthese biostratigraphique sur Pectinides du Neogene Mediterraneen. Proc. VIIth Intern. Congress on Mediterranean Neogene, Athens. Ann. Geol. Pays Helieniques Hors Ser. 1979, 1, 305–307. [Google Scholar]
- Bohn-Havas, M.; Báldi, T.; Kókay, J.; Halmai, J. Pectinid assemblage zones of the Miocene in Hungary. Ann. Inst. Geol. Publ. Hung. 1987, 70, 441–446. [Google Scholar]
- Demarcq, G. Pectinides Neogenes: Proposition déchelle biostratigraphique pour la Mediterranean. Geobios. Paleont. Stratigr. Paleoecolog. 1990, 23, 149–159. [Google Scholar] [CrossRef]
- Demarcq, G. Biostratigraphic scale of Mediterranean Neogene pectinids. Global events and Neogene evolution of the Mediterranean. In Proceedings of the IXth Congress R.C.M.N.S., Barcelona, Spain, 19–24 November 1990; pp. 123–124. [Google Scholar]
- Studencka, B. Remarks on Miocene bivalve zonation in the Polish part of the Carpathian Foredeep. Geol. Q. 1999, 43, 467–477. [Google Scholar]
- Mandic, O. Pectinid Bivalve Zonation of the Central Paratethys. Joannea Geol. Paläont. 2007, 9, 59–60. [Google Scholar]
- Rögl, F. Palaeogeographic Considerations for Mediterranean and Paratethys Seaways (Oligocene to Miocene). Ann. Des Nat. Mus Wien 1998, 99A, 279–310. [Google Scholar]
- Piller, W.E.; Harzhauser, M.; Mandic, O. Miocene Central Paratethys stratigraphy—Current status and future directions. Stratigraphy 2007, 4, 151–168. [Google Scholar] [CrossRef]
- Pavelić, D.; Kovačić, M. Sedimentology and stratigraphy of the Neogene rift-type North Croatian Basin (Pannonian Basin System, Croatia): A review. Mar. Pet. Geol. 2018, 91, 455–469. [Google Scholar] [CrossRef]
- Kováč, M.; Andreyeva-Grigorovich, A.; Bajraktarević, Z.; Brzobohatý, R.; Filipescu, S.; Fodor, L.; Harzhauser, M.; Nagymarosy, A.; Oszczypko, N.; Pavelić, D.; et al. Badenian evolution of the Central Paratethys Sea: Paleogeography, climate and eustatic sea-level changes. Geol. Carpathica 2007, 58, 579–606. [Google Scholar]
- Kováč, M.; Hudáčková, N.; Halásová, E.; Kováčová, M.; Holcová, K.; Oszczypko-Clowes, M.; Báldi, K.; Less, G.; Nagymarosy, A.; Ruman, A.; et al. The Central Paratethys palaeoceanography: A water circulation model based on microfossil proxies, climate, and changes of depositional environment. Acta Geol. Slovaca 2017, 9, 75–114. [Google Scholar]
- Raffi, I.; Wade, B.S.; Pällike, H.; Beu, A.G.; Cooper, R.; Crundwell, M.P.; Krijgsman, W.; Moore, T.; Raine, I.; Sardella, R.; et al. Chapter 29—The Neogene period. In Geologic Time Scale 2020; Gradstein, F.M., Oogg, J.G., Schmitz, M.D., Ogg, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1141–1215. [Google Scholar] [CrossRef]
- Kováč, M.; Halásová, E.; Hudáčková, N.; Holcová, K.; Hyžný, M.; Jamrich, M.; Ruman, A. Towards better correlation of the Central Paratethys regional time scale with the standard geological time scale of the Miocene Epoch. Geol. Carpathica 2018, 69, 283–300. [Google Scholar] [CrossRef]
- Hohenegger, J.; Ćorić, S.; Wagreich, M. Timing of the Middle Miocene Badenian stage of the Central Paratethys. Geol. Carpathica 2014, 65, 56–66. [Google Scholar] [CrossRef]
- Rögl, F.; Ćorić, S.; Hohenegger, J.; Pervesler, P.; Roetzel, R.; Scholger, R.; Spezzaferri, S.; Stingl, K. Cyclostratigraphy and Transgressions at the Early/Middle Miocene (Karpatian/Badenian) Boundary in the Austrian Neogene Basins (Central Paratethys). Scr. Fac. Sci. Natur. Univ. Masaryk. Brun. Geol. 2007, 36, 7–13. [Google Scholar]
- Mandic, O.; Hajek-Tadesse, V.; Bakrač, K.; Reichenbacher, B.; Grizelj, A.; Miknić, M. Multiproxy reconstruction of the middle Miocene Požega palaeolake in the Southern Pannonian Basin (NE Croatia) prior to the Badenian transgression of the Central Paratethys Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 516, 203–219. [Google Scholar] [CrossRef]
- Palcu, D.V.; Maris, I.; de Leeuw, A.; Melinte-Dobrinescu, M.; Anton, E.; Frunzescu, D.; Popov, S.; Stoica, M.; Jovane, L.; Krijgsman, W. The legacy of the Tethys Ocean: Anoxic seas, evaporitic basins, and megalakes in the Cenozoic of Central Europe. Earth-Sci. Rev. 2023, 246, 104594. [Google Scholar] [CrossRef]
- Mandic, O.; Sant, K.; Kallanxhi, M.E.; Ćorić, S.; Theobalt, D.; Grunert, P.; de Leeuw, A.; Krijgsman, W. Integrated bio-magnetostratigraphy of the Badenian reference section Ugljevik in southern Pannonian Basin—Implications for the Paratethys history (middle Miocene, Central Europe). Glob. Planet. Change 2019, 172, 374–395. [Google Scholar] [CrossRef]
- Šikić, K.; Basch, O.; Šimunić, A. Basic Geological Map of SFRY 1:100.000, Geology of the Zagreb Sheet, L33–80; Institute of Geology: Zagreb, Croatia; Federal Institute of Geology: Belgrade, Serbia, 1979; pp. 1–81. (In Croatian) [Google Scholar]
- Basch, O. Basic Geological Map of SFRY 1:100.000, Geology of the Ivanić-Grad Sheet, L33–81; Institute of Geology: Zagreb, Croatia, 1980; Federal Institute of Geology: Belgrade, Serbia, 1983; pp. 1–66. (In Croatian) [Google Scholar]
- Pikija, M. Basic Geological Map of SFRY 1:100.000, Geology of the Sisak Sheet, L33–93; Institute of Geology: Zagreb, Croatia, 1986; Federal Institute of Geology: Belgrade, Serbia, 1987; pp. 1–55. (In Croatian) [Google Scholar]
- Šimunić, A.; Pikija, M.; Hećimović, I.; Šimunić, A. Basic Geological Map of SFRY 1:100.000, Geology of the Varaždin Sheet, L33–69; Institute of Geology: Zagreb, Croatia, 1982; Federal Institute of Geology: Belgrade, Serbia, 1981; pp. 1–75. (In Croatian) [Google Scholar]
- Jamičić, D.; Vragović, M.; Matičec, D. Basic Geological Map of SFRY 1:100.000, Geology of the Daruvar Sheet, L33–95; Institute of Geology: Zagreb, Croatia, 1988; Federal Institute of Geology: Belgrade, Serbia, 1989; pp. 1–63. (In Croatian) [Google Scholar]
- Roger, J. Le genre Chlamys dans le Formation Néogènes de l’Europe. Mém. Soc. Geol. France Nouv. Ser. 1939, 40, 1–249. [Google Scholar]
- Mandic, O. Oligocene to Early Miocene Pectinid Bivalves of Western Tethys (N-Greece, S-Turkey, Central Iran and NE-Egypt)—Taxonomy and Paleobiogeography. Ph.D. Thesis, University of Vienna, Vienna, Austria, 2000. [Google Scholar]
- Mandic, O. Pectinid bivalves from the Grund Formation (Lower Badenian, Middle Miocene, Alpine-Carpathian Foredeep)—Taxonomic revision and stratigraphic significance. Geol. Carpathica 2004, 55, 129–146. [Google Scholar]
- Bieler, R.; Carter, J.G.; Coan, E.V. Classification of Bivalve Families, pp. 113–133. In Nomenclator of Bivalve Families; Bouchet, P., Rocroi, J.-P., Eds.; Malacologia: Haddonfield, NJ, USA, 2010; Volume 52, pp. 1–184. [Google Scholar]
- Waller, T.R. New Phylogenies of the Pectinidae (Mollusca: Bivalvia): Reconciling Morphological and Molecular Approaches. In Scallops: Biology, Ecology and Aquaculture; Shumway, S.E., Parson, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–44. [Google Scholar]
- Studencka, B. Note on Gigantopecten nodosiformis (Pusch, 1837), the logo of the 8th International Workshop on Neogene of Central and South-Eastern Europe. In Abstract Volume, Field Trip Guidebook, Proceedings of the 8th International Workshop Neogene of Central and South-Eastern Europe, Chęciny, Poland, 27–31 May 2019; Studencka, B., Ed.; University of Warsaw, Polish Academy of Sciences: Warsaw, Poland, 2019; pp. 11–16. [Google Scholar]
- Mandic, O. Bivalves of the Karpatian in the Central Paratethys. In The Karpatian—A Lower Miocene Stage of the Central Paratethys; Brzobohaty, R., Cicha, I., Kováč, M., Rögl, F., Eds.; Masaryk University Brno: Brno, Czech Republic, 2003; pp. 217–227. [Google Scholar]
- Bongrain, M. Révision de Flabellipecten solarium (Lamarck, 1819) (Mollusca, Bivalvia, Pectinidae) du Miocène moyen et supérieur de l’Ancien Monde. Geodiversitas 2003, 25, 657–679, (In French with English Abstract). [Google Scholar]
- Ćorić, S.; Pavelić, D.; Rögl, F.; Mandic, O.; Vrabac, S.; Avanić, R.; Jerković, L.; Vranjković, A. Revised Middle Miocene datum for initial marine flooding of North Croatian Basins (Pannonian Basin System, Central Paratethys). Geol. Croat. 2009, 62, 31–43. [Google Scholar] [CrossRef]
- Schultz, O. Bivalvia neogenica (Nuculacea-Unionacea). In Catalogus Fossilium Austriae; Piller, W.E., Ed.; Verlag der Österreichischen Akademie der Wissenschaften: Wien, Austria, 2001; Band 1/Teil 1; pp. 1–379. [Google Scholar]
- Steininger, F.; Švagrovský, J.; Muldini-Mamužić, S.; Kochansky, V. Pectinidae, pp. 340–347. In Chronostratigraphie und Neostratotypen, Miozän der Zentralen Paratethys. Volume 6, M4—Badenien (Moravien, Wielicien, Kosovien); Papp, A., Cicha, I., Seneš, J., Steininger, F.F., Eds.; Slowakische Akademie der Wissenschaften: Bratislava, Slovakia, 1978; pp. 1–594. [Google Scholar]
- Harzhauser, M.; Reuter, M.; Mandic, O.; Schneider, S.; Piller, W.E.; Brandano, M. „Pseudo-Sarmatian“ mollusc assemblages from the Early Messinian oolite shoals of Sicily (Italy). Riv. It. Paleontol. Strat. 2013, 119, 351–386. [Google Scholar]
- Bongrain, M.; Cahuzac, B. Cristatopecten nov. gen. benoisti (Pectinidae, Bivalvia) du Chattien d’Aquitaine (France). Révision systématique et réflexions sur l’origine et l’évolution des Cristatopecten. Geobios 2004, 37, 488–515. [Google Scholar]
- Kautsky, F. Die biostratigraphische Bedeutung der Pectiniden des NÖ Miozäns. Ann. Naturhist. Mus. Wien 1928, 42, 245–273. [Google Scholar]
- Csepreghy-Meznerics, I. Pectinides du Neogene de la Hongrie et leur importance Stratigraphique. Mém. Soc. Geol. France Nouv. Ser. 1960, 92, 1–58. [Google Scholar]
- Studencka, B.; Gontsharova, I.A.; Popov, S.V. The bivalve faunas as a basis for reconstruction of the Middle Miocene history of the Paratethys. Acta Geol. Pol. 1998, 48, 285–342. [Google Scholar]
- Mandic, O.; Schneider, S.; Harzhauser, M.; Danninger, W. Bivalves from the Innviertel Group of Allerding in the North Alpine Foreland Basin (lower Miocene, Upper Austria). N. Jb. Geol. Paläont. Abh. 2020, 297, 47–100. [Google Scholar] [CrossRef]
- Studencka, B. Bivalves from the Badenian (Middle Miocene) marine sandy facies of southern Poland. Palaeontol. Pol. 1986, 47, 1–128. [Google Scholar]
- Studencka, B. Middle Miocene bivalves from the Carpathian Foredeep basin: Specimens from the Busko (Mlyny) PIG-1 and Kazimierza Wielka (Donosy) PIG-1 boreholes—Stratigraphy and taxonomy. Biul. Pañstwowego Inst. Geol. 2015, 461, 305–324, (In Polish with English abstract and summary). [Google Scholar]
- Dijkstra, H.H.; Warén, A.; Gudmundsson, G. Pectinoidea (Mollusca: Bivalvia) from Iceland. Mar. Biol. Res. 2009, 5, 207–243. [Google Scholar] [CrossRef]
- Dijkstra, H.H.; Kilburn, R.N. The family Pectinidae in South Africa and Mozambique (Mollusca: Bivalvia: Pectinoidea). Afr. Invertebr. 2001, 42, 263–321. [Google Scholar]
- Dijkstra, H.H.; Goud, J. Pectinoidea (Bivalvia, Propeamussiidae & Pectinidae) collected during the Dutch CANCAP and MAURITANIA expeditions in the south-eastern region of the North Atlantic Ocean. Basteria 2002, 66, 31–82. [Google Scholar]
- Dijkstra, H.H.; Gofas, S. Pectinoidea (Bivalvia: Propeamussiidae and Pectinidae) from some norteastern Atlantic seamounts. Sarsia 2004, 89, 33–78. [Google Scholar] [CrossRef]
- Filek, T.; Hofmayer, F.; Feichtinger, I.; Berning, B.; Pollerspöck, J.; Zwicker, J.; Smrzka, D.; Peckmann, J.; Kranner, M.; Mandic, O.; et al. Environmental conditions during the late Oligocene transgression in the North Alpine Foreland Basin (Eferding Formation, Egerian)—A multidisciplinary approach. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 580, 110527. [Google Scholar] [CrossRef]
- De Porta, J. Distribución geográfica y cronoestratigráfica de Palliolum (Lissochlamys) excisum (Mollusca, Pectinidae) en España. Acta Geológica Hispánica Homenatge Lluís Solé Sabarís 1979, 14, 370–374. [Google Scholar]
- Dollfus, G.F.; Dautzenberg, P. Conchyologie du Miocène moyen du Bassin de la Loire. Premiére Partie: Pélécypodes (Suite et fin). Mém. Soc. Geol. France 1920, 27, 379–500. [Google Scholar]
- Schneider, S.; Berning, B.; Bitner, M.A.; Carriol, R.-P.; Jäger, M.; Kriwet, J.; Kroh, A.; Werner, W. A parautochthonous shallow marine fauna from the Late Burdigalian (early Ottnangian) of Gurlarn (Lower Bavaria, SE Germany): Macrofaunal inventory and paleoecology. Neues Jahrb. Geol. Paläontologie Abh. 2009, 254, 63–103. [Google Scholar] [CrossRef]
- Sacco, F. I Molluschi dei Terreni Terziarii del Piemonte e della Liguria. Parte XXIV (Pectinidae); Carlo Clausen: Torino, Italy, 1897; pp. 1–71. [Google Scholar]
- Kranjec, V.; Hernitz, Z.; Prelogović, E. Prilog poznavanju mlađih tercijarnih naslaga Medvednice (SZ Hrvatska) (Ein Beitrag zur Kenntnis jungerer Tertiärschichten des Medvednica-Gebirges (Nordwest-Kroatien)). Geol. Vjesn. 1973, 25, 65–99, (In Croatian with German summary). [Google Scholar]
- Bošnjak, M.; Sremac, J.; Vrsaljko, D.; Aščić, Š.; Bosak, L. Miocene “Pteropod event” in the SW part of the Central Paratethys (Medvednica Mt., northern Croatia). Geol. Carpathica 2017, 68, 329–349. [Google Scholar] [CrossRef]
- Crnko, J. Basic Geological Map of SFRY 1:100, Geology of the Varaždin Sheet Kutina L33-69; Croatian Geological Survey: Zagreb, Croatia, 1991. [Google Scholar]
- Basso, D.; Vrsaljko, D.; Grgasović, T. The coralline flora of a Miocene maërl: The Croatian “Litavac”. Geol. Croat. 2008, 61, 333–340. [Google Scholar] [CrossRef]
- Coletti, G.; Basso, D. Coralline algae as depth indicators in the Miocene carbonates of the Eratosthenes Seamount (ODP Leg 160, Hole 966F). Geobios 2020, 60, 29–46. [Google Scholar] [CrossRef]
- Cinelli, F.; Tunesi, L. The corraligenous domain. In Marine bioconstructions. Nature’s Architectural Seascapes; Relini, G., Ed.; Museo Friulano di Storia Naturale: Udine, Italy, 2011; pp. 13–28. [Google Scholar]
- Riegl, B.; Piller, W.E. Biostromal coral facies—A Miocene example from the Leitha Limestone (Austria) and its Actualistic Interpretation. Palaios 2000, 15, 399–413. [Google Scholar] [CrossRef]
- Coastal Depositional Landforms: Beaches, Dunes, Barrier Bars, Lagoons. Available online: https://pwonlyias.com/udaan/coastal-depositional-landforms/ (accessed on 7 July 2024).
- Ocean Zones. Available online: https://www.ck12.org/earth-science/ocean-zones-1501910316.92/lesson/Ocean-Zones-HS-ES/ (accessed on 7 July 2024).
- El-Hedeny, M. Encrustation and bioerosion on Middle Miocene bivalve shells and echinoid skeletons: Paleoenvironmental implications. Rev. Paléobiologie 2007, 26, 381–389. [Google Scholar]
- Sremac, J.; Bošnjak, M.; Velić, J.; Kovačić, M. Hunting for the Paratethyan acorn barnacles (Cirripedia: Balanomorpha: Balanidae). In Abstract Volume, 10th International Workshop Neogene of Central and South-Eastern Europe, Podčetrtek, Slovenia, 27–31 May 2024; Bartol, M., Ivančić, K., Horvat, A., Eds.; Geološki zavod Slovenije: Ljubljana, Slovenia, 2024; pp. 74–75. [Google Scholar]
- Zuschin, M.; Stachowitsch, M.; Stanton, R.J. Patterns and processes of shell fragmentation in modern and ancient marine environments. Earth-Sci. Rev. 2003, 63, 33–82. [Google Scholar] [CrossRef]
- Carriker, M.R. Shell penetration and feeding by Naticacean and Muricacaean predatory gastropods: A synthesis. Malacologia 1981, 20, 403–422. [Google Scholar]
- Kanat, A.R. Predatory ecology of naticid gastropods with a review of shell boring predation. Malacologia 1990, 32, 155–193. [Google Scholar]
- Kelley, P.H.; Hansen, T.A. Evolution of the Naticid Gastropod Predator-Prey System: An Evaluation of the Hypothesis of Escalation. Palaios 1993, 8, 358–375. [Google Scholar] [CrossRef]
- Kowalewski, M. A hermeneutic analysis of the shell-drilling gastropod predation on mollusks in the Korytnica Clays (Middle Miocene; Holy Cross Mountains, Central Poland). Acta Geol. Pol. 1990, 40, 183–213. [Google Scholar]
- Kowalewski, M. The fossil record of predation: An overview of analytical methods. In The Fossil Record of Predation; Kowalewski, M., Kelley, P.H., Eds.; Paleontological Special Papers, 8; Yale University: New Haven, CT, USA, 2002; pp. 3–42. [Google Scholar]
- Arnold, J.M.; Arnold, K.O. Some Aspects of Hole-Boring Predation by Octopus vulgaris. Am. Zool. 1966, 9, 991–996. [Google Scholar] [CrossRef]
- Hiemistra, A.F. Recognizing cephalopod boreholes in shells and the northward spread of Octopus vulgaris Cuvier, 1797 (Cephalopoda, Octopodoidea). Vita Malacol. 2015, 13, 53–56. [Google Scholar]
- McMenamin, M.; Lapic, W.; Rabinow, S.; Curtis Hill, A.; Rivers, H.L.; Lapic, S.M. Shell stacks at calvert cliffs: Evidence for cephalopod midden construction. In Proceedings of the Geological Society of America Abstracts with Programs, GSA Annual Meeting, Indianapolis, IN, USA, 4–7 November 2018; Volume 50, p. 6. [Google Scholar] [CrossRef]
- Hoffmeister, A.P.; Kowalewski, M. Spatial and Environmental Variation in the Fossil Record of Drilling Predation: A Case Study from the Miocene of Central Europe. Palaoios 2001, 16, 566–579. [Google Scholar] [CrossRef]
- Brom, K.R.; Szopa, K.; Krzykawski, T.; Brachaniec, T.; Salamon, M.A. Anti-predator adaptations in a great scallop (Pecten maximus)—A palaeontological perspective. Geosci. Rec. 2015, 1, 16–20. [Google Scholar] [CrossRef]
- Allmon, W.D.; Nieh, J.C.; Norris, R.D. Drilling and peeling of turritelline gastropods since the late Cretaceous. Palaeontology 1990, 33, 595–611. [Google Scholar]
- Mandic, O.; Piller, W.E. Pectinid coquinas and their palaeoenvironmental implications—Examples from the early Miocene of northeastern Egypt. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 172, 171–191. [Google Scholar] [CrossRef]
- Kelley, P.H.; Hansen, T.A. Comparisons of class- and lower taxon-level patterns in naticid gastropod predation, Cretaceous to Pleistocene of the U.S. Coastal Plain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 236, 302–320. [Google Scholar] [CrossRef]
- Naimi, M.N.; Vinn, O.; Cherif, A. Bioerosion in Ostrea lamellosa shells from the Messinian of the Tafna basin (NW Algeria). Carnets Geol. 2021, 21, 127–135. [Google Scholar] [CrossRef]
- Hoffman, A.; Pisera, A.; Ryszkiewicz, M. Predation by muricid and naticid gastropods on the Lower Tortonian mollusks from the Korytnica clays. Acta Geol. Pol. 1974, 24, 249–260. [Google Scholar]
- Dávid, Á. Trace fossils on molluscs from the Molluscan Clay (Late Oligocene, Egerian)—A comparison between two localities (Wind Brickyard, Eger, and Nyárjas Hill, Novaj, NE Hungary). Scr. Geol. Spec. Issue 1993, 2, 75–82. [Google Scholar]
- Dávid, Á. Predation by Naticid Gastropods on Late—Oligocene (Egerian) Molluscs Collected from Wind Brickyard, Eger, Hungary. Malacol. Newsl. 1999, 17, 11–19. [Google Scholar]
- Sawyer, J.A.; Zuschin, M. Drilling predation in mollusks from the Lower and Middle Miocene of the Central Paratethys. Palaios 2011, 26, 284–297. [Google Scholar] [CrossRef]
- Zagyvai, A.; Demeter, G. Tracing prey-predatory interactions in the Early Sarmatian (Mid-Miocene) shelly community from Rollsdorf Formation, Waldhof, Austria based on bioerosional observations. Acta GGM Debrecina Geol. Geomorphol. Phys. Geogr. Ser. 2008, 3, 51–60. [Google Scholar]
- Ganić, M.; Radović, P.; Rundić, L.J.; Bradić, K.; Knežević, S. Traces of drilling predation in the Upper Badenian (Middle Miocene) molluscs from the Rakovica stream (Belgrade). Geol. Croat. 2016, 69, 205–212. [Google Scholar] [CrossRef]
- Bošnjak, M.; Sremac, J.; Karaica, B.; Mađerić, I.; Jarić, A. Middle Miocene serial killers: Drilled gastropods from the south-western margin of the Central Paratethys, Croatia. Geol. Croat. 2021, 74, 225–235. [Google Scholar] [CrossRef]
- Marshall, C.R.; Finnegan, S.; Clites, E.C.; Holroyd, P.A.; Bonuso, N.; Cortez, C.; Davis, E.; Dietl, G.P.; Druckenmiller, P.S.; Eng, R.C.; et al. Quantifying the dark data in museum fossil collections as palaeontology undergoes a second digital revolution. Biol. Lett. 2018, 14, 20180431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).