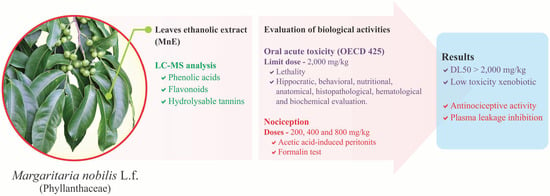
Margaritaria nobilis L.f. (Phyllanthaceae) Ethanolic Extract: Low Acute Oral Toxicity and Antinociceptive Activity
Abstract
:1. Introduction
2. Results
2.1. MnE Phytochemical Composition
2.2. MnE Acute Oral Toxicity
2.2.1. MnE Does Not Change Hippocratic Signs, Motor Behavior, or Emotionality, or Cause Deaths
2.2.2. MnE Does Not Alter Water or Feed Consumption, Weight Gain, or Organ Histology
2.2.3. MnE Does Not Interfere with the WBC or Liver and Kidney Function Markers
2.3. MnE Antinociceptive and Antiinflamatory Activity
2.3.1. MnE Reduces ACA-Induced Abdominal Writhing and Plasma Leakage
2.3.2. MnE Reduces the Nociception in the Inflammatory Phase of the Formalin Test
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Collection and Extract Preparation Protocol
4.3. Phytochemical Analysis
4.4. Animals
4.5. Drug Solutions and Administration
4.6. Acute Oral Toxicity
4.6.1. Treatment and Hippocratic Screening
4.6.2. Open Field Test (OF)
4.6.3. Blood Count
4.6.4. Biochemical Assays
Sample
Hepatic Function Assays
Kidney Function Assays
4.6.5. Histopathological Analysis
4.7. Antinociceptive Activity
4.7.1. Acetic-Acid-Induced Peritonitis
4.7.2. Formalin Test (FT)
4.7.3. Open Field (OF) Test
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karshikoff, B.; Tadros, M.A.; Mackey, S.; Zouikr, I. Neuroimmune Modulation of Pain across the Developmental Spectrum. Curr. Opin. Behav. Sci. 2019, 28, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular Mechanism of the Anti-Inflammatory Effects of Plant Essential Oils: A Systematic Review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef] [PubMed]
- Zobdeh, F.; Eremenko, I.I.; Akan, M.A.; Tarasov, V.V.; Chubarev, V.N.; Schiöth, H.B.; Mwinyi, J. Pharmacogenetics, and Pain Treatment with a Focus on Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Antidepressants: A Systematic Review. Pharmaceutics 2022, 14, 1190. [Google Scholar] [CrossRef]
- Kim, K.-H.; Seo, H.-J.; Abdi, S.; Huh, B. All about Pain Pharmacology: What Pain Physicians Should Know. Korean J. Pain 2020, 33, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- WFO. Margaritaria nobilis L.f. Published on the Internet. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000236325 (accessed on 8 December 2022).
- Santiago, J.C.C.; Albuquerque, C.A.B.; Muribeca, A.D.J.B.; Sá, P.R.C.; Pamplona, S.D.G.S.R.; Silva, C.Y.Y.E.; Ribera, P.C.; Fontes-Júnior, E.D.A.; Da Silva, M.N. Margaritaria nobilis L.F. (Phyllanthaceae): Ethnopharmacology and Application of Computational Tools in the Annotation of Bioactive Molecules. Metabolites 2022, 12, 681. [Google Scholar] [CrossRef]
- Moraes, L.S.; Donza, M.R.H.; Rodrigues, A.P.D.; Silva, B.J.M.; Brasil, D.S.B.; Zoghbi, M.D.G.B.; Andrade, E.H.A.; Guilhon, G.M.S.P.; Silva, E.O. Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae). Molecules 2015, 20, 22157–22169. [Google Scholar] [CrossRef]
- Arbain, D.; Byrne, L.; Cannon, J.; Engelhardt, L.; White, A. The Alkaloids of Margaritaria indica (Euphorbiaceae). The Crystal Structure and Absolute Configuration of the Hydrobromide of (+)-15α-Methoxy-14,15-Dihydrophyllochrysine. Aust. J. Chem. 1990, 43, 439. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Sofidiya, M.O.; Afolayan, A.J. Anti-Inflammatory and Analgesic Activities of the Aqueous Extracts of Margaritaria discoidea (Euphorbiaceae) Stem Bark in Experimental Animal Models. Regist. Behav. Tech. 2008, 57, 4. [Google Scholar] [CrossRef]
- Dickson, R.; Fleischer, T.; Ekuadzi, E.; Mensah, A.; Annan, K.; Woode, E. Antibacterial, Antioxidant and Anti-Inflammatory Properties of Margaritaria discoidea, a Wound Healing Remedy from Ghana. Pharmacogn. J. 2010, 2, 32–39. [Google Scholar] [CrossRef]
- Ekuadzi, E.; Dickson, R.; Fleischer, T.; Annan, K.; Pistorius, D.; Oberer, L.; Gibbons, S. Flavonoid Glycosides from the Stem Bark of Margaritaria discoidea Demonstrate Antibacterial and Free Radical Scavenging Activities: Antibacterial and Antioxidant Flavonoid Glycosides. Phytother. Res. 2014, 28, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.-W. Cytotoxic Effects of Stem Bark Extracts and Pure Compounds from Margaritaria discoidea on Human Ovarian Cancer Cell Lines. Phytomedicine 2015, 22, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hell, T.; Rutz, A.; Dürr, L.; Dobrzyński, M.; Reinhardt, J.K.; Lehner, T.; Keller, M.; John, A.; Gupta, M.; Pertz, O.; et al. Combining Activity Profiling with Advanced Annotation to Accelerate the Discovery of Natural Products Targeting Oncogenic Signaling in Melanoma. Natl. Prod. Acad. Sci. USA 2022, 85, 1540–1554. [Google Scholar] [CrossRef] [PubMed]
- Benrahou, K.; Mrabti, H.N.; Assaggaf, H.M.; Mortada, S.; Salhi, N.; Rouas, L.; El Bacha, R.; Dami, A.; Masrar, A.; Alshahrani, M.M.; et al. Acute and Subacute Toxicity Studies of Erodium guttatum Extracts by Oral Administration in Rodents. Toxins 2022, 14, 735. [Google Scholar] [CrossRef] [PubMed]
- Kpemissi, M.; Metowogo, K.; Melila, M.; Veerapur, V.P.; Negru, M.; Taulescu, M.; Potârniche, A.-V.; Suhas, D.S.; Puneeth, T.A.; Vijayakumar, S.; et al. Acute and Subchronic Oral Toxicity Assessments of Combretum micranthum (Combretaceae) in Wistar Rats. Toxicol. Rep. 2020, 7, 162–168. [Google Scholar] [CrossRef]
- OECD. Test No. 420: Acute Oral Toxicity—Fixed Dose Procedure. OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Pairs, France, 2002. [Google Scholar] [CrossRef]
- Ministério da Saúde. Resolução-Re N° 90. 16 March 2004. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2004/rdc0090_16_03_2004.html (accessed on 5 March 2023).
- Jothy, S.L.; Zakaria, Z.; Chen, Y.; Lau, Y.L.; Latha, L.Y.; Sasidharan, S. Acute Oral Toxicity of Methanolic Seed Extract of Cassia Fistula in Mice. Molecules 2011, 16, 5268–5282. [Google Scholar] [CrossRef]
- Shahed-Al-Mahmud, M.; Lina, S.M.M. Evaluation of Sedative and Anxiolytic Activities of Methanol Extract of Leaves of Persicaria hydropiper in Mice. Clin. Phytosci. 2017, 3, 20. [Google Scholar] [CrossRef]
- Baldo, B.A. Toxicities of Opioid Analgesics: Respiratory Depression, Histamine Release, Hemodynamic Changes, Hypersensitivity, Serotonin Toxicity. Arch. Toxicol. 2021, 95, 2627–2642. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Lopes, K.; Oliveira, J.; Sousa-Junior, F.J.C.; Santos, T.D.F.; Andrade, D.; Andrade, S.L.; Pereira, W.L.; Gomes, P.W.P.; Monteiro, M.C.; E Silva, C.Y.Y.; et al. Chemical Composition, Toxicity, Antinociceptive, and Anti-Inflammatory Activity of Dry Aqueous Extract of Varronia multispicata (Cham.) Borhidi (Cordiaceae) Leaves. Front. Pharmacol. 2019, 10, 1376. [Google Scholar] [CrossRef]
- Falcão, T.R.; Araújo, A.A.D.; Soares, L.A.L.; Farias, I.B.D.; Silva, W.A.V.D.; Ferreira, M.R.A.; Araújo, R.F.D., Jr.; Medeiros, J.S.D.; Lopes, M.L.D.D.S.; Guerra, G.C.B. Libidibia ferrea Fruit Crude Extract and Fractions Show Anti-Inflammatory, Antioxidant, and Antinociceptive Effect In Vivo and Increase Cell Viability In Vitro. Evid.-Based Complement. Altern. Med. 2019, 2019, 6064805. [Google Scholar] [CrossRef] [PubMed]
- Dantas, L.L.S.F.R.; Fonseca, A.G.; Pereira, J.R.; Furtado, A.A.; Gomes, P.A.T.M.; Fernandes-Pedrosa, M.F.; Leite, A.C.L.; Rêgo, M.J.B.M.; Pitta, M.G.R.; Lemos, T.M.A.M. Anti-Inflammatory and Antinociceptive Effects of the Isatin Derivative (Z)-2-(5-Chloro-2-Oxoindolin-3-Ylidene)-N-Phenyl-Hydrazinecarbothioamide in Mice. Braz. J. Med. Biol. Res. 2020, 53, e10204. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Shajib, S.; Rashid, R.B.; Khan, M.F.; Al-Mansur, A.; Datta, B.K.; Rashid, M.A. Antinociceptive Activities of Artocarpus lacucha Buch-Ham (Moraceae) and Its Isolated Phenolic Compound, Catechin, in Mice. BMC Complement. Altern. Med. 2019, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Valle-Dorado, M.G.; Hernández-León, A.; Nani-Vázquez, A.; Ángeles-López, G.E.; González-Trujano, M.E.; Ventura-Martínez, R. Antinociceptive Effect of Mansoa alliacea Polar Extracts Involves Opioid Receptors and Nitric Oxide in Experimental Nociception in Mice. Biomed. Pharmacother. 2022, 152, 113253. [Google Scholar] [CrossRef]
- De Lima, M.N.N.; Guimarães, B.A.; De Castro, A.L.S.; Ribeiro, K.B.; Miller, D.C.; Da Silva, P.I.C.; Freitas, J.J.S.; De Lima, A.B.; Setzer, W.N.; Da Silva, J.K.R.; et al. Chemical Composition and Antinociceptive and Anti-Inflammatory Activity of the Essential Oil of Hyptis crenata Pohl Ex Benth. from the Brazilian Amazon. J. Ethnopharmacol. 2023, 300, 115720. [Google Scholar] [CrossRef]
- Da Cruz De Moraes, S.Z.; Shan, A.Y.K.V.; Oliveira Melo, M.A.; Pereira Da Silva, J.; Rocha Santos Passos, F.; De Souza Graça, A.; Araújo, B.S.D.; Quintans, J.D.S.S.; Quintans Júnior, L.J.; Oliveira Barreto, E.D.; et al. Antinociceptive and Anti-Inflammatory Effect of Poincianella pyramidalis (Tul.) L.P. Queiroz. J. Ethnopharmacol. 2020, 254, 112563. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.W.; Sohng, J.K.; Pandey, R.P.; Park, Y.I. The Immunostimulating Activity of Quercetin 3-O-Xyloside in Murine Macrophages via Activation of the ASK1/MAPK/NF-ΚB Signaling Pathway. Int. Immunopharmacol. 2016, 31, 88–97. [Google Scholar] [CrossRef]
- Seo, J.Y.; Pandey, R.P.; Lee, J.; Sohng, J.K.; Namkung, W.; Park, Y.I. Quercetin 3-O-Xyloside Ameliorates Acute Pancreatitis in Vitro via the Reduction of ER Stress and Enhancement of Apoptosis. Phytomedicine 2019, 55, 40–49. [Google Scholar] [CrossRef]
- Ojo, O.A.; Rotimi, D.E.; Ojo, A.B.; Ogunlakin, A.D.; Ajiboye, B.O. Gallic Acid Abates Cadmium Chloride Toxicity via Alteration of Neurotransmitters and Modulation of Inflammatory Markers in Wistar Rats. Sci. Rep. 2023, 13, 1577. [Google Scholar] [CrossRef] [PubMed]
- Corbett, S.; Daniel, J.; Drayton, R.; Field, M.; Steinhardt, R.; Garrett, N. Evaluation of the Anti-Inflammatory Effects of Ellagic Acid. J. PeriAnesth. Nurs. 2010, 25, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a Potential Cytostatic and Cure for Inflammatory Disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.J.; Rasool, M. Dietary component p-coumaric acid suppresses monosodium urate crystal-induced inflammation in rats. Inflamm. Res. 2013, 62, 489–498. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Feng, Y.-M.; Kong, W.-S.; Li, S.-N.; Sun, X.-J.; Zhou, G.; Xie, R.-F.; Zhou, X. Gallic Acid Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice via Inhibiting NLRP3 Inflammasome. Front. Pharmacol. 2023, 14, 1095721. [Google Scholar] [CrossRef]
- Zhou, P.; Lai, J.; Li, Y.; Deng, J.; Zhao, C.; Huang, Q.; Yang, F.; Yang, S.; Wu, Y.; Tang, X.; et al. Methyl Gallate Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Inhibiting TLR4/NF-ΚB Pathway. Int. J. Mol. Sci. 2022, 23, 14024. [Google Scholar] [CrossRef]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure; Organisation for Economic Co-operation and Development: Paris, France, 2022. [Google Scholar] [CrossRef]
- Malone, M.H. Pharmacological Approaches to Natural Product Screening and Evaluation. In New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity; Wagner, H., Wolff, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 23–53. [Google Scholar] [CrossRef]
- Thrall, M.A.; Weiser, G.; Allison, R.; Campbell, T.W. Veterinary Hematology and Clinical Chemistry, 2nd ed.; Wiley: Somerset, UK, 2012. [Google Scholar]
- Hamada, H.; Ohkura, Y. A New Photometric Method for the Determination of Serum Glutamate Pyruvate Transaminase Activity Using Pyruvate and Glutamate as Substrates. Chem. Pharm. Bull. 1976, 24, 1865–1869. [Google Scholar] [CrossRef]
- Arnold, P.M.; Parslow, G.R. Designing a Coupled Assay System for Aspartate Aminotransferase. Biochem. Educ. 1995, 23, 40–41. [Google Scholar] [CrossRef]
- Yagi, T.; Kagamiyama, H.; Ohtawara, S.; Soda, K.; Nozaki, M. A New Assay for L-Aspartate: 2-Oxoglutarate Aminotransferase. Anal. Biochem. 1979, 100, 20–24. [Google Scholar] [CrossRef]
- Bartels, H.; Böhmer, M. Micro-determination of creatinine. Clin. Chim. Acta 1971, 32, 81–85. [Google Scholar] [PubMed]
- Hallett, C.J.; Cook, J.G.H. Reduced Nicotinamide Adenine Dinucleotide-Coupled Reaction for Emergency Blood Urea Estimation. Clin. Chim. Acta 1971, 35, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–417. [Google Scholar]
- Lowry Oliver, H.; Rosebrough, N.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Hunskaar, S.; Fasmer, O.B.; Hole, K. Formalin Test in Mice, a Useful Technique for Evaluating Mild Analgesics. J. Neurosci. Methods 1985, 14, 69–76. [Google Scholar] [CrossRef] [PubMed]






| Peak | RT (min) | Compound | Formula | [M−H]−Exp. | Error (ppm) |
|---|---|---|---|---|---|
| 1 | 1.84 | Gallic acid | C7H6O5 | 169.0138 | 0.6 |
| 2 | 4.51 | O-Coumaroylgalactaric acid | C15H16O10 | 355.0661 | 1.1 |
| 3 | 4.91 | O-Feruloylgalactaric acid | C16H18O11 | 385.0766 | 1.3 |
| 4 | 5.17 | Methyl gallate | C8H8O5 | 183.0285 | 4.4 |
| 5 | 5.92 | Galloyl-DHHDP-HHDP-glucose | C41H28O27 | 951.0703 | 3.9 |
| 6 | 6.09 | Galloyl-HHDP-glucose | C27H22O18 | 633.0710 | 2.8 |
| 7 | 6.56 | Galloyl-Che-HHDP-glucose Isomer I | C41H30O27 | 953.0888 | 0.8 |
| 8 | 6.56 | Trigalloyl-glucose | C27H24O18 | 635.0866 | 2.8 |
| 9 | 6.92 | p-Coumaric acid | C9H8O3 | 163.0389 | 3.7 |
| 10 | 6.98 | Quercetin 3-O-glucosyl-glucoside | C27H30O17 | 625.1368 | 5.9 |
| 11 | 7.18 | Ethyl gallate | C9H10O5 | 197.0445 | 2.5 |
| 12 | 7.24 | Phyllanthusiin C Isomer | C40H30O26 | 925.0983 | 3.6 |
| 13 | 7.55 | Ellagic acid O-xyloside | C19H14O12 | 433.0410 | 0.7 |
| 14 | 7.67 | Quercetin 3-O-xylosyl-glucoside | C26H28O16 | 595.1321 | 3.7 |
| 15 | 7.84 | Quercetin 3-O-rhamnosyl-glucoside | C27H30O16 | 609.1427 | 4.8 |
| 16 | 7.87 | Ellagic acid O-rhamnoside | C20H16O12 | 447.0585 | 4.7 |
| 17 | 7.96 | Galloyl-Che-HHDP-glucose Isomer II | C41H30O27 | 953.0904 | 0.8 |
| 18 | 8.01 | Ellagic acid | C14H6O8 | 300.9972 | 4.0 |
| 19 | 8.39 | Digalloyl-HHDP-glucose | C34H26O22 | 785.0847 | 1.3 |
| 20 | 8.39 | Methyl neochebulagate Isomer | C42H34O28 | 985.1155 | 0.3 |
| 21 | 8.62 | Quercetin 3-O-glucoside Isomer I | C21H20O12 | 463.0890 | 2.8 |
| 22 | 8.73 | Excoecariphenol C Isomer | C37H30O24 | 857.1077 | 3.3 |
| 23 | 8.73 | Tetragalloyl-glucose | C34H28O22 | 787.0977 | 2.2 |
| 24 | 8.76 | Kaempferol 3-O-rhamnosyl-glucoside | C27H30O15 | 593.1528 | 3.7 |
| 25 | 8.87 | Kaempferol 3-O-xylosyl-glucoside | C26H28O15 | 579.1376 | 4.0 |
| 26 | 8.87 | Quercetin 3-O-glucoside Isomer II | C21H20O12 | 463.0898 | 4.5 |
| 27 | 8.93 | Di-O-Methyl ellagic acid O-glucoside | C22H20O13 | 491.0852 | 5.3 |
| 28 | 9.41 | Quercetin 3-O-rhamnosyl-xyloside | C26H28O15 | 579.1350 | 0.0 |
| 29 | 9.61 | Quercetin 3-O-xyloside | C20H18O11 | 433.0765 | 1.4 |
| 30 | 9.70 | Kaempferol 3-O-glucoside Isomer I | C21H20O11 | 447.0935 | 1.8 |
| 31 | 9.95 | Galloyl-HHDP-di-deoxyglucose | C27H24O16 | 603.0945 | 6.8 |
| 32 | 10.15 | Trigalloyl-dideoxyglucose | C27H24O16 | 603.1013 | 4.5 |
| 33 | 10.24 | Kaempferol 3-O-glucoside Isomer II | C21H20O11 | 447.0914 | 2.9 |
| 34 | 10.61 | Kaempferol 3-O-rhamnosyl-xyloside | C26H27O14 | 563.1431 | 5.3 |
| 35 | 10.69 | Kaempferol 3-O-xyloside | C20H18O10 | 417.0836 | 3.4 |
| 36 | 10.78 | Methylellagic acid O-rhamnoside | C21H18O12 | 461.0736 | 3.5 |
| 37 | 11.01 | Phyllanthusiin A Isomer | C41H28O27 | 951.0743 | 0.3 |
| 38 | 12.10 | Trigalloyl-HHDP-glucose | C41H30O26 | 937.0962 | 1.6 |
| 39 | 12.29 | Phyllanthusiin U Isomer | C40H28O26 | 923.0801 | 1.1 |
| 40 | 14.00 | Quercetin | C15H10O7 | 301.0334 | 4.7 |
| 41 | 14.91 | Methylquercetin 3-O-glucoside | C22H22O12 | 477.1018 | 3.1 |
| 42 | 16.91 | Kaempferol | C15H10O6 | 285.0399 | 0.0 |
| 43 | 18.14 | Galloyl-Cinnamoyl-HHDP-glucose | C36H28O19 | 763.1154 | 0.9 |
| 44 | 19.04 | Tri-O-methyl ellagic acid | C17H12O8 | 343.0450 | 1.2 |
| Leukocytes | Control (%) | MnE 2000 mg/kg (%) | p |
|---|---|---|---|
| Segmented neutrophils | 37.50 ± 0.64 | 33.60 ± 2.09 | 0.153 |
| Banded neutrophils | 0.50 ± 0.29 | 0.20 ± 0.20 | 0.407 |
| Lymphocytes | 59.50 ± 1.19 | 64.40 ± 2.48 | 0.147 |
| Monocytes | 2.50 ± 0.65 | 1.80 ± 0.37 | 0.356 |
| Basophils | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.000 |
| Eosinophils | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camara, F.M.S.; da Conceição, B.C.; Cardoso, E.K.S.; Santiago, J.C.C.; Albuquerque, C.A.B.; Pereira, W.L.; Monteiro, M.C.; Yoshioka e Silva, C.Y.; da Silva, M.N.; Maia, C.F.; et al. Margaritaria nobilis L.f. (Phyllanthaceae) Ethanolic Extract: Low Acute Oral Toxicity and Antinociceptive Activity. Pharmaceuticals 2023, 16, 689. https://doi.org/10.3390/ph16050689
Camara FMS, da Conceição BC, Cardoso EKS, Santiago JCC, Albuquerque CAB, Pereira WL, Monteiro MC, Yoshioka e Silva CY, da Silva MN, Maia CF, et al. Margaritaria nobilis L.f. (Phyllanthaceae) Ethanolic Extract: Low Acute Oral Toxicity and Antinociceptive Activity. Pharmaceuticals. 2023; 16(5):689. https://doi.org/10.3390/ph16050689
Chicago/Turabian StyleCamara, Fabiana Menezes S., Brenda Costa da Conceição, Eloise Karoline S. Cardoso, Johan Carlos C. Santiago, Carlos Alberto B. Albuquerque, Washington L. Pereira, Marta C. Monteiro, Consuelo Y. Yoshioka e Silva, Milton Nascimento da Silva, Cristiane F. Maia, and et al. 2023. "Margaritaria nobilis L.f. (Phyllanthaceae) Ethanolic Extract: Low Acute Oral Toxicity and Antinociceptive Activity" Pharmaceuticals 16, no. 5: 689. https://doi.org/10.3390/ph16050689
APA StyleCamara, F. M. S., da Conceição, B. C., Cardoso, E. K. S., Santiago, J. C. C., Albuquerque, C. A. B., Pereira, W. L., Monteiro, M. C., Yoshioka e Silva, C. Y., da Silva, M. N., Maia, C. F., & Fontes-Junior, E. A. (2023). Margaritaria nobilis L.f. (Phyllanthaceae) Ethanolic Extract: Low Acute Oral Toxicity and Antinociceptive Activity. Pharmaceuticals, 16(5), 689. https://doi.org/10.3390/ph16050689