Differential Accumulation of Particulate Pollutants in Gills and Gastrointestinal Tracts in Sphoeroides Fish from Tropical and Subtropical Estuaries in Brazil
Abstract
1. Introduction
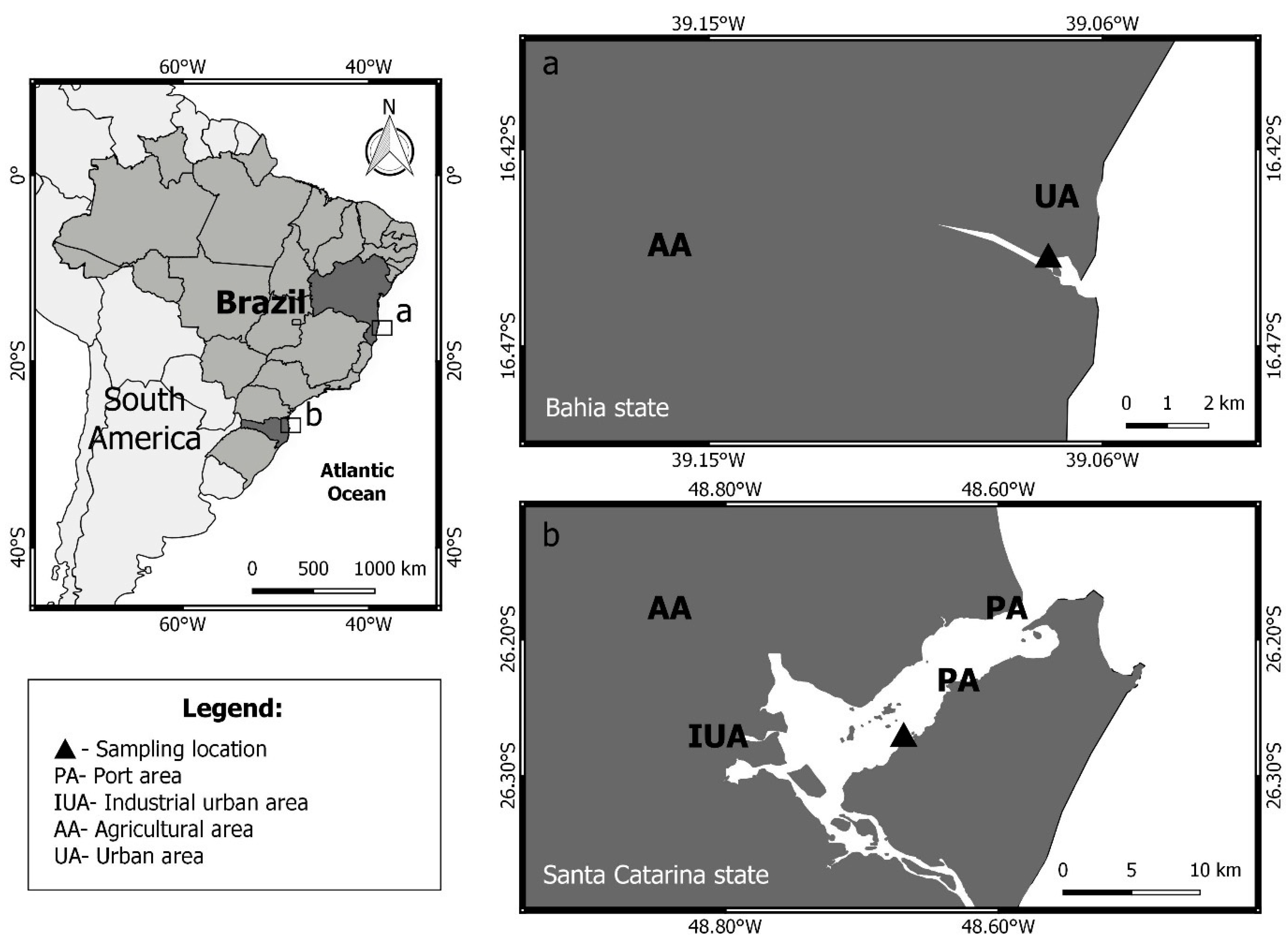
2. Material and Methods
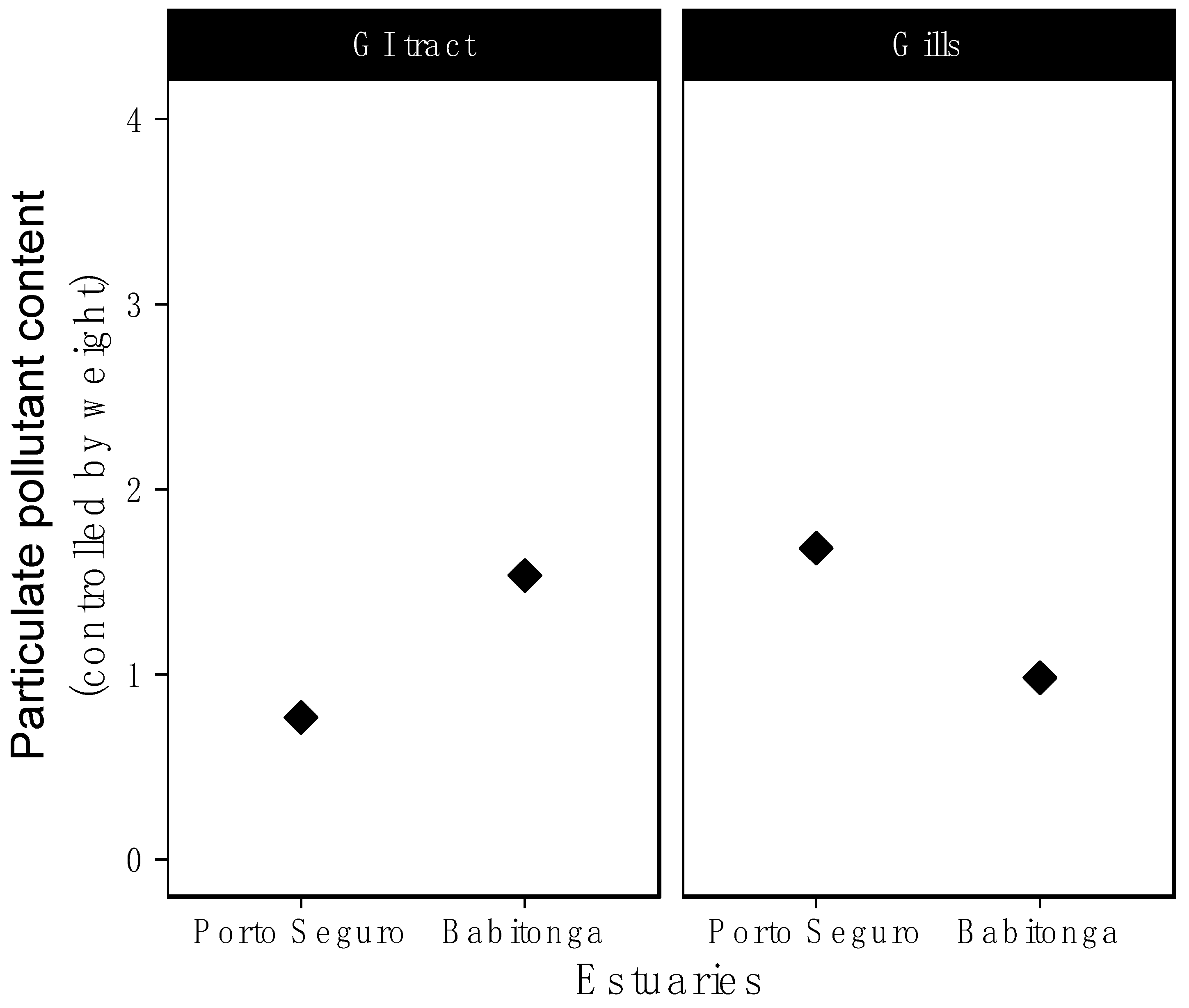
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Einoder, L.D.; MacLeod, C.K.; Coughanowr, C. Metal and isotope analysis of bird feathers in a contaminated estuary reveals bioaccumulation, biomagnification, and potential toxic effects. Arch. Environ. Contam. Toxicol. 2018, 75, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Lana, P.C.; Bernardino, A.F. (Eds.) Brazilian Estuaries: A Benthic Perspective; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Paitach, P.L.; Simões-Lopes, P.C.; Cremer, M.J. Tidal and seasonal influences in dolphin habitat use in a southern Brazilian estuary. Sci. Mar. 2017, 81, 49–56. [Google Scholar] [CrossRef]
- Vasconcelos, R.P.; Reis-Santos, P.; Costa, M.J.; Cabral, H.N. Connectivity between estuaries and marine environment: Integrating metrics to assess estuarine nursery function. Ecol. Indic. 2011, 11, 1123–1133. [Google Scholar] [CrossRef]
- Santos, T.A.; Bonfim, T.M.; Silva, F.S.; Silva, A.G.; Bandeira, M.L.S.F.; Jesus, R.M.; Nascimento, L.D. Determination of metals in an estuarine system: Laguncularia racemosa as a potential indicator of contamination. Braz. J. Environ. Sci. 2018, 49, 51–65. [Google Scholar] [CrossRef]
- Trevizani, T.H.; Domit, C.; Santos, M.C.O.; Figueira, R.C.L. Bioaccumulation of heavy metals in estuaries in the southwest Atlantic Ocean. Environ. Sci. Pollut. Res. 2023, 30, 26703–26717. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Lusher, A.L.; O’Donnell, C.; Officer, R.; O’Connor, I. Microplastic interactions with North Atlantic mesopelagic fish. ICES J. Mar. Sci. 2016, 73, 1214–1225. [Google Scholar] [CrossRef]
- Gokul, T.; Ramesh Kumar, K.; Prema, P.; Arun, A.; Balaji, P.; Faggio, C. Particulate pollution and its toxicity to fish: An overview. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 270, 109646. [Google Scholar] [CrossRef]
- Haward, M. Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nat. Commun. 2018, 9, 667. [Google Scholar] [CrossRef]
- Li, H.X.; Ma, L.S.; Lin, L.; Ni, Z.X.; Xu, X.R.; Shi, H.H.; Yan, Y.; Zheng, G.M.; Rittschof, D. Microplastics in oysters Saccostrea cucullata along the Pearl River Estuary, China. Environ. Pollut. 2018, 236, 619–625. [Google Scholar] [CrossRef] [PubMed]
- van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Bouças da Silva, D.L.; Gil, J.; Nascimento, E.P.; Costa, H.A.; Paixão, R. Poluição plástica no litoral brasileiro: Percepções de gestores de meios de hospedagem sobre consumo de descartáveis. Rev. Bras. Pesqui. Em Tur. 2022, 16, e2481. [Google Scholar] [CrossRef]
- Global Plastic Production. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 31 March 2025).
- Hollman, P.C.H.; Bouwmeester, H.; Peters, R.J.B. Microplastics in Aquatic Food Chain: Sources, Measurement, Occurrence and Potential Health Risks; RIKILT Report; Wageningen UR: Wageningen, The Netherlands, 2013; 28p. [Google Scholar]
- Krelling, A.P.; Possatto, F.E.; Maia, G.R.A.; Araki, K.; Olivieri, G.B.R.; Nogueira, H.P. First Evidence of High Microplastic Concentrations in Estuarine Litter Windrows of a World Heritage Site: The Paranaguá Estuarine Complex, Brazil. Water Air Soil Pollut. 2025, 236, 266. [Google Scholar] [CrossRef]
- Menezes, K.M.; da Costa, M.B.; Schuab, J.M.; Dalbó, G.Z.; Alves, M.M.; Ocarisd, E.R.Y.; Rodrigues, F.L. Microplastic contamination in the mangroves of Piraquê-Açu and Piraquê-Mirim rivers, Aracruz (Brazil): An analysis in sediment, water, and biota. Mar. Pollut. Bull. 2025, 214, 117696. [Google Scholar] [CrossRef]
- Parra, D.F.; Gimiliani, G.T.; Dos Santos, J.L.; Wetter, N.U.; Schepis, W.R.; Bereczki, A.; Cotrim, M.E.B. Microplastics in Santos São Vicente estuarine–Hotspot in sediments caused by low energy hydrodynamic events in strongly populated areas. Mar. Pollut. Bull. 2025, 210, 117286. [Google Scholar] [CrossRef]
- Pegado, T.S.S.; Schmid, K.; Winemiller, K.O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018, 133, 814–821. [Google Scholar] [CrossRef]
- Possatto, F.E.; Barletta, M.; Costa, M.F.; Ivar do Sul, J.A.; Dantas, D.V. Plastic debris ingestion by marine catfish: An unexpected fisheries impact. Mar. Pollut. Bull. 2011, 62, 1098–1102. [Google Scholar] [CrossRef]
- Ribeiro, V.V.; Casado-Coy, N.; Rangel, D.F.; Sanz-Lazaro, C.; Castro, Í.B. Microplastic in bivalves of an urbanized Brazilian estuary: Human modification, population density and vegetation influence. J. Hazard. Mater. 2025, 482, 136546. [Google Scholar] [CrossRef]
- Vendel, A.L.; Bessa, F.; Alves, V.E.N.; Amorim, A.L.A.; Patrício, J.; Palma, A.R.T. Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar. Pollut. Bull. 2017, 117, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef]
- Kabir, A.E.; Michon, E.; Mingelbier, M.; Robert, D.; Soubaneh, Y.D.; Xie, H.; Lu, Z. Microplastics in the benthic fish from the Canadian St. Lawrence River and Estuary: Occurrence, spatial distribution and ecological risk assessment. Mar. Pollut. Bull. 2025, 212, 117509. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Paz, A.; Encinas-García, T.; Ávila-Félix, L.; Mendoza-Cano, F. Microplastics in surface waters surrounding a touristic beach at the Gulf of California, Mexico. Int. J. Environ. Health Res. 2025, 1–13. [Google Scholar] [CrossRef]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.G.L.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Wang, W.; Guo, S.; Li, B.; Xu, D.; Gao, B. Microplastics in Pearl River Estuary and Yangtze River Estuary, China: Occurrence, fragmentation and pollution risk. J. Hazard. Mater. Adv. 2025, 17, 100529. [Google Scholar] [CrossRef]
- Goswami, P.; Vinithkumar, N.V.; Dharani, G. First evidence of microplastics bioaccumulation by marine organisms in the Port Blair Bay, Andaman Islands. Mar. Pollut. Bull. 2020, 155, 111163. [Google Scholar] [CrossRef]
- Kalangutkar, N.; Mhapsekar, S.; Rajagopal, P. Comparative assessment of microplastic pollution in Terekhol and Sal estuaries, Goa, India. Environ. Earth Sci. 2025, 84, 48. [Google Scholar] [CrossRef]
- Foekema, E.M.; de Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.T.J.; Koelmans, A.A. Plastic in North Sea fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
- Romeo, T.; Battaglia, P.; Pedá, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Shi, Y.; Zhao, Y.; Zheng, S.; Liang, J.; Liu, T.; Tian, Z. Characteristics and retention of microplastics in the digestive tracts of fish from the Yellow Sea. Environ. Pollut. 2019, 249, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.R.M.; Ying, P.X.; Zainuddin, A.H.; Razak, M.R.; Aris, A.Z. Occurrence, abundance, and distribution of microplastics pollution: An evidence in surface tropical water of Klang River estuary, Malaysia. Environ. Geochem. Health 2021, 43, 3733–3748. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.R.M.; Zaid, S.H.M.; Zainuddin, A.H.; Aris, A.Z. Microplastic pollution in tropical estuary gastropods: Abundance, distribution and potential sources of Klang River estuary, Malaysia. Mar. Pollut. Bull. 2021, 162, 111866. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro de Sena, S.; de Souza Veiga, R.; De Amorim Silva, V. Análise de áreas de risco à degradação ambiental no município de Porto Seguro, Bahia, Brasil. Braz. J. Phys. Geogr. 2023, 16, 3059–3072. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef]
- Sinha, P.; Saini, V.; Varshney, N.; Pandey, R.K.; Jha, H.C. The Infiltration of Microplastics in Human Systems: Gastrointestinal Accumulation and Pathogenic Impacts. Heliyon 2025, 11, e42606. [Google Scholar] [CrossRef]
- Wang, J.; Peng, J.; Tian, Z.; Gao, Y.; Zhan, Z.; Chen, Q.; Cai, L. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar] [CrossRef]
- Wang, L.C.; Lin, J.C.; Dong, C.; Chen, C.; Liu, T. The sorption of persistent organic pollutants in microplastics from the coastal environment. J. Hazard. Mater. 2021, 420, 126658. [Google Scholar] [CrossRef] [PubMed]
- Cverenkárová, K.; Valachovicová, M.; Mackul’ak, T.; Žemlicka, L.; Bírošová, L. Microplastics in the food chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Smith, K.L., Jr. Plastics on the Sargasso Sea surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Sheraz, M.; Kim, J.; Kim, J. Nano/microplastics in indoor air: A critical review of synthesis routes for toxicity testing and preventative measure strategies. Process Saf. Environ. Prot. 2023, 180, 274–304. [Google Scholar] [CrossRef]
- VishnuRadhan, R.; Thresyamma, D.D.; Eldho, T.I.; Bhagat, J. Atmospheric plastics- a potential airborne fomite with an emerging climate signature. J. Clim. Change Health 2021, 3, 100037. [Google Scholar] [CrossRef]
- IBGE—Brazilian Institute of Geography and Statistics. Demographic Census 2022; IBGE: Rio de Janeiro, Brazil, 2022. [Google Scholar]
- Filgueras, A.S.; Silva, T.S.; Corrêa, I.C.S. Impacts of sea level rise and land use on coastal wetlands: Methodology applied to Baía da Babitonga (SC). Soc. Nat. 2023, 36, e69403. [Google Scholar] [CrossRef]
- Mazzer, A.M.; Gonçalves, M.L. Aspectos geomorfológicos da Baía da Babitonga, Santa Catarina, Brazil: Caracterização morfométrica. Rev. Bras. Geomorfol. 2011, 12, 115–120. [Google Scholar] [CrossRef][Green Version]
- Figueiredo, J.L.; Menezes, N.A. Manual de Peixes Marinhos do Sudeste do Brasil. IV. Teleostei (5); Museu de Zoologia-USP: São Paulo, Brazil, 2000. [Google Scholar]
- Vargas, J.G.M.; Silva, V.B.; Oliveira, L.K.; Molina, E.F. Microplásticos: Uso na indústria cosmética e impactos no ambiente aquático. Química Nova 2022, 45, 705–711. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Ho, B.Y.; Larat, V.; Salamatinia, B. Microplastics in eviscerated flesh and excised organs of dried fish. Sci. Rep. 2017, 7, 5473. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.N.; Robert, K.C. Permanova+ for Primer: Guide to Software and Statistical Methods; Primer-E: Plymouth, UK, 2008. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 31 March 2025).
- Marinsek, G.P.; Gusso-Choueri, P.K.; Choueri, R.B.; Abessa, D.M.S.; Gonçalves, A.R.N.; Bortolotto, L.B.; Mari, R.B. Integrated analysis of fish intestine biomarkers: Complementary tools for pollution assessment. Mar. Pollut. Bull. 2022, 178, 113590. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef]
- Arias, A.H.; Ronda, A.C.; Oliva, A.L. Evidence of microplastic ingestion by fish from the Bahía Blanca Estuary in Argentina, South America. Bull. Environ. Contam. Toxicol. 2019, 102, 750–756. [Google Scholar] [CrossRef]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Poh, F. Seafloor microplastic hotspots controlled by deep-sea circulation. Science 2020, 368, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Löder, M.G.J.; Fricke, N.F.; Lang, T.; Griebeler, E.M.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Davison, P.; Asch, R.G. Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. Mar. Ecol. Prog. Ser. 2011, 432, 173–180. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H. Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci. Rep. 2016, 6, 34351. [Google Scholar] [CrossRef]
- Cozar, A.; Echevarría, F.; Gonzalez-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernandez-León, S.; Palma, A.T.; Navarro, S.; Garcia-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.L.; Tonkin, A.; Galloway, T.; Thompson, R.C. Accumulations of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- IPPUJ. Joinville—City in Data; Research and Planning Institute for the Sustainable Development of Joinville: Joinville, Brazil, 2011. [Google Scholar]
- IBGE—Brazilian Institute of Geography and Statistics. Demographic Census 2010; IBGE: Rio de Janeiro, Brazil, 2010. [Google Scholar]
- Silva, A.C.R.S.; Bernardes, M.E.C.; Assireu, A.T.; Siegle, E.; Sousa, P.H.G.O.; Brown, D. Hydrodynamics of a tropical estuary: Buranhém River, Porto Seguro, Brazil. Braz. J. Water Resour. 2018, 23, 1–9. [Google Scholar] [CrossRef]
- Batel, A.; Borchert, F.; Reinwald, H.; Erdinger, L.; Braunbeck, T. Microplastic accumulation patterns and transfer of benzo[a]pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 2018, 235, 918–930. [Google Scholar] [CrossRef]
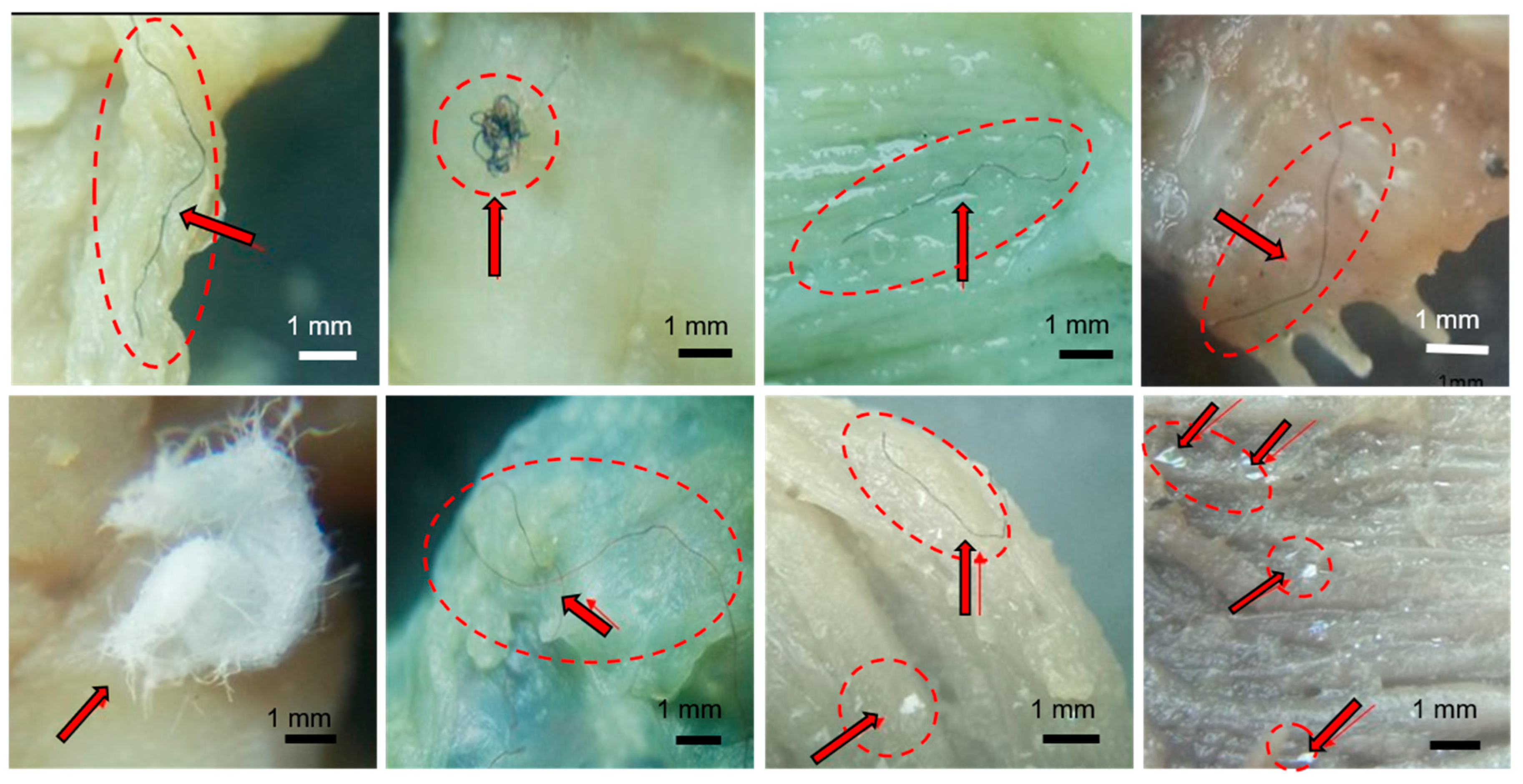
| Estuaries | PP Number GI | PP Number Gills | Total PP Number |
|---|---|---|---|
| Porto Seguro | 80 (3.6) | 419 (19.0) | 499 (11.3) |
| Babitonga Bay | 170 (10.0) | 76 (5.1) | 246 (7.3) |
| GI FO% of PP | |||
|---|---|---|---|
| Estuaries | Fragment (n) | Fiber (n) | Film (n) |
| Porto Seguro | 13.6% | 90.9% | 13.6% |
| Babitonga Bay | 64.7% | 94.1% | 35.3% |
| Gill FO% of PP | |||
| Estuaries | Fragment (n) | Fiber (n) | Film (n) |
| Porto Seguro | 4.5% | 63.6% | 72.8% |
| Babitonga Bay | 33.3% | 86.7% | 13.3% |
| GI—Types of PP | ||||
|---|---|---|---|---|
| Estuaries | Fragment (n) | Fiber (n) | Film (n) | Total (n) |
| Porto Seguro | 4 (5%) | 70 (87.5%) | 6 (7.5%) | 80 |
| Babitonga Bay | 47 (27.7%) | 99 (58.2%) | 24 (14.1%) | 170 |
| Gill—Types of PP | ||||
| Estuaries | Fragment (n) | Fiber (n) | Film (n) | Total |
| Porto Seguro | 1 (0.2%) | 41 (9.8%) | 377 (90%) | 419 |
| Babitonga Bay | 10 (13.2%) | 45 (59.2%) | 21 (27.6%) | 76 |
| GI FO% of the Sizes (mm) of PP Types | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fragment | Fiber | Film | |||||||
| Estuary | ≤1 | 1.1–3 | 3.1–5 | ≤1 | 1.1–3 | 3.1–5 | ≤1 | 1.1–3 | 3.1–5 |
| Porto Seguro | 9% | 0 | 4.5% | 59.1% | 59.1% | 54.5% | 13.6% | 4.5% | 0 |
| Babitonga Bay | 41.2% | 29.4% | 11.8% | 70.6% | 70.6% | 64.7% | 0 | 35.3% | 11.8% |
| Gill FO% of the Sizes (mm) of PP Types | |||||||||
| Fragment | Fiber | Film | |||||||
| Estuary | ≤1 | 1.1–3 | 3.1–5 | ≤1 | 1.1–3 | 3.1–5 | ≤1 | 1.1–3 | 3.1–5 |
| Porto Seguro | 0 | 4.5% | 0 | 27.3% | 45.5% | 50% | 63.6% | 54.5% | 40.9% |
| Babitonga Bay | 33.3% | 6.7% | 6.7% | 73.3% | 33.3% | 53.3% | 0 | 13.3% | 6.7% |
| GI—Sizes of the PP Types (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fragment | Fiber | Film | |||||||
| Estuary | ≤1 | 1–3 | 3.1–5 | ≤1 | 1–3 | 3.1–5 | ≤1 | 1–3 | 3.1–5 |
| Porto Seguro | 3 (75%) | 0 | 1 (25%) | 29 (41.4%) | 25 (35.8%) | 16 (22.8%) | 5 (83.3%) | 1 (16.7%) | 0 |
| Babitonga Bay | 22 (46.8%) | 14 (29.8%) | 11 (23.4%) | 47 (47.5%) | 23 (23.2%) | 29 (29.3%) | 0 | 20 (83.3%) | 4 (16.7%) |
| Gill—Sizes of the PP types (mm) | |||||||||
| Fragment | Fiber | Film | |||||||
| Estuary | ≤1 | 1–3 | 3.1–5 | ≤1 | 1–3 | 3.1–5 | ≤1 | 1–3 | 3.1–5 |
| Porto Seguro | 0 | 1 (100%) | 0 | 11 (26.8%) | 18 (43.9%) | 12 (29.3%) | 194 (51.4%) | 162 (43%) | 21 (5.6%) |
| Babitonga Bay | 6 (60%) | 3 (30%) | 1 (10%) | 19 (42.2%) | 17 (37.8%) | 9 (20%) | 0 | 15 (71.4%) | 6 (28.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filho, S.M.d.S.; Grassi, M.T.; dos Santos, M.P.; Morimoto, J.; Soeth, M.; Fávaro, L.F. Differential Accumulation of Particulate Pollutants in Gills and Gastrointestinal Tracts in Sphoeroides Fish from Tropical and Subtropical Estuaries in Brazil. Diversity 2025, 17, 300. https://doi.org/10.3390/d17040300
Filho SMdS, Grassi MT, dos Santos MP, Morimoto J, Soeth M, Fávaro LF. Differential Accumulation of Particulate Pollutants in Gills and Gastrointestinal Tracts in Sphoeroides Fish from Tropical and Subtropical Estuaries in Brazil. Diversity. 2025; 17(4):300. https://doi.org/10.3390/d17040300
Chicago/Turabian StyleFilho, Sérgio Murilo de Souza, Marco Tadeu Grassi, Mayara Padovan dos Santos, Juliano Morimoto, Marcelo Soeth, and Luís Fernando Fávaro. 2025. "Differential Accumulation of Particulate Pollutants in Gills and Gastrointestinal Tracts in Sphoeroides Fish from Tropical and Subtropical Estuaries in Brazil" Diversity 17, no. 4: 300. https://doi.org/10.3390/d17040300
APA StyleFilho, S. M. d. S., Grassi, M. T., dos Santos, M. P., Morimoto, J., Soeth, M., & Fávaro, L. F. (2025). Differential Accumulation of Particulate Pollutants in Gills and Gastrointestinal Tracts in Sphoeroides Fish from Tropical and Subtropical Estuaries in Brazil. Diversity, 17(4), 300. https://doi.org/10.3390/d17040300








