Enhancement of Cognitive Function by Andrographolide-Loaded Lactose β-Cyclodextrin Nanoparticles: Synthesis, Optimization, and Behavioural Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Lactose-Appended β-Cyclodextrin
2.2. Andrographolides Loaded Lactose β-Cyclodextrin Nanoparticles (ALN-βCD Nanoparticles)
2.3. Optimization of Formulation
2.4. FTIR Spectroscopy
2.5. Drug Loading and Entrapment Efficacy
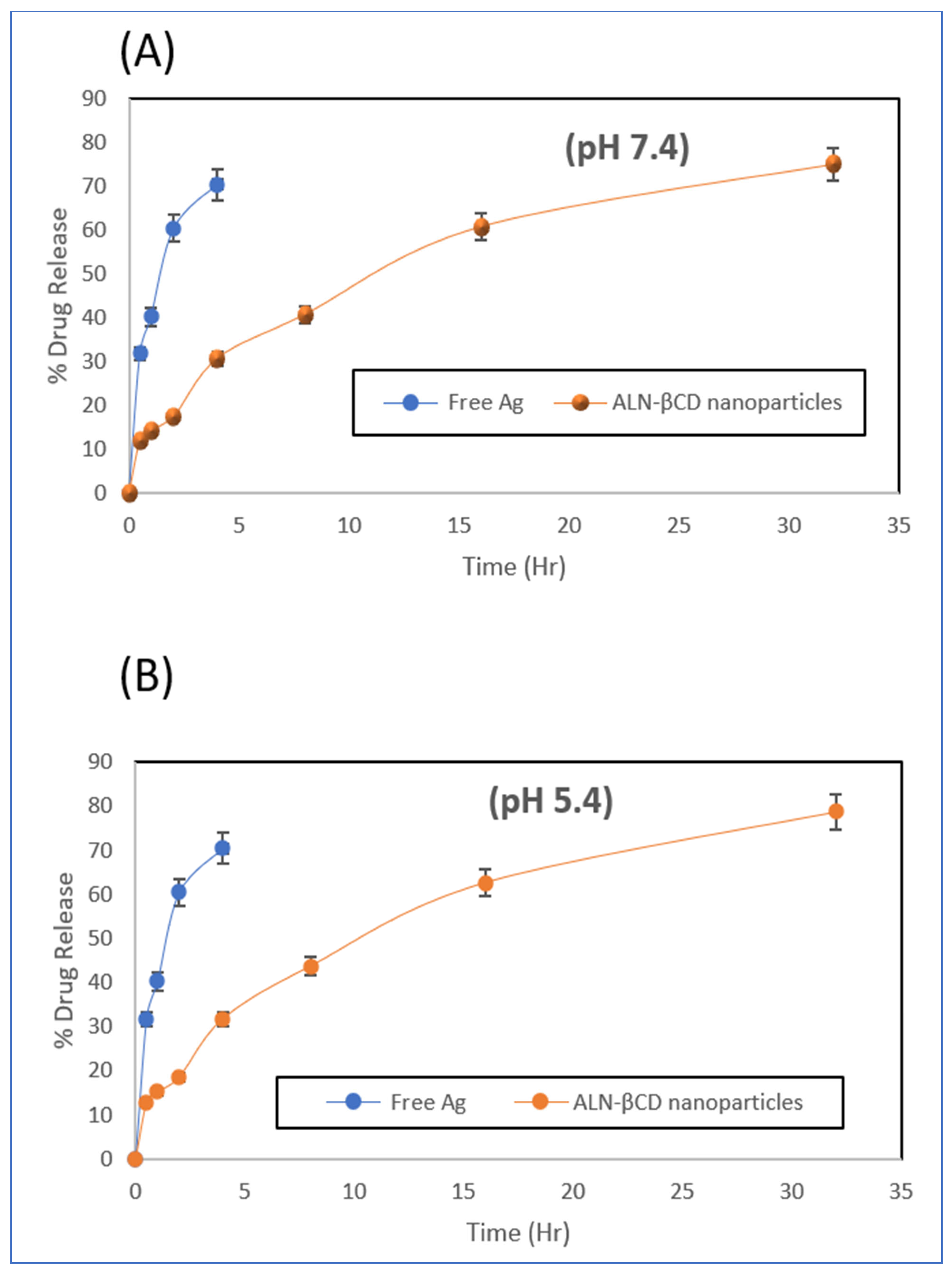
2.6. In Vitro Drug Release
2.7. Cytotoxicity Assay
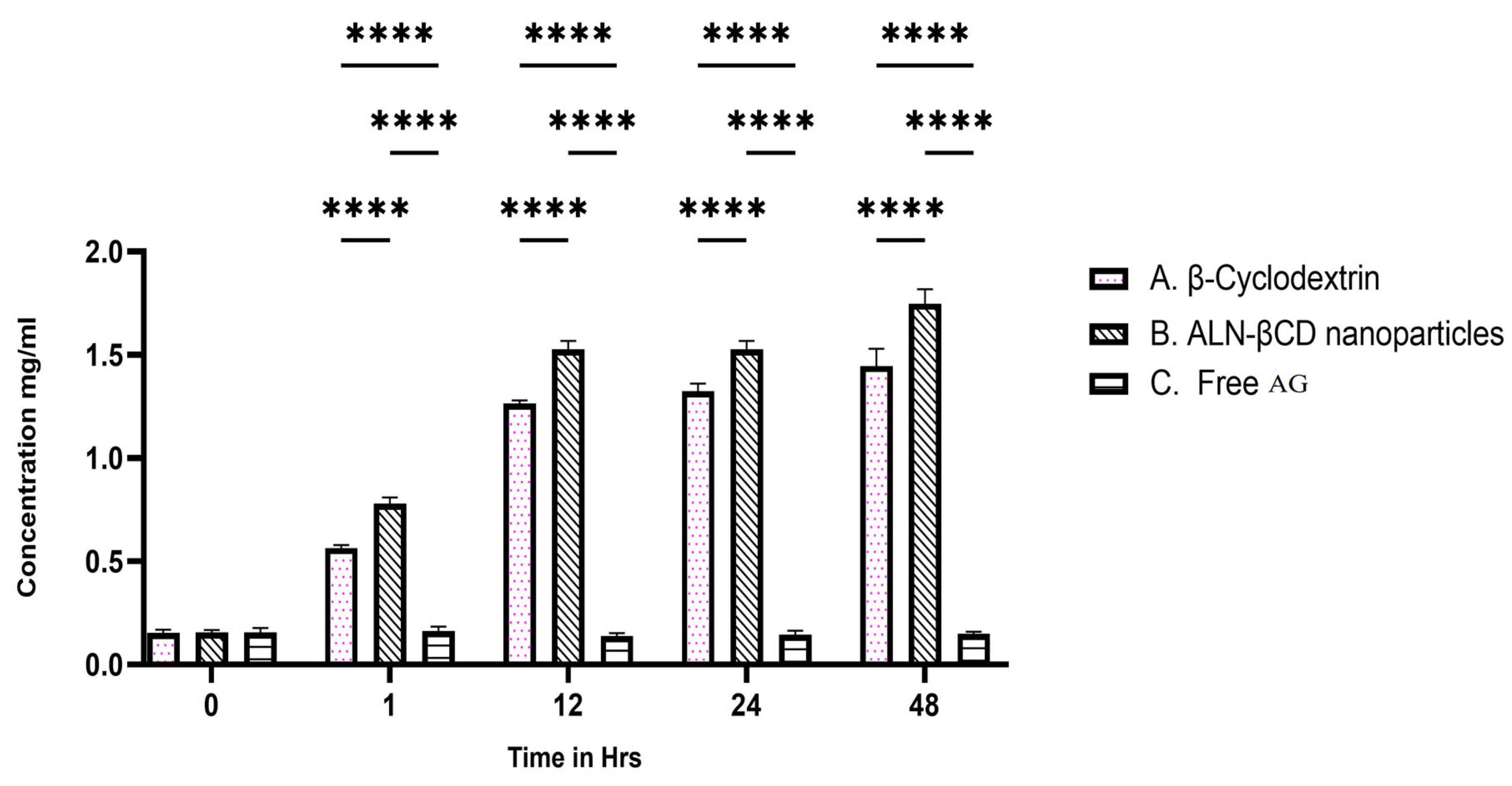
2.8. In Vitro BBB Permeability
2.9. In Vitro Cellular Uptake
2.10. Assessment of Behavioural Activity
2.10.1. Assessing the Elevated Plus Maze’s (EPM) Capacity to Improve Memory
2.10.2. Evaluation of Memory-Enhancing Potential by Using Y-Maze
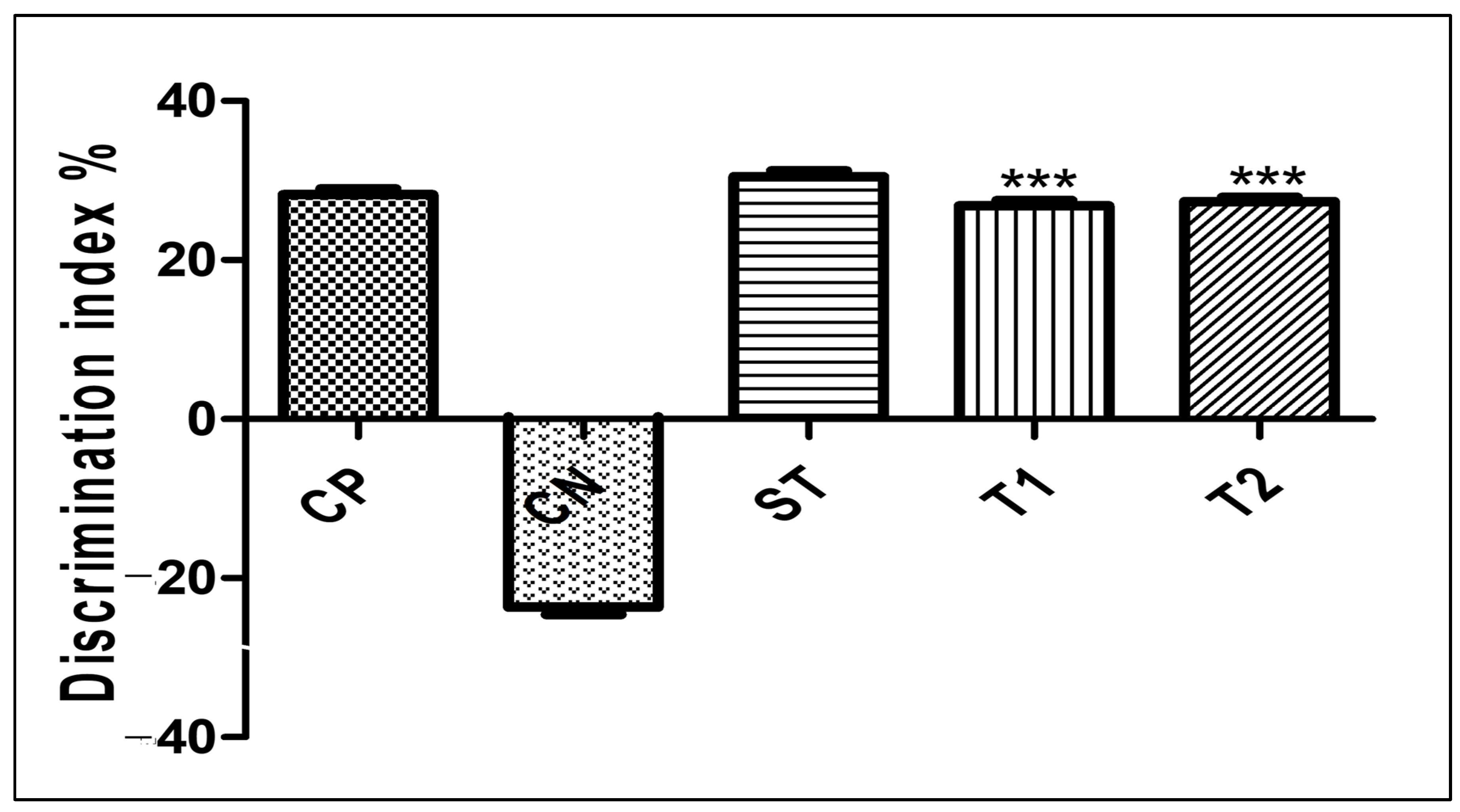
2.10.3. Evaluation of Memory-Enhancing Potential by Using the Object Recognition Test
2.10.4. Evaluation of Motor Coordination Test by Using Rota-Rod
2.10.5. Evaluation of Locomotor Activity by Using an Actophotometer
2.11. Assessment of Biochemical Estimation
2.11.1. Effect on Acetylcholinesterase Level
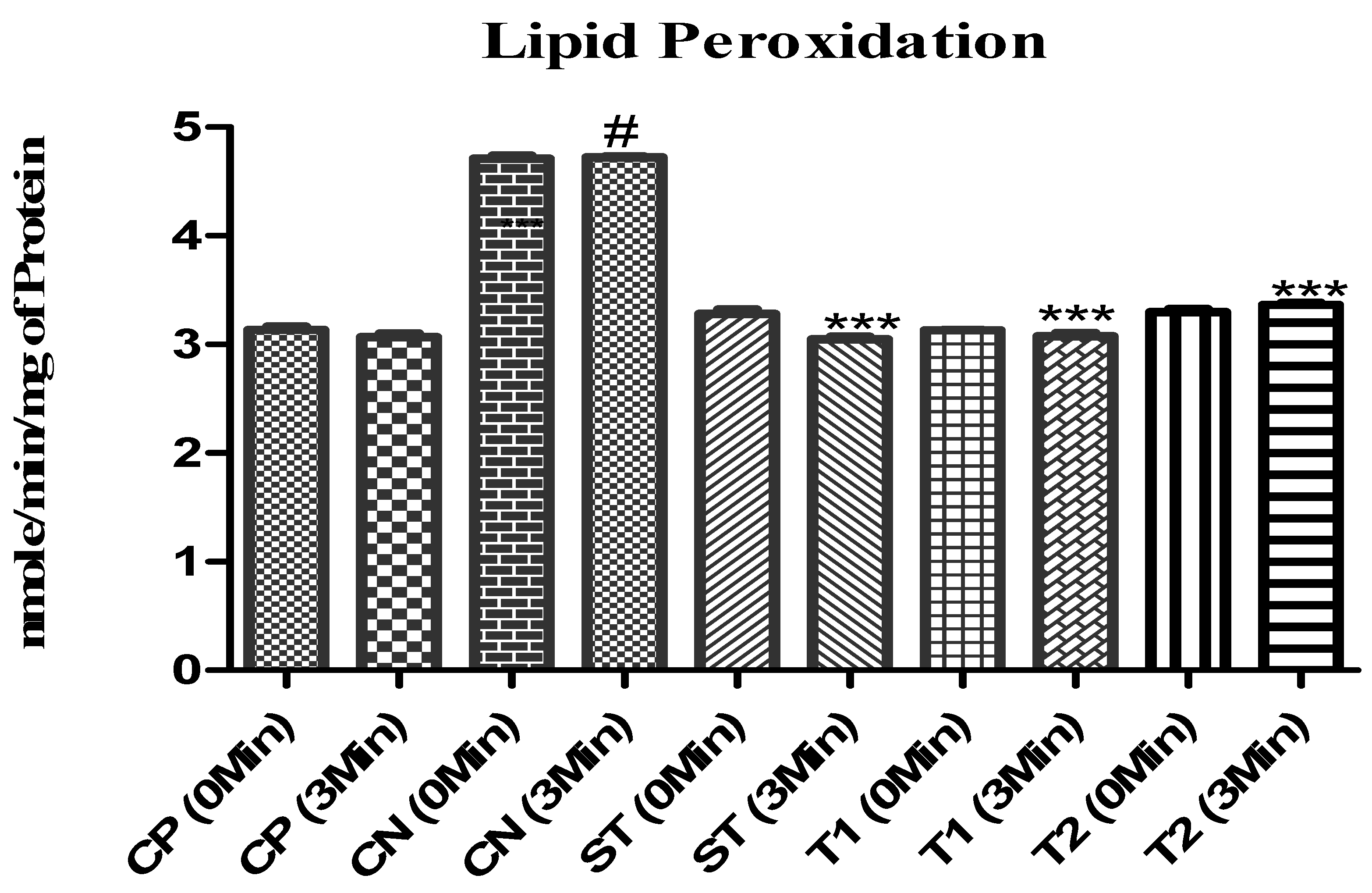
2.11.2. Effect on Lipid Peroxidation
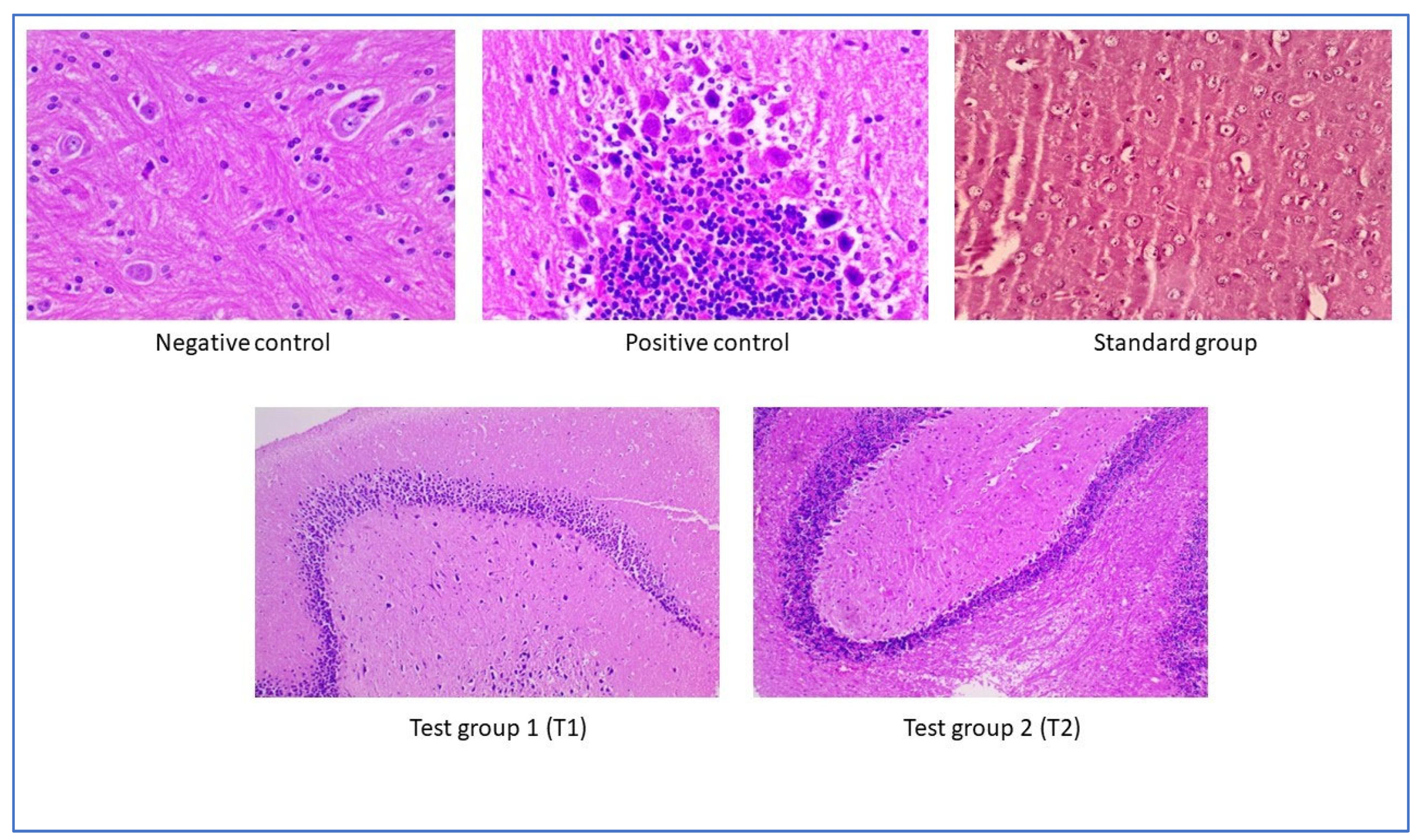
2.12. Assessment of Histopathological Examination
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Lactose-Appended β-Cyclodextrin
3.3. Preparation of Andrographolide-Loaded Nanoparticles (ALN)
3.4. Optimization of Formulation
3.5. Characterization of ALN-βCD Nanoparticles
3.6. Drug Loading and Entrapment Efficacy
3.7. Fourier Transform Infrared Spectroscopy (FT-IR)
3.8. In Vitro Drug Release
3.9. Cytotoxicity Assay
3.10. In Vitro BBB Permeability
3.11. In Vitro Cellular Uptake
3.12. Experimental Animals
3.13. Treatment Protocol
3.14. Behavioural Assessment of Prepared Formulation
3.14.1. Elevated Plus Maze Test
3.14.2. Y-Maze Test
3.14.3. Object Recognition Test
3.14.4. Motor Coordination Test by Rota-Rod Apparatus
3.14.5. Locomotor Activity Test by Actophotometer
3.15. Biochemical Estimation
3.15.1. Homogenization of Brain
3.15.2. Protein Quantification
3.15.3. Estimation of Acetylcholinesterase Activity
3.15.4. Determination of Lipid Peroxidation
3.16. Estimation of Protein
Preparation of Calibration Standard Curve of Protein
3.17. Histopathological Examination
3.18. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayeni, E.A.; Aldossary, A.M.; Ayejoto, D.A.; Gbadegesin, L.A.; Alshehri, A.A.; Alfassam, H.A.; Afewerky, H.K.; Almughem, F.A.; Bello, S.M.; Tawfik, E.A. Neurodegenerative Diseases: Implications of Environmental and Climatic Influences on Neurotransmitters and Neuronal Hormones Activities. Int. J. Environ. Res. Public Health 2022, 19, 12495. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2020 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-Based Therapy for Alzheimer’s Disease: Challenges, Successes and Future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.A.; Alshamari, A.K.; Hassan, A.A.; Elharrif, M.G.; Alhajri, A.M.; Sattam, M.; Khattab, R.R. Advances on Therapeutic Strategies for Alzheimer’s Disease: From Medicinal Plant to Nanotechnology. Molecules 2022, 27, 4839. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Sharma, A.K.; Singh, R.K. Pharmacological Activities and Molecular Mechanisms of Pure and Crude Extract of Andrographis Paniculata: An Update. Phytomed. Plus 2021, 1, 100085. [Google Scholar] [CrossRef]
- Majumdar, M.; Misra, T.K.; Roy, D.N. In Vitro Anti-Biofilm Activity of 14-Deoxy-11,12-Didehydroandrographolide from Andrographis Paniculata against Pseudomonas Aeruginosa. Brazilian J. Microbiol. 2020, 51, 15–27. [Google Scholar] [CrossRef]
- Baru Venkata, R.; Prasanth, D.S.N.B.K.; Pasala, P.K.; Panda, S.P.; Tatipamula, V.B.; Mulukuri, S.; Kota, R.K.; Rudrapal, M.; Khan, J.; Aldosari, S.; et al. Utilizing Andrographis Paniculata Leaves and Roots by Effective Usage of the Bioactive Andrographolide and Its Nanodelivery: Investigation of Antikindling and Antioxidant Activities through in Silico and in Vivo Studies. Front. Nutr. 2023, 10, 1185236. [Google Scholar] [CrossRef] [PubMed]
- Serrano, F.G.; Tapia-Rojas, C.; Carvajal, F.J.; Hancke, J.; Cerpa, W.; Inestrosa, N.C. Andrographolide Reduces Cognitive Impairment in Young and Mature AβPPswe/PS-1 Mice. Mol. Neurodegener. 2014, 9, 61. [Google Scholar] [CrossRef]
- Guccione, C.; Oufir, M.; Piazzini, V.; Eigenmann, D.E.; Jähne, E.A.; Zabela, V.; Faleschini, M.T.; Bergonzi, M.C.; Smiesko, M.; Hamburger, M.; et al. Andrographolide-Loaded Nanoparticles for Brain Delivery: Formulation, Characterisation and in Vitro Permeability Using HCMEC/D3 Cell Line. Eur. J. Pharm. Biopharm. 2017, 119, 253–263. [Google Scholar] [CrossRef]
- Thingale, A.D.; Shaikh, K.S.; Channekar, P.R.; Galgatte, U.C.; Chaudhari, P.D.; Bothiraja, C. Enhanced Hepatoprotective Activity of Andrographolide Complexed with a Biomaterial. Drug Deliv. 2015, 22, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K. Non-Invasive Drug Delivery across the Blood–Brain Barrier: A Prospective Analysis. Pharmaceutics 2023, 15, 2599. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.G.R.; Coutinho, A.J.; Pinheiro, M.; Neves, A.R. Nanoparticles for Targeted Brain Drug Delivery: What Do We Know? Int. J. Mol. Sci. 2021, 22, 11654. [Google Scholar] [CrossRef] [PubMed]
- Duelli, R.; Kuschinsky, W. Brain Glucose Transporters: Relationship to Local Energy Demand. Physiology 2001, 16, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, D.; Das Paul, S.; Paliwal, R.; Satapathy, T. Development, QbD Based Optimization and in Vitro Characterization of Diacerein Loaded Nanostructured Lipid Carriers for Topical Applications. J. Radiat. Res. Appl. Sci. 2023, 16, 100565. [Google Scholar] [CrossRef]
- Farheen, M.; Akhter, M.H.; Chitme, H.; Suliman, M.; Jaremko, M.; Emwas, A.-H. Surface-Modified Biobased Polymeric Nanoparticles for Dual Delivery of Doxorubicin and Gefitinib in Glioma Cell Lines. ACS Omega 2023, 8, 28165–28184. [Google Scholar] [CrossRef]
- Dashputre, N.L.; Laddha, U.D.; Darekar, P.P.; Kadam, J.D.; Patil, S.B.; Sable, R.R.; Udavant, P.B.; Tajanpure, A.B.; Kakad, S.P.; Kshirsagar, S.J. Potential Therapeutic Effects of Naringin Loaded PLGA Nanoparticles for the Management of Alzheimer’s Disease: In Vitro, Ex Vivo and in Vivo Investigation. Heliyon 2023, 9, e19374. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ali Reza, A.S.M.; Abdullah-Al-Mamun, M.; Kabir, M.S.H.; Nasrin, M.S.; Akhter, S.; Arman, M.S.I.; Rahman, M.A. Antinociceptive and Anxiolytic and Sedative Effects of Methanol Extract of Anisomeles Indica: An Experimental Assessment in Mice and Computer Aided Models. Front. Pharmacol. 2018, 9, 00246. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; El-Feky, Y.A.; Al-Sawahli, M.M.; Ali, M.E.; Fayez, A.M.; Abbas, H. A Brain-Targeted Approach to Ameliorate Memory Disorders in a Sporadic Alzheimer’s Disease Mouse Model via Intranasal Luteolin-Loaded Nanobilosomes. Pharmaceutics 2022, 14, 576. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Z.; Blanchard, J.; Dai, C.-L.; Sun, S.; Lee, M.H.; Grundke-Iqbal, I.; Iqbal, K.; Liu, F.; Gong, C.-X. A Non-Transgenic Mouse Model (Icv-STZ Mouse) of Alzheimer’s Disease: Similarities to and Differences from the Transgenic Model (3xTg-AD Mouse). Mol. Neurobiol. 2013, 47, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Nardiello, P.; Piazzini, V.; Leri, M.; Bergonzi, M.C.; Bucciantini, M.; Casamenti, F. Successful Brain Delivery of Andrographolide Loaded in Human Albumin Nanoparticles to TgCRND8 Mice, an Alzheimer’s Disease Mouse Model. Front. Pharmacol. 2019, 10, 00910. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liu, W.; Gao, J.; Jiang, C.; Fan, T.; Sun, Y.; Qin, Z.; Xu, Q.; Guo, W.; Gao, J. Andrographolide Alleviates Parkinsonism in MPTP-PD Mice via Targeting Mitochondrial Fission Mediated by Dynamin-related Protein 1. Br. J. Pharmacol. 2019, 176, 4574–4591. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Taharabaru, T.; Nishida, T.; Ohno, Y.; Maeda, Y.; Sato, M.; Ishikura, K.; Yanagihara, K.; Takagi, H.; Nakamura, T.; et al. Lactose-Appended β-Cyclodextrin as an Effective Nanocarrier for Brain Delivery. J. Control. Release 2020, 328, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, N.; Hirai, H.; Aizawa, K.; Takada, M. Production of Water-Soluble Indigestible Polysaccharides Using Activated Carbon. J. Appl. Glycosci. 2015, 62, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Das, S.; Bera, T.; Mondol, S.; Mukherjee, A. Andrographolide Nanoparticles in Leishmaniasis: Characterization and in Vitro Evaluations. Int. J. Nanomed. 2010, 2010, 1113–1121. [Google Scholar] [CrossRef]
- Farheen, M.; Akhter, M.H.; Chitme, H.; Akhter, M.S.; Tabassum, F.; Jaremko, M.; Emwas, A.-H. Harnessing Folate-Functionalized Nasal Delivery of Dox–Erlo-Loaded Biopolymeric Nanoparticles in Cancer Treatment: Development, Optimization, Characterization, and Biodistribution Analysis. Pharmaceuticals 2023, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Brandbu, E. Nursing Practice—A Conflict-Filled Reality. How Can Qualifications Be Met? Vard Nord. Utveckl. Forsk. 1994, 14, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Oseni, B.A.; Azubuike, C.P.; Okubanjo, O.O.; Igwilo, C.I.; Panyam, J. Encapsulation of Andrographolide in Poly(Lactide-Co-Glycolide) Nanoparticles: Formulation Optimization and in Vitro Efficacy Studies. Front. Bioeng. Biotechnol. 2021, 9, 639409. [Google Scholar] [CrossRef]
- Tyagi, N.; Gupta, P.; Khan, Z.; Neupane, Y.R.; Mangla, B.; Mehra, N.; Ralli, T.; Alhalmi, A.; Ali, A.; Al Kamaly, O. Superparamagnetic Iron-Oxide Nanoparticles Synthesized via Green Chemistry for the Potential Treatment of Breast Cancer. Molecules 2023, 28, 2343. [Google Scholar] [CrossRef]
- Kulsirirat, T.; Sathirakul, K.; Kamei, N.; Takeda-Morishita, M. The in Vitro and in Vivo Study of Novel Formulation of Andrographolide PLGA Nanoparticle Embedded into Gelatin-Based Hydrogel to Prolong Delivery and Extend Residence Time in Joint. Int. J. Pharm. 2021, 602, 120618. [Google Scholar] [CrossRef] [PubMed]
- Zhongyu, X.; Jiangmeng, R.; Qiufang, J.; Fuzheng, R.; Mengting, H.; Wenrui, D.; Bubing, Z. Andrographolide-Loaded Silk Fibroin Nanoparticles. RSC Adv. 2018, 8, 34726–34732. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.Z.; Hassan, Z.; Che Has, A.T. Morris Water Maze: A Versatile and Pertinent Tool for Assessing Spatial Learning and Memory. Exp. Anim. 2022, 71, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Suri, A.; Katz, J.D. Dissecting the Role of CD4+ T Cells in Autoimmune Diabetes through the Use of TCR Transgenic Mice. Immunol. Rev. 1999, 169, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [PubMed]
- Howard, R.B. Medical Education: An Unwelcome Guest at the Healthcare Table. Postgrad. Med. 1995, 98, 23–25+29. [Google Scholar] [CrossRef] [PubMed]
- Carter, R. The Mask of Thomas Mann (1875–1955): Medical Insights and Last Illness. Ann. Thorac. Surg. 1998, 65, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Jahan, I.; Ishaq, M.; Alhalmi, A.; Khan, R.; Noman, O.M.; Hasson, S.; Mothana, R.A. Neuroprotective Effects of Trigonelline in Kainic Acid-Induced Epilepsy: Behavioral, Biochemical, and Functional Insights. Saudi Pharm. J. 2023, 31, 101843. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Hao, L.; Mei, T.; Zhongchuan, L.; Ganggang, W. Quantitation of Nucleoprotein Complexes by UV Absorbance and Bradford Assay. Biophys. Rep. 2021, 7, 429–436. [Google Scholar] [CrossRef]
- Halder, S.; Anand, U.; Nandy, S.; Oleksak, P.; Qusti, S.; Alshammari, E.M.; El-Saber Batiha, G.; Koshy, E.P.; Dey, A. Herbal Drugs and Natural Bioactive Products as Potential Therapeutics: A Review on pro-Cognitives and Brain Boosters Perspectives. Saudi Pharm. J. 2021, 29, 879–907. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Fabian, O.; Bajer, L. Histopathological Assessment of the Microscopic Activity in Inflammatory Bowel Diseases: What Are We Looking For? World J. Gastroenterol. 2022, 28, 5300–5312. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, T.; Pathak, D.; Virmani, T.; Kumar, G.; Alhalmi, A. Antidepressive-Like Effect of Aegle Marmelos Leaf Extract in Chronic Unpredictable Mild Stress-Induced Depression-Like Behaviour in Rats. Biomed Res. Int. 2022, 2022, 6479953. [Google Scholar] [CrossRef] [PubMed]



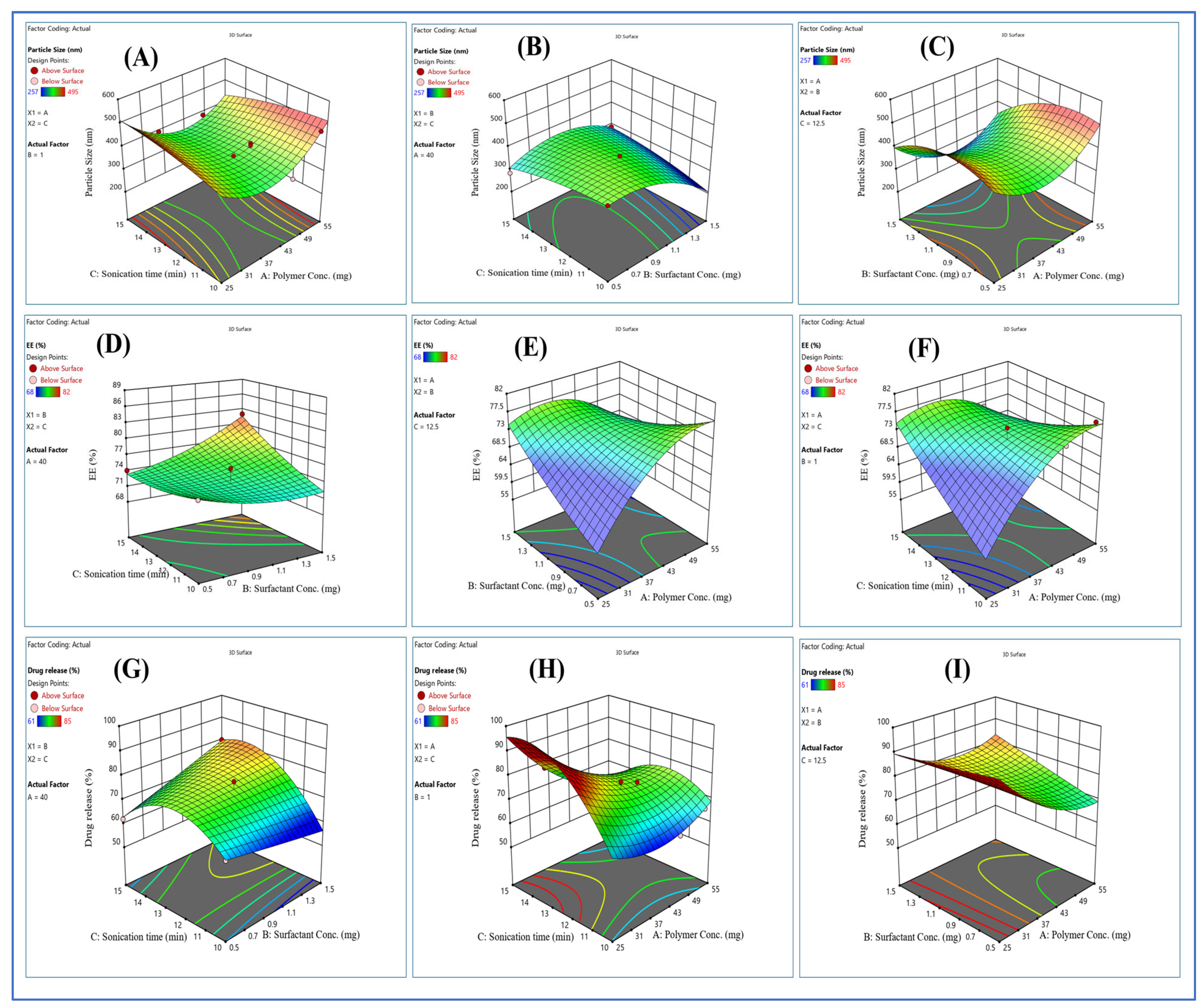





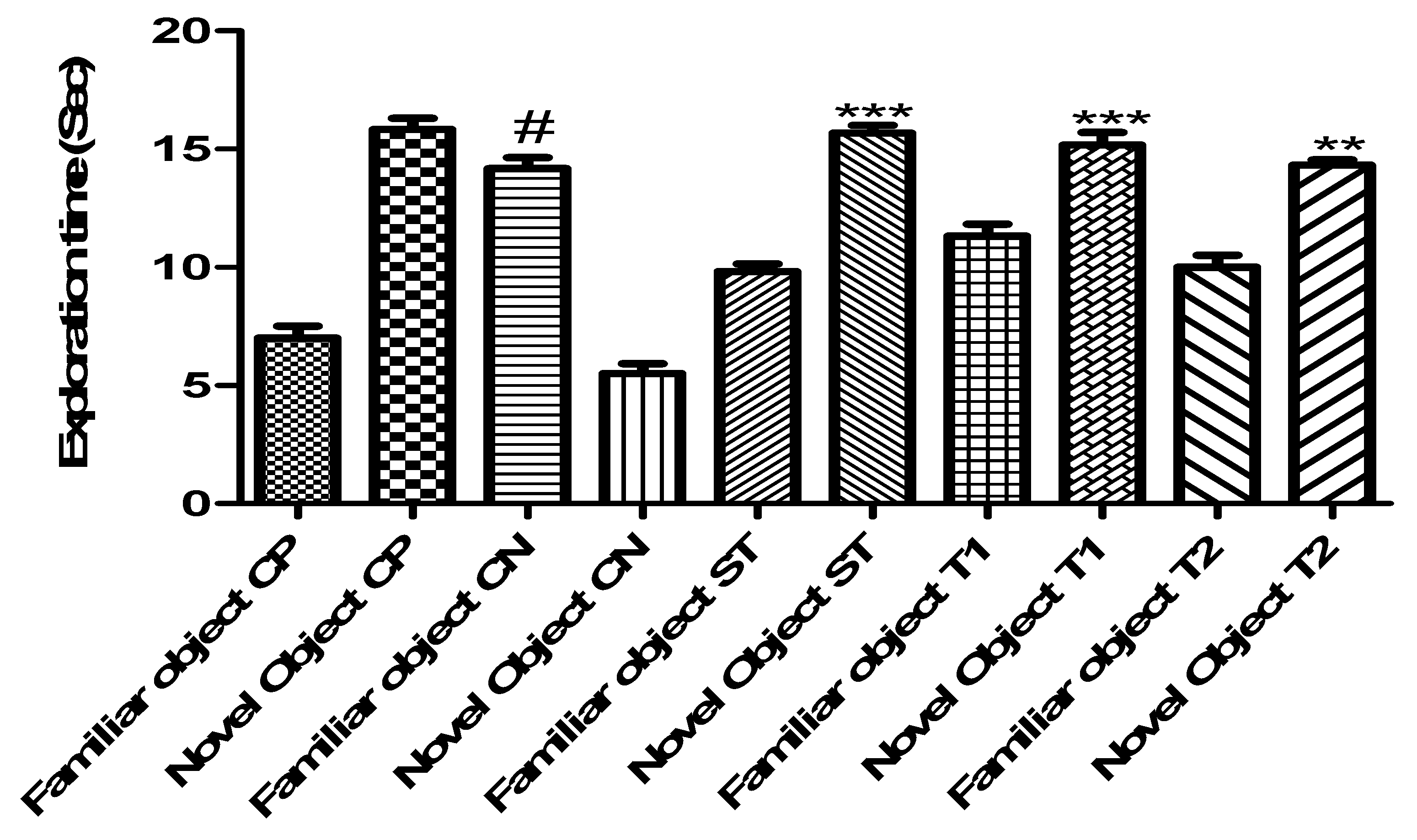

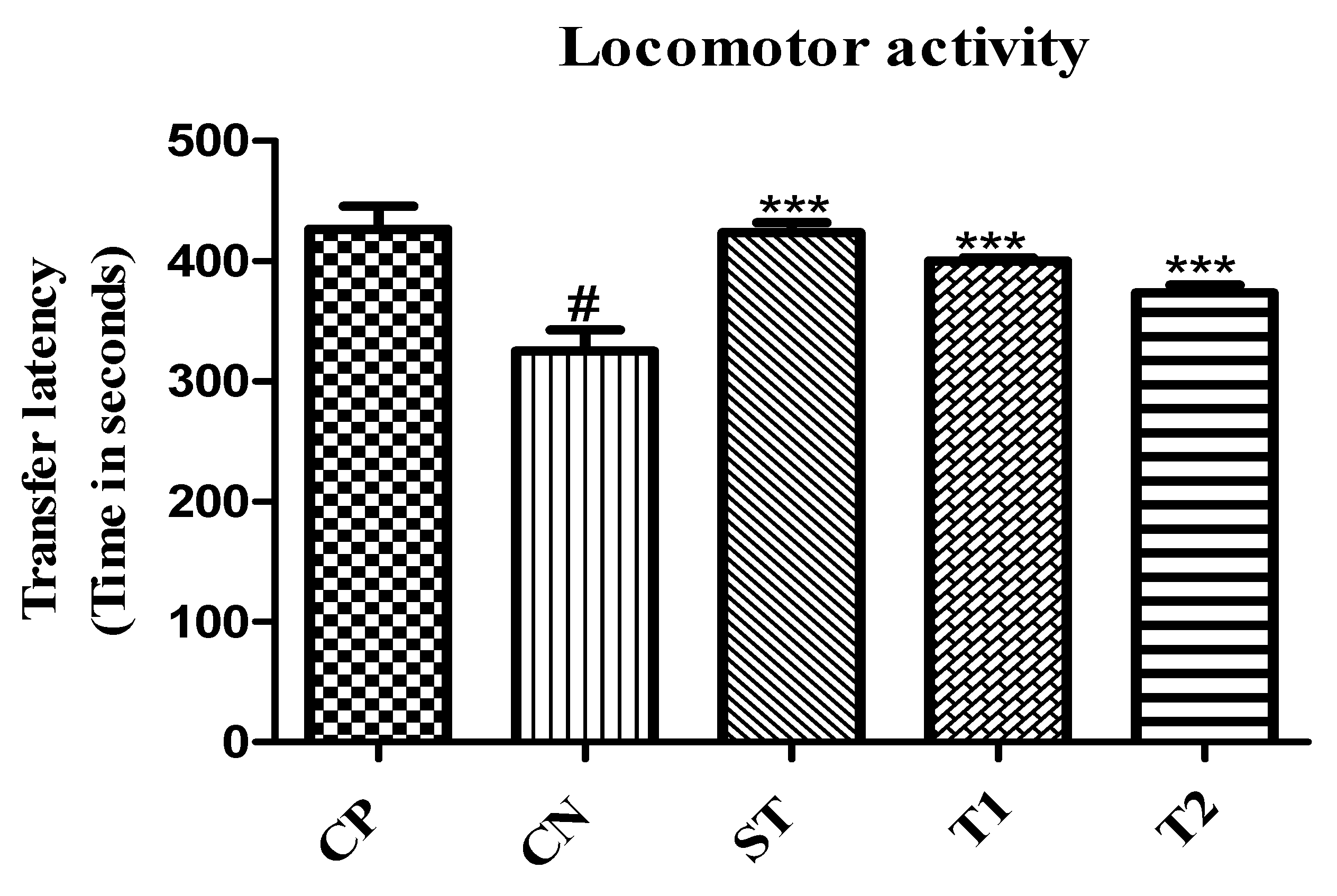

| STD | Run | Factor 1 Polymer Concentration (mg) | Factor 2 Surfactant Concentration (mg) | Factor 3 Sonication Time (min) | Response 1 Particle Size | Response 2 EE% | Response 3 Drug Release % |
|---|---|---|---|---|---|---|---|
| 16 | 1 | 35 | 0.5 | 12 | 378 | 70 | 85 |
| 17 | 2 | 40 | 1 | 12 | 389 | 76 | 73 |
| 15 | 3 | 45 | 1 | 12 | 398 | 73 | 76 |
| 7 | 4 | 35 | 1 | 15 | 398 | 76 | 78 |
| 1 | 5 | 35 | 0.5 | 12 | 387 | 68 | 75 |
| 8 | 6 | 48 | 1 | 15 | 387 | 72 | 64 |
| 5 | 7 | 45 | 1 | 10 | 345 | 75 | 61 |
| 13 | 8 | 40 | 1 | 12 | 378 | 73 | 79 |
| 6 | 9 | 53 | 1 | 10 | 489 | 78 | 67 |
| 14 | 10 | 45 | 1 | 12 | 408 | 73 | 73 |
| 3 | 11 | 29 | 1.5 | 12 | 317 | 74 | 81 |
| 11 | 12 | 40 | 0.5 | 15 | 285 | 74 | 62 |
| 10 | 13 | 47 | 1.5 | 10 | 278 | 72 | 62 |
| 4 | 14 | 50 | 1.5 | 12 | 257 | 71 | 78 |
| 12 | 15 | 40 | 1.5 | 15 | 287 | 82 | 80 |
| 9 | 16 | 40 | 0.5 | 10 | 398 | 75 | 65 |
| 2L | 17 | 53 | 0.5 | 12 | 495 | 79 | 71 |
| Tube | Concentration (µg/mL) | Absorbance | Drug Entrapment (mg) | Drug Loading | Drug Entrapment % | Drug Loading % |
|---|---|---|---|---|---|---|
| 1 | 5 | 0.128 | 3.90 | 1.09 | 78.08 | 21.91 |
| 2 | 10 | 0.238 | 7.40 | 2.59 | 74.07 | 25.92 |
| 3 | 15 | 0.356 | 11.16 | 3.83 | 74.43 | 25.56 |
| 4 | 20 | 0.524 | 16.51 | 3.48 | 82.57 | 17.42 |
| 5 | 25 | 0.625 | 19.73 | 5.26 | 78.92 | 21.07 |
| 6 | 30 | 0.745 | 23.55 | 6.44 | 78.51 | 21.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramanick, D.; Rani, K.N.; Singh, V.K.; Basist, P.; Khan, R.; Al-Tamimi, J.H.; Noman, O.M.; Ibrahim, M.N.; Alhalmi, A. Enhancement of Cognitive Function by Andrographolide-Loaded Lactose β-Cyclodextrin Nanoparticles: Synthesis, Optimization, and Behavioural Assessment. Pharmaceuticals 2024, 17, 966. https://doi.org/10.3390/ph17070966
Paramanick D, Rani KN, Singh VK, Basist P, Khan R, Al-Tamimi JH, Noman OM, Ibrahim MN, Alhalmi A. Enhancement of Cognitive Function by Andrographolide-Loaded Lactose β-Cyclodextrin Nanoparticles: Synthesis, Optimization, and Behavioural Assessment. Pharmaceuticals. 2024; 17(7):966. https://doi.org/10.3390/ph17070966
Chicago/Turabian StyleParamanick, Debashish, Kagithala Naga Rani, Vijay Kumar Singh, Parakh Basist, Rahmuddin Khan, Jameel H. Al-Tamimi, Omar M. Noman, Mansour N. Ibrahim, and Abdulsalam Alhalmi. 2024. "Enhancement of Cognitive Function by Andrographolide-Loaded Lactose β-Cyclodextrin Nanoparticles: Synthesis, Optimization, and Behavioural Assessment" Pharmaceuticals 17, no. 7: 966. https://doi.org/10.3390/ph17070966







