Modeling the Present and Future Geographical Distribution Potential of Dipteronia dyeriana, a Critically Endangered Species from China
Abstract
:1. Introduction
2. Experimental Procedures and Methodology
2.1. Species Occurrence Data
2.2. Environmental Data Sources
2.3. RF Modeling and Potential Habitat Evaluation
2.4. MaxEnt Modeling and Potential Habitat Evaluation
2.5. Model Evaluation
3. Results
3.1. Evaluation of the Accuracy of Simulation Results
3.2. Environmental Factors in Determining the Distribution of D. dyeriana
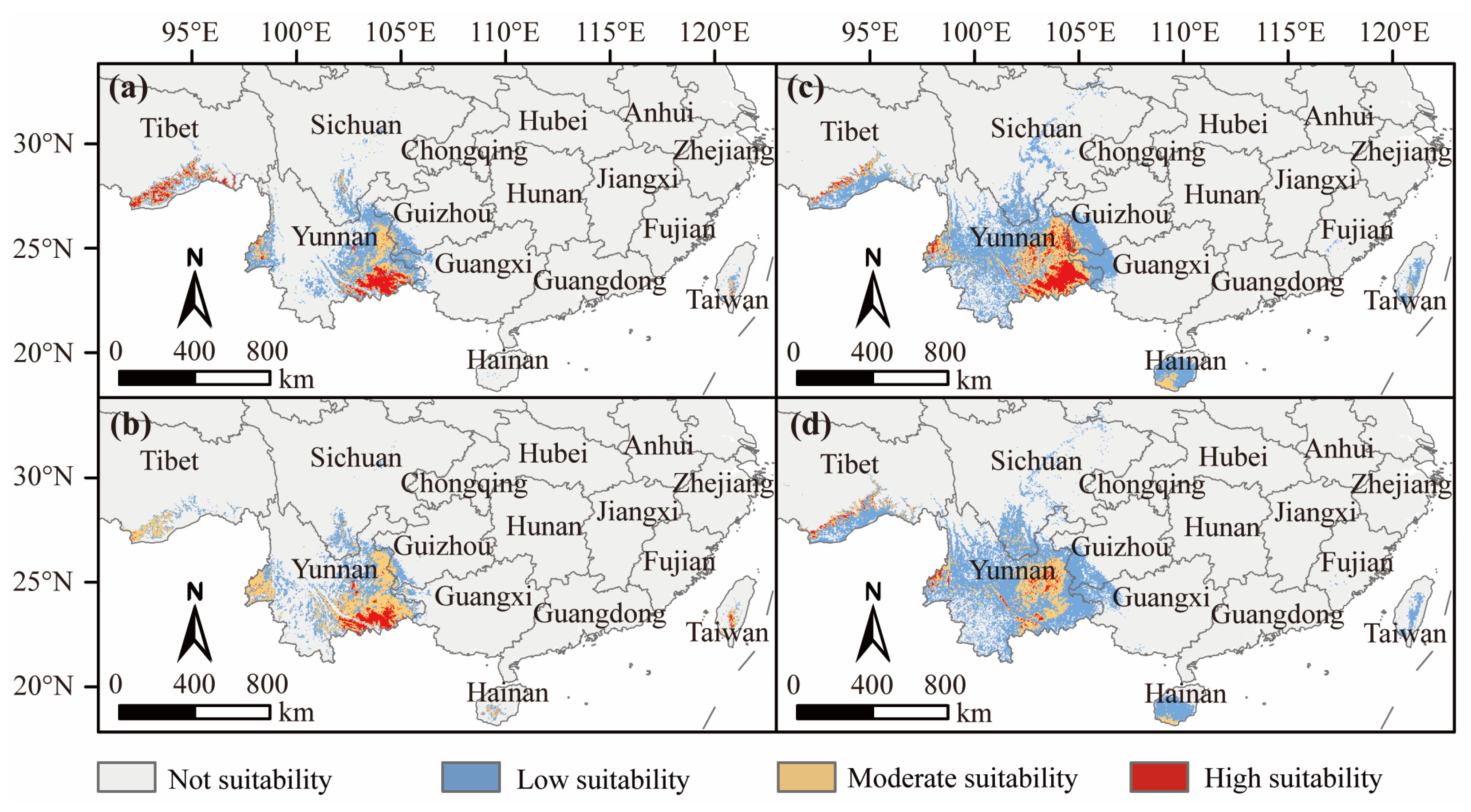
3.3. Suitable D. dyeriana Distribution Areas in the Current Climate
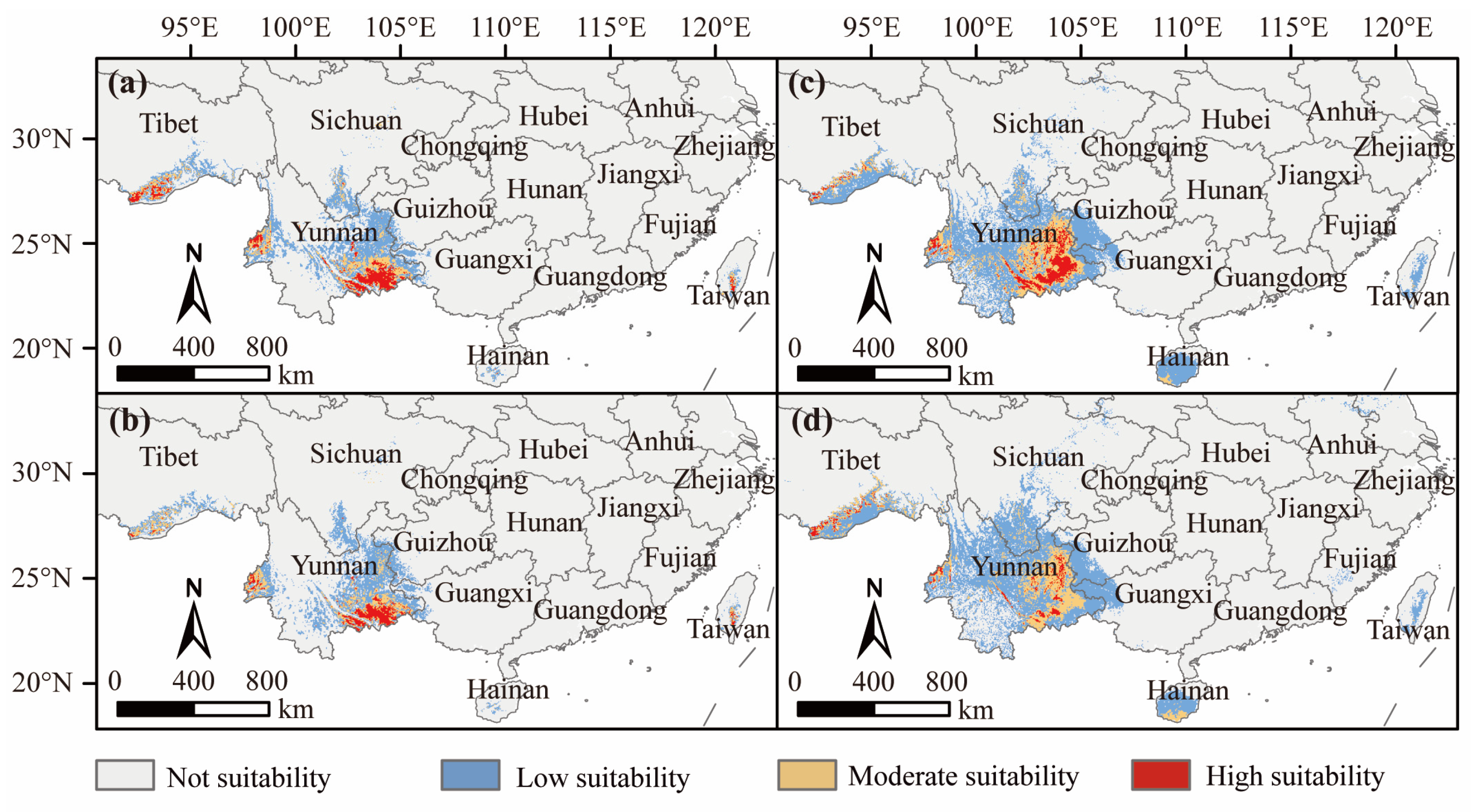
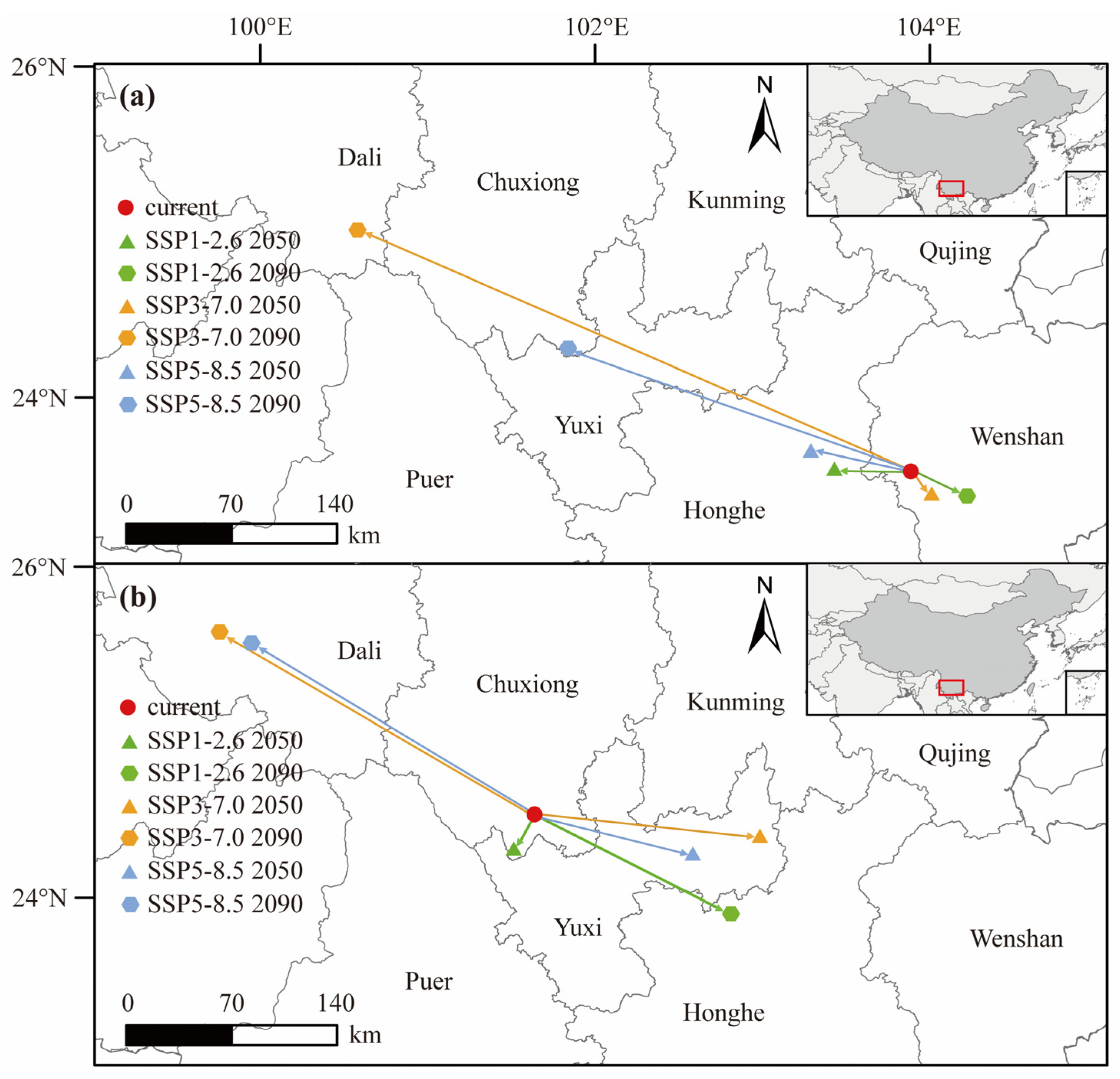
3.4. Suitable D. dyeriana Distribution Areas in the Future
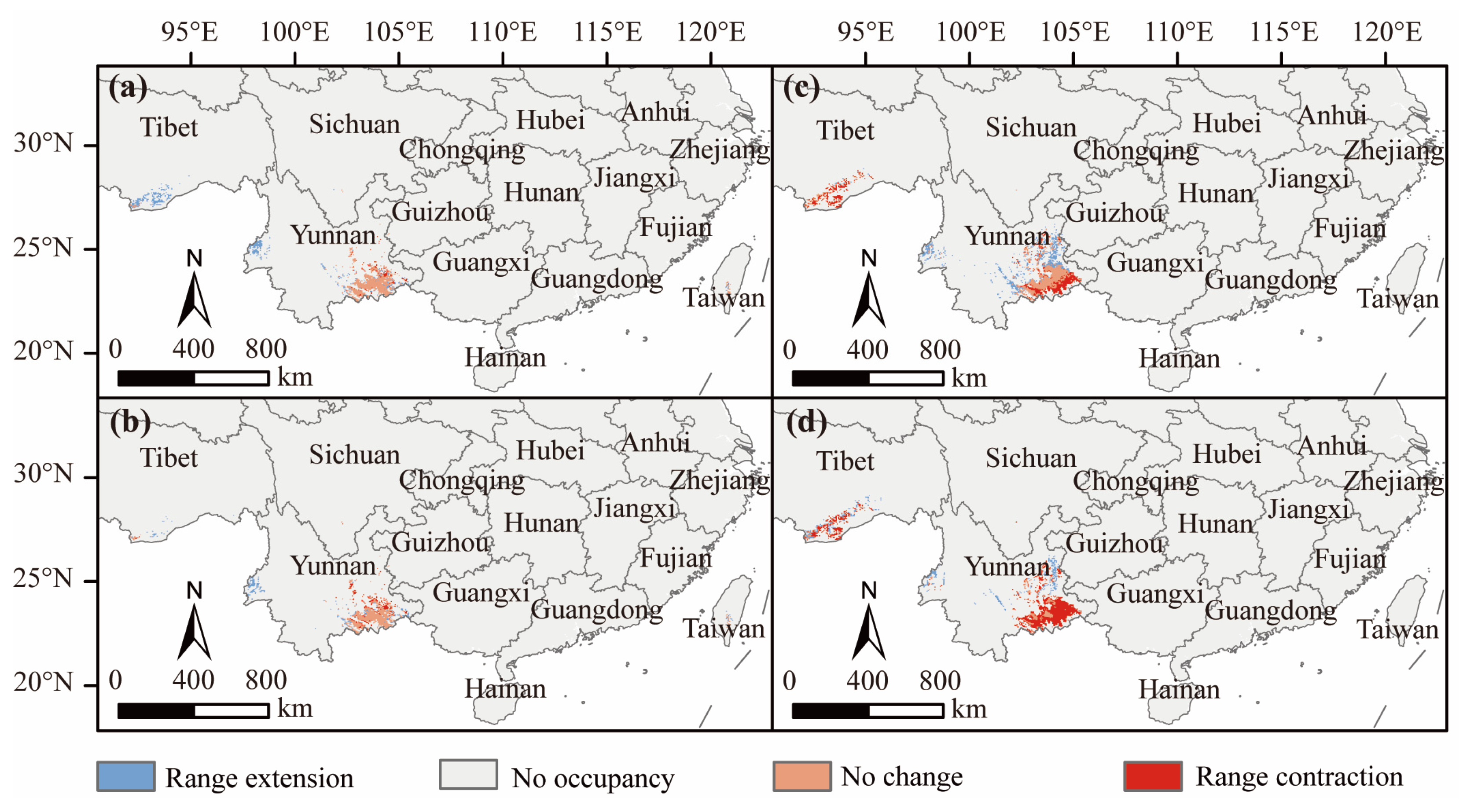
3.5. Changes in Practical Available Planting Areas under Different Climate Scenarios
4. Discussion
4.1. Assessment of Model Performance
4.2. Significant Climatic Factors Influencing the Distribution of D. dyeriana
4.3. Geographical Distribution and Temporal Dynamics of D. dyeriana
4.4. Protection Measures for D. dyeriana
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahecha, M.D.; Bastos, A.; Bohn, F.J.; Eisenhauer, N.; Feilhauer, H.; Hickler, T.; Kalesse-Los, H.; Migliavacca, M.; Otto, F.E.L.; Peng, J.; et al. Biodiversity and climate extremes: Known interactions and research gaps. Earths Future 2024, 12, e2023EF003963. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Scholes, R.J.; Arneth, A.; Barnes, D.K.A.; Burrows, M.T.; Diamond, S.E.; Duarte, C.M.; Kiessling, W.; Leadley, P.; Managi, S.; et al. Overcoming the coupled climate and biodiversity crises and their societal impacts. Science 2023, 380, eabl4881. [Google Scholar] [CrossRef]
- Bhadra, P.; Maitra, S.; Shankar, T.; Hossain, A.; Praharaj, S.; Aftab, T. Climate Change Impact on Plants: Plant Responses and Adaptations. In Plant Perspectives to Global Climate Changes; Academic Press: Cambridge, MA, USA, 2022; Volume 32, pp. 1–24. [Google Scholar] [CrossRef]
- APG. An ordinal classification for the families of flowering plants. Ann. Mo. Bot. Gard. 1998, 85, 531–553. [Google Scholar] [CrossRef]
- APGII. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003, 141, 399–436. [Google Scholar] [CrossRef]
- Harrington, M.G.; Edwards, K.J.; Johnson, S.A.; Chase, M.W.; Gadek, P.A. Phylogenetic inference in Sapindaceae sensu lato using plastid matK and rbcL DNA sequences. Syst. Bot. 2005, 30, 366–382. [Google Scholar] [CrossRef]
- Wu, Z.; Raven, P. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1996; Volume 15, pp. 1–387. [Google Scholar]
- Zhou, T.; Chen, C.; Wei, Y.; Chang, Y.; Bai, G.; Li, Z.; Kanwal, N.; Zhao, G. Comparative transcriptome and chloroplast genome analyses of two related Dipteronia species. Front. Plant Sci. 2016, 7, 1512. [Google Scholar] [CrossRef]
- Rivers, M.C.; Barstow, M.; Crowley, D. Dipteronia dyeriana. The IUCN Red List of Threatened Species 2017, 2017, e.T32340A2815531. [Google Scholar] [CrossRef]
- Ouyang, Z.; Cheng, Q.; Zhang, X.; Yang, X.; Wang, J. Status of Rare Dipteronia dyeriana and Countermeasure for its Protection. For. Inventory Plan. 2007, 32, 143–145. (In Chinese) [Google Scholar]
- Chen, C.; Lu, R.; Zhu, S.; Tamaki, I.; Qiu, Y. Population structure and historical demography of Dipteronia dyeriana (Sapindaceae), an extremely narrow palaeoendemic plant from China: Implications for conservation in a biodiversity hot spot. Heredity 2017, 119, 95–106. [Google Scholar] [CrossRef]
- Guo, R.; Luo, M.; Long, C.; Li, M.; Ouyang, Z.; Zhou, Y.; Wang, Y.; Li, X.; Shi, Y. Triterpenoid ester saponins from Dipteronia dyeriana. Helv. Chim. Acta. 2008, 91, 1728–1735. [Google Scholar] [CrossRef]
- Harris, A.J.; Frawley, E.; Wen, J. The utility of single-copy nuclear genes for phylogenetic resolution of Acer and Dipteronia (Acereae, Sapindaceae). Ann. Botan. Fenn. 2017, 54, 209–222. [Google Scholar] [CrossRef]
- Zhou, T.; Li, Z.; Bai, G.; Feng, L.; Chen, C.; Wei, Y.; Chang, Y.; Zhao, G. Transcriptome Sequencing and Development of Genic SSR Markers of an Endangered Chinese Endemic Genus Dipteronia Oliver (Aceraceae). Molecules 2016, 21, 166. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Li, S.; Zhao, G. What is the phylogenetic placement of Dipteronia dyeriana Henry? An example of plant species placement based on nucleotide sequences. Plant Biosyst. 2010, 144, 634–643. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. S. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paz, S.; Frelich, L.E.; Jagodzinski, A.M. How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Qazi, A.W.; Saqib, Z.; Zaman-ul-Haq, M. Trends in Species distribution modelling in context of rare and endemic plants: A systematic review. Ecol. Process. 2022, 11, 40. [Google Scholar] [CrossRef]
- Phillips, S.; Anderson, R.; Schapire, R. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.; Dudík, M. Modeling of species distributions with MaxEnt: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Bar Massada, A.; Syphard, A.; Stewart, S.; Radeloff, V. Wildfire ignition-distribution modelling: A comparative study in the Huron–Manistee National Forest, Michigan, USA. Int. J. Wildland Fire 2013, 22, 174–183. [Google Scholar] [CrossRef]
- Bai, G.; Yang, J.; Li, Z.; Zhao, G. Genetic Diversity and Implications for Conservation of Dipteronia Oliv. Acta Bot. Boreali-Occident. Sin. 2014, 34, 1975–1980. (In Chinese) [Google Scholar]
- Bai, G.; Li, S.; Li, W.; Li, Z.; Yang, J.; Zhao, G. Interspecific Differentiation of the Endemic Plant Genus Dipteronia Oliv. Acta Bot. Boreali-Occident. Sin. 2015, 35, 1123–1128. (In Chinese) [Google Scholar]
- Gao, J.; Du, F.; Li, J. Systematic position of Dipteronia dyeriana inferred from eight gene regions. J. Beijing For. Univ. 2017, 39, 24–29. (In Chinese) [Google Scholar]
- Ouyang, Z.; Wang, B.; Dang, X.; Zhang, X.; Ding, K. Allozyme-based Genetic Diversity of Dipteronia dyeriana in China. J. Wuhan Bot. Res. 2009, 27, 461–466. (In Chinese) [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Siabi, E.K.; Awafo, E.A.; Kabo-bah, A.T.; Derkyi, N.S.A.; Akpoti, K.; Mortey, E.M.; Yazdanie, M. Assessment of Shared Socioeconomic Pathway (SSP) Climate Scenarios and Its Impacts on the Greater Accra Region. Urban Clim. 2023, 49, 101432. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- FAO; IIASA. Harmonized World Soil Database Version 2.0; FAO: Rome, Italy; IIASA: Laxenburg, Austria, 2023. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Zhang, S.; Li, R.; Yan, C.; Wu, S. China’s Multi-Period Land Use/Cover Change Monitoring Dataset, Resource and Environment Science and Data Center [Data Set]. 2020. Available online: https://www.resdc.cn/DOI/doi.aspx?DOIid=54 (accessed on 7 January 2024).
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. MaxEnt modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Yi, Y.; Cheng, X.; Yang, Z.; Zhang, S. MaxEnt modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan. Chin. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudik, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of MaxEnt. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Heydari, M.; Ahmadi, K.; Khwarahm, N.R.; Karami, O.; Almasieh, K.; Naderi, B.; Bernard, P.; Mosavi, A. The current and future potential geographical distribution of Nepeta crispa Willd., an endemic, rare and threatened aromatic plant of Iran: Implications for ecological conservation and restoration. Ecol. Indic. 2022, 137, 108752. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Yan, H.; Feng, L.; Zhao, Y.; Feng, L.; Wu, D.; Zhu, C. Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob. Ecol. Conserv. 2020, 21, e00856. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. Ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Wunderlich, R.; Lin, Y.; Anthony, J.; Petway, J. Two alternative evaluation metrics to replace the true skill statistic in the assessment of species distribution models. Nat. Conserv. 2019, 35, 97–116. [Google Scholar] [CrossRef]
- Lesa, H.; Michael, J. Multilevel models for the experimental psychologist: Foundations and illustrative examples. Behav. Res. Methods 2007, 39, 101–117. [Google Scholar] [CrossRef]
- Zhang, S.; Kubota, K. Accounting for dispersal and intraspecific variation in forecasts of species distribution under climate change. Insect Conserv. Divers. 2023, 16, 902–908. [Google Scholar] [CrossRef]
- Feng, L.; Sun, J.; El-Kassaby, Y.A.; Yang, X.; Tian, X.; Wang, T. Predicting potential habitat of a plant species with small populations under climate change: Ostrya rehderiana. Forests 2022, 13, 129. [Google Scholar] [CrossRef]
- Buonincontri, M.P.; Bosso, L.; Smeraldo, S.; Chiusano, M.L.; Pasta, S.; Di Pasquale, G. Shedding light on the effects of climate and anthropogenic pressures on the disappearance of Fagus sylvatica in the Italian lowlands: Evidence from archaeo-anthracology and spatial analyses. Sci. Total Environ. 2023, 877, 162893. [Google Scholar] [CrossRef]
- Xiao, J.; Eziz, A.; Zhang, H.; Wang, Z.; Tang, Z.; Fang, J. Responses of four dominant dryland plant species to climate change in the Junggar Basin, northwest China. Ecol. Evol. 2019, 9, 13596–13607. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Bertola, L.; Hoskin, C. Species distribution modelling of the endangered mahogany glider (Petaurus gracilis) reveals key areas for targeted survey and conservation. Austral Ecol. 2022, 48, 289–312. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Lei, Y.; Wu, Q.; Liu, Y.; Shi, X. Potentially Suitable distribution areas of Populus Euphratica and Tamarix Chinensis by MaxEnt and Random Forest Model in the lower reaches of the Heihe river, China. Environ. Monit. Assess. 2023, 195, 1519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, N.; Shen, M.; Li, J. Comparison between optimized maxent and random forest modeling in predicting potential distribution: A case study with Quasipaa Boulengeri in China. Sci. Total Environ. 2022, 842, 156867. [Google Scholar] [CrossRef]
- Kim, S.; Lim, C.; Kim, G.; Lee, J.; Geiger, T.; Rahmati, O.; Lee, W. Multi-temporal analysis of forest fire probability using socio-economic and environmental variables. Remote Sens. 2019, 11, 86. [Google Scholar] [CrossRef]
- Wouyou, H.; Lokonon, B.; Idohou, R.; Zossou-Akete, A.; Assogbadjo, A.; Glèlè Kakaï, R. Predicting the potential impacts of climate change on the endangered Caesalpinia bonduc (L.) Roxb in Benin (West Africa). Heliyon 2022, 8, e09022. [Google Scholar] [CrossRef]
- Mtengwana, B.; Dube, T.; Mudereri, B.; Shoko, C. Modeling the geographic spread and proliferation of invasive alien plants (IAPs) into new ecosystems using multi-source data and multiple predictive models in the Heuningnes catchment, South Africa. GISci. Remote Sens. 2021, 58, 483–500. [Google Scholar] [CrossRef]
- Gao, B.; Wei, H.; Guo, Y.; Gu, W. Using GIS and MaxEnt to analyze the potential distribution of Abies chensiensis. Chin. J. Ecol. 2015, 34, 843–852. (In Chinese) [Google Scholar]
- Jiang, X.; Liu, W.; Zhu, Y.; Cao, Y.; Yang, X.; Geng, Y.; Zhang, F.; Sun, R.; Jia, R.; Yan, C.; et al. Impacts of Climate Changes on Geographic Distribution of Primula filchnerae, an Endangered Herb in China. Plants 2023, 12, 3561. [Google Scholar] [CrossRef]
- Huang, E.; Chen, Y.; Fang, M.; Zheng, Y.; Yu, S. Environmental Drivers of Plant Distributions at Global and Regional Scales. Global Ecol. Biogeogr. 2021, 30, 697–709. [Google Scholar] [CrossRef]
- Yu, F.; Groen, T.A.; Wang, T.; Skidmore, A.K.; Huang, J.; Ma, K. Climatic niche breadth can explain variation in geographical range size of alpine and subalpine plants. Int. J. Geog. Inf. Sci. 2016, 31, 190–212. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Islam, H.M.T.; Ahmed, S.; Bhattacharjya, D.K.; Khan, M.S.K.; Mahmud, G.I.; Almazroui, M.; Shahid, S. Evaluating the Effects of Climate Change on Thermal Bioclimatic indices in a tropical region using climate projections from the bias-corrected CMIP6 model. Earth Syst. Environ. 2023, 7, 699–722. [Google Scholar] [CrossRef]
- Fu, Y.H.; Jiang, D.B. Climate change over China with a 2 °C global warming. Chin. J. Atmos. Sci. 2012, 36, 234–246. (In Chinese) [Google Scholar] [CrossRef]
- Mo, X.G.; Hu, S.; Lu, H.J.; Lin, Z.H.; Liu, S.X. Drought trends over the terrestrial China in the 21st century in climate change scenarios with ensemble GCM projections. J. Nat. Resour. 2018, 33, 1244–1256. [Google Scholar] [CrossRef]
- Deng, L.; Zhu, H.H.; Jiang, Z.H. Projection of climate change in China under carbon neutral scenarios. Trans. Atmos. Sci. 2022, 45, 364–375. [Google Scholar] [CrossRef]
- Su, Z.K.; Li, W.X.; Zhang, J.Y.; Jin, J.L.; Xue, Q.; Wang, Y.T.; Wang, G.Q. Historical changes and future trends of extreme precipitation and high temperature in China. Strateg. Study Chin. Acad. Eng. 2022, 24, 116–125. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Chen, B.; Zou, H.; Zhang, B.; Zhang, X.; Jin, X.; Wang, C.; Zhang, X. Distribution pattern and change prediction of Saposhnikovia divaricata suitable area in China under climate change. Ecol. Indic. 2022, 143, 109311. [Google Scholar] [CrossRef]
- Rubenstein, M.A.; Weiskopf, S.R.; Bertrand, R.; Carter, S.L.; Comte, L.; Eaton, M.J.; Johnson, C.G.; Lenoir, J.; Lynch, A.J.; Miller, B.W.; et al. Climate Change and the Global Redistribution of Biodiversity: Substantial Variation in Empirical Support for Expected Range Shifts. Environ. Evid. 2023, 12, 7. [Google Scholar] [CrossRef]
- Dong, X.D.; Li, J.H.; Yang, X.X. Surver of Yunnans Trachycarpus nanu and its biological features. Dep. Biol. Chem. 2002, 21, 338–341. (In Chinese) [Google Scholar]
- Zhang, K.; Zhang, Y.; Jia, D.; Tao, J. Species Distribution Modeling of Sassafras Tzumu and Implications for Forest Management. Sustainability 2020, 12, 4132. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zhou, J.; Huang, Q.; Li, D. Ex situ conservation of Dipteronia dyeriana, a rare and endangered plant. Yunnan For. Sci. Technol. 2001, 96, 24–27. (In Chinese) [Google Scholar]

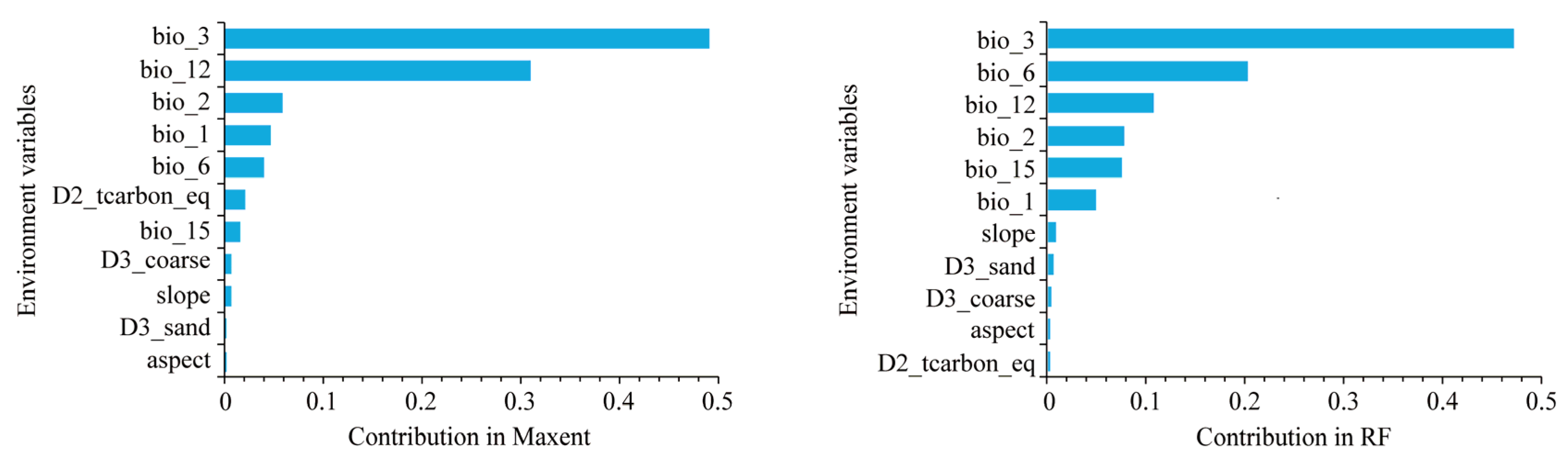
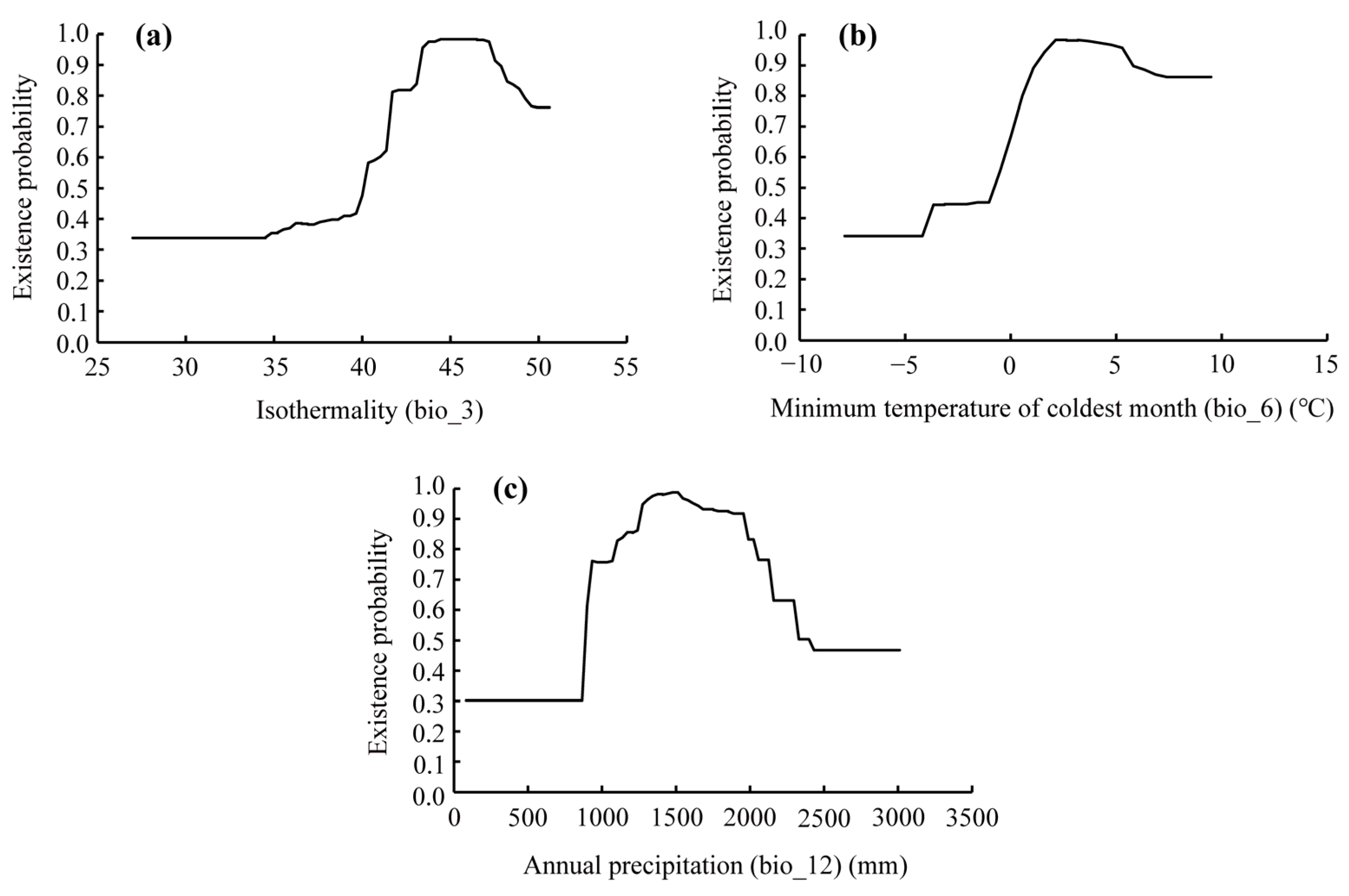
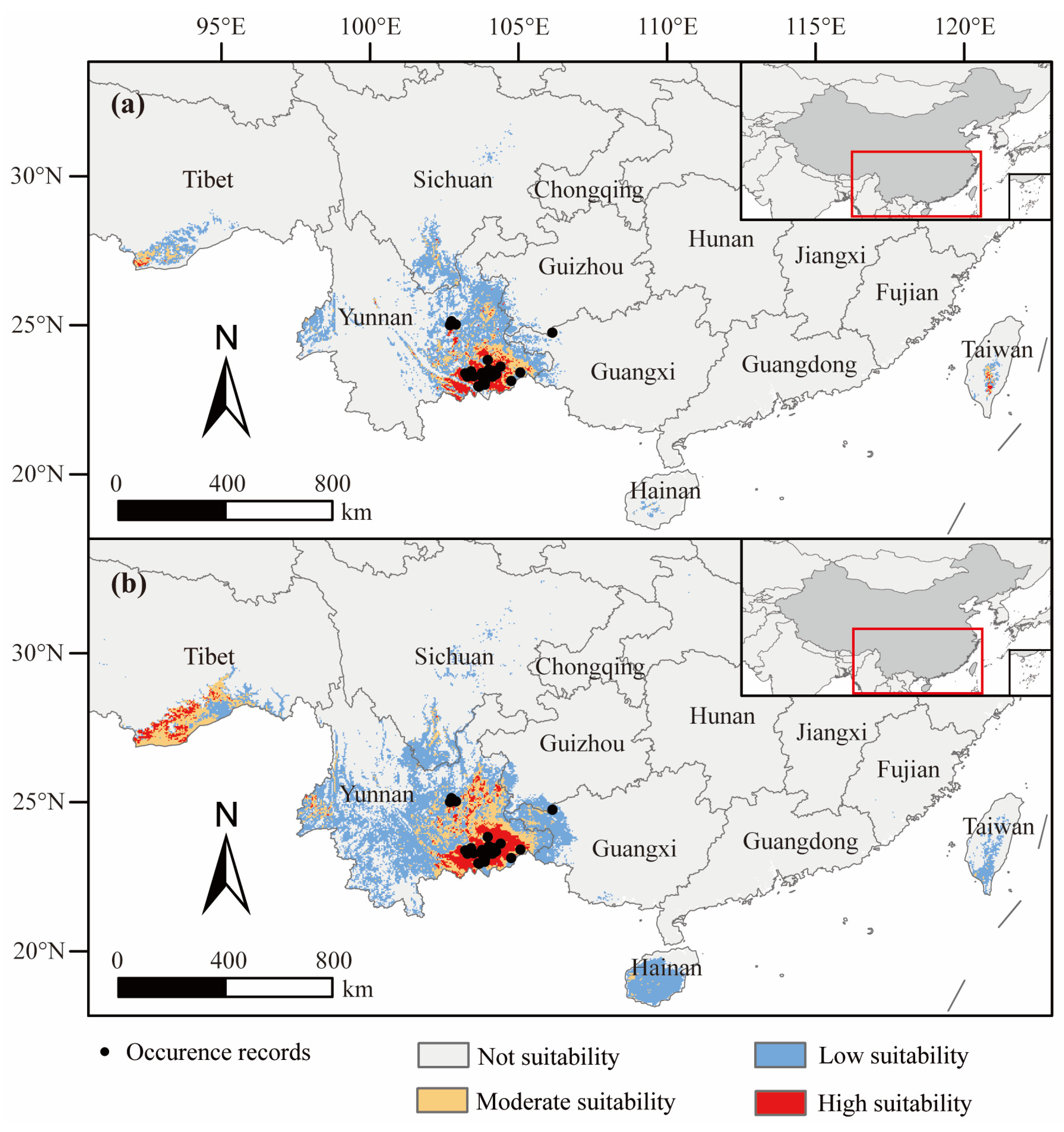
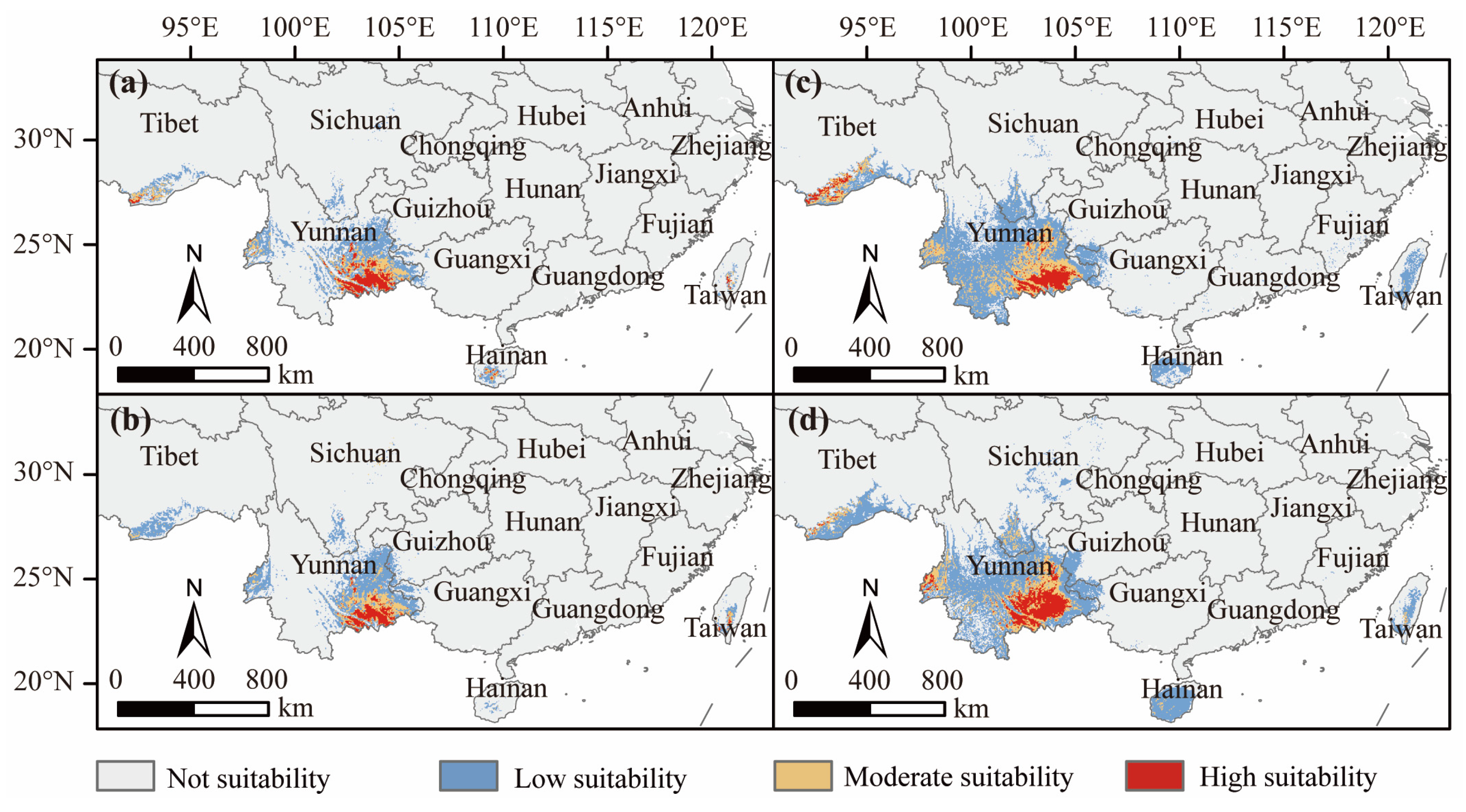


| Type | Factor | Impact Factor | Tolerance | VIF | Source |
|---|---|---|---|---|---|
| Bioclimatic factors | bio_1 | Annual mean temperature | 0.431 | 2.320 | WorldClim |
| bio_2 | Mean diurnal range (mean of monthly (max temp–min temp)) | 0.658 | 1.520 | WorldClim | |
| bio_3 | Isothermality (bio_2/bio_7) × (100) | 0.126 | 7.937 | WorldClim | |
| bio_6 | Minimum temperature of coldest month | 0.231 | 4.329 | WorldClim | |
| bio_12 | Annual precipitation | 0.714 | 1.401 | WorldClim | |
| bio_15 | Precipitation seasonality (coefficient of variation) | 0.431 | 2.320 | WorldClim | |
| Topographic factors | aspect | Aspect | 0.79 | 1.265 | WorldClim |
| slope | Slope | 0.592 | 1.69 | WorldClim | |
| Soil factors | D2_tcarbon_eq | Calcium carbonate content | 0.231 | 4.333 | HSWD 2.0 |
| D3_coarse | Coarse fragments | 0.216 | 4.619 | HSWD 2.0 | |
| D3_sand | Sand | 0.208 | 4.801 | HSWD 2.0 |
| Model Type | AUC | TSS | Kappa |
|---|---|---|---|
| MaxEnt | 0.994 ± 0.008 | 0.831 ± 0.012 | 0.672 ± 0.029 |
| RF | 0.998 ± 0.002 | 0.990 ± 0.023 | 0.913 ± 0.017 |
| p | 0.097 | 0.001 | 0.001 |
| Model | Climate Scenarios | High Suitability Area | Middle Suitability Area | Low Suitability Area | Total Suitability Area |
|---|---|---|---|---|---|
| MaxEnt | current | 1.96 | 2.78 | 10.46 | 15.20 |
| SSP1-2.6 2050 | 2.28 | 3.18 | 9.83 | 15.29 | |
| SSP1-2.6 2090 | 1.65 | 2.63 | 10.62 | 14.89 | |
| SSP3-7.0 2050 | 2.97 | 5.00 | 12.91 | 20.88 | |
| SSP3-7.0 2090 | 1.88 | 8.46 | 9.77 | 20.11 | |
| SSP5-8.5 2050 | 2.84 | 4.06 | 13.36 | 20.26 | |
| SSP5-8.5 2090 | 2.07 | 3.63 | 12.84 | 18.54 | |
| RF | current | 3.53 | 6.56 | 21.27 | 31.36 |
| SSP1-2.6 2050 | 2.70 | 7.00 | 24.66 | 34.37 | |
| SSP1-2.6 2090 | 3.82 | 6.10 | 26.06 | 35.99 | |
| SSP3-7.0 2050 | 3.84 | 8.23 | 26.20 | 38.27 | |
| SSP3-7.0 2090 | 1.24 | 7.00 | 30.10 | 38.34 | |
| SSP5-8.5 2050 | 3.34 | 7.76 | 27.03 | 38.13 | |
| SSP5-8.5 2090 | 1.50 | 8.14 | 31.80 | 41.45 |
| Model | Climate Scenarios | Farmland | Woodland | Grassland | Bare Land |
|---|---|---|---|---|---|
| Maxent | SSP1-2.6 2050 | 2.45 | 9.33 | 3.06 | 0.01 |
| SSP1-2.6 2090 | 2.60 | 8.49 | 3.29 | 0.01 | |
| SSP3-7.0 2050 | 3.75 | 11.89 | 4.45 | 0.02 | |
| SSP3-7.0 2090 | 3.38 | 12.02 | 4.18 | 0.01 | |
| SSP5-8.5 2050 | 3.23 | 12.35 | 4.08 | 0.01 | |
| SSP5-8.5 2090 | 3.15 | 11.03 | 3.84 | 0.01 | |
| RF | SSP1-2.6 2050 | 6.29 | 20.57 | 6.61 | 0.03 |
| SSP1-2.6 2090 | 6.92 | 21.00 | 7.01 | 0.03 | |
| SSP3-7.0 2050 | 7.52 | 22.12 | 7.52 | 0.03 | |
| SSP3-7.0 2090 | 6.91 | 22.49 | 7.77 | 0.03 | |
| SSP5-8.5 2050 | 7.15 | 22.28 | 7.64 | 0.03 | |
| SSP5-8.5 2090 | 7.54 | 24.10 | 8.47 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.-H.; Liu, B.-W.; Tiamiyu, B.B.; Zhang, Y.; Ning, W.-Y.; Si, J.-Y.; Dong, N.-C.; Lv, X.-L. Modeling the Present and Future Geographical Distribution Potential of Dipteronia dyeriana, a Critically Endangered Species from China. Diversity 2024, 16, 545. https://doi.org/10.3390/d16090545
Yan M-H, Liu B-W, Tiamiyu BB, Zhang Y, Ning W-Y, Si J-Y, Dong N-C, Lv X-L. Modeling the Present and Future Geographical Distribution Potential of Dipteronia dyeriana, a Critically Endangered Species from China. Diversity. 2024; 16(9):545. https://doi.org/10.3390/d16090545
Chicago/Turabian StyleYan, Ming-Hui, Bin-Wen Liu, Bashir B. Tiamiyu, Yin Zhang, Wang-Yang Ning, Jie-Ying Si, Nian-Ci Dong, and Xin-Lan Lv. 2024. "Modeling the Present and Future Geographical Distribution Potential of Dipteronia dyeriana, a Critically Endangered Species from China" Diversity 16, no. 9: 545. https://doi.org/10.3390/d16090545







