The Effects of Salinity on the Growth, Survival, and Feeding of Sanderia malayensis (Cnidaria: Scyphozoa) Ephyrae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acquisition of Sanderia malayensis Ephyrae
2.2. Sanderia malayensis Ephyrae Growth, Survival, and Feeding Response to Salinity
2.3. Statistical Analysis
3. Results
3.1. Effect of Salinity Environmental Factors on the Growth of Sanderia malayensis Ephyrae
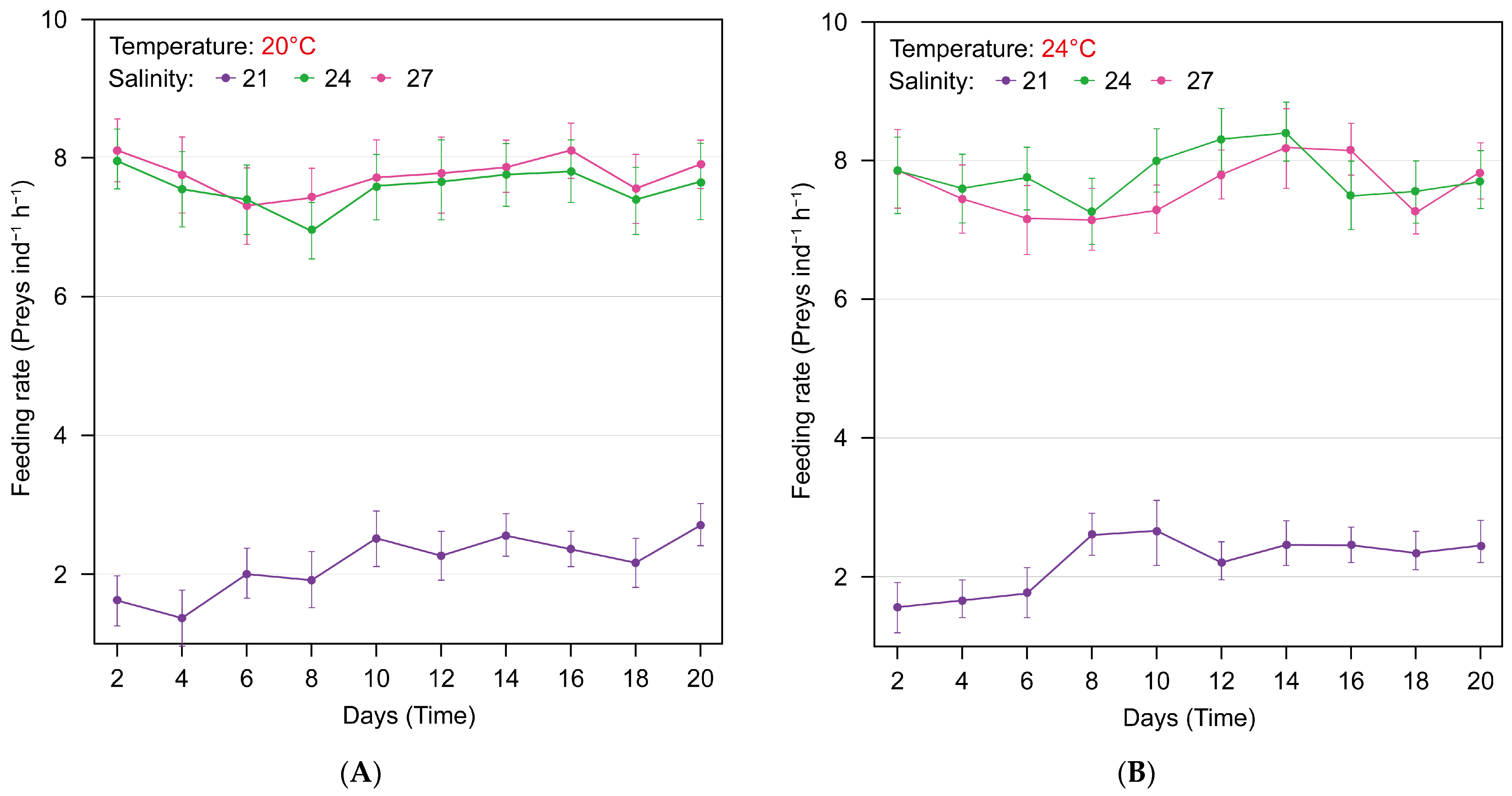
3.2. Effect of Salinity Environmental Factors on the Feeding of Sanderia malayensis Ephyrae
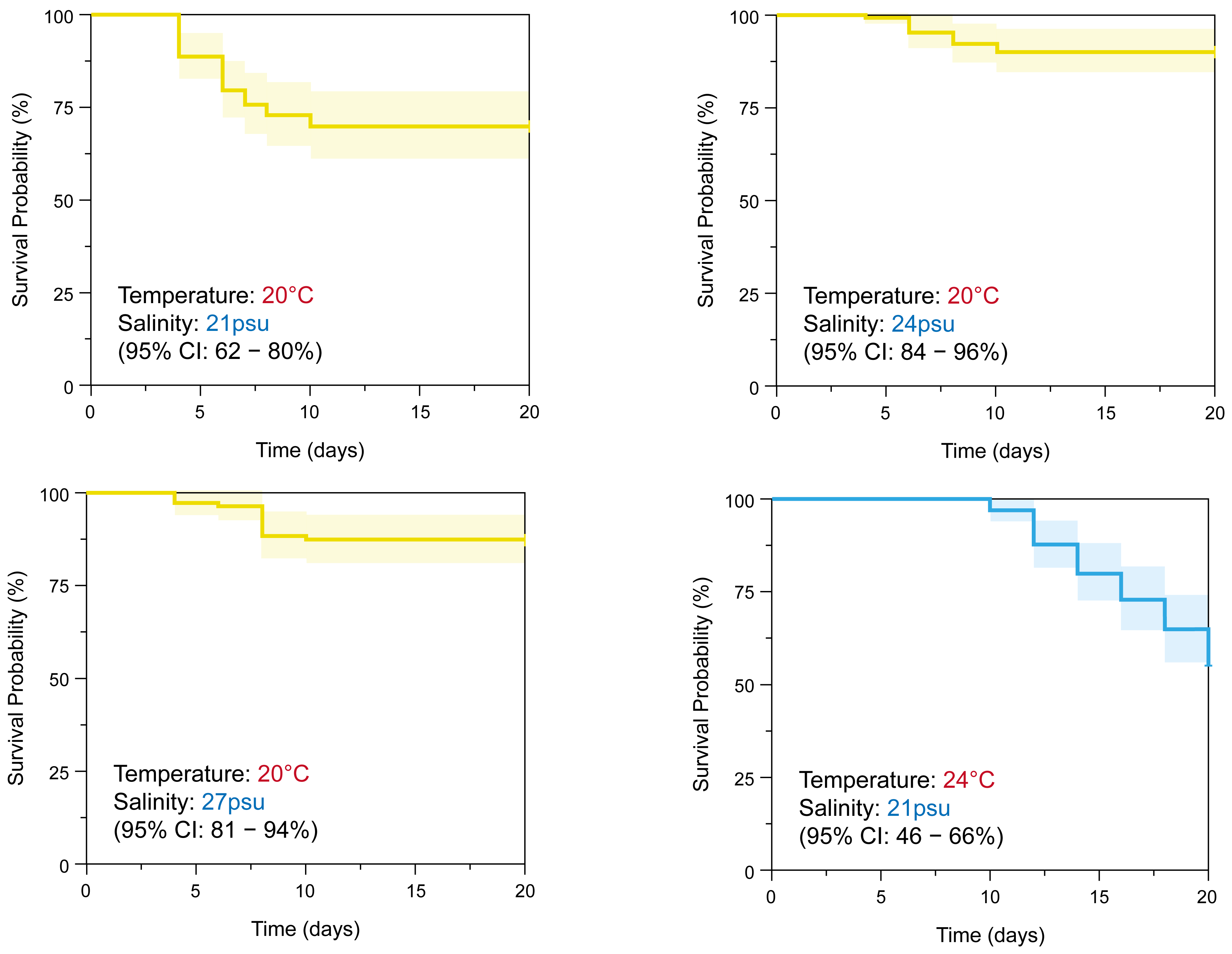
3.3. Effect of Salinity Environmental Factors on the Survival of Sanderia malayensis Ephyrae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purcell, J.E.; Uye, S.; Lo, W.-T. Anthropogenic causes of jellyfish blooms their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Purcell, J.E.; Atienza, D.; Fuentes, V.; Olariaga, A.; Tilves, U.; Colahan, C.; Gili, J.M. Temperature effects on asexual reproduction rates of scyphozoan species from the northwest Mediterranean Sea. In Jellyfish Blooms IV; Springer: Dordrecht, The Netherlands, 2012; Volume 220, pp. 169–180. [Google Scholar] [CrossRef]
- Pitt, K.A.; Lucas, C.H.; Condon, R.H.; Duarte, C.M.; Stewart-Koster, B. Claims That Anthropogenic Stressors Facilitate Jellyfish Blooms Have Been Amplified Beyond the Available Evidence: A Systematic Review. Front. Mar. Sci. 2018, 5, 451. [Google Scholar] [CrossRef]
- Lee, S.H.; Scotti, M.; Jung, S.J.; Hwang, J.S. Jellyfish blooms challenge the provisioning of ecosystem services in the Korean coastal waters. Hydrobiologia 2023, 850, 2855–2870. [Google Scholar] [CrossRef]
- Bosch-Belmar, M.; Milisenda, G.; Basso, L.; Doyle, T.K. Jellyfish Impacts on Marine Aquaculture and Fisheries. Rev. Fish. Sci. Aquac. 2023, 29, 242–259. [Google Scholar] [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Ismael, J.; Sabah, S.; Pérez-Ruzafa, A. Population dynamics and growth in three Scyphozoan jellyfishes, and their relationship with environmental conditions in a coastal lagoon. Estuar. Coast. Shelf. Sci. 2020, 243, 106901. [Google Scholar] [CrossRef]
- Quiñones, J.; Monroy, A.; Acha, E.M.; Mianzan, H. Jellyfish bycatch diminishes profit in an anchovy fishery off Peru. Fish. Res. 2013, 139, 47–50. [Google Scholar] [CrossRef]
- Purcell, J.E.; Baxter, E.J.; Fuentes, V. 13-Jellyfish as products and problems of aquaculture. In Advances in Aquaculture Hatchery Technology, 1st ed.; Allan, G., Burnell, G., Eds.; Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2013; pp. 404–430. [Google Scholar] [CrossRef]
- De Donno, A.; Idolo, A.; Bagordo, F.; Grassi, T.; Leomanni, A.; Serio, F.; Guido, M.; Canitano, M.; Zampardi, S.; Boero, F.; et al. Impact of stinging jellyfish proliferations along south Italian coasts: Human health hazards, treatment and social costs. Int. J. Environ. Res. Public Health 2014, 1, 2488–2503. [Google Scholar] [CrossRef]
- Milisenda, G.; Martinez-Quintana, A.; Fuentes, V.L.; Bosch-Belmar, M.; Aglieri, G.; Boero, F.; Piraino, S. Reproductive and bloom patterns of Pelagia noctiluca in the Strait of Messina, Italy. Estuar. Coast. Shelf Sci. 2018, 201, 29–39. [Google Scholar] [CrossRef]
- Tilves, U.; Fuentes, V.L.; Milisenda, G.; Parrish, C.C.; Vizzini, S.; Sabatés, A. Trophic interactions of the jellyfish Pelagia noctiluca in the NW Mediterranean: Evidence from stable isotope signatures and fatty acid composition. Mar. Ecol. Prog. Ser. 2018, 591, 101–116. [Google Scholar] [CrossRef]
- Frolova, A.; Miglietta, M.P. Insights on Bloom Forming Jellyfish (Class: Scyphozoa) in the Gulf of Mexico: Environmental Tolerance Ranges and Limits Suggest Differences in Habitat Preference and Resistance to Climate Change Among Congeners. Front. Mar. Sci. 2020, 7, 93. [Google Scholar] [CrossRef]
- Tang, C.; Sun, S.; Zhang, F. Natural predators of polyps of three scyphozoans: Nemopilema nomurai, Aurelia coerulea, and Rhopilema esculentum. J. Oceanol. Limnol. 2021, 39, 598–608. [Google Scholar] [CrossRef]
- Ishii, H. The influence of environmental changes upon the coastal plankton ecosystems, with special reference to mass occurrence of jellyfish. Bull. Plankt. Soc. Jpn. 2001, 48, 55–61. [Google Scholar]
- Wang, S.; Zhang, G.; Sun, S.; Wang, Y.; Zhao, Z. Population dynamics of three Scyphozoan jellyfish species during summer of 2011 in Jiaozhou Bay. Oceanol. Limnol. Sin. 2012, 43, 471–479. [Google Scholar]
- Sun, M.; Dong, J.; Purcell, J.E.; Li, Y.; Duan, Y.; Wang, A.; Wang, B. Testing the influence of previous–year temperature and food supply on development of Nemopilema nomurai blooms. Hydrobiologia 2015, 754, 85–86. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.S.; Kim, K.Y.; Molinero, J.C. Contrasting Effects of Regional and Local Climate on the Interannual Variability and Phenology of the Scyphozoan, Aurelia coerulea and Nemopilema nomurai in the Korean Peninsula. Diversity 2021, 13, 214. [Google Scholar] [CrossRef]
- Yao, L.; He, P.; Xia, Z.; Li, J.; Liu, J. Typical Marine Ecological Disasters in China Attributed to Marine Organisms and Their Significant Insights. Biology 2024, 13, 678. [Google Scholar] [CrossRef]
- Helm, R.R. Evolution and development of scyphozoan jellyfish. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1228–1250. [Google Scholar] [CrossRef]
- Feng, S.; Wang, S.; Sun, S.; Zhang, F.; Zhang, G.; Mengtan, L.; Uye, S.-I. Strobilation of three scyphozoans (Aurelia coelurea, Nemopilema nomurai and Rhopilema esculentum) in the field at Jiaozhou Bay, China. Mar. Ecol. Prog. Ser. 2018, 591, 141–153. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, Q.; Zhang, M.; Zhen, Y.; Mi, T.; Yu, Z. Effects of temperature and salinity on the asexual reproduction of Aurelia coerulea polyps. J. Oceanol. Limnol. 2019, 38, 133–142. [Google Scholar] [CrossRef]
- Loveridge, A.; Lucas, C.H.; Pitt, K.A. Shorter, warmer winters may inhibit production of ephyrae in a population of the moon jellyfish Aurelia aurita. Hydrobiologia 2021, 848, 739–749. [Google Scholar] [CrossRef]
- Takauchi, S.; Miyake, H.; Hirata, N.; Nagai, M.; Suzuki, N.; Ogiso, S.; Ikeguchi, S. Morphological characteristics of ephyrae of Aurelia coerulea derived from planula strobilation. Fish. Sci. 2021, 87, 617–679. [Google Scholar] [CrossRef]
- Schäfer, S.; Gueroun, S.K.M.; Andrade, C.; Canning-Clode, J. Combined Effects of Temperature and Salinity on Polyps and Ephyrae of Aurelia solida (Cnidaria: Scyphozoa). Diversity 2021, 13, 573. [Google Scholar] [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Perez-Ruzafa, A. Reconstructing the Biogeographic History of the Genus Aurelia Lamarck, 1816 (Cnidaria, Scyphozoa), and Reassessing the Nonindigenous Status of A. solida and A. coerulea in the Mediterranean Sea. Diversity 2023, 15, 1181. [Google Scholar] [CrossRef]
- Scorrano, S.; Aglieri, G.; Boero, F.; Dawson, M.N.; Piraino, S. Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. 2017, 180, 243–267. [Google Scholar] [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Pérez-Ruzafa, A. The unpredictability of scyphozoan jellyfish blooms. Front. Mar. Sci. 2024, 11, 1349956. [Google Scholar] [CrossRef]
- Fernández-Alías, A.; Marcos, C.; Perez-Ruzafa, A. Larger scyphozoan species dwelling in temperate, shallow waters show higher blooming potential. Mar. Pollut. Bull. 2021, 173, 113100. [Google Scholar] [CrossRef]
- Boero, F.; Brotz, L.; Gibbons, M.J.; Piraino, S.; Zampardi, S. 3.10 Impacts and Effects of Ocean Warming on Jellyfish. In Explaining Ocean Warming: Causes, Scale, Effects and Consequences; IUCN: Gland, Switzerland, 2016; pp. 213–237. Available online: http://www.researchgate.net/publication/309209466 (accessed on 5 January 2025).
- Avian, M.; Motta, G.; Prodan, M.; Tordoni, E.; Macaluso, V.; Beran, A.; Goruppi, A.; Bacaro, G.; Tirelli, V. Asexual reproduction and strobilation of S. malayensis (Scyphozoa, Pelagiidae) in relation to temperature: Experimental evidence and implications. Diversity 2021, 13, 37. [Google Scholar] [CrossRef]
- Adler, L.; Jarms, G. New insights into reproductive traits of scyphozoans: Special methods of propagation in S. malayensis Goette, 1886 (Pelagiidae, Semaeostomeae) enable establishing a new classification of asexual reproduction in the class Scyphozoa. Mar. Biol. 2009, 156, 1411–1420. [Google Scholar] [CrossRef]
- Morandini, A.C.; Gul, S. Rediscovery of S. malayensis and remarks on Rhopilema nomadica record in Pakistan (Cnidaria: Scyphozoa). Pap. Avulsos Zool. 2016, 56, 171–175. [Google Scholar] [CrossRef]
- Kramp, P.L. Synopsis of the medusae of the world. J. Mar. Biol. Assoc. UK 1961, 40, 7–382. [Google Scholar]
- Gershwin, L.A.; Zeidler, W. Two new jellyfishes (Cnidaria: Scyphozoa) from tropical Australian waters. Zootaxa 2008, 1764, 41–52. [Google Scholar] [CrossRef]
- Uchida, T. Medusae in the vicinity of the Amakusa Marine Biological Station. Bull. Biogeogr. Soc. Japan 1938, 8, 143–149. [Google Scholar]
- Miyake, H.; Hashimoto, J.; Chikuchishin, M.; Miura, T. Scyphopolyps of Sanderia malayensis and Aurelia aurita attached to tubes of vestimentiferan tubeworm (Lamellibrachia satsuma) at submarine fumaroles in Kagoshima Bay. Mar. Biotechnol. 2004, 6, S174–S178. [Google Scholar]
- Straehler-Pohl, I.; Widmer, C.L.; Morandini, A.C. Characterizations of juvenile stages of some semaeostome Scyphozoa (Cnidaria), with recognition of a new family (Phacellophoridae). Zootaxa 2011, 2741, 1–37. [Google Scholar] [CrossRef]
- Schiariti, A.; Morandini, A.C.; Jarms, G.; Paes, R.V.G.; Franke, S.; Mianzan, H. Asexual reproduction strategies and blooming potential in Scyphozoa. Mar. Ecol. Prog. Ser. 2014, 510, 241–253. [Google Scholar] [CrossRef]
- Shin, K.H.; Choi, K.H. Effects of the seawater temperature and salinity on the survival and growth of the Sanderia malayensis (Cnidaria: Scyphozoa) ephyrae. Mar. Biol. Res. 2025; in press (accepted). [Google Scholar] [CrossRef]
- Kubota, S. Appearance of Sanderia malayensis at Shirahama, Wakayama Prefecture, Japan after half a century absence. Nanki Biol. Soc. 2012, 54, 147–148. [Google Scholar]
- Minemizu, R.; Kobota, S.; Hirano, Y.; Lindsay, D.J. A Photographic Guide to the Jellyfishes of Japan [Japanese]; Heibonsha: Tokyo, Japan, 2015; p. 268. [Google Scholar]
- Toshino, S.; Yamashiro, H.; Tanimoto, M. New record of Sanderia malayensis from the Ryukyu Archipelago, southern Japan. Fauna Ryukyuana 2018, 43, 19–25. [Google Scholar]
- Roabalais, N.N.; Turner, R.E.; Diaz, R.J.; Justic, D. Global change and eutrophication of coastal waters. Ices J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Båmstedt, U.; Lane, J.; Martinussen, M.B. Bioenergetics of ephyra larvae of the scyphozoan jellyfish Aurelia aurita in relation to temperature and salinity. Mar. Biol. 1999, 135, 89–98. [Google Scholar] [CrossRef]
- Widmer, C.L. Influences of Temperature and Salinity on Asexual Reproduction and Development of Scyphozoan Jellyfish from the British Isles. Ph.D. Thesis, University of St Andrews, St Andrews, UK, 2014; pp. 72–85. [Google Scholar]
- Fuchs, B.; Wang, W.; Graspeuntner, S.; Li, Y.; Insua, S.; Herbst, E.-M.; Dirksen, P.; Boehm, A.-M.; Hemmrich, G.; Sommer, F.; et al. Regulation of polyp-to-jellyfish transition in Aurelia aurita. Curr. Biol. 2014, 24, 263–273. [Google Scholar] [CrossRef]
- Uchida, T.; Sugiura, Y. On the ephyra and postephyra of Semaeostome medusa, Sanderia malayensis Goette, 1886. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1975, 19, 879–881. [Google Scholar]
- Uchida, T.; Sugiura, Y. On the polyp of the Scyphomedusa, Sanderia malayensis and its reproduction. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1978, 21, 279–286. [Google Scholar]
- Riisgård, H.U. Superfluous Feeding and Growth of Jellyfish Aurelia aurita. J. Mar. Sci. Eng. 2022, 10, 1368. [Google Scholar] [CrossRef]
- Straehler-Pohl, I.; Jarms, G. Identification key for young ephyrae: A first step for early detection of jellyfish blooms. Hydrobiologia 2010, 645, 3–21. [Google Scholar] [CrossRef]
- Båmstedt, U.; Ishii, H.; Martinussen, M.B. Is the scyphomedusa Cyanea capillata (L.) dependent on gelatinous prey for its early development? Sarsia 1997, 82, 269–273. [Google Scholar]
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only Anova Procedures. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 143–146. [Google Scholar] [CrossRef]
- Elkin, L.A.; Kay, M.; Higgins, J.J.; Wobbrock, J.O. An Aligned Rank Transform procedure for multifactor contrast tests. In Proceedings of the 34th Annual ACM Symposium on User Interface Software and Technology, Virtual, 10–14 October 2021. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. Therneau, T.M.; Grambsch, P.M. The cox model. In Modeling Survival Data: Extending the Cox Model; Springer: Berlin/Heidelberg, Germany, 2000; pp. 39–77. [Google Scholar] [CrossRef]
- Heins, A.; Sötje, I.; Holst, S. Assessment of investigation techniques for scyphozoan statoliths, with focus on early development of the jellyfish Sanderia malayensis. Mar. Ecol. Prog. Ser. 2018, 591, 37–56. [Google Scholar] [CrossRef]
- Ballesteros, A.; Siles, P.; Jourdan, E.; Gili, J.-M. Versatile aquarium for jellyfish: A rearing system for the biomass production of early life stages in flow-through or closed systems. Front. Mar. Sci. 2022, 9, 942094. [Google Scholar] [CrossRef]
- Crow, J.; Howard, M.; Lévesque, V.; Matsushige, L.; Spina, S.; Schaadt, M.; Sowinski, N.; Widmer, C.L.; Upton, B. AZA Aquatic Invertebrate TAG. In Jellyfish (Cnidaria/Ctenophora) Care Manual; Schaadt, M., Ed.; Association of Zoos and Aquariums (AZA): Silver Spring, MD, USA, 2013; pp. 5–79. [Google Scholar]
- Purcell, J.E.; White, J.R.; Nemazie, D.A.; Wright, D.A. Temperature, salinity and food effects on asexual reproduction and abundance of the scyphozoan Chrysaora quinquecirrha. Mar. Ecol. Prog. Ser. 1999, 180, 187–196. [Google Scholar] [CrossRef]
- Fu, Z.; Li, J.; Wang, J.; Lai, J.; Liu, Y.; Sun, M. Combined effects of temperature and salinity on the growth and pulsation of moon jellyfish (Aurelia coerulea) ephyrae. Am. J. Life Sci. 2020, 8, 144–151. [Google Scholar] [CrossRef]
- Yu, S.; Song, D.; Fan, M.; Xie, C. Effects of temperature and salinity on growth of Aurelia aurita. Ecol. Model. 2023, 476, 110229. [Google Scholar] [CrossRef]
- Duarte, I.M.; Marques, S.C.; Leandro, S.M.; Calado, R. An overview of jellyfish aquaculture: For food, feed, pharma and fun. Rev. Aquac. 2022, 14, 265–287. [Google Scholar] [CrossRef]
- Miranda, F.A.S.; Chambel, J.; Almeida, C.; Pires, D.; Dauarte, I.; Esteves, L.; Maranhão, P. Effect of different diets on growth and survival of the white-spotted jellyfish, Phyllorhiza punctata. In Proceedings of the International Meeting on Marine Research 2016, Peniche, Portugal, 14–15 July 2016; p. 3. [Google Scholar] [CrossRef]
- Widmer, C.L. Effects of temperature on growth of north-east Pacific moon jellyfish ephyrae, Aurelia labiata (Cnidaria: Scyphozoa). J. Mar. Biol. Assoc. UK 2005, 85, 569–573. [Google Scholar] [CrossRef]
- Kitajima, S.; Hasegawa, T.; Nishiuchi, K.; Kiyomoto, Y.; Taneda, T.; Yamada, H. Temporal fluctuations in abun dance and size of the giant jellyfish Nemopilema nomurai medusae in the northern East China Sea, 2006–2017. Mar. Biol. 2020, 167, 75. [Google Scholar] [CrossRef]
- Kitajima, S.; Iguchi, N.; Honda, N.; Watanabe, T.; Katoh, O. Distribution of Nemopilema nomurai in the southwestern Sea of Japan related to meandering of the Tsushima Warm Current. J. Oceanogr. 2015, 71, 287–296. [Google Scholar] [CrossRef]
- Yoon, W.D.; Yang, J.Y.; Shim, M.B.; Kang, H.K. Physical processes influencing the occurrence of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) around Jeju Island, Korea. J. Plankton Res. 2008, 30, 251–260. [Google Scholar] [CrossRef]

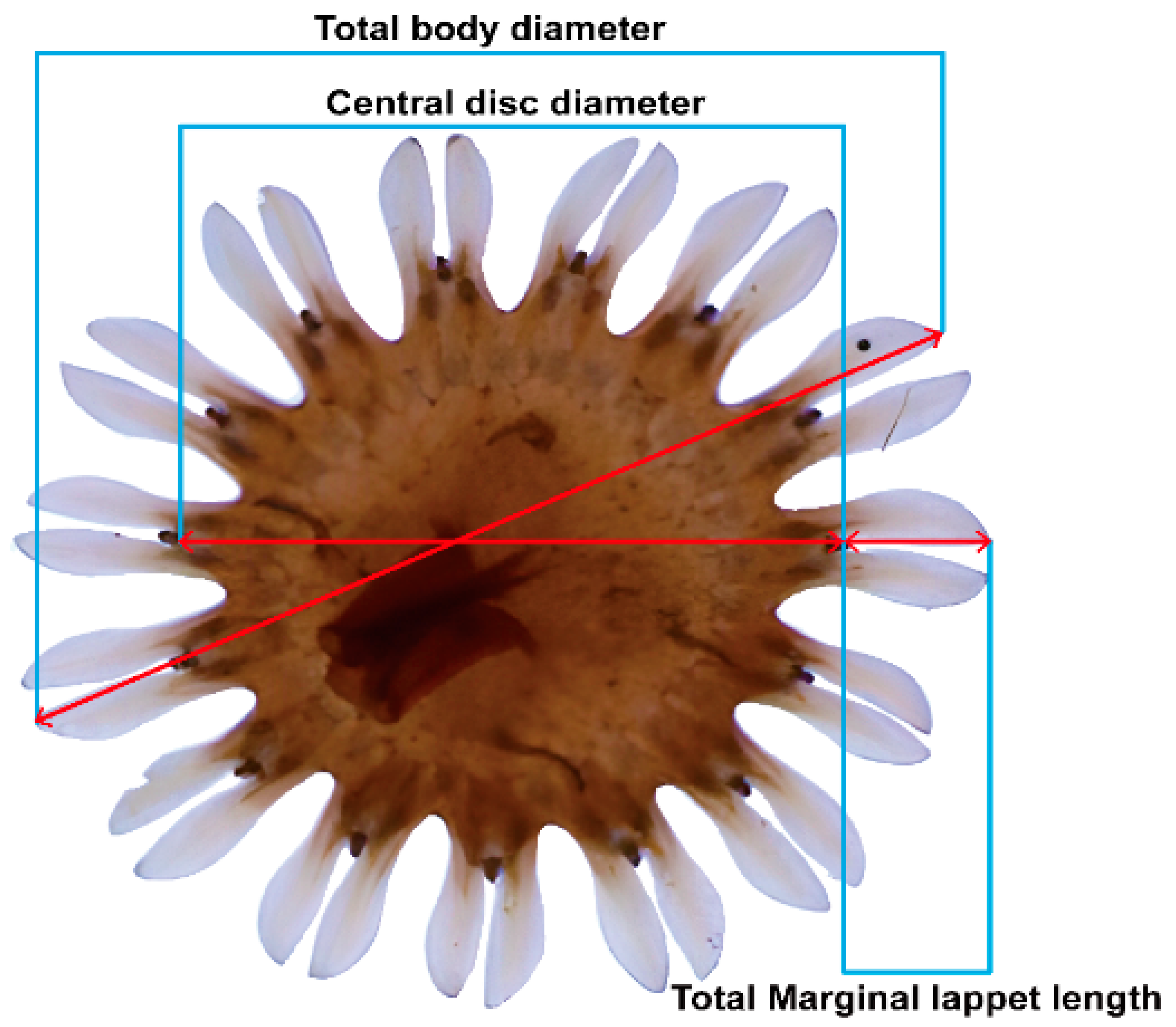




| Temperature (°C) | Salinity (PSU) | Size Increase (mm, n = 10) | 95% Confidence Interval | ||
|---|---|---|---|---|---|
| Mean | SD | Lower Limit (CI) | Upper Limit (CI) | ||
| 20 | 21 | 6.34 a | 0.14 | 6.1 | 6.7 |
| 24 | 8.47 b | 0.15 | 8.2 | 8.7 | |
| 27 | 8.51 b | 0.13 | 8.3 | 8.7 | |
| 24 | 21 | 6.35 a | 0.14 | 6.1 | 6.6 |
| 24 | 8.50 b | 0.13 | 8.3 | 8.7 | |
| 27 | 8.50 b | 0.13 | 8.3 | 8.7 | |
| Temperature (°C) | Salinity (PSU) | 95% Confidence Interval | ||
|---|---|---|---|---|
| Growth Rate (% d−1, n = 10) | Lower Limit (CI) | Upper Limit (CI) | ||
| 20 | 21 | 10.31 | 10.19 | 10.71 |
| 24 | 14.88 | 14.83 | 14.86 | |
| 27 | 14.86 | 14.83 | 15.05 | |
| 24 | 21 | 10.24 | 10.19 | 10.47 |
| 24 | 14.8 | 14.38 | 15.05 | |
| 27 | 14.94 | 14.38 | 15.05 | |
| Temperature (°C) | Source | Size Increase (mm, n = 10) | |||
|---|---|---|---|---|---|
| Degrees of Freedom | Sum of Squares | F-Value | p-Value | ||
| 20 | Time | 2 | 15,988,153.54 | 633.97 | <0.001 |
| Salinity | 10 | 23,703,043.10 | 6023.03 | <0.001 | |
| Salinity × Days | 20 | 20,791,713.35 | 234.03 | <0.001 | |
| 24 | Time | 2 | 15,974,650.91 | 633.98 | <0.001 |
| Salinity | 10 | 23,715,381.33 | 6284.18 | <0.001 | |
| Salinity × Days | 20 | 20,437,093.72 | 207.09 | <0.001 | |
| Temperature (°C) | Salinity (PSU) | Feeding Rate (Artemia ind−1 h−1, n = 10) | 95% Confidence Interval | ||
|---|---|---|---|---|---|
| Mean | SD | Lower Limit (CI) | Upper Limit (CI) | ||
| 20 | 21 | 2.10 a | 0.908 | 0.00 | 4.00 |
| 24 | 7.58 b | 1.138 | 5.00 | 10.00 | |
| 27 | 7.69 b | 1.165 | 4.00 | 10.00 | |
| 24 | 21 | 2.16 a | 0.869 | 0.00 | 5.00 |
| 24 | 7.75 b | 1.118 | 5.00 | 10.00 | |
| 27 | 7.64 b | 1.136 | 5.00 | 11.00 | |
| Source | Feeding Rate (Artemia ind−1 h−1, n = 10) | ||||
|---|---|---|---|---|---|
| Sum of Squares | Degrees of Freedom | Mean of Squares | F-Value | p-Value | |
| Time | 4489 | 2 | 2245 | 2029.04 | <0.001 |
| Salinity | 35 | 10 | 3 | 3.16 | <0.001 |
| Salinity × Days | 33 | 20 | 2 | 1.47 | 0.085 |
| Source | Feeding Rate (Artemia ind−1 h−1, n = 10) | |||
|---|---|---|---|---|
| Degrees of Freedom | Sum of Squares | F-Value | p-Value | |
| Time | 2 | 15,983,505.68 | 633.18 | <0.001 |
| Salinity | 10 | 1,186,144.58 | 3.27 | <0.001 |
| Salinity × Days | 20 | 1,927,731.32 | 2.75 | <0.001 |
| Temperature (°C) | Salinity (PSU) | Survival Rate (%) | Observed Event | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Limit (CI) | Upper Limit (CI) | ||||
| 20 | 21 a | 70 | 30 | 0.62 | 0.80 |
| 24 b | 90 | 10 | 0.84 | 0.96 | |
| 27 c | 87 | 13 | 0.81 | 0.94 | |
| 24 | 21 a | 55 | 45 | 0.46 | 0.66 |
| 24 b | 79 | 21 | 0.69 | 0.86 | |
| 27 c | 86 | 14 | 0.79 | 0.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, K.-H.; Choi, K.-H. The Effects of Salinity on the Growth, Survival, and Feeding of Sanderia malayensis (Cnidaria: Scyphozoa) Ephyrae. Diversity 2025, 17, 239. https://doi.org/10.3390/d17040239
Shin K-H, Choi K-H. The Effects of Salinity on the Growth, Survival, and Feeding of Sanderia malayensis (Cnidaria: Scyphozoa) Ephyrae. Diversity. 2025; 17(4):239. https://doi.org/10.3390/d17040239
Chicago/Turabian StyleShin, Kyong-Ho, and Keun-Hyung Choi. 2025. "The Effects of Salinity on the Growth, Survival, and Feeding of Sanderia malayensis (Cnidaria: Scyphozoa) Ephyrae" Diversity 17, no. 4: 239. https://doi.org/10.3390/d17040239
APA StyleShin, K.-H., & Choi, K.-H. (2025). The Effects of Salinity on the Growth, Survival, and Feeding of Sanderia malayensis (Cnidaria: Scyphozoa) Ephyrae. Diversity, 17(4), 239. https://doi.org/10.3390/d17040239







