Vernonia amygdalina Leaf Extract Induces Apoptosis in HeLa Cells: A Metabolomics and Proteomics Study
Abstract
:1. Introduction
2. Results and Discussion
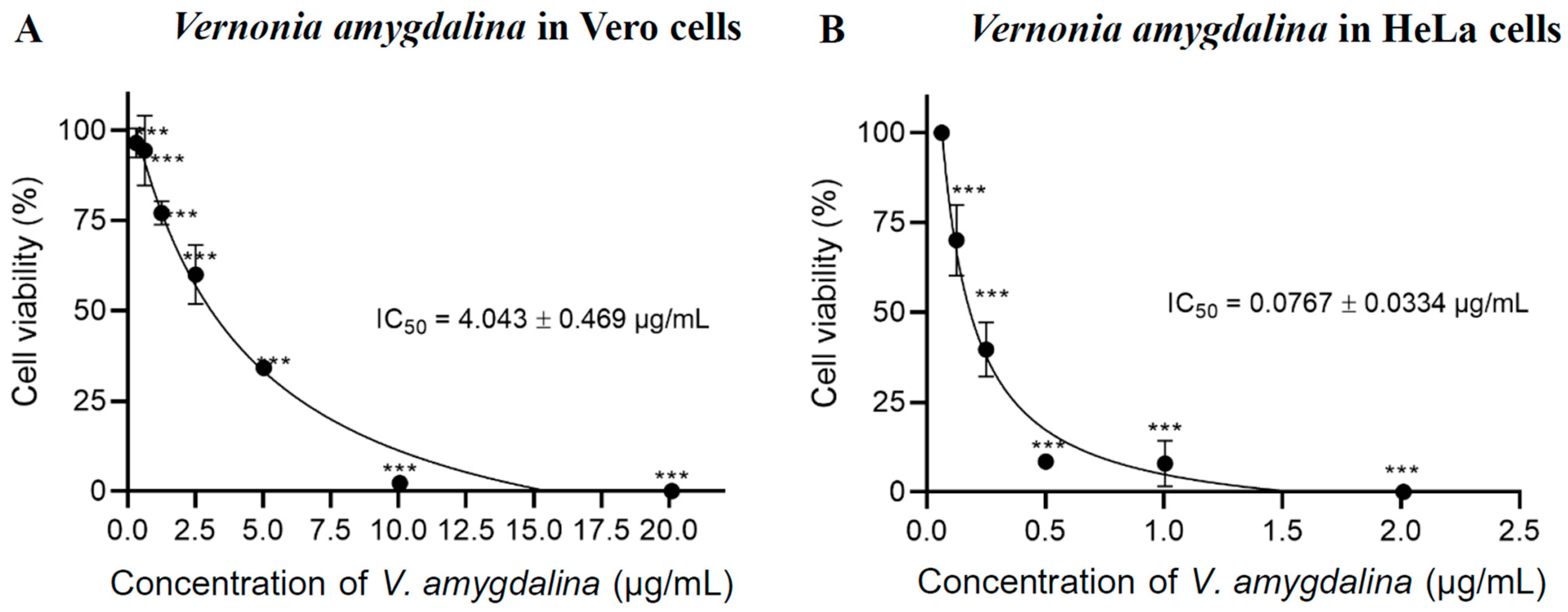
2.1. Cell Cytotoxicity
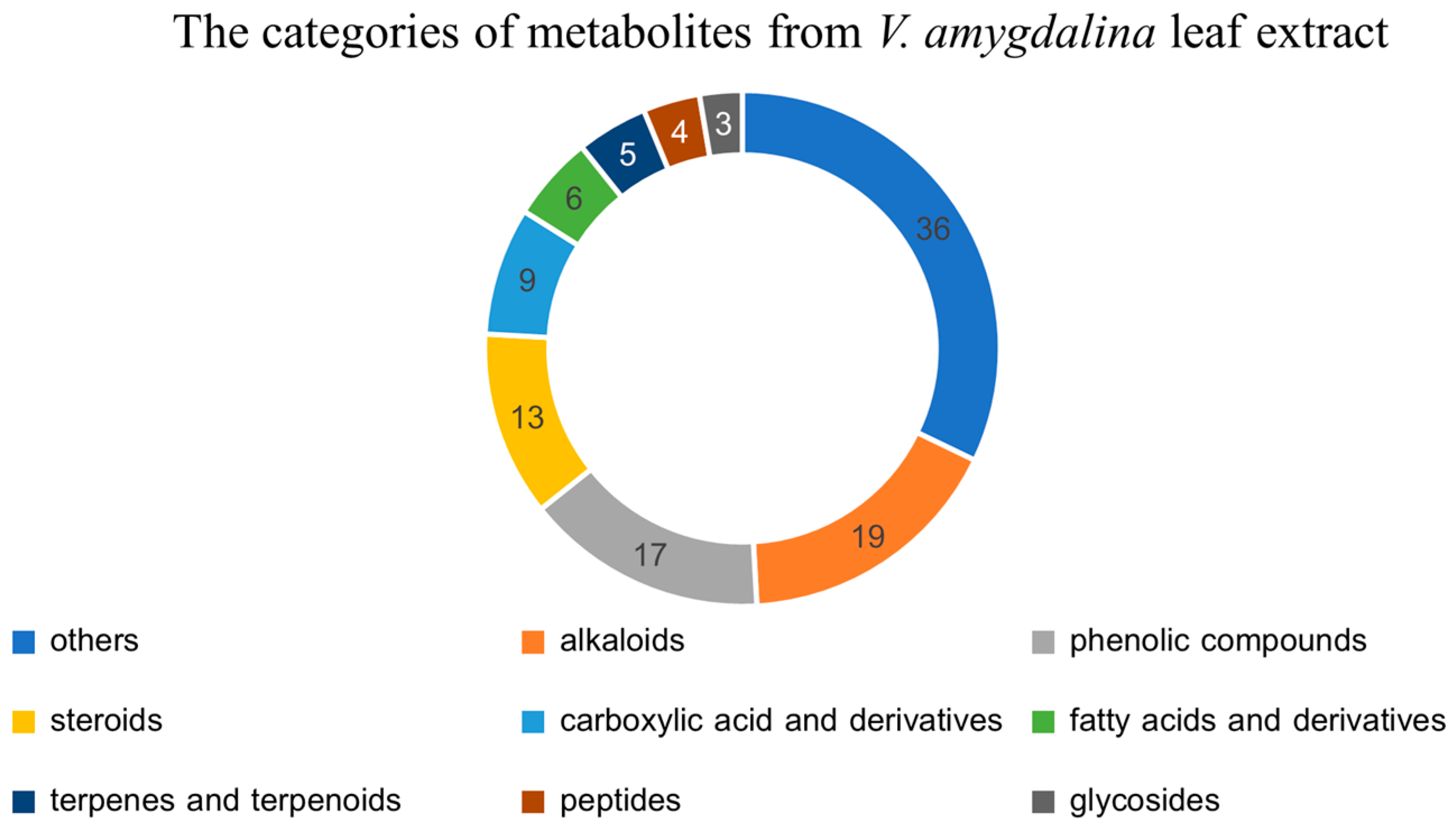
2.2. The Metabolomics Profile of V. amygdalina Leaf Extract
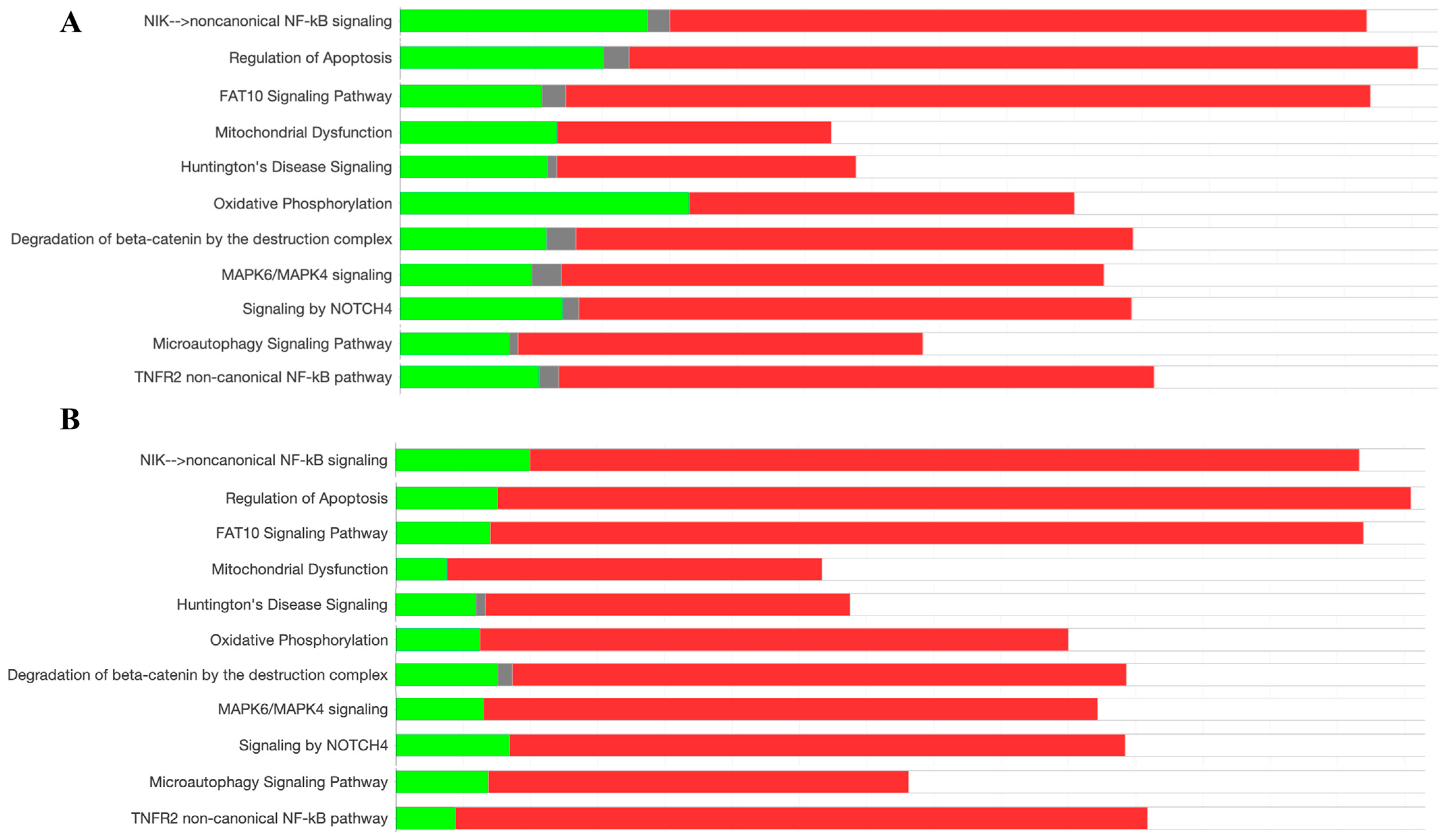
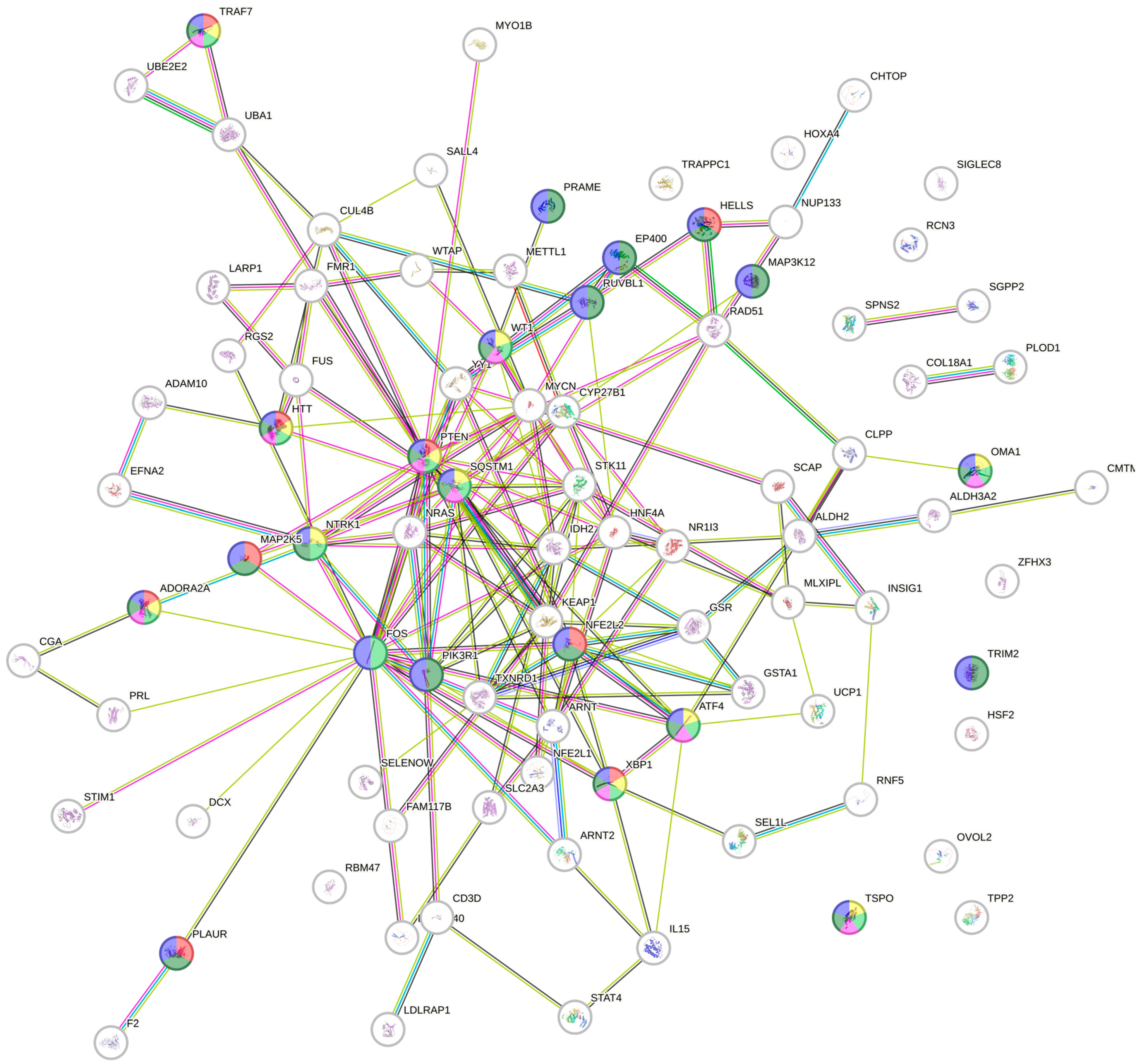
2.3. Protein Pathway Analysis
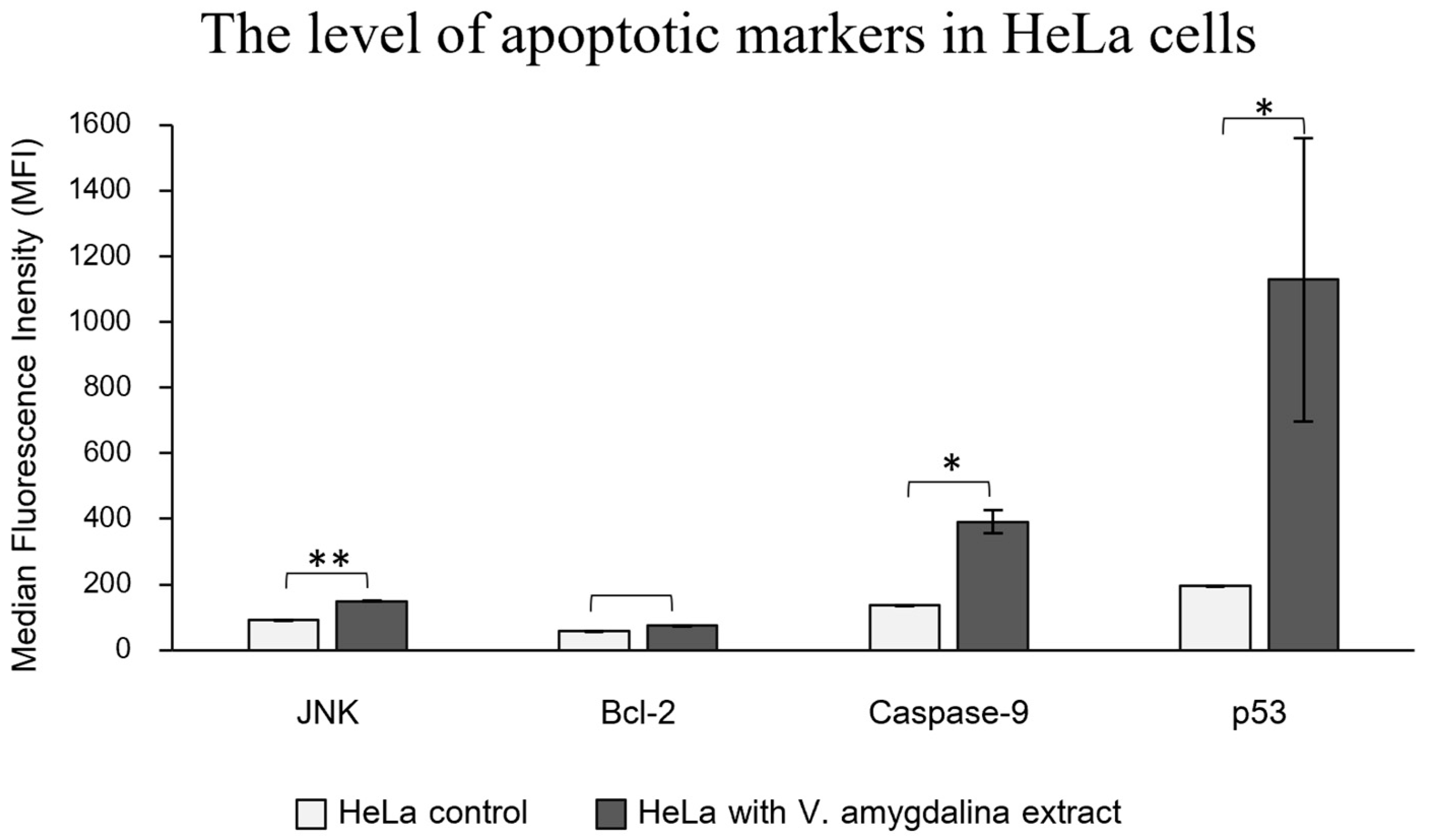
2.4. Apoptotic Cell Imaging and Apoptotic Protein Level Quantification
3. Materials and Methods
3.1. The Extraction of V. amygdalina Leaves
3.2. Cell Cultures and Cytotoxicity Test
3.3. The Metabolomics Profiling of V. amygdalina Leaf Extract
3.4. Sample Preparation for Label-Free Proteomics Analysis
3.5. LC-MS/MS Settings and Configurations for Proteomics Analysis
3.6. Biological Pathway Analysis
3.7. Imaging of Apoptotic Cells and Immuno-Based Early Apoptosis Protein Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, K.; Aktar, A.; Roy, P.; Biswas, B.; Hossain, M.E.; Sarkar, K.K.; Bachar, S.C.; Ahmed, F.; Monjur-Al-Hossain, A.S.M.; Fukase, K. A Review on Mechanistic Insight of Plant Derived Anticancer Bioactive Phytocompounds and Their Structure-Activity Relationship. Molecules 2022, 27, 3036. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, H. The Program Cell Death (Apoptosis) and the Therapy of Cancer. In Regulation and Dysfunction of Apoptosis; Tutar, Y., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Kim, G.D. Ursolic Acid Decreases the Proliferation of MCF-7 Cell-Derived Breast Cancer Stem-Like Cells by Modulating the ERK and PI3K/AKT Signaling Pathways. Prev. Nutr. Food Sci. 2021, 26, 434. [Google Scholar] [CrossRef]
- Leong, K.H.; Mahdzir, M.A.; Din, M.F.M.; Awang, K.; Tanaka, Y.; Kulkeaw, K.; Ishitani, T.; Sugiyama, D. Induction of Intrinsic Apoptosis in Leukaemia Stem Cells and in Vivo Zebrafish Model by Betulonic Acid Isolated from Walsura pinnata Hassk (Meliaceae). Phytomedicine 2017, 26, 11–21. [Google Scholar] [CrossRef]
- Clark, P.A.; Bhattacharya, S.; Elmayan, A.; Darjatmoko, S.R.; Thuro, B.A.; Yan, M.B.; Van Ginkel, P.R.; Polans, A.S.; Kuo, J.S. Resveratrol Targeting of AKT and P53 in Glioblastoma and Glioblastoma Stem-like Cells to Suppress Growth and Infiltration. J. Neurosurg. 2017, 126, 1448–1460. [Google Scholar] [CrossRef]
- Fu, H.; Wang, C.; Yang, D.; Wei, Z.; Xu, J.; Hu, Z.; Zhang, Y.; Wang, W.; Yan, R.; Cai, Q. Curcumin Regulates Proliferation, Autophagy, and Apoptosis in Gastric Cancer Cells by Affecting PI3K and P53 Signaling. J. Cell Physiol. 2018, 233, 4634–4642. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Li, Z.; Yuan, Z. Effect of Berberine on the Proliferation and Apoptosis of Lung Cancer Stem Cells and the Possible Mechanism. Chin. J. Tissue Eng. Res. 2017, 21, 1313. [Google Scholar] [CrossRef]
- Patathananone, S.; Pothiwan, M.; Uapipatanakul, B.; Kunu, W. Inhibitory Effects of Vernonia amygdalina Leaf Extracts on Free Radical Scavenging, Tyrosinase, and Amylase Activities. Prev. Nutr. Food Sci. 2023, 28, 302. [Google Scholar] [CrossRef]
- Usunobun, U.; Ngozi, O. Phytochemical Analysis and Proximate Composition of Vernonia amygdalina. Int. J. Sci. World 2016, 4, 11–14. [Google Scholar] [CrossRef]
- Ugbogu, E.A.; Emmanuel, O.; Dike, E.D.; Agi, G.O.; Ugbogu, O.C.; Ibe, C.; Iweala, E.J. The Phytochemistry, Ethnobotanical, and Pharmacological Potentials of the Medicinal Plant—Vernonia amygdalina L. (Bitter Leaf). Clin. Complement. Med. Pharmacol. 2021, 1, 100006. [Google Scholar] [CrossRef]
- Lai, C.C.; Zhou, X.; Wang, H.K.; Lin, Y.C.; Lin, H.Y.; Way, T.D.; Liu, B.L. Vernonia amygdalina Extract Induces Apoptosis and Inhibits Epithelial-Mesenchymal Transition in Hep 3B Cells through the Inhibition of PI3k/Akt Signaling Pathway. Int. J. Appl. Sci. Eng. 2022, 19, 35–41. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Johnson, W.; Tchounwou, S.S.; Dasari, S.; Njiki, S.; Tchounwou, P.B. Vernonia amygdalina Delile Induces Apoptotic Effects of PC3 Cells: Implication in the Prevention of Prostate Cancer. J. Biomed. Res. Environ. Sci. 2022, 3, 1118. [Google Scholar] [CrossRef] [PubMed]
- Syahputra, R.A.; Harahap, U.; Harahap, Y.; Gani, A.P.; Dalimunthe, A.; Ahmed, A.; Zainalabidin, S. Vernonia amygdalina Ethanol Extract Protects against Doxorubicin-Induced Cardiotoxicity via TGFβ, Cytochrome c, and Apoptosis. Molecules 2023, 28, 4305. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shen, L. Advances and Trends in Omics Technology Development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef] [PubMed]
- Ivanisevic, T.; Sewduth, R.N. Multi-Omics Integration for the Design of Novel Therapies and the Identification of Novel Biomarkers. Proteomes 2023, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Harefa, H.S.; Hasibuan, P.A.Z.; Harahap, U. Cytotoxic and Apoptotic Activities of Vernonia Amygdalina Extract in HepG2 Cell Line. Indones. J. Pharm. Clin. Res. 2022, 5, 24–29. [Google Scholar] [CrossRef]
- Nkono, B.L.N.Y.; Rouamba, A.; Duceac, I.A.; Verestiuc, L. Antihyperglycemic Effect of Vernonia amygdalina and in Vitro Evaluation of Its Antiproliferative Activity on Human Osteosarcoma MG-63. Pan Afr. Med. J. 2022, 42, 222. [Google Scholar] [CrossRef] [PubMed]
- Dumas, N.G.E.; Anderson, N.T.Y.; Godswill, N.N.; Thiruvengadam, M.; Ana-Maria, G.; Ramona, P.; Crisan, G.C.; Laurian, V.; Shariati, M.A.; Tokhtarov, Z.; et al. Secondary Metabolite Contents and Antimicrobial Activity of Leaf Extracts Reveal Genetic Variability of Vernonia amygdalina and Vernonia calvoana Morphotypes. Biotechnol. Appl. Biochem. 2021, 68, 938–947. [Google Scholar] [CrossRef]
- Bashir, R.A.; Mukhtar, Y.; Chimbekujwo, I.B.; Aisha, D.M.; Fatima, S.U.; Salamatu, S.U. Phytochemical Screening and Fourier Transform Infrared Spectroscopy (FT-IR) Analysis of Vernonia amygdalina Del. (Bitter Leaf) Methanol Leaf Extract. FUTY J. Environ. 2020, 14, 35–41. [Google Scholar]
- Sasaki, Y.; Kato, D.; Boger, D.L. Asymmetric Total Synthesis of Vindorosine, Vindoline, and Key Vinblastine Analogues. J. Am. Chem. Soc. 2010, 132, 13533–13544. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, M.; Wen, W.; Yu, R. Biosynthesis and Regulation of Terpenoid Indole Alkaloids in Catharanthus Roseus. Pharmacogn. Rev. 2015, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, Y.; Li, S.; Jiang, H.; Chen, N.; Xiao, H.; Liu, J.; Liang, D.; Zheng, Q.; Tang, J.; et al. Licochalcone B Confers Protective Effects against LPS-Induced Acute Lung Injury in Cells and Mice through the Keap1/Nrf2 Pathway. Redox Rep. 2023, 28, 2243423. [Google Scholar] [CrossRef]
- Wang, J.; Liao, A.M.; Thakur, K.; Zhang, J.G.; Huang, J.H.; Wei, Z.J. Licochalcone B Extracted from Glycyrrhiza Uralensis Fisch Induces Apoptotic Effects in Human Hepatoma Cell HepG2. J. Agric. Food Chem. 2019, 67, 3341–3353. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Konorev, E.A.; Kotamraju, S.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Doxorubicin Induces Apoptosis in Normal and Tumor Cells Via distinctly different mechanisms. intermediacy of H2O2- and p53-dependent pathways. J. Biol. Chem. 2004, 279, 25535–25543. [Google Scholar] [CrossRef] [PubMed]
- Naderali, E.; Khaki, A.A.; Rad, J.S.; Ali-Hemmati, A.; Rahmati, M.; Charoudeh, H.N. Regulation and Modulation of PTEN Activity. Mol. Biol. Rep. 2018, 45, 2869–2881. [Google Scholar] [CrossRef]
- Bononi, A.; Bonora, M.; Marchi, S.; Missiroli, S.; Poletti, F.; Giorgi, C.; Pandolfi, P.P.; Pinton, P. Identification of PTEN at the ER and MAMs and Its Regulation of Ca2+ Signaling and Apoptosis in a Protein Phosphatase-Dependent Manner. Cell Death Differ. 2013, 20, 1631–1643. [Google Scholar] [CrossRef]
- Shen, T.; Li, Y.; Liang, S.; Chen, Z. XBP1 Negatively Regulates CENPF Expression via Recruiting ATF6α to the Promoter during ER Stress. Cancer Cell Int. 2020, 20, 459. [Google Scholar] [CrossRef]
- Allagnat, F.; Christulia, F.; Ortis, F.; Pirot, P.; Lortz, S.; Lenzen, S.; Eizirik, D.L.; Cardozo, A.K. Sustained Production of Spliced X-Box Binding Protein 1 (XBP1) Induces Pancreatic Beta Cell Dysfunction and Apoptosis. Diabetologia 2010, 53, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, A. Role of JNK Activation in Apoptosis: A Double-Edged Sword. Cell Res. 2005, 15, 36–42. [Google Scholar] [CrossRef]
- Oleinik, N.V.; Krupenko, N.I.; Krupenko, S.A. Cooperation between JNK1 and JNK2 in Activation of P53 Apoptotic Pathway. Oncogene 2007, 26, 7222–7230. [Google Scholar] [CrossRef]
- Schroeter, H.; Boyd, C.S.; Ahmed, R.; Spencer, J.P.E.; Duncan, R.F.; Rice-Evans, C.; Cadenas, E. C-Jun N-Terminal Kinase (JNK)-Mediated Modulation of Brain Mitochondria Function: New Target Proteins for JNK Signalling in Mitochondrion-Dependent Apoptosis. Biochem. J. 2003, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M. The Apoptosome: Heart and Soul of the Cell Death Machine. Neoplasia 1999, 1, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Willforss, J.; Chawade, A.; Levander, F. NormalyzerDE: Online Tool for Improved Normalization of Omics Expression Data and High-Sensitivity Differential Expression Analysis. J. Proteome Res. 2019, 18, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Degryse, S.; De Bock, C.E.; Demeyer, S.; Govaerts, I.; Bornschein, S.; Verbeke, D.; Jacobs, K.; Binos, S.; Skerrett-Byrne, D.A.; Murray, H.C.; et al. Mutant JAK3 Phosphoproteomic Profiling Predicts Synergism between JAK3 Inhibitors and MEK/BCL2 Inhibitors for the Treatment of T-Cell Acute Lymphoblastic Leukemia. Leukemia 2018, 32, 788–800. [Google Scholar] [CrossRef]

| Name | Formula | Molecular Weight | Peak Area | Categories |
|---|---|---|---|---|
| Nandrolone decanoate | C28H44O3 | 428.3216 | 8.07 × 108 | Steroids |
| Deacetylvindoline | C23H30N2O5 | 414.2143 | 7.28 × 108 | Alkaloids |
| Rubiarbonone C | C32H50O5 | 536.3454 | 5.03 × 108 | Terpenes and terpenoids |
| Glycyrrhetinic acid | C30H46O4 | 492.3183 | 4.49 × 108 | Terpenes and terpenoids |
| Testosterone decanoate | C29H46O3 | 442.3378 | 2.23 × 108 | Steroids |
| Psoralidin | C20H16O5 | 336.1055 | 1.82 × 108 | Steroids |
| Ursolic acid | C30H48O3 | 456.3540 | 1.65 × 108 | Terpenes and terpenoids |
| 3-Hydroxybupivicaine | C18H28N2O2 | 304.2111 | 1.64 × 108 | Alkaloids |
| Phenylglyoxylic acid | C8H6O3 | 150.0316 | 1.53 × 108 | Phenolic compounds |
| 2-Hydroxyoctanoic acid | C8H16O3 | 182.0888 | 1.17 × 108 | Fatty acids and derivatives |
| Deidaclin | C12H17NO6 | 271.1056 | 7.48 × 107 | Glycosides |
| Prostaglandin F2 | C21H36O5 | 368.2553 | 5.63 × 107 | Steroids |
| Licochalcone B | C16H14O5 | 308.0693 | 5.09 × 107 | Phenolic compounds |
| Prostaglandin E2-biotin | C35H58N4O6S | 684.4016 | 3.75 × 107 | Steroids |
| Cafestol | C20H28O3 | 316.2089 | 2.44 × 107 | Terpenes and terpenoids |
| Upstream Regulator | Molecule Type | z-Score | p-Value |
|---|---|---|---|
| HNF4A | Transcription regulator | 2.67 | 2.44 × 10−108 |
| LARP1 | Translation regulator | 5.229 | 1.34 × 10−68 |
| MYCN | Transcription regulator | −3.682 | 1.76 × 10−56 |
| NFE2L2 | Transcription regulator | 5.012 | 3.11 × 10−41 |
| MLXIPL | Transcription regulator | −4.433 | 4.52 × 10−37 |
| XBP1 | Transcription regulator | 3.704 | 3.06 × 10−28 |
| STK11 | Kinase | 2.964 | 1.22 × 10−27 |
| CLPP | Peptidase | −2.411 | 1.31 × 10−27 |
| PTEN | Phosphatase | 2.149 | 4.7 × 10−16 |
| IL15 | Cytokine | 3.184 | 1.03 × 10−14 |
| GO Term | Biological Process | False Discovery Rate |
|---|---|---|
| GO:0042981 | Regulation of apoptotic process | 0.00046 |
| GO:0010941 | Regulation of cell death | 0.00073 |
| GO:0010942 | Positive regulation of cell death | 0.0025 |
| GO:0043068 | Positive regulation of programed cell death | 0.0037 |
| GO:2001233 | Regulation of apoptotic signaling pathway | 0.0062 |
| GO:0043065 | Positive regulation of apoptotic process | 0.0119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samutrtai, P.; Yingchutrakul, Y.; Faikhruea, K.; Vilaivan, T.; Chaikeeratisak, V.; Chatwichien, J.; Krobthong, S.; Aonbangkhen, C. Vernonia amygdalina Leaf Extract Induces Apoptosis in HeLa Cells: A Metabolomics and Proteomics Study. Pharmaceuticals 2024, 17, 1079. https://doi.org/10.3390/ph17081079
Samutrtai P, Yingchutrakul Y, Faikhruea K, Vilaivan T, Chaikeeratisak V, Chatwichien J, Krobthong S, Aonbangkhen C. Vernonia amygdalina Leaf Extract Induces Apoptosis in HeLa Cells: A Metabolomics and Proteomics Study. Pharmaceuticals. 2024; 17(8):1079. https://doi.org/10.3390/ph17081079
Chicago/Turabian StyleSamutrtai, Pawitrabhorn, Yodying Yingchutrakul, Kriangsak Faikhruea, Tirayut Vilaivan, Vorrapon Chaikeeratisak, Jaruwan Chatwichien, Sucheewin Krobthong, and Chanat Aonbangkhen. 2024. "Vernonia amygdalina Leaf Extract Induces Apoptosis in HeLa Cells: A Metabolomics and Proteomics Study" Pharmaceuticals 17, no. 8: 1079. https://doi.org/10.3390/ph17081079





