Diversity of Rotifera in Freshwaters of Bolivia: An Updated Checklist
Abstract
1. Introduction
2. Materials and Methods
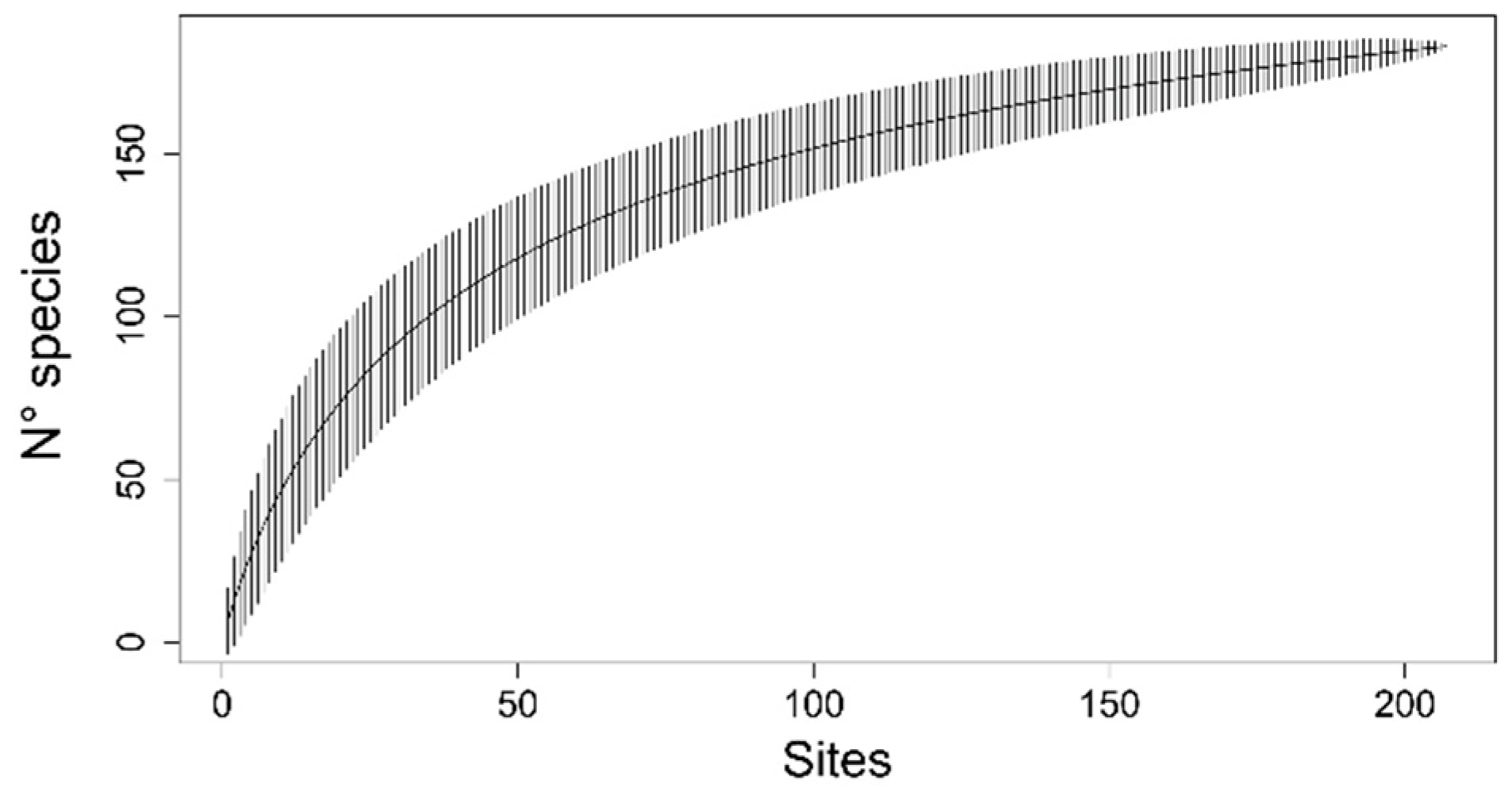
3. Results
3.1. Literature Review
3.2. Sample Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arndt, H. Rotifers as predators on components of the microbial web (bacteria, heterotrophic flagellates, ciliates)—A review. In Proceedings of the Rotifer Symposium VI: Proceedings of the Sixth International Rotifer Symposium, Banyoles, Spain, 3–8 June 1991; pp. 231–246. [Google Scholar]
- Rejas, D.; Muylaert, K.; De Meester, L. Trophic interactions within the microbial food web in a tropical floodplain lake (Laguna Bufeos, Bolivia). Rev. Biol. Trop. 2005, 53, 85–96. [Google Scholar] [PubMed]
- Park, G.; Marshall, H. The trophic contributions of rotifers in tidal freshwater and estuarine habitats. Estuar. Coast. Shelf Sci. 2000, 51, 729–742. [Google Scholar] [CrossRef]
- Saksena, D.N. Rotifers as Indicators of Water Quality. Acta Hydrochim. Hydrobiol. 1987, 15, 481–485. [Google Scholar] [CrossRef]
- Marneffe, Y.; Comblin, S.; Thomé, J.-P. Ecological water quality assessment of the Bütgenbach lake (Belgium) and its impact on the River Warche using rotifers as bioindicators. In Proceedings of the Rotifera VIII: A Comparative Approach: Proceedings of the VIIIth International Rotifer Symposium, Collegeville, MN, USA, 22–27 June 1997; pp. 459–467. [Google Scholar]
- Aoyagui, A.S.; Bonecker, C.C. Rotifers in different environments of the Upper Paraná River floodplain (Brazil): Richness, abundance and the relationship with connectivity. Hydrobiologia 2004, 522, 281–290. [Google Scholar] [CrossRef]
- Segers, H. Global diversity of rotifers (Rotifera) in freshwater. In Freshwater Animal Diversity Assessment; Balian, E.V., Lévêque, C., Segers, H., Martens, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 49–59. [Google Scholar]
- Jézéquel, C.; Tedesco, P.A.; Darwall, W.; Dias, M.S.; Frederico, R.G.; Hidalgo, M.; Hugueny, B.; Maldonado-Ocampo, J.; Martens, K.; Ortega, H.; et al. Freshwater fish diversity hotspots for conservation priorities in the Amazon Basin. Conserv. Biol. 2020, 34, 956–965. [Google Scholar] [CrossRef]
- Mariac, C.; Duponchelle, F.; Miranda, G.; Ramallo, C.; Wallace, R.; Tarifa, G.; Garcia-Davila, C.; Ortega, H.; Pinto, J.; Renno, J.-F. Unveiling biogeographical patterns of the ichthyofauna in the Tuichi basin, a biodiversity hotspot in the Bolivian Amazon, using environmental DNA. PLoS ONE 2022, 17, e0262357. [Google Scholar] [CrossRef]
- De Paggi, J.S.; Paggi, J.C. El zooplancton de los grandes ríos sudamericanos con planicie de inundación. Fabicib 2015, 18, 166–194. [Google Scholar] [CrossRef]
- Ferrando, N.S.; Claps, M.C. A Revised and Updated Checklist of Monogononta Rotifers from Argentina. 2016. Available online: https://ri.conicet.gov.ar/handle/11336/23554 (accessed on 11 September 2024).
- García-Morales, A.E.; Gutiérrez, M.E. The Rotifer fauna of Guatemala and Belize: Survey and biogeographical affinities. Rev. De Biol. Trop. 2007, 55, 569–584. [Google Scholar]
- Koste, W.; De Paggi, S.J. Rotifera of the Superorder Monogononta recorded from Neotropis. Gewässer Abwässer 1982, 68, 71–102. [Google Scholar]
- Nogrady, T.; Pourriot, R.; Segers, H. Rotifera 3. Notommatidae and Scaridiidae. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 8; Dumont, H., Nogrady, T., Eds.; SPB Academic Publishing BV: Amsterdam, The Netherlands, 1995; 248p. [Google Scholar]
- Nogrady, T.; Segers, H. Asplanchnidae, Gastropodidae, Lindiidae, Microcodidae, Synchaetidae, Trochosphaeridae and Filinia. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; HJF Dumont. Kingston Ont. Canada Gent University, Belgium Backhuys Publisher: Leiden, The Netherlands, 2002; Volume 6, 264p. [Google Scholar]
- Segers, H.; Dumont, H. 102+ rotifer species (Rotifera: Monogononta) in Broa reservoir (SP., Brazil) on 26 August 1994, with the description of three new species. Hydrobiologia 1995, 316, 183–197. [Google Scholar] [CrossRef]
- Shiel, R.; Koste, W. Rotifera from Australian inland waters. VIII: Trichocercidae (Monogononta). Trans. R. Soc. South Aust. 1992, 116, 1–27. [Google Scholar]
- De Paggi, S.B.J.; Wallace, R.; Fontaneto, D.; Marinone, M.C. Phylum rotifera. In Thorp and Covich’s Freshwater Invertebrates; Elsevier: Amsterdam, The Netherlands, 2020; pp. 145–200. [Google Scholar]
- Kriska, G. Rotifers: Rotifera. In Freshwater Invertebrates in Central Europe: A Field Guide; Springer: Berlin/Heidelberg, Germany, 2023; pp. 55–81. [Google Scholar]
- Sarma, S.; Jiménez-Santos, M.A.; Nandini, S. Rotifer species diversity in Mexico: An updated checklist. Diversity 2021, 13, 291. [Google Scholar] [CrossRef]
- Segers, H. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 2007, 1564, 1–104. [Google Scholar] [CrossRef]
- Wilke, T.; Ahlrichs, W.H.; Bininda-Emonds, O.R. A weighted taxonomic matrix key for species of the rotifer genus Synchaeta (Rotifera, Monogononta, Synchaetidae). ZooKeys 2019, 871, 1–40. [Google Scholar] [CrossRef]
- Jersabek, C.D.; De Smet, W.H.; Hinz, C.; Fontaneto, D.; Hussey, C.G.; Michaloudi, E.; Wallace, R.L.; Segers, H. List of Available Names in Zoology, Candidate Part Phylum Rotifera, Genus-Group Names Established before 1 January 2000. Available online: https://archive.org/details/LANCandidatePartGeneraRotifera (accessed on 11 September 2024).
- Jersabek, C.D.; De Smet, W.H.; Hinz, C.; Fontaneto, D.; Hussey, C.G.; Michaloudi, E.; Wallace, R.L.; Segers, H. List of Available Names in Zoology, Candidate Part Phylum Rotifera, Species-Group Names Established before 1 January 2000. 2018. Available online: https://archive.org/details/LANCandidatePartSpeciesRotifera (accessed on 11 September 2024).
- Segers, H.; De Smet, W.H.; Fischer, C.; Fontaneto, D.; Michaloudi, E.; Wallace, R.L.; Jersabek, C.D. Towards a list of available names in zoology, partim Phylum Rotifera. Zootaxa 2012, 3179, 61–68. [Google Scholar] [CrossRef]
- International Commission on Zoological Nomenclature, I.C.Z.N. Opinion 2430–Parts of the List of Available Names in Zoology for phylum Rotifera: Accepted. Bull. Zool. Nomencl. 2019, 76, 74–76. [Google Scholar]
- Chiu, C.-H.; Wang, Y.-T.; Walther, B.A.; Chao, A. An improved nonparametric lower bound of species richness via a modified good–turing frequency formula. Biometrics 2014, 70, 671–682. [Google Scholar] [CrossRef]
- Segers, H.; Meneses, L.; Del Castillo, M. Rotifera (Monogononta) from Lake Kothia, a high-altitude lake in the Bolivian Andes. Arch. Für Hydrobiol. 1994, 132, 227–236. [Google Scholar]
- Segers, H.; Ferrufino, N.L.; de Meester, L. Diversity and Zoogeography of Rotifera (Monogononta) in a Flood Plain Lake of the Ichilo River, Bolivia, with Notes on Little-Known Species. Int. Rev. Hydrobiol. 1998, 83, 439–448. [Google Scholar] [CrossRef]
- Rejas, D.; de Meester, L.; Ferrufino, L.; Maldonado, M.; Ollevier, F. Diel vertical migration of zooplankton in an Amazonian várzea lake (Laguna Bufeos, Bolivia). Stud. Neotrop. Fauna Environ. 2007, 42, 71–81. [Google Scholar] [CrossRef]
- Iltis, A.; Risacher, F.; Servant Vildary, S. Contribution à l’étude hydrobiologique des lacs salés du sud de l’Altiplano bolivien. 1984. Available online: https://agris.fao.org/search/en/providers/122415/records/647368cc2c1d629bc980712f (accessed on 11 September 2024).
- Acosta, F. Caracterización del zooplancton de lagunas en la llanura inundable del río Ichilo (Cochabamba-Bolivia). Rev. Boliv. De Ecol. 2005, 17, 1–14. [Google Scholar]
- Aguilera, X.; Crespo, G.; Declerck, S.; De Meester, L. Diel vertical migration of zooplankton in tropical high mountain lakes (Andes, Bolivia). Pol. J. Ecol. 2006, 3, 453–464. [Google Scholar]
- Pinto, J. Distribución del zooplancton en la parte boliviana del lago. In El Lago Titicaca: Síntesis Del Conoc. Limnológico Actual; Hisbol-ORSTOM: La Paz, Bolivia, 1991; pp. 277–283. [Google Scholar]
- Uéno, M. Zooplankton of Lake Titicaca on the Bolivian side. Hydrobiologia 1967, 29, 547–568. [Google Scholar] [CrossRef]
- Barbosa, F.V.V. A Biological Assessment of the Aquatic Ecosystems of the Upper Río Orthon Basin, Pando, Bolivia; Conservation International: Washington, DC, USA, 1999. [Google Scholar]
- Ibañez, C.; Paggi, J.C.; Molina, C.; Pinto, J.; Koste, W. Zooplancton de las lagunas. In Diversidad Biológica en la Llanura de Inundación del Río Mamoré. Importancia Ecológica de la Dinámica Fluvial; Pouilly, M., Beek, S.G., Moraes, M., Ibañez, C., Eds.; Centro de Ecología Simón I. Patiño: Santa Cruz, Bolivia, 2004. [Google Scholar]
- Williams, W.D.; Carrick, T.R.; Bayly, I.A.E.; Green, J.; Herbst, D.B. Invertebrates in salt lakes of the Bolivian Altiplano. Int. J. Salt Lake Res. 1995, 4, 65–77. [Google Scholar] [CrossRef]
- De Paggi, J. Ecological and biogeographical remarks on the rotifer fauna of Argentina. Rev. Hydrobiol. Trop 1990, 23, 297–311. [Google Scholar]
- Martínez, J.C.C.; Canesin, A.; Bonecker, C.C. Species composition of rotifers in different habitats of an artificial lake, Mato Grosso do Sul State, Brazil. Acta Sci. 2000, 22, 343–346. [Google Scholar]
- Sørensen, M.V.; Giribet, G. A modern approach to rotiferan phylogeny: Combining morphological and molecular data. Mol. Phylogenetics Evol. 2006, 40, 585–608. [Google Scholar] [CrossRef]
- Oyedotun, T.D.T.; Ally, N. Environmental issues and challenges confronting surface waters in South America: A review. Environ. Chall. 2021, 3, 100049. [Google Scholar] [CrossRef]

| Subclass | Family | References 1965–2009 | Own Observations | Total Spp. | Not Identified/Not Included Morphospecies | New Additions |
|---|---|---|---|---|---|---|
| Bdelloidea | Habrotrochidae | 1 | 0 | 1 | 0 | 0 |
| NI * | 1 | 1 | 1 | 9 | 0 | |
| Monogononta | Asplanchnidae | 3 | 2 | 3 | 6 | 0 |
| Brachionidae | 21 | 38 | 51 | 8 | 30 | |
| Collothecidae | 1 | 0 | 1 | 0 | 0 | |
| Conochilidae | 2 | 1 | 2 | 1 | 0 | |
| Dicranophoridae | 8 | 1 | 9 | 0 | 1 | |
| Epiphanidae | 2 | 5 | 7 | 0 | 5 | |
| Euchlanidae | 6 | 0 | 6 | 0 | 0 | |
| Flosculariidae | 6 | 1 | 7 | 0 | 1 | |
| Gastropodidae | 4 | 1 | 4 | 6 | 0 | |
| Hexarthridae | 3 | 3 | 3 | 12 | 0 | |
| Lecanidae | 28 | 28 | 43 | 13 | 15 | |
| Lepadellidae | 27 | 20 | 28 | 19 | 1 | |
| Mytilinidae | 7 | 6 | 12 | 1 | 5 | |
| Notommatidae | 19 | 6 | 19 | 12 | 0 | |
| Philodinidae | 7 | 0 | 7 | 0 | 0 | |
| Proalidae | 1 | 0 | 1 | 0 | 0 | |
| Scaridiidae | 1 | 1 | 2 | 0 | 1 | |
| Synchaetidae | 10 | 4 | 10 | 5 | 0 | |
| Testudinellidae | 7 | 5 | 11 | 1 | 4 | |
| Trichocercidae | 19 | 18 | 36 | 1 | 17 | |
| Trichotriidae | 3 | 4 | 5 | 2 | 2 | |
| Trochosphaeridae | 8 | 8 | 10 | 6 | 2 | |
| TOTAL | 192 | 153 | 279 | 102 | 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, C.E.; Campero, M.; Acosta, F.; Prado, P.E.; Maldonado, M.; Goitia, E.; Stamou, G.; Michaloudi, E.; López, C. Diversity of Rotifera in Freshwaters of Bolivia: An Updated Checklist. Diversity 2024, 16, 589. https://doi.org/10.3390/d16090589
Fernández CE, Campero M, Acosta F, Prado PE, Maldonado M, Goitia E, Stamou G, Michaloudi E, López C. Diversity of Rotifera in Freshwaters of Bolivia: An Updated Checklist. Diversity. 2024; 16(9):589. https://doi.org/10.3390/d16090589
Chicago/Turabian StyleFernández, Carla E., Melina Campero, Francisca Acosta, Pablo E. Prado, Mabel Maldonado, Edgar Goitia, Georgia Stamou, Evangelia Michaloudi, and Carlos López. 2024. "Diversity of Rotifera in Freshwaters of Bolivia: An Updated Checklist" Diversity 16, no. 9: 589. https://doi.org/10.3390/d16090589
APA StyleFernández, C. E., Campero, M., Acosta, F., Prado, P. E., Maldonado, M., Goitia, E., Stamou, G., Michaloudi, E., & López, C. (2024). Diversity of Rotifera in Freshwaters of Bolivia: An Updated Checklist. Diversity, 16(9), 589. https://doi.org/10.3390/d16090589









