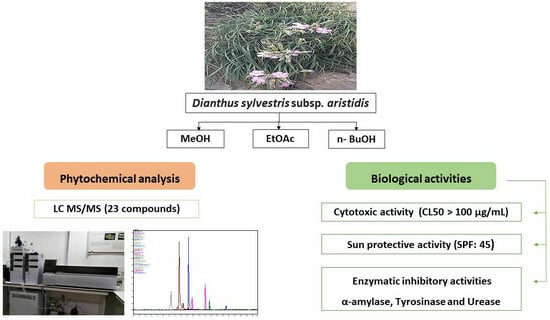
Phytochemical Profiling and Biological Evaluation of Dianthus sylvestris subsp. aristidis: A Chromatographic and Mass Spectrometry Approach to Uncovering Bioactive Metabolites for Dermatological and Metabolic Disorder Management
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Composition of HMeOH Extract: LC-MS/MS Results
2.2. Brine Shrimp Cytotoxicity
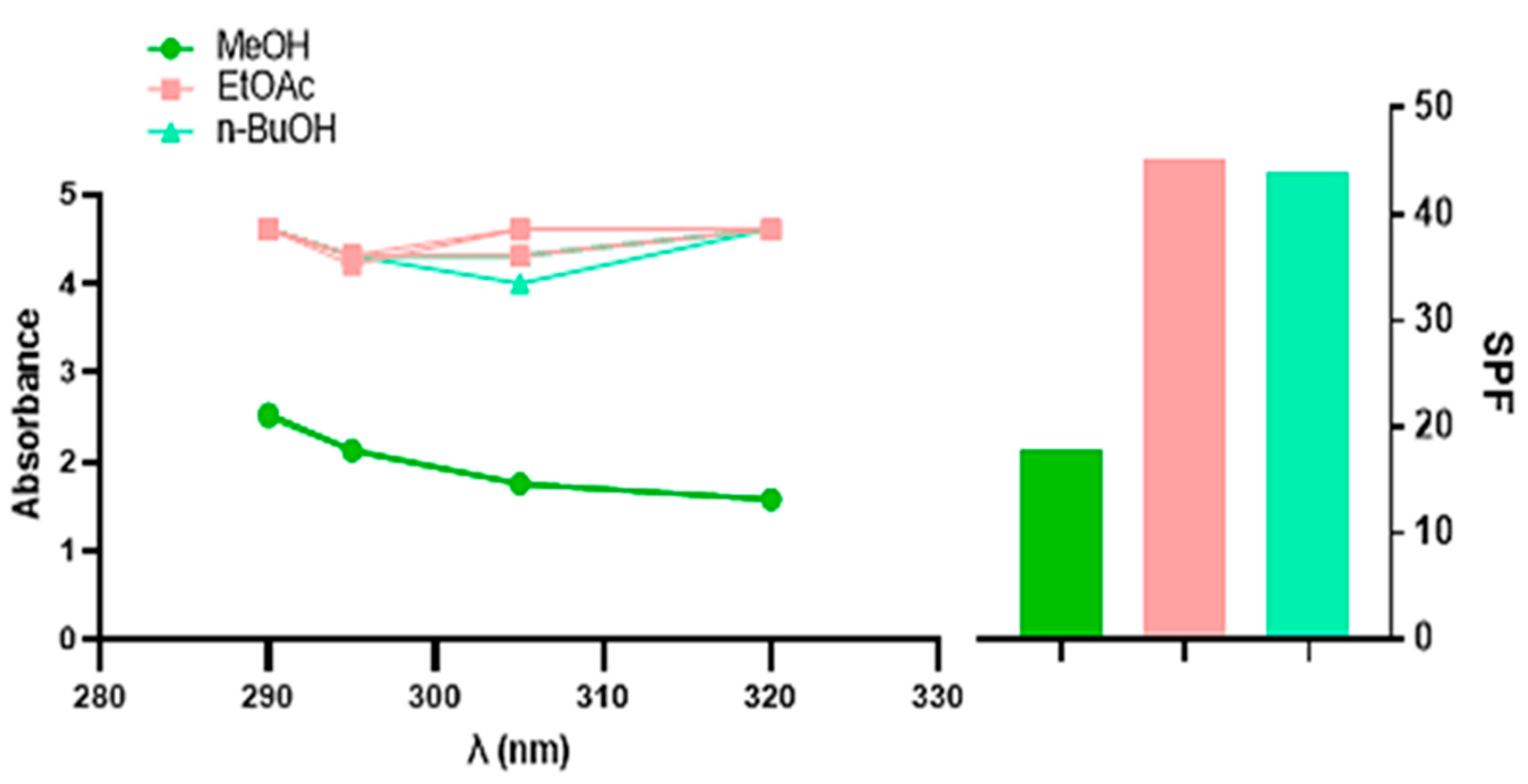
2.3. Sun Protection Factor Activity (SPF)
2.4. Enzymatic Inhibitory Activities
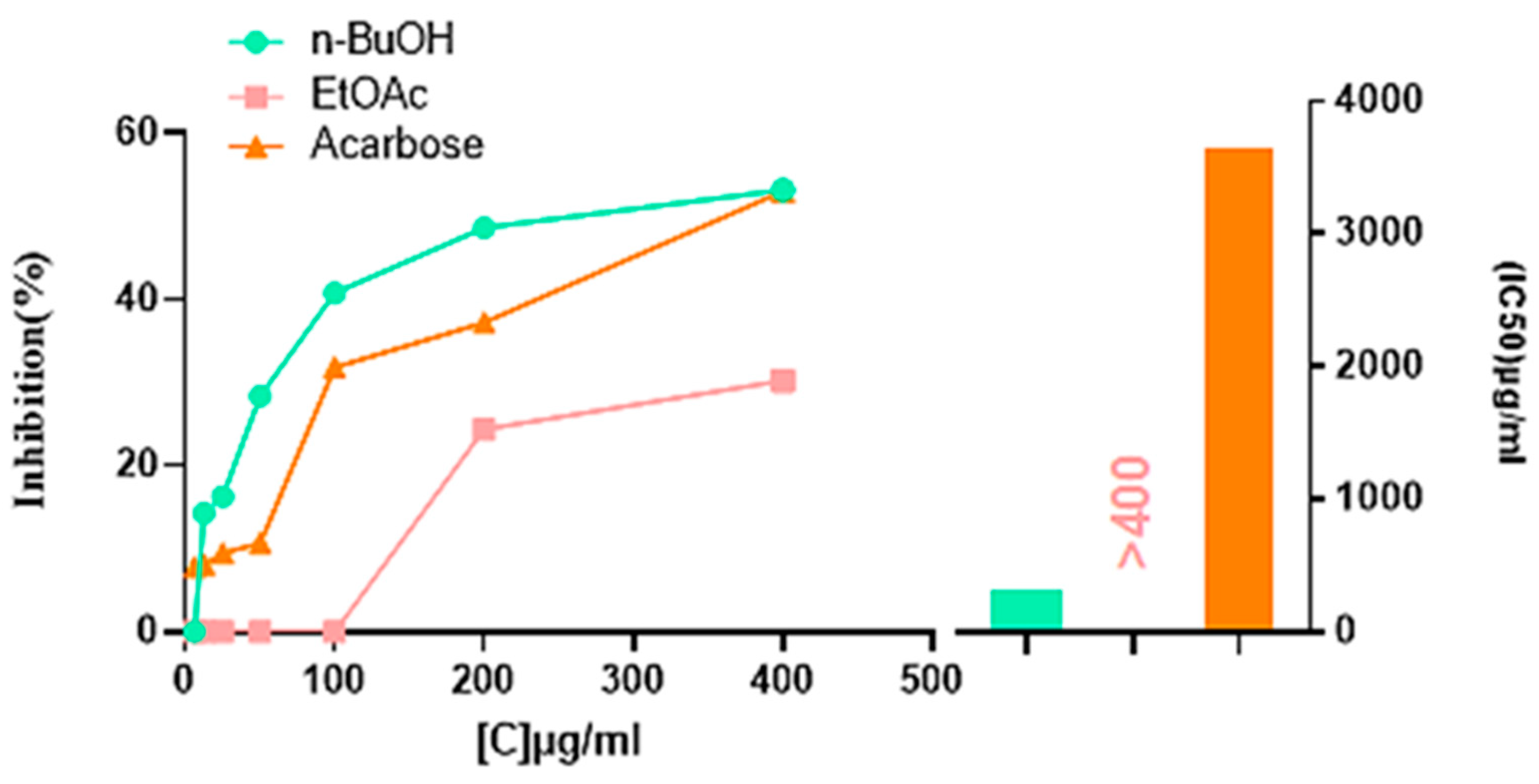
2.4.1. Alpha-Amylase Inhibitory Activity
2.4.2. Urease Inhibitory Activity
2.4.3. Tyrosinase Inhibitory Activity
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. Phytochemical Profile of HMeOH Extract via LC-ESI-MS/MS
3.3. Brine Shrimp Lethality Bioassay
3.4. Sun Protection Factor (SPF)
3.5. Inhibition of Enzymatic Activities
3.5.1. Inhibition of Alpha-Amylase Activity
3.5.2. Inhibition of Urease Activity
3.5.3. Inhibition of Tyrosinase Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kilic, M. The Healing Power of Plants for Health. In Medicinal Plants-Harnessing the Healing Power of Plants; InTechOpen: London, UK, 2024. [Google Scholar]
- Kaur, P.; Sonkar, R.; Chaudhari, R.S.; Jadhav, V.A.; Kulkarni, N.; Patil, S.V. A review of medicinal plants: Phytochemicals, molecular docking, and bioactive properties. World J. Adv. Res. Rev. 2024, 23, 141–160. [Google Scholar]
- Zouied, D.; Chettah, W.; Khelfaoui, M.; Bouzenad, N.; Abdennouri, A.; Dob, K.; Zouaoui, E. The use of arum italicum root extracts for protection against corrosion and green synthesis of silver nanoparticles: Investigation of their photocatalytic and antimicrobial properties. Glob. Nest J. 2024, 27, 206931. [Google Scholar]
- Otero, C.; Martínez-Araya, J.I.; del Campo, J.M.; Gordillo-Fuenzalida, F. Editorial: Endemic Plants: Experimental and Theoretical Insights Into Properties of Bioactive Metabolites With Therapeutic Potential. Front. Chem. 2021, 9, 786865. [Google Scholar]
- Sakhraoui, N.; Chefrour, A.; Zohra, C.; Hadef, A. Nouvelle station de Dianthus sylvestris subsp. aristidis (Batt.) Greuter et Burdet dans la région de Skikda (Nord-Est algérien). Bull. Société Linnéenne Provence 2021, 72, 81–84. [Google Scholar]
- Quezel, P.; Santa, S. Nouvelle Flore de l’Algerie et des Regions Desertiques Meridionales; CNRS, Edition; Centre National de la Recherche Scientifique: Paris, France, 1962–1963; Volume 1–2. [Google Scholar]
- Dobignard, A.; Chatelain, C. Synonymic Index to the Flora of North Africa. Volume 3: Dicotyledoneae: Balsaminaceae-Euphorbiaceae. 2011. Available online: https://www.cabdirect.org/cabdirect/abstract/20113309798 (accessed on 29 September 2023).
- Ertürk, Ö. Antibacterial and antifungal activity of ethanolic extracts from eleven spice plants. Biologia 2006, 61, 275–278. [Google Scholar]
- Yu, J.-Q.; Yin, Y.; Lei, J.-C.; Zhang, X.-Q.; Chen, W.; Ding, C.-L.; Wu, S.; He, X.-Y.; Liu, Y.-W.; Zou, G.-L. Activation of apoptosis by ethyl acetate fraction of ethanol extract of Dianthus superbus in HepG2 cell line. Cancer Epidemiol. 2012, 36, e40–e45. [Google Scholar]
- Kazeem, M.I.; Ashafa, A.O.T. In-vitro antioxidant and antidiabetic potentials of Dianthus basuticus Burtt Davy whole plant extracts. J. Herb. Med. 2015, 5, 158–164. [Google Scholar]
- Ahmed, V.A.; Rahman, H.S.; Mohammed Raheem, M.O.; Othman, H.H.; Algarawi, M.; Ibnaouf, K.H. Antidiabetic, Antihyperlipidemic, Antioxidant Effects and Regulation of miRNA Expression by Dianthus orientalis Adams Extract in Diabetic rat Model. Nat. Prod. Commun. 2024, 19, 1934578X241259377. [Google Scholar]
- Celik, A.K.; Usta, N.C.; Baba, Y.; Cimen, A.; Turker, A.U. Phenolic characterization, antimutagenic, antioxidant and antibacterial capacities of seven endemic Dianthus species from Turkey. S. Afr. J. Bot. 2024, 164, 39–49. [Google Scholar]
- Chandra, S.; Rawat, D.S. Medicinal plants of the family Caryophyllaceae: A review of ethno-medicinal uses and pharmacological properties. Integr. Med. Res. 2015, 4, 123–131. [Google Scholar]
- Al-Snafi, A.E. Chemical contents and medical importance of Dianthus caryophyllus—A review. IOSR J. Pharm. 2017, 7, 61–71. [Google Scholar]
- Mutlu, K.; Sarikahya, N.B.; Nalbantsoy, A.; Kirmizigul, S. Chemical constituents and biological activities of Dianthus elegans var. elegans. Nat. Prod. Res. 2018, 32, 1245–1253. [Google Scholar]
- Zhou, X.; Wang, M.; Li, H.; Ye, S.; Tang, W. Widely targeted metabolomics reveals the antioxidant and anticancer activities of different colors of Dianthus caryophyllus. Front. Nutr. 2023, 10, 1166375. [Google Scholar]
- Zhou, X.; Yang, X.; Sun, R.; Wang, J.; Mao, Y.; Cao, G.; Wang, M. Identification of chemical components in Dianthus determined by widely targeted metabolomics. Hortic. Sci. 2022, 49, 71–77. [Google Scholar]
- Koike, A.C.R. Antioxidant activity of Dianthus chinensis flowers processed by ionizing radiation. Braz. J. Radiat. Sciences. 2019, 7, 2A. [Google Scholar]
- Bouzana, A.; Zohra, C.; Becheker, I.; Sakhraoui, N.; Bouzenad, N.; Bensouici, C. Phytochemical analysis by LC MS/MS and in vitro antioxidant activity of the Algerian endemic plant Dianthus sylvestris subsp. aristidis (Batt.) Greuter & Burdet. Glob. NEST J. 2023, 25, 113–119. [Google Scholar]
- Bouzana, A.; Becheker, I.; Chekroud, Z.; Boudjellab, Z.E.; Sakhraoui, N. Antibacterial and Antifungal Activities of Various Extracts of Dianthus sylvestris subsp. aristidis (Batt.) Greuter & Burdet. Glob. NEST J. 2025, 27, 1–8. [Google Scholar]
- Kumar, A.; Khan, F.; Saikia, D. Phenolic Compounds and Their Biological and Pharmaceutical Activities. Chem. Inside Spices Herbs Res. Dev. 2022, 31, 204–234. [Google Scholar]
- Sehrawat, R.; Rathee, P.; Akkol, E.K.; Khatkar, S.; Lather, A.; Redhu, N.; Khatkar, A. Phenolic acids-versatile natural moiety with numerous biological applications. Curr. Top. Med. Chem. 2022, 22, 1472–1484. [Google Scholar]
- Aouzal, B.; Slimani, S.; Kamah, F.; Ounissi, I.; Bouacha, A. LC MS/MS Phytochemical analysis of Ruta montana and the Hepatic Preventive Effects in male Rats exposed to Tebuconazole. Glob. NEST J. 2024, 26, 1–11. [Google Scholar]
- Chahna, R.; Nina, S.; Bouzana, A.; Bougouizi, A.; Haouame, I.; Rebbas, K.; Garzoli, S.; Bendif, H. Chemical profiling by HPLC-DAD and GC-MS analysis and antimicrobial potential of Achillea santolina plant extracts against ESBL-Producing E. coli and fungal pathogens. Chem. Biodivers. 2024, e202403064. [Google Scholar] [CrossRef]
- Jabbar, S.S.; Ali Salman, N.; Ali Dawood, F.; Al-Fahham, A. The Chemical Structure and Clinical Significance of Phenolic Compounds. Int. J. Health Med. Res. 2024, 3, 741–745. [Google Scholar]
- Mihaylova, D.; Dimitrova-Dimova, M.; Popova, A. Dietary Phenolic Compounds Wellbeing and Perspective Applications. Int. J. Mol. Sci. 2024, 25, 4769. [Google Scholar] [CrossRef]
- Liu, Q.; Zang, E.-H.; Wang, C.-C.; Liu, Y.-C.; Niu, H.; Gao, Y.; Li, M.-H. Dianthi herba: A comprehensive review of its botany, traditional use, phytochemistry, and pharmacology. Chin. Med. 2022, 17, 15. [Google Scholar]
- Wang, M.; Shen, Q.; Pang, J.; Mao, Y.; Li, X.; Tao, Y.; Tang, W.; Sun, R.; Zhou, X. Study on chemical constituents and antioxidant activities of Dianthus caryophyllus L. Front. Plant Sci. 2024, 15, 1438967. [Google Scholar]
- Aliyazıcıoğlu, R.; Demir, S.; Badem, M.; Şener, S.Ö.; Korkmaz, N.; Demir, E.A.; Özgen, U.; Karaoğlu, Ş.A.; Aliyazıcıoğlu, Y. Antioxidant, antigenotoxic, antimicrobial activities and phytochemical analysis of Dianthus carmelitarum. Rec. Nat. Prod. 2017, 11, 270–284. [Google Scholar]
- Ding, C.; Zhang, W.; Li, J.; Lei, J.; Yu, J. Cytotoxic constituents of ethyl acetate fraction from Dianthus superbus. Nat. Prod. Res. 2013, 27, 1691–1694. [Google Scholar]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar]
- Becheker, I.; Becheker, A.; Melakhessou, M.A.; Marref, S.E.; Berredjem, H. Antibacterial, Antifungal, Cytotoxic and Genotoxic Activities of Different Extracts of Arabic and Myrrh Gums. Int. J. Pharm. Investig. 2022, 12, 20–27. [Google Scholar]
- Karbakhsh-Ravari, S.; Nili-Ahmadabadi, A.; Mahboobian, M.M.; Ghaffari, F. Chlorogenic acid as a protector against iron overload-induced hepatotoxicity: The importance of iron chelation and free radical scavenging. Proc. Indian Natl. Sci. Acad. Part A Phys. Sci. 2024, 1–10. Available online: https://link.springer.com/10.1007/s43538-024-00359-x (accessed on 4 March 2025).
- Yang, L.-C.; Chang, Y.-C.; Yeh, K.-L.; Huang, F.-M.; Su, N.-Y.; Kuan, Y.-H. Protective Effect of Rutin on Triethylene Glycol Dimethacrylate-Induced Toxicity through the Inhibition of Caspase Activa-tion and Reactive Oxygen Species Generation in Macrophages. Int. J. Mol. Sci. 2022, 23, 11773. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-I.; Hu, W.-S.; Hung, M.-Y.; Ou, H.-C.; Huang, S.-H.; Hsu, P.-T.; Day, C.-H.; Lin, K.H.; Viswa-Nadha, V.P.; Kuo, W.W.; et al. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1032–1043. [Google Scholar] [CrossRef]
- Alqrad, M.A.I.; El-Agamy, D.S.; Ibrahim, S.R.M.; Sirwi, A.; Abdallah, H.M.; Abdel-Sattar, E.; El-Halawany, A.M.; Elsaed, W.M.; & Mohamed, G.A. SIRT1/Nrf2/NF-κB Signaling Mediates Anti-Inflammatory and Anti-Apoptotic Activities of Oleanolic Acid in a Mouse Model of Acute Hepatorenal Damage. Medicina 2023, 59, 1351. [Google Scholar] [CrossRef]
- Ortiz-Andrade, R.; Araujo-León, J.A.; Sánchez-Recillas, A.; Navarrete-Vazquez, G.; González-Sánchez, A.A.; Hidalgo-Figueroa, S.; Alonso-Castro, Á.J.; Aranda-González, I.; Hernández-Núñez, E.; Coral-Martínez, T.I. Toxicological screening of four bioactive citroflavonoids: In vitro, in vivo, and in silico approaches. Molecules 2020, 25, 5959. [Google Scholar] [CrossRef] [PubMed]
- Lamula, S.N.; Ashafa, A.T. Antimicrobial and cytotoxic potential of Dianthus basuticus used in Basotho traditional practice. Bangladesh J. Pharmacol. 2014, 9, 105–111. [Google Scholar]
- Chisvert, A.; Salvador, A. Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Commission of European Communities. Recommendation of 22 September 2006. On sunscreen products and manufacturers claims. (2006/647/EC). Off. J. Eur. Union 2006, L265/39. [Google Scholar]
- Sax, B.W. Educating Consumers about sun protection. Pharm. Times 2000, 66, 48–54. [Google Scholar]
- Latha, M.S.; Martis, J.; Shobha, V.; Shinde, R.S.; Bangera, S.; Krishnankutty, B.; Bellary, S.; Varughese, S.; Rao, P.; Kumar, B.N. Sunscreening agents: A review. J. Clin. Aesthetic Dermatol. 2013, 6, 16. [Google Scholar]
- Donglikar, M.M.; Deore, S.L. Development and evaluation of herbal sunscreen. Pharmacogn. J. 2017, 9, 83–97. [Google Scholar]
- Martínez, A.; Estévez, J.C.; Silva-Pando, F.J. Antioxidant activity, total phenolic content and skin care properties of 35 selected plants from Galicia (NW Spain). Front. Life Sci. 2012, 6, 7–86. [Google Scholar]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Stevanato, R.; Bertelle, M.; Fabris, S. Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharmacol. 2014, 69, 71–77. [Google Scholar]
- Muzaffer, U.; Paul, V.I.; Prasad, N.R.; Karthikeyan, R.; Agilan, B. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine 2018, 42, 100–111. [Google Scholar]
- Vadivelan, R.; Krishnan, R.G.; Kannan, R. Antidiabetic potential of Asparagus racemosus Willd leaf extracts through inhibition of α-amylase and α-glucosidase. J. Tradit. Complement. Med. 2019, 9, 1–4. [Google Scholar]
- Pieper-Bigelow, C.; Strocchi, A.; Levitt, M.D. Where does serum amylase come from and where does it go? Gastroenterol. Clin. N. Am. 1990, 19, 793–810. [Google Scholar]
- Nguyen, V.B.; Nguyen, A.D.; Nguyen, Q.V.; Wang, S.-L. Porcine pancreatic α-amylase inhibitors from Euonymus laxiflorus Champ. Res. Chem. Intermed. 2017, 43, 259–269. [Google Scholar]
- Mechchate, H.; Es-Safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alharbi, M.S.; Alqahtani, A.; Bari, A. In vitro alpha-amylase and alpha-glucosidase inhibitory activity and in vivo antidiabetic activity of Withania frutescens L. Foliar extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. [Google Scholar]
- Khan, M.; Alam, A.; Khan, K.M.; Salar, U.; Chigurupati, S.; Wadood, A.; Ali, F.; Mohammad, J.I.; Riaz, M.; Perveen, S. Flurbiprofen derivatives as novel α-amylase inhibitors: Biology-oriented drug synthesis (BIODS), in vitro, and in silico evaluation. Bioorganic Chem. 2018, 81, 157–167. [Google Scholar]
- Wresdiyati, T.; Sa’diah, S.; Winarto, A.D.I.; Febriyani, V. Alpha-glucosidase inhibition and hypoglycemic activities of Sweitenia mahagoni seed extract. J. Biosci. 2015, 22, 73–78. [Google Scholar]
- Bljajić, K.; Brajković, A.; Čačić, A.; Vujić, L.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Končić, M. Chemical composition, antioxidant, and α-glucosidase-inhibiting activity of aqueous and hydroethanolic extracts of traditional antidiabetics from Croatian ethnomedicine. Horticulturae 2021, 7, 15. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human alpha-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [PubMed]
- Proença, C.; Ribeiro, D.; Freitas, M.; Fernandes, E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3137–3207. [Google Scholar]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary polyphenols as natural inhibitors of α-amylase and α-glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Kamah, F.; Basli, A.; Richard, T.; Bensouici, C.; Bounaas, J. Algerian vine canes as a good source of phenolic compounds for several in vitro biological activities. Glob. NEST J. 2024, 26, 5320. [Google Scholar]
- Kamah, F.; Basli, A.; Erenler, R.; Bouzana, A.; Richard, T.; Al-Mekhlafi, F.; Wadaan, M.; Boulkenafet, F. Phenolic compounds and biological activities of grape (Vitis vinifera L.) seeds at different ripening stages: Insights from Algerian varieties. Arq. Bras. Med. Veterinária Zootec. 2025, 77, 13361. [Google Scholar]
- Shin, J.-E.; Kim, J.-M.; Bae, E.-A.; Hyun, Y.-J.; Kim, D.-H. In Vitro Inhibitory Effect of Flavonoids on Growth, Infection and Vacuolation of Helicobacter pylori. Planta Med. 2005, 71, 197–201. [Google Scholar]
- Wu, D.-W.; Yu, X.-D.; Xie, J.-H.; Su, Z.-Q.; Su, J.-Y.; Tan, L.-R.; Huang, X.-Q.; Chen, J.-N.; Su, Z.-R. Inactivation of jack bean urease by scutellarin: Elucidation of inhibitory efficacy, kinetics and mechanism. Fitoterapia 2013, 91, 60–67. [Google Scholar]
- Modolo, L.V.; de Souza, A.X.; Horta, L.P.; Araujo, D.P.; de Fatima, A. An overview on the potential of natural products as ureases inhibitors: A review. J. Adv. Res. 2015, 6, 35–44. [Google Scholar]
- Al-Rooqi, M.M.; Mughal, E.U.; Raja, Q.A.; Hussein, E.M.; Naeem, N.; Sadiq, A.; Ahmed, S.A. Flavonoids and related privileged scaffolds as potential urease inhibitors: A review. RSC Adv. 2023, 13, 3210–3233. [Google Scholar]
- Bari, A.; Ghani, U.; Syed, S.A. Thiosemicarbazide binds with the dicopper center in the competitive inhibition of mushroom tyrosinase enzyme: Synthesis and molecular modeling of theophylline analogues. Bioorg. Med. Chem. Lett. 2021, 36, 127826. [Google Scholar]
- Qu, Y.; Zhan, Q.; Du, S.; Ding, Y.; Fang, B.; Du, W.; Wu, Q.; Yu, H.; Li, L.; Huang, W. Catalysis-based specific detection and inhibition of tyrosinase and their application. J. Pharm. Anal. 2020, 10, 414–425. [Google Scholar] [PubMed]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar]
- Mirmortazavi, S.S.; Farvandi, M.; Ghafouri, H.; Mohammadi, A.; Shourian, M. Evaluation of novel pyrimidine derivatives as a new class of mushroom tyrosinase inhibitor. Drug Des. Dev. Ther. 2019, 13, 2169–2178. [Google Scholar]
- Gong, C.-F.; Wang, Y.-X.; Wang, M.-L.; Su, W.-C.; Wang, Q.; Chen, Q.-X.; Shi, Y. Evaluation of the Structure and Biological Activities of Condensed Tannins from Acanthus ilicifolius Linn and Their Effect on Fresh-Cut Fuji Apples. Appl. Biochem. Biotechnol. 2019, 189, 855–870. [Google Scholar]
- Kubo, I.; Kinst-Hori, I. Flavonols from Saffron Flower: Tyrosinase Inhibitory Activity and Inhibition Mechanism. J. Agric. Food Chem. 1999, 47, 4121–4125. [Google Scholar]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural Phenolic Compounds from Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar]
- Dwibedi, V.; Jain, S.; Singhal, D.; Mittal, A.; Rath, S.K.; Saxena, S. Inhibitory activities of grape bioactive compounds against enzymes linked with human diseases. Appl. Microbiol. Biotechnol. 2022, 106, 1399–1417. [Google Scholar]
- Upson, T.M.; Grayer, R.J.; Greenham, J.R.; Williams, C.A.; Al-Ghamdi, F.; Chen, F.-H. Leaf flavonoids as systematic characters in the genera Lavandula and Sabaudia. Biochem. Syst. Ecol. 2000, 28, 991–1007. [Google Scholar]
- Global Biodiversity Information Facility (GBIF). Dianthus aristidis Batt. Checklist Dataset. 2023. Available online: https://www.gbif.org/fr/occurrence/1424544781 (accessed on 17 February 2023).
- Finney Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Benjamin, L. Hlina, Written in ‘Programming Language R’, version 3.4.3 (2017-11-30)—“Kite-Eating Tree”; Wilfrid Laurier University: Waterloo, ON, Canada, 2009. [Google Scholar]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.; d’Ascençäo Azulay, R.D. Determinaçäo do fator de proteçäo solar por espectrofotometria. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crops Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. J. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Gouzi, H.; Benmansour, A. Partial Purification and Characterization of Polyphenol Oxidase Extracted from Agaricus bisporus (J.E. Lange) Imbach. Int. J. Chem. React. Eng. 2007, 5. [Google Scholar] [CrossRef]
- Deveci, E.; Tel-Çayan, G.; Duru, M.E. Phenolic profile, antioxidant, anticholinesterase, and anti-tyrosinase activities of the various extracts of Ferula elaeochytris and Sideritis stricta. Int. J. Food Prop. 2018, 21, 771–783. [Google Scholar] [CrossRef]
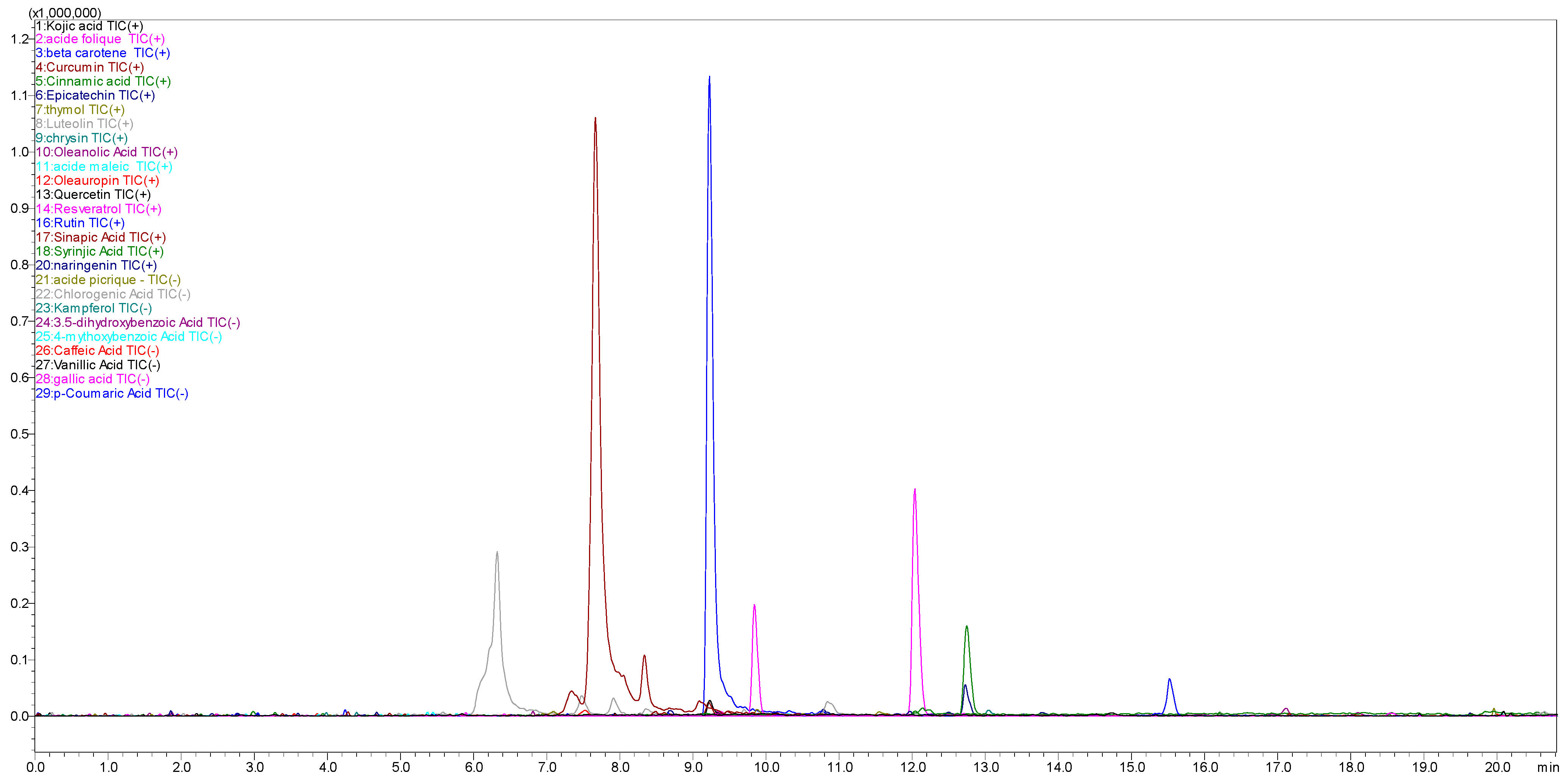

| ID | Name | Molecular Formula | Molecular Weight | ESI Charge (+/−) | m/z | Ret. Time | Height | Area |
|---|---|---|---|---|---|---|---|---|
| 1 | Ferulic acid | C10H10O4 | 194.18 | (+) | 194.8000 > 177.0500 | 8.610 | 3,736,345 | 19,847,102 |
| 2 | Chlorogenic Acid | C16H18O9 | 354.31 | (−) | 353.0500 > 191.1000 | 6.323 | 257,094 | 2,536,296 |
| 3 | Sinapic Acid | C11H12O5 | 224.21 | (+) | 225.0000 > 91.1000 | 9.215 | 13,522 | 63,556 |
| 4 | Caffeic Acid | C9H8O4 | 180.16 | (−) | 179.1500 > 135.0000 | 7.521 | 8033 | 53,166 |
| 5 | Cinnamic acid | C9H8O2 | 148.16 | (+) | 149.0500 > 117.0000 | 12.141 | 11,812 | 56,679 |
| 6 | Syringic Acid | C9H10O5 | 198.17 | (+) | 199.0000 > 155.1500 | 20.210 | 5779 | 12,301 |
| 7 | p-Coumaric Acid | C9H8O3 | 164.16 | (−) | 163.0500 > 118.9500 | 8.660 | 3291 | 9547 |
| 8 | Vanillic Acid | C8H8O4 | 168.15 | (−) | 166.9500 > 151.9000 | 7.372 | 966 | 5209 |
| 9 | Rutin | C27H30O16 | 610.5 | (+) | 611.0000 > 465.2000 | 9.226 | 208,513 | 1,157,023 |
| 10 | Luteolin | C15H10O6 | 286.24 | (+) | 286.7500 > 153.0000 | 10.844 | 11,315 | 85,663 |
| 11 | Epicatechin | C15H14O6 | 290.27 | (+) | 290.9000 > 123.1000 | 11.966 | 7332 | 36,849 |
| 12 | Naringenin | C15H12O5 | 272.25 | (+) | 273.0500 > 153.0000 | 10.781 | 5456 | 35,475 |
| 13 | Chrysin | C15H10O4 | 254.24 | (+) | 255.1000 > 68.8500 | 11.332 | 1667 | 6144 |
| 14 | β carotene | C40H56 | 536.87 | (+) | 537.2000 > 23.1000 | 15.521 | 63,553 | 393,908 |
| 15 | Oleanolic Acid | C30H48O3 | 456.7 | (+) | 457.3000 > 411.5000 | 17.117 | 9012 | 47,730 |
| 16 | Riboflavin | C17H20N4O6 | 376.4 | (+) | 377.9000 > 361.3500 | 14.721 | 4,026,753 | 25,896,378 |
| 17 | Folic Acid | C19H19N7O6 | 441 | (+) | 442.9000 > 323.4500 | 18.539 | 4865 | 31,602 |
| 18 | Curcumin | C21H20O6 | 368.4 | (+) | 368.9000 > 145.0500 | 7.672 | 148,455 | 1,135,080 |
| 19 | Resveratrol | C14H12O3 | 228.24 | (+) | 229.0500 > 135.1000 | 9.842 | 115,557 | 575,817 |
| 20 | Thymol | C10H14O | 150.22 | (+) | 151.1000 > 109.0500 | 11.547 | 3160 | 13,242 |
| 21 | 4-Methoxybenzoic Acid | C8H8O3 | 152.15 | (+) | 151.0500 > 107.0500 | 8.348 | 2403 | 10,040 |
| 22 | Oleuropein | C25H32O13 | 540.5 | (−) | 541.3000 > 524.5500 | 19.765 | 1244 | 7789 |
| Tested Extracts | Concentrations (µg/mL) | Initial Number of Nauplii | Total Death | Percentage of Letality | LC50 (µg/mL) | Confidence Interval (LCL-UCL) | ||
|---|---|---|---|---|---|---|---|---|
| HMeOH | 1000 | 10 | 2 | 5 | 3 | 33.33 | 6320 | 1.18 × 103–6.01 × 109 |
| 500 | 10 | 3 | 3 | 5 | 36.66 | |||
| 250 | 10 | 1 | 2 | 3 | 20 | |||
| 125 | 10 | 0 | 1 | 4 | 16.66 | |||
| 62.5 | 10 | 1 | 2 | 2 | 16.66 | |||
| 31.25 | 10 | 1 | 0 | 1 | 6.66 | |||
| EtOAc | 1000 | 10 | 4 | 4 | 4 | 40 | 2500 | 6.75 × 102–1.44 × 107 |
| 500 | 10 | 3 | 4 | 5 | 40 | |||
| 250 | 10 | 3 | 2 | 3 | 30 | |||
| 125 | 10 | 3 | 3 | 0 | 20 | |||
| 62.5 | 10 | 3 | 2 | 2 | 23.33 | |||
| 31.25 | 10 | 2 | 2 | 1 | 16.66 | |||
| n-BuOH | 1000 | 10 | 5 | 5 | 6 | 53.33 | 1272 | 436–9.21 × 105 |
| 500 | 10 | 4 | 4 | 3 | 36.66 | |||
| 250 | 10 | 2 | 2 | 3 | 23.33 | |||
| 125 | 10 | 1 | 3 | 2 | 20 | |||
| 62.5 | 10 | 1 | 4 | 1 | 20 | |||
| 31.25 | 10 | 1 | 2 | 2 | 16.66 | |||
| Control | / | 10 | 0 | 1 | 0 | 3.33 | / | / |
| Extracts | HMeOH | EtOAc | n-BuOH | Venus | Uriage |
|---|---|---|---|---|---|
| SPF | 17.82 ± 0.45 a | 45.19 ± 0.73 b | 43.81 ± 0.59 b | 50.11 ± 0.53 c | 44.22 ± 0.3 b |
| Extracts | % Inhibition of α-Amylase | IC50 (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 6.25 µg | 12.5 µg | 25 µg | 50 µg | 100 µg | 200 µg | 400 µg | IC50 (µg/mL) | |
| HMeOH | NA | NA | NA | NA | NA | NA | NA | NA |
| EtOAc | NA | NA | NA | NA | NA | 24.40 ± 0.36 | 30.24 ± 1.74 | ˃400 |
| n-BuOH | NA | 14.27 ± 1.04 | 16.27 ± 2.87 | 28.36 ± 0.78 | 40.75 ± 0.79 | 48.64 ± 0.42 | 53.19 ± 0.04 | 307.08 ± 1.13 b |
| 62.5 µg | 125 µg | 250 µg | 500 µg | 1000 µg | 2000 µg | 4000 µg | IC50 (µg/mL) | |
| Acarbose | 7.76 ± 0.17 | 8.08 ± 0.30 | 9.46 ± 0.11 | 10.70 ± 0.96 | 31.81 ± 2.89 | 37.21 ± 3.54 | 53.05 ± 1.59 | 3650.93 ± 10.70 a |
| Extracts | IC50 µg/mL |
|---|---|
| HMeOH | NA |
| EtOAc | NA |
| n-BuOH | NA |
| Thiourea | 11.57 ± 0.68 |
| Extracts | IC50 µg/mL |
|---|---|
| HMeOH | NA |
| EtOAc | NA |
| n-BuOH | NA |
| Kojic acid | 19.43 ± 0.98 |
| Wavelength λ (nm) | EE (λ) × I(λ) (Norms) |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0837 |
| 320 | 0.0180 |
| Total | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzana, A.; Chekroud, Z.; Becheker, I.; Kamah, F.; Sakhraoui, N.; Bensouici, C.; Boufahja, F.; Alsalamah, S.A.; Alghonaim, M.I.; Garzoli, S.; et al. Phytochemical Profiling and Biological Evaluation of Dianthus sylvestris subsp. aristidis: A Chromatographic and Mass Spectrometry Approach to Uncovering Bioactive Metabolites for Dermatological and Metabolic Disorder Management. Pharmaceuticals 2025, 18, 578. https://doi.org/10.3390/ph18040578
Bouzana A, Chekroud Z, Becheker I, Kamah F, Sakhraoui N, Bensouici C, Boufahja F, Alsalamah SA, Alghonaim MI, Garzoli S, et al. Phytochemical Profiling and Biological Evaluation of Dianthus sylvestris subsp. aristidis: A Chromatographic and Mass Spectrometry Approach to Uncovering Bioactive Metabolites for Dermatological and Metabolic Disorder Management. Pharmaceuticals. 2025; 18(4):578. https://doi.org/10.3390/ph18040578
Chicago/Turabian StyleBouzana, Amina, Zohra Chekroud, Imène Becheker, Fatima Kamah, Nora Sakhraoui, Chawki Bensouici, Fehmi Boufahja, Sulaiman A. Alsalamah, Mohammed I. Alghonaim, Stefania Garzoli, and et al. 2025. "Phytochemical Profiling and Biological Evaluation of Dianthus sylvestris subsp. aristidis: A Chromatographic and Mass Spectrometry Approach to Uncovering Bioactive Metabolites for Dermatological and Metabolic Disorder Management" Pharmaceuticals 18, no. 4: 578. https://doi.org/10.3390/ph18040578
APA StyleBouzana, A., Chekroud, Z., Becheker, I., Kamah, F., Sakhraoui, N., Bensouici, C., Boufahja, F., Alsalamah, S. A., Alghonaim, M. I., Garzoli, S., & Bendif, H. (2025). Phytochemical Profiling and Biological Evaluation of Dianthus sylvestris subsp. aristidis: A Chromatographic and Mass Spectrometry Approach to Uncovering Bioactive Metabolites for Dermatological and Metabolic Disorder Management. Pharmaceuticals, 18(4), 578. https://doi.org/10.3390/ph18040578