Comparative Forced Degradation Study of Anticomplement C5 Biosimilar and Originator Monoclonal Antibodies
Abstract
1. Introduction
2. Results and Discussion
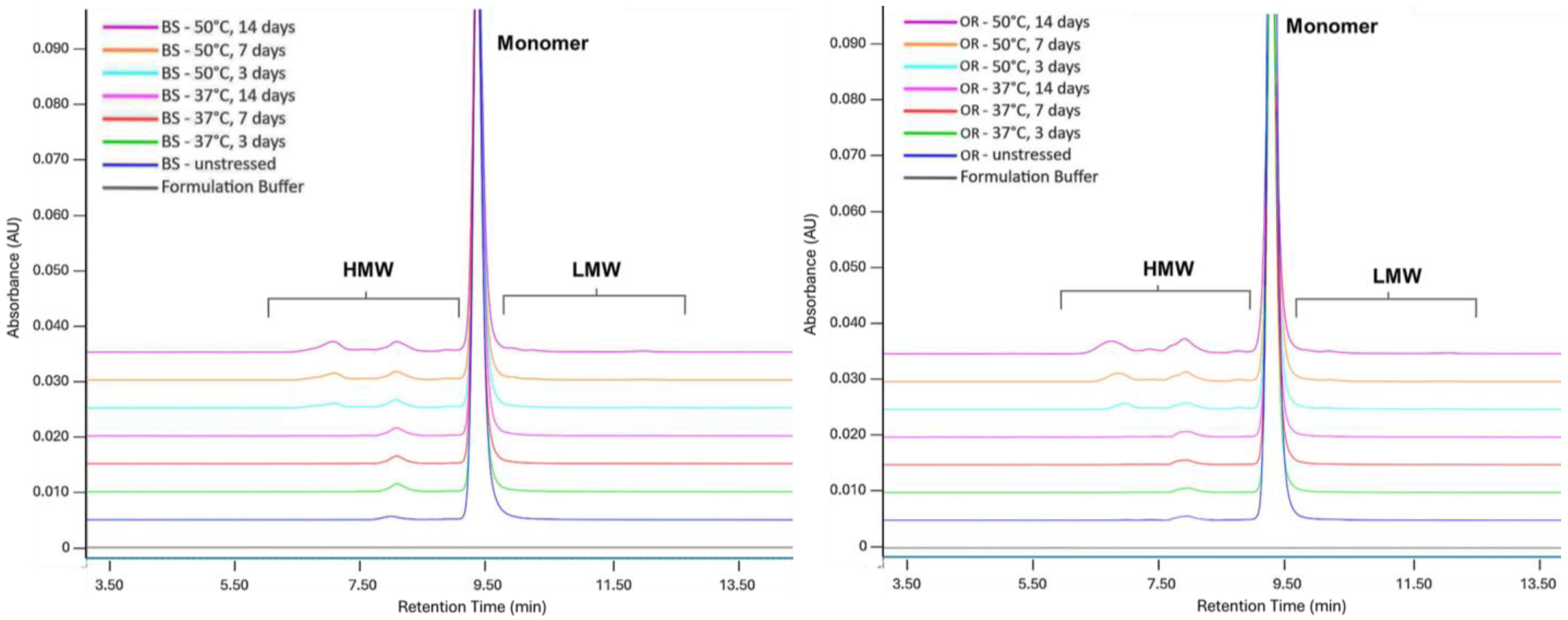
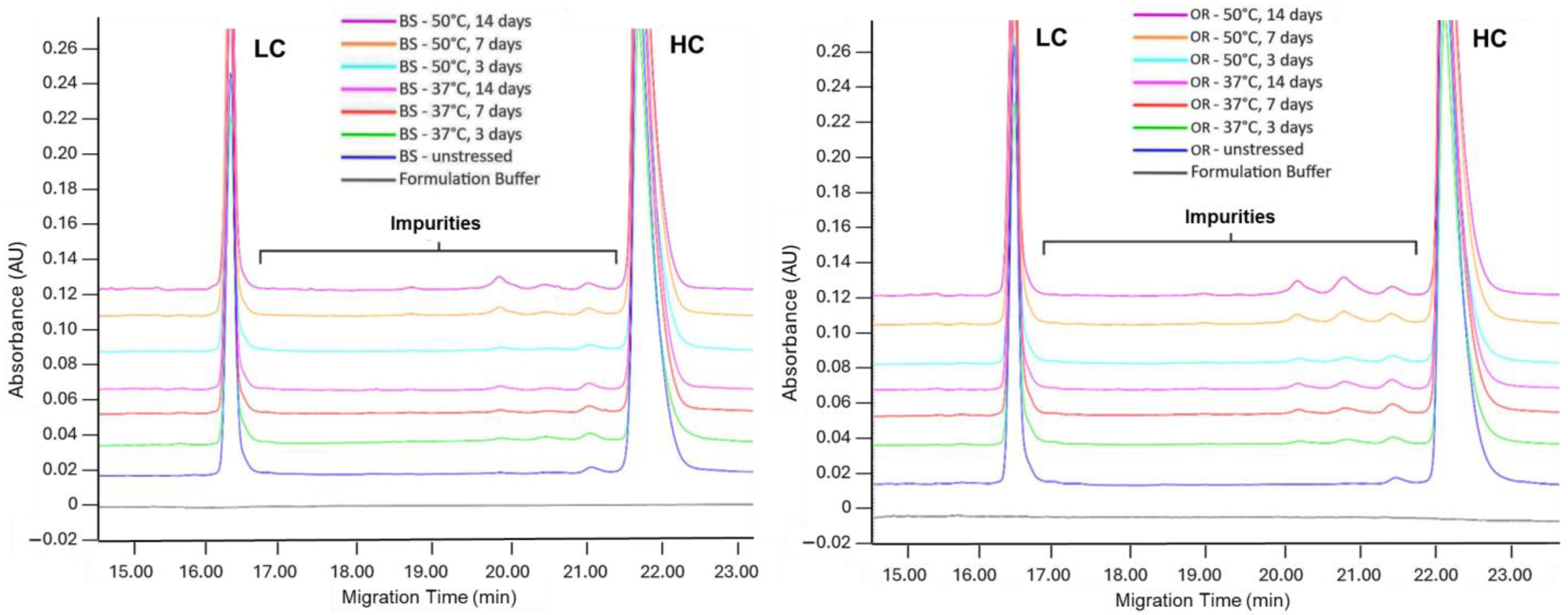
2.1. Thermal Stress
2.2. Agitation Stress
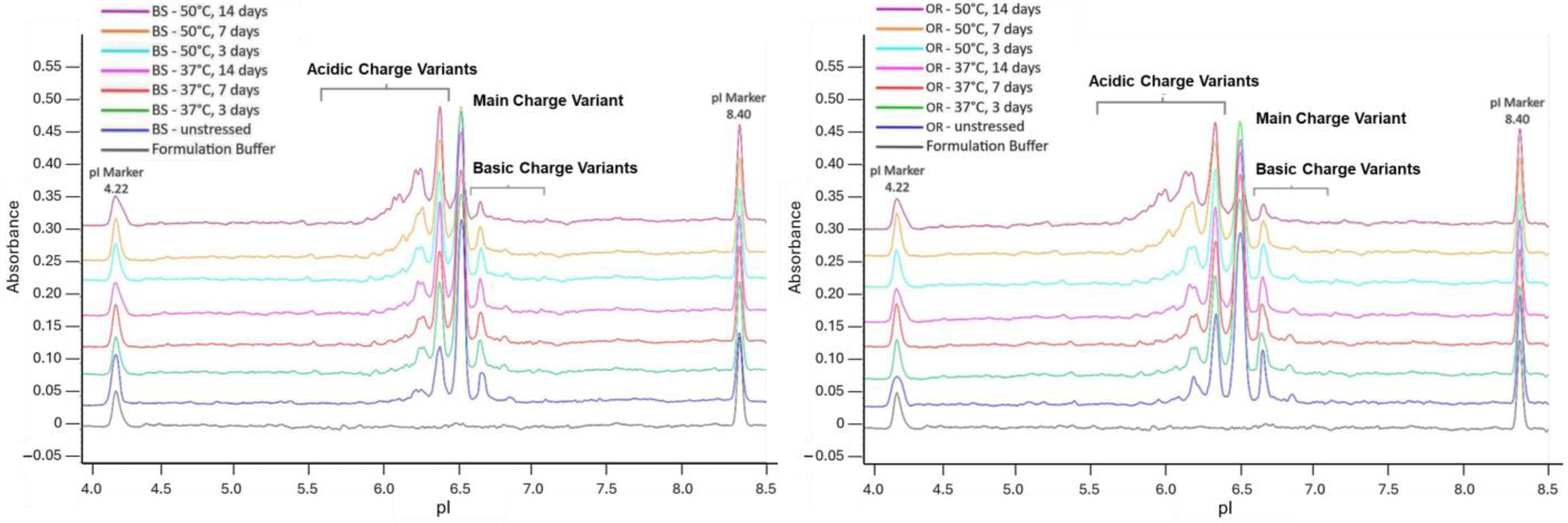
2.3. pH Stress
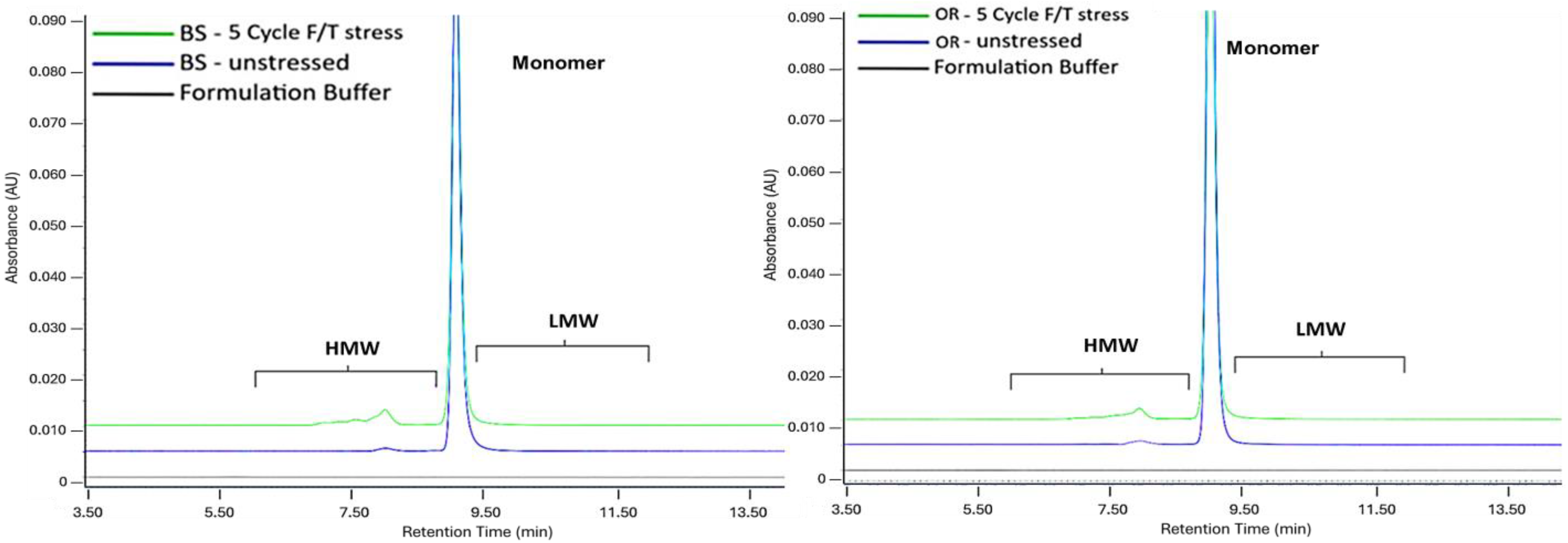
2.4. Freeze/Thaw Stress
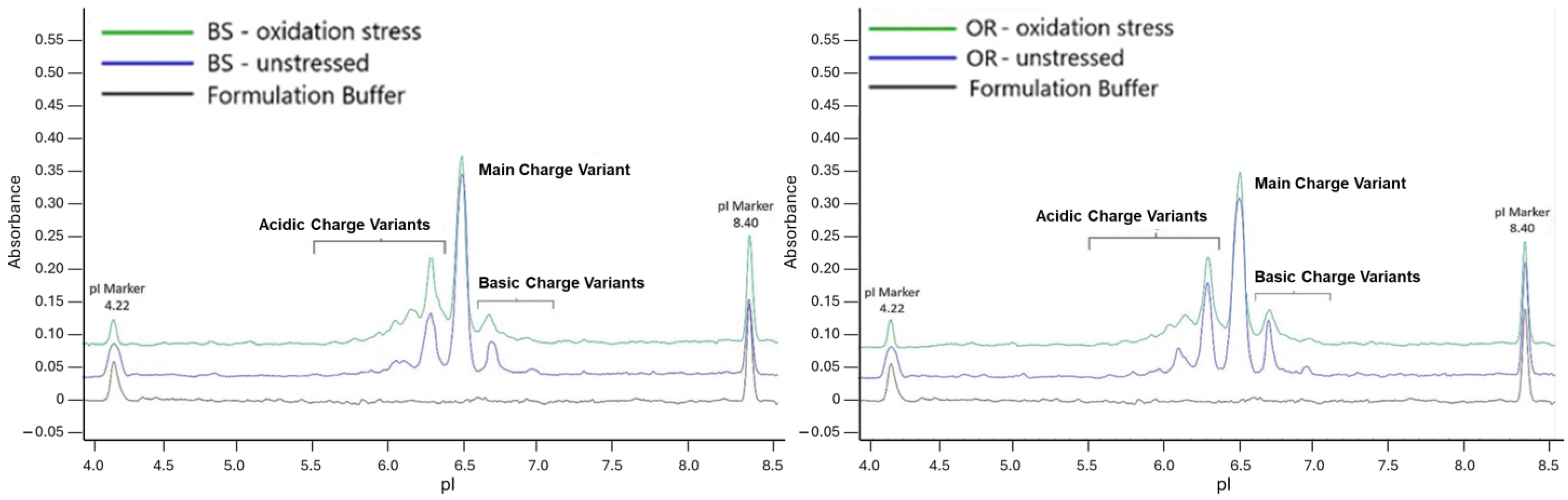
2.5. Oxidation Stress
3. Materials and Methods
3.1. Sample Details
3.2. Sample Preparation
3.2.1. Thermal Stress
3.2.2. Agitation Stress
3.2.3. pH Stress
3.2.4. Freeze/Thaw Stress
3.2.5. Oxidation Stress
3.3. Size Exclusion Ultra-Performance Liquid Chromatography (SE-UPLC)
3.4. Imaged Capillary Isoelectric Focusing (icIEF)
3.5. Capillary Electrophoresis-Sodium Dodecyl Sulfate (CE-SDS)
3.6. Complement Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BS | Biosimilar monoclonal antibody |
| CE-SDS | Capillary Electrophoresis Sodium Dodecyl Sulfate |
| F/T | Freeze/thaw |
| HC | Heavy Chain |
| HMW | High Molecular Weight |
| icIEF | Imaged Capillary Isoelectric Focusing |
| LC | Light Chain |
| LMW | Light Molecular Weight |
| mAb | Monoclonal antibody |
| nrCE-SDS | Non-Reduced Capillary Electrophoresis-Sodium Dodecyl Sulfate |
| OR | Originator Monoclonal Antibody |
| rCE-SDS | Reduced Capillary Electrophoresis-Sodium Dodecyl Sulfate |
| SD | Standard Deviation |
| SE-UPLC | Size Exclusion Ultra High-Performance Liquid Chromatography |
References
- Zhong, X.; D’Antona, A.M. Recent Advances in the Molecular Design and Applications of Multispecific Biotherapeutics. Antibodies 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, W. The Current Status, Trend, and Development Strategies of Chinese Biopharmaceutical Industry with a Challenging Perspective. Sage Open. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Ingram, B.; Lumsden, R.S.; Radosavljevic, A.; Kobryn, C. Analysis of the Regulatory Science Applied to a Single Portfolio of Eight Biosimilar Product Approvals by Four Key Regulatory Authorities. Pharmaceuticals 2021, 14, 306. [Google Scholar] [CrossRef]
- Agrawal, P. Biopharmaceuticals: An emerging trend in Drug Development. SOJ Pharm. Pharm. Sci. 2015, 2, 1–2. [Google Scholar] [CrossRef][Green Version]
- Parr, M.K.; Montacir, O.; Montacir, H. Physicochemical characterization of biopharmaceuticals. J. Pharm. Biomed. 2016, 130, 366–389. [Google Scholar] [CrossRef] [PubMed]
- Singh Sekhon, B. Biopharmaceuticals: An overview. Thai J. Pharm. Sci. 2010, 34, 1–19. [Google Scholar] [CrossRef]
- Mekala, J.R.; Nalluri, H.P.; Reddy, P.N.; Sainath, S.B.; Kumar, N.S.S.; Sai Kiran, G.V.S.D.; Dhiman, R.; Chamarthy, S.; Rao, K.R.; Manyam, R.R.; et al. Emerging trends and therapeutic applications of monoclonal antibodies. Gene 2024, 925, 148607. [Google Scholar] [CrossRef]
- Berger, M.; Shankar, V.; Vafai, A. Therapeutic Applications of Monoclonal Antibodies. AJMS 2002, 324, 14–30. [Google Scholar] [CrossRef]
- Aboul-Ella, H.; Gohar, A.; Ali, A.A.; Ismail, L.M.; Mahmoud, A.E.E.-R.; Elkhatib, W.F. Monoclonal antibodies: From magic bullet to precision weapon. Mol. Biomed. 2024, 5, 47. [Google Scholar]
- Moorkens, E.; Jonker-Exler, C.; Huys, I.; Declerck, P.; Simoens, S.; Vulto, A.G. Overcoming barriers to the market access of biosimilars in the European union: The case of biosimilar monoclonal antibodies. Front. Pharmacol. 2016, 7, 193. [Google Scholar] [CrossRef]
- Vulto, A.G.; Jaquez, O.A. The process defines the product: What really matters in biosimilar design and production? Rheumatology. 2017, 56, 14–29. [Google Scholar] [CrossRef]
- Wang, J.; Chow, S.-C. On the Regulatory Approval Pathway of Biosimilar Products. Pharmaceuticals 2012, 5, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Kesik-Brodacka, M. Progress in Biopharmaceutical Development. Biotechnol. Appl. Biochem. 2018, 65, 306–322. [Google Scholar] [CrossRef]
- Mellstedt, H.; Niederwieser, D.; Ludwig, H. The challenge of biosimilars. Ann. Oncol. 2008, 19, 411–419. [Google Scholar] [CrossRef]
- Sivendran, R.; Ramírez, J.; Ramchandani, M.; Liu, J. Scientific and statistical considerations in evaluating the analytical similarity of ABP 501 to adalimumab. Immunotherapy 2018, 10, 1011–1021. [Google Scholar] [CrossRef]
- Erdemgil, Y.; Celik Yamaci, M.; Pamukcu, C.; Unalp, F.; Yildirim Keles, Z.Z.; Emin Atik, A.; Serdar, M.A. Effects of thermal treatment on quality of biosimilar and originator monoclonal antibodies. Adv. Sample Prep. 2024, 10, 100109. [Google Scholar] [CrossRef]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of forced degradation and stability indicating studies of drugs—A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Pisupati, K.; Benet, A.; Tian, Y.; Okbazghi, S.; Kang, J.; Ford, M.; Saveliev, S.; Sen, K.I.; Carlson, E.; Tolbert, T.J.; et al. Biosimilarity under stress: A forced degradation study of Remicade and Remsima. mAbs 2017, 9, 1197–1209. [Google Scholar] [CrossRef]
- Laptoš, T.; Omersel, J. The importance of handling high-value biologicals: Physico-chemical instability and immunogenicity of monoclonal antibodies. Exp. Ther. Med. 2018, 15, 3161–3168. [Google Scholar] [CrossRef]
- Tamizi, E.; Jouyban, A. Forced degradation studies of biopharmaceuticals: Selection of stress conditions. Eur. J. Pharm. Biopharm. 2016, 98, 26–46. [Google Scholar] [CrossRef]
- Kang, J.; Halseth, T.; Vallejo, D.; Najafabadi, Z.I.; Sen, K.I.; Ford, M.; Ruotolo, B.T.; Schwendeman, A. Assessment of biosimilarity under native and heat-stressed conditions: Rituximab, bevacizumab, and trastuzumab originators and biosimilars. Anal. Bioanal. Chem. 2020, 412, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Halley, J.; Chou, Y.R.; Cicchino, C.; Huang, M.; Sharma, V.; Tan, N.C.; Thakkar, S.; Zhou, L.L.; Al-Azzam, W.; Cornen, S.; et al. An Industry Perspective on Forced Degradation Studies of Biopharmaceuticals: Survey Outcome and Recommendations. J. Pharm. Sci. 2020, 109, 6–21. [Google Scholar] [CrossRef]
- Hutterer, K.M.; Ip, A.; Kuhns, S.; Cao, S.; Wikström, M.; Liu, J. Analytical Similarity Assessment of ABP 959 in Comparison with Eculizumab Reference Product. BioDrugs 2021, 35, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Torrente-López, A.; Hermosilla, J.; Salmerón-García, A.; Cabeza, J.; Navas, N. Comprehensive Analysis of Nivolumab, A Therapeutic Anti-Pd-1 Monoclonal Antibody: Impact of Handling and Stress. Pharmaceutics 2022, 14, 692. [Google Scholar] [CrossRef]
- Zhang, A.; Singh, S.K.; Shirts, M.R.; Kumar, S.; Fernandez, E.J. Distinct aggregation mechanisms of monoclonal antibody under thermal and freeze-thaw stresses revealed by hydrogen exchange. Pharm. Res. 2012, 29, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jiménez, J.; Martínez-Ortega, A.; Salmerón-García, A.; Cabeza, J.; Prados, J.C.; Ortíz, R.; Navas, N. Study of aggregation in therapeutic monoclonal antibodies subjected to stress and long-term stability tests by analyzing size exclusion liquid chromatographic profiles. Int. J. Biol. Macromol. 2018, 118, 511–524. [Google Scholar] [CrossRef]
- Haberger, M.; Bomans, K.; Diepold, K.; Hook, M.; Gassner, J.; Schlothauer, T.; Zwick, A.; Spick, C.; Kepert, J.F.; Hienz, B.; et al. Assessment of chemical modifications of sites in the CDRs of recombinant antibodies. mAbs 2014, 6, 327–339. [Google Scholar] [CrossRef]
- Schmid, I.; Bonnington, L.; Gerl, M.; Bomans, K.; Thaller, A.L.; Wagner, K.; Schlothauer, T.; Falkenstein, R.; Zimmermann, B.; Kopitz, J.; et al. Assessment of susceptible chemical modification sites of trastuzumab and endogenous human immunoglobulins at physiological conditions. Commun. Biol. 2018, 1, 28. [Google Scholar] [CrossRef]
- Khawli, L.A.; Goswami, S.; Hutchinson, R.; Kwong, Z.W.; Yang, J.; Wang, X.; Yao, Z.; Sreedhara, A.; Cano, T.; Tesar, D.B.; et al. Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. mAbs 2010, 2, 613–624. [Google Scholar] [CrossRef]
- Sreenivasan, S.; Schöneich, C.; Rathore, A.S. Aggregation of therapeutic monoclonal antibodies due to thermal and air/liquid interfacial agitation stress: Occurrence, stability assessment strategies, aggregation mechanism, influencing factors, and ways to enhance stability. Int. J. Pharm. 2024, 666, 124735. [Google Scholar] [CrossRef]
- Gurel, B.; Capkin, E.; Parlar, A.; Ozkan, A.; Corbacioglu, M.; Daglikoca, D.E.; Yuce, M. Optimized Methods for Analytical and Functional Comparison of Biosimilar mAb Drugs: A Case Study for Avastin, Mvasi, and Zirabev. Sci. Pharm. 2022, 90, 36. [Google Scholar] [CrossRef]
- Kaur, H. Stability testing in monoclonal antibodies. Crit. Rev. Biotechnol. 2021, 41, 692–714. [Google Scholar] [CrossRef] [PubMed]
- Mahler, H.C.; Friess, W.; Grauschopf, U.; Kiese, S. Protein aggregation: Pathways, induction factors and analysis. J. Pharm. Sci. 2009, 98, 2909–2934. [Google Scholar] [CrossRef] [PubMed]
- Farjami, A.; Siahi-Shadbad, M.; Akbarzadehlaleh, P.; Roshanzamir, K.; Molavi, O. Evaluation of the physicochemical and biological stability of cetuximab under various stress condition. J. Pharm. Pharm. Sci. 2019, 22, 171–190. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.J.; Wang, D.Q. Dual effects of Tween 80 on protein stability. Int. J. Pharm. 2008, 347, 31–38. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.J.; Cao, M.; de Mel, N.; Albarghouthi, M.; Miller, K.; Bee, J.S.; Wang, J.; Wang, X.; Albarghouthi, M. Analytical characterization of coformulated antibodies as combination therapy. mAbs 2020, 12, 1738691. [Google Scholar] [CrossRef]
- Bansal, R.; Dash, R.; Rathore, A.S. Impact of mAb Aggregation on Its Biological Activity: Rituximab as a Case Study. J. Pharm. Sci. 2020, 109, 2684–2698. [Google Scholar] [CrossRef]
- Vázquez-Rey, M.; Lang, D.A. Aggregates in monoclonal antibody manufacturing processes. Biotechnol. Bioeng. 2011, 108, 1494–1508. [Google Scholar] [CrossRef]
- Shukla, A.A.; Gupta, P.; Han, X. Protein aggregation kinetics during Protein A chromatography. Case study for an Fc fusion protein. J. Chromatogr. A 2007, 1171, 22–28. [Google Scholar] [CrossRef]
- Mazzer, A.R.; Perraud, X.; Halley, J.; O’Hara, J.; Bracewell, D.G. Protein A chromatography increases monoclonal antibody aggregation rate during subsequent low pH virus inactivation hold. J. Chromatogr. A 2015, 1415, 83–90. [Google Scholar] [CrossRef]
- Kueltzo, L.A.; Wang, W.; Randolph, T.W.; Carpenter, J.F. Effects of solution conditions, processing parameters, and container materials on aggregation of a monoclonal antibody during freeze-thawing. J. Pharm. Sci. 2008, 97, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Hassan, L.A.; Al-Ghobashy, M.A.; Abbas, S.S. Evaluation of the pattern and kinetics of degradation of adalimumab using a stability-indicating orthogonal testing protocol. Biomed. Chromatogr. 2019, 33, e4676. [Google Scholar] [CrossRef] [PubMed]
- Hawe, A.; Kasper, J.C.; Friess, W.; Jiskoot, W. Structural properties of monoclonal antibody aggregates induced by freeze-thawing and thermal stress. Eur. J. Pharm. Sci. 2009, 38, 79–87. [Google Scholar] [CrossRef]
- Gómez, G.; Pikal, M.J.; Rodríguez-Hornedo, N. Effect of initial buffer composition on pH changes during far-from-equilibrium freezing of sodium phosphate buffer solutions. Pharm. Res. 2001, 18, 90–97. [Google Scholar] [CrossRef]
- Chi, E.Y.; Krishnan, S.; Randolph, T.W.; Carpenter, J.F. Physical Stability of Proteins in Aqueous Solution: Mechanism and Driving Forces in Nonnative Protein Aggregation. Pharm. Res. 2003, 20, 1325–1336. [Google Scholar] [CrossRef]
- Alsante, K.M.; Ando, A.; Brown, R.; Ensing, J.; Hatajik, T.D.; Kong, W.; Tsuda, Y. The role of degradant profiling in active pharmaceutical ingredients and drug products. Adv. Drug Deliv. Rev. 2007, 59, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chumsae, C.; Gaza-Bulseco, G.; Sun, J.; Liu, H. Comparison of methionine oxidation in thermal stability and chemically stressed samples of a fully human monoclonal antibody. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2007, 850, 285–294. [Google Scholar] [CrossRef]
- Gaza-Bulseco, G.; Faldu, S.; Hurkmans, K.; Chumsae, C.; Liu, H. Effect of methionine oxidation of a recombinant monoclonal antibody on the binding affinity to protein A and protein G. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2008, 870, 55–62. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Wang, Y.; Zhao, J.; Liu, Y.H.; Richardson, D.; Li, H.; Shameem, M.; Yang, X. High throughput peptide mapping method for analysis of site specific monoclonal antibody oxidation. J. Chromatogr. A 2016, 1460, 51–60. [Google Scholar] [CrossRef]
- Sokolowska, I.; Mo, J.; Dong, J.; Lewis, M.J.; Hu, P. Subunit mass analysis for monitoring antibody oxidation. mAbs 2017, 9, 498–505. [Google Scholar] [CrossRef]
- Liu, H.; Gaza-Bulseco, G.; Xiang, T.; Chumsae, C. Structural effect of deglycosylation and methionine oxidation on a recombinant monoclonal antibody. Mol. Immunol. 2008, 45, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ren, D.; Huang, H.; Dankberg, J.; Rosenfeld, R.; Cocco, M.J.; Li, L.; Brems, D.N.; Remmele, R.L. Structure and Stability Changes of Human IgG1 Fc as a Consequence of Methionine Oxidation. Biochem. 2008, 47, 5088–5100. [Google Scholar] [CrossRef] [PubMed]
- Shabestari, A.B.; Mostafavi, S.M.; Malekzadeh, H. Force Degradation Comparative Study on Biosimilar Adalimumab and Humira. Rev. Latinoam. Hipertens. 2018, 13, 498–509. [Google Scholar]
- Leblanc, Y.; Ramon, C.; Bihoreau, N.; Chevreux, G. Charge variants characterization of a monoclonal antibody by ion exchange chromatography coupled on-line to native mass spectrometry: Case study after a long-term storage at +5 °C. J. Chromatogr. B. 2017, 1048, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, S.; Rathore, A.S. Impact of Various Forced Oxidative Stress Factors in Rapid Degradation of mAb: Trastuzumab as a Case Study. Pharm. Res. 2025, 42, 335–351. [Google Scholar] [CrossRef]
- Sarin, D.; Chakraborty, D.; Sreenivasan, S.; Mishra, A.; Rathore, A.S. Higher concentration of trehalose dihydrate stabilizes recombinant IgG1 under forced stress conditions. J. Pharm. Sci. 2025, 114, 1398–1409. [Google Scholar] [CrossRef]


| SE-UPLC | CE-SDS | icIEF | ||||||
|---|---|---|---|---|---|---|---|---|
| Monomer | HMW | LC + HC | IgG | Main Variant | Acidic Variants | Basic Variants | ||
| BS control | 97.9 ± 0.01 | 1.2 ± 0.01 | 99.4 ± 0.04 | 97.8 ± 0.2 | 59.6 ± 0.24 | 28.6 ± 0.30 | 11.8 ± 0.53 | |
| Thermal Stress | 37 °C, 3 d | 97.1 ± 0.01 | 2.0 ± 0.01 | 98.3 ± 0.00 | 97.6 ± 0.04 | 58.1 ± 0.02 | 30.8 ± 0.05 | 11.1 ± 0.07 |
| p-value | >0.99 | 0.49 | 0.98 | >0.99 | >0.99 | >0.99 | >0.99 | |
| 37 °C, 7 d | 97.0 ± 0.01 | 2.2 ± 0.01 | 97.9 ± 0.05 | 97.5 ± 0.02 | 55.6 ± 0.11 | 33.8 ± 0.01 | 10.6 ± 0.11 | |
| p-value | 0.22 | 0.22 | 0.49 | >0.99 | >0.99 | >0.99 | 0.77 | |
| 37 °C, 14 d | 96.6 ± 0.01 | 2.4 ± 0.01 | 97.2 ± 0.04 | 96.4 ± 0.02 | 49.8 ± 0.18 | 40.1 ± 0.03 | 10.1 ± 0.16 | |
| p-value | 0.01 * | 0.01 * | 0.01 * | 0.361 | 0.286 | 0.066 | 0.458 | |
| 50 °C, 3 d | 94.6 ± 0.01 | 4.4 ± 0.01 | 97.2 ± 0.16 | 97.6 ± 0.03 | 49.1 ± 0.04 | 41.4 ± 0.14 | 9.5 ± 0.18 | |
| p-value | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | |
| 50 °C, 7 d | 92.7 ± 0.01 | 6.2 ± 0.01 | 96.2 ± 0.07 | 97.0 ± 0.04 | 37.6 ± 0.26 | 53.8 ± 0.12 | 8.6 ± 0.14 | |
| p-value | 0.98 | 0.98 | 0.38 | >0.99 | 0.54 | 0.54 | 0.62 | |
| 50 °C, 14 d | 89.6 ± 0.02 | 9.0 ± 0.02 | 92.2 ± 0.50 | 96.4 ± 0.06 | 25.1 ± 0.01 | 67.6 ± 0.28 | 7.4 ± 0.29 | |
| p-value | 0.09 | 0.09 | 0.01 * | 0.12 | 0.07 | 0.07 | 0.34 | |
| Agitation Stress | 24 h | 97.3 ± 0.01 | 1.9 ± 0.01 | 98.4 ± 0.03 | 97.4 ± 0.07 | 57.2 ± 0.16 | 30.4 ± 0.02 | 12.3 ± 0.13 |
| p-value | 0.08 | 0.08 | 0.49 | >0.99 | >0.99 | >0.99 | >0.99 | |
| 72 h | 97.3 ± 0.02 | 1.9 ± 0.01 | 98.3 ± 0.09 | 97.2 ± 0.02 | 56.0 ± 0.21 | 31.3 ± 0.30 | 12.7 ± 0.09 | |
| p-value | 0.02 * | 0.02 * | 0.36 | 0.54 | >0.99 | >0.99 | >0.99 | |
| pH Stress | pH 4, 72 h | 53.6 ± 0.02 | 45.6 ± 0.03 | 97.1 ± 0.08 | 96.8 ± 0.04 | 51.6 ± 0.15 | 26.2 ± 0.04 | 22.2 ± 0.18 |
| p-value | 0.98 | 0.98 | 0.08 | 0.08 | 0.19 | 0.53 | 0.19 | |
| pH 9, 72 h | 96.9 ± 0.04 | 2.2 ± 0.02 | 97.7 ± 0.12 | 97.2 ± 0.02 | 55.9 ± 0.08 | 32.5 ± 0.51 | 11.6 ± 0.43 | |
| p-value | 0.08 | 0.08 | 0.08 | 0.41 | >0.99 | >0.99 | >0.99 | |
| Freeze/Thaw Stress | F & T | 91.9 ± 0.01 | 7.2 ± 0.01 | 98.4 ± 0.23 | 97.3 ± 0.06 | 57.6 ± 0.01 | 30.2 ± 0.03 | 12.2 ± 0.04 |
| p-value | 0.08 | 0.08 | 0.22 | 0.50 | >0.99 | >0.99 | >0.99 | |
| Oxidation Stress | 24 h | 96.4 ± 0.01 | 2.8 ± 0.02 | 98.5 ± 0.06 | 91.2 ± 0.07 | 47.5 ± 0.27 | 40.7 ± 0.11 | 12.0 ± 0.16 |
| p-value | 0.08 | 0.08 | 0.13 | 0.08 | 0.19 | 0.19 | >0.99 | |
| SE-UPLC | CE-SDS | icIEF | ||||||
|---|---|---|---|---|---|---|---|---|
| Monomer | HMW | LC + HC | IgG | Main Variant | Acidic Variants | Basic Variants | ||
| OR control | 98.1 ± 0.02 | 1.0 ± 0.02 | 98.5 ± 0.02 | 98.6 ± 0.03 | 52.7 ± 0.21 | 34.6 ± 0.29 | 12.7 ± 0.23 | |
| Thermal Stress | 37 °C, 3 d | 97.3 ± 0.01 | 1.8 ± 0.02 | 98.0 ± 0.62 | 98.5 ± 0.14 | 51.2 ± 0.11 | 36.6 ± 0.16 | 12.3 ± 0.11 |
| p-value | 0.8308 | 0.83 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | |
| 37 °C, 7 d | 97.1 ± 0.01 | 1.9 ± 0.01 | 97.3 ± 0.09 | 97.8 ± 0.10 | 49.3 ± 0.13 | 39.2 ± 0.08 | 11.5 ± 0.09 | |
| p-value | 0.02 * | 0.08 | 0.04 * | 0.49 | 0.27 | 0.66 | 0.41 | |
| 37 °C, 14 d | 96.9 ± 0.01 | 2.3 ± 0.04 | 96.7 ± 0.06 | 97.5 ± 0.14 | 43.5 ± 0.25 | 45.4 ± 0.28 | 11.1 ± 0.08 | |
| p-value | <0.0001 * | <0.0001 * | 0.0002 * | 0.001 * | 0.03 * | 0.03 * | 0.01 * | |
| 50 °C, 3 d | 94.4 ± 0.01 | 4.6 ± 0.02 | 96.3 ± 0.20 | 98.1 ± 0.13 | 43.6 ± 0.56 | 45.0 ± 0.43 | 11.4 ± 0.27 | |
| p-value | 0.2892 | 0.2902 | 0.6007 | >0.99 | >0.99 | >0.99 | >0.99 | |
| 50 °C, 7 d | 92.1 ± 0.01 | 6.9 ± 0.02 | 94.2 ± 0.08 | 97.4 ± 0.17 | 32.4 ± 0.31 | 58.5 ± 0.44 | 9.0 ± 0.16 | |
| p-value | 0.003 * | 0.003 * | 0.02 * | 0.02 * | 0.10 | 0.10 | 0.10 | |
| 50 °C, 14 d | 86.4 ± 0.01 | 12.2 ± 0.01 | 90.6 ± 0.21 | 96.8 ± 0.07 | 19.6 ± 0.15 | 73.3 ± 0.14 | 7.1 ± 0.04 | |
| p-value | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * | 0.002 * | 0.002 * | 0.0004 * | |
| Agitation Stress | 24 h | 97.6 ± 0.01 | 1.5 ± 0.02 | 97.6 ± 0.31 | 98.0 ± 0.05 | 50.4 ± 0.04 | 36.3 ± 0.08 | 13.3 ± 0.06 |
| p-value | 0.19 | 0.14 | 0.01 * | 0.60 | >0.99 | >0.99 | 0.66 | |
| 72 h | 97.5 ± 0.01 | 1.6 ± 0.01 | 97.5 ± 0.34 | 97.8 ± 0.07 | 48.6 ± 0.20 | 37.7 ± 0.04 | 13.7 ± 0.23 | |
| p-value | 0.005 * | 0.01 * | 0.003 * | 0.02 * | 0.1363 | 0.1359 | 0.0571 | |
| pH Stress | pH 4, 72 h | 30.3 ± 0.02 | 68.8 ± 0.01 | 96.3 ± 0.06 | 97.4 ± 0.09 | 46.2 ± 0.13 | 26.2 ± 0.18 | 27.6 ± 0.07 |
| p-value | 0.0002 * | 0.0002 * | 0.01 * | 0.01 * | 0.04 * | 0.01 * | 0.04 * | |
| pH 9, 72 h | 97.0 ± 0.01 | 2.0 ± 0.01 | 96.6 ± 0.28 | 97.8 ± 0.05 | 48.7 ± 0.14 | 38.7 ± 0.18 | 12.6 ± 0.13 | |
| p-value | 0.01 * | 0.01 * | 0.01 * | 0.04 * | 0.23 | 0.23 | >0.99 | |
| Freeze/Thaw Stress | F & T | 92.1 ± 0.01 | 7.1 ± 0.01 | 97.6 ± 0.17 | 97.9 ± 0.06 | 51.2 ± 0.16 | 35.8 ± 0.14 | 13.1 ± 0.03 |
| p-value | 0.01 * | 0.01 * | 0.02 * | 0.05 * | 0.23 | 0.23 | 0.23 | |
| Oxidation Stress | 24 h | 96.8 ± 0.19 | 2.4 ± 0.21 | 98.0 ± 0.42 | 92.1 ± 0.14 | 40.5 ± 0.17 | 46.3 ± 0.25 | 13.1 ± 0.09 |
| p-value | 0.01 * | 0.01 * | 0.0216 * | 0.01 * | 0.04 * | 0.04 * | 0.23 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celik Yamaci, M.; Pamukcu, C.; Erdemgil, Y.; Atik, A.E.; Keles, Z.Z.Y.; Can, O. Comparative Forced Degradation Study of Anticomplement C5 Biosimilar and Originator Monoclonal Antibodies. Pharmaceuticals 2025, 18, 579. https://doi.org/10.3390/ph18040579
Celik Yamaci M, Pamukcu C, Erdemgil Y, Atik AE, Keles ZZY, Can O. Comparative Forced Degradation Study of Anticomplement C5 Biosimilar and Originator Monoclonal Antibodies. Pharmaceuticals. 2025; 18(4):579. https://doi.org/10.3390/ph18040579
Chicago/Turabian StyleCelik Yamaci, Merve, Ceren Pamukcu, Yigit Erdemgil, Ahmet Emin Atik, Zeynep Zulfiye Yildirim Keles, and Ozge Can. 2025. "Comparative Forced Degradation Study of Anticomplement C5 Biosimilar and Originator Monoclonal Antibodies" Pharmaceuticals 18, no. 4: 579. https://doi.org/10.3390/ph18040579
APA StyleCelik Yamaci, M., Pamukcu, C., Erdemgil, Y., Atik, A. E., Keles, Z. Z. Y., & Can, O. (2025). Comparative Forced Degradation Study of Anticomplement C5 Biosimilar and Originator Monoclonal Antibodies. Pharmaceuticals, 18(4), 579. https://doi.org/10.3390/ph18040579







