Extracellular Vesicles Isolated from Equine Adipose-Derived Stromal Stem Cells (ASCs) Mitigate Tunicamycin-Induced ER Stress in Equine Corneal Stromal Stem Cells (CSSCs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model and Subject Details
2.2. Cornea Tissue Harvesting and CSSC Isolation
2.3. Phenotypic Characterization of Corneal Stromal Stem Cells
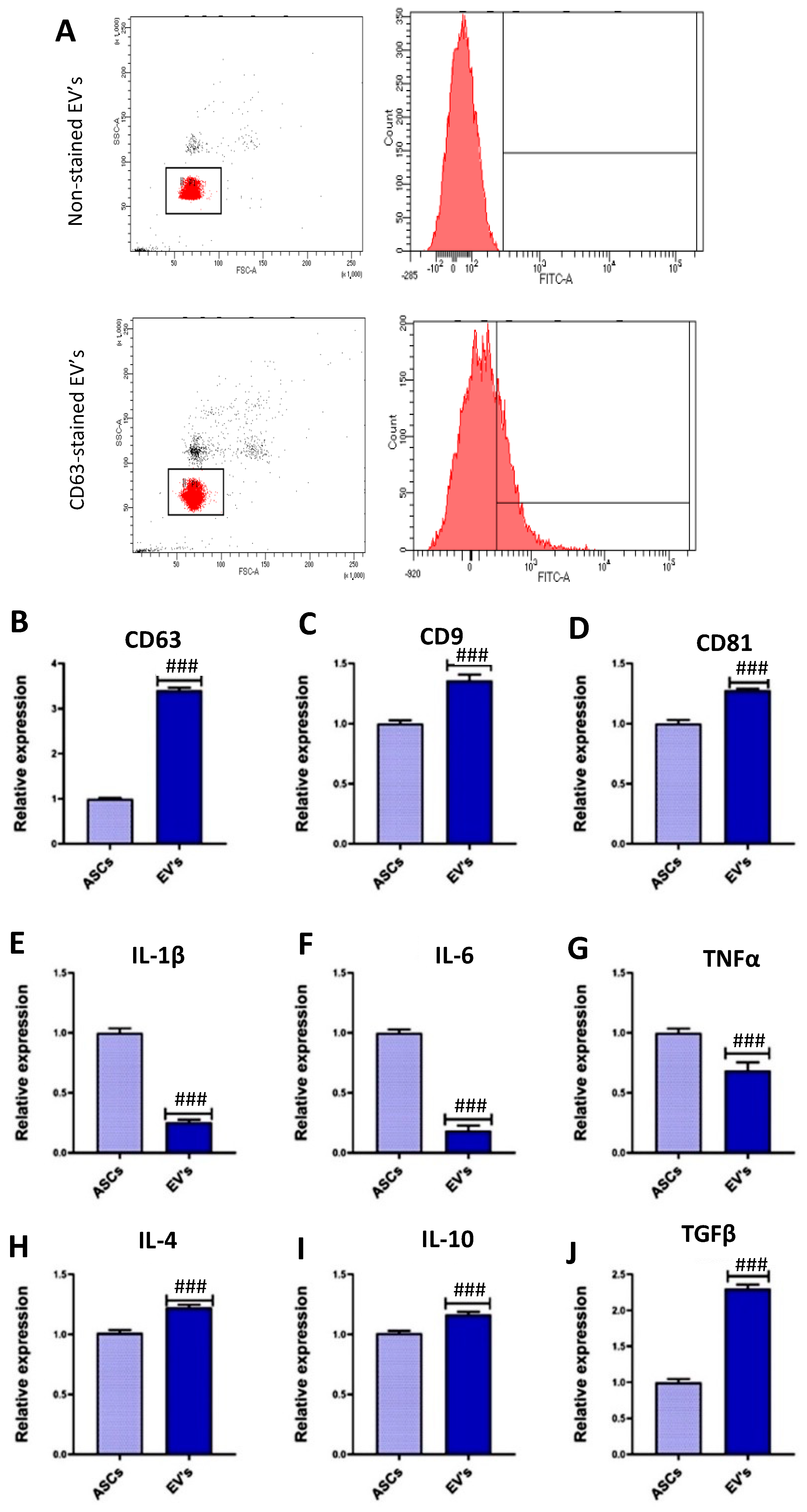
2.4. ASC-EVs Isolation and Characterization
2.5. Experimental Model Setting
2.6. Proliferation Assay
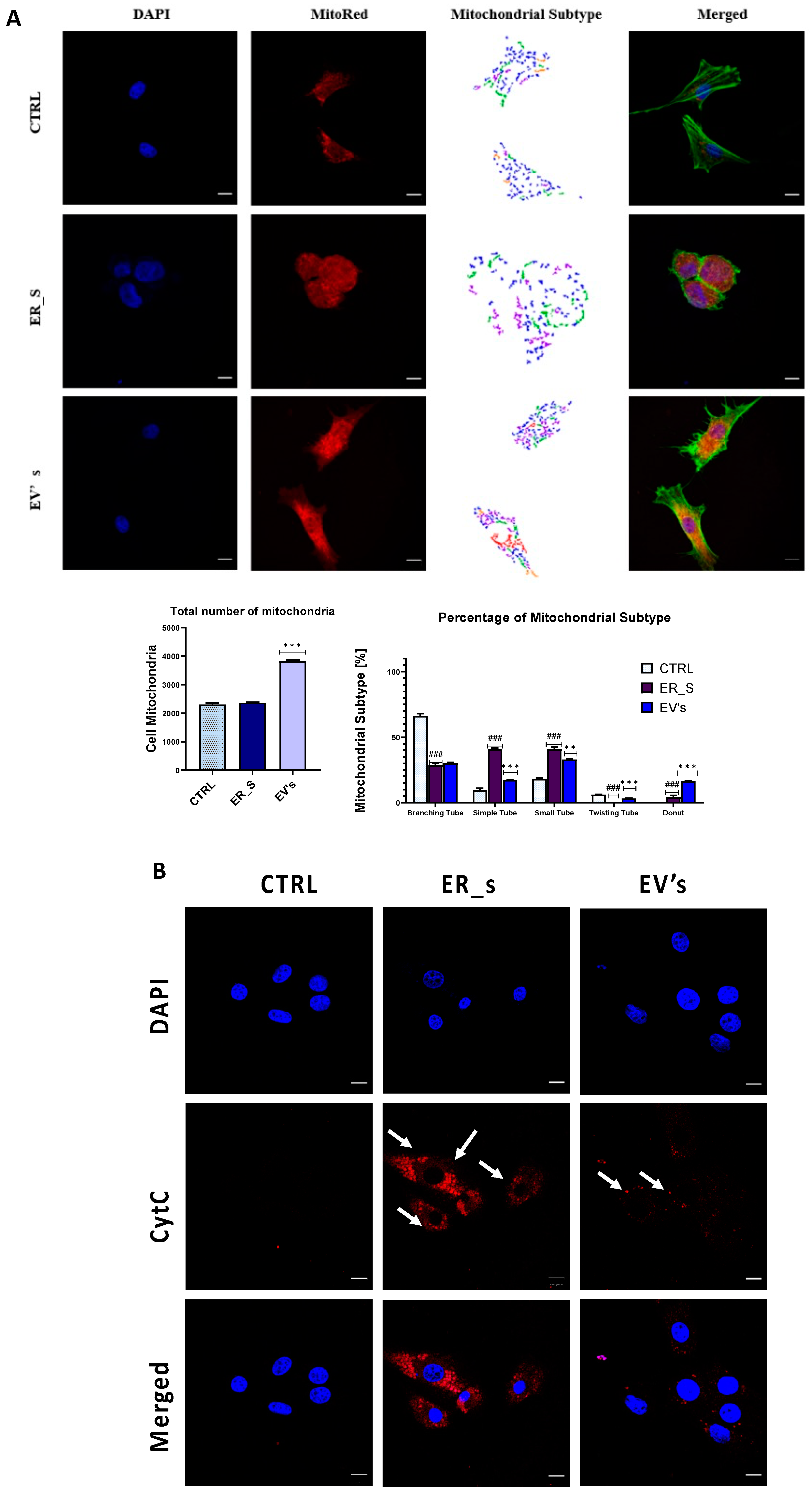
2.7. Visualization of Cells’ Mitochondria and Cytoskeleton
2.8. Immunofluorescence Staining
2.9. mRNA Expression Analysis
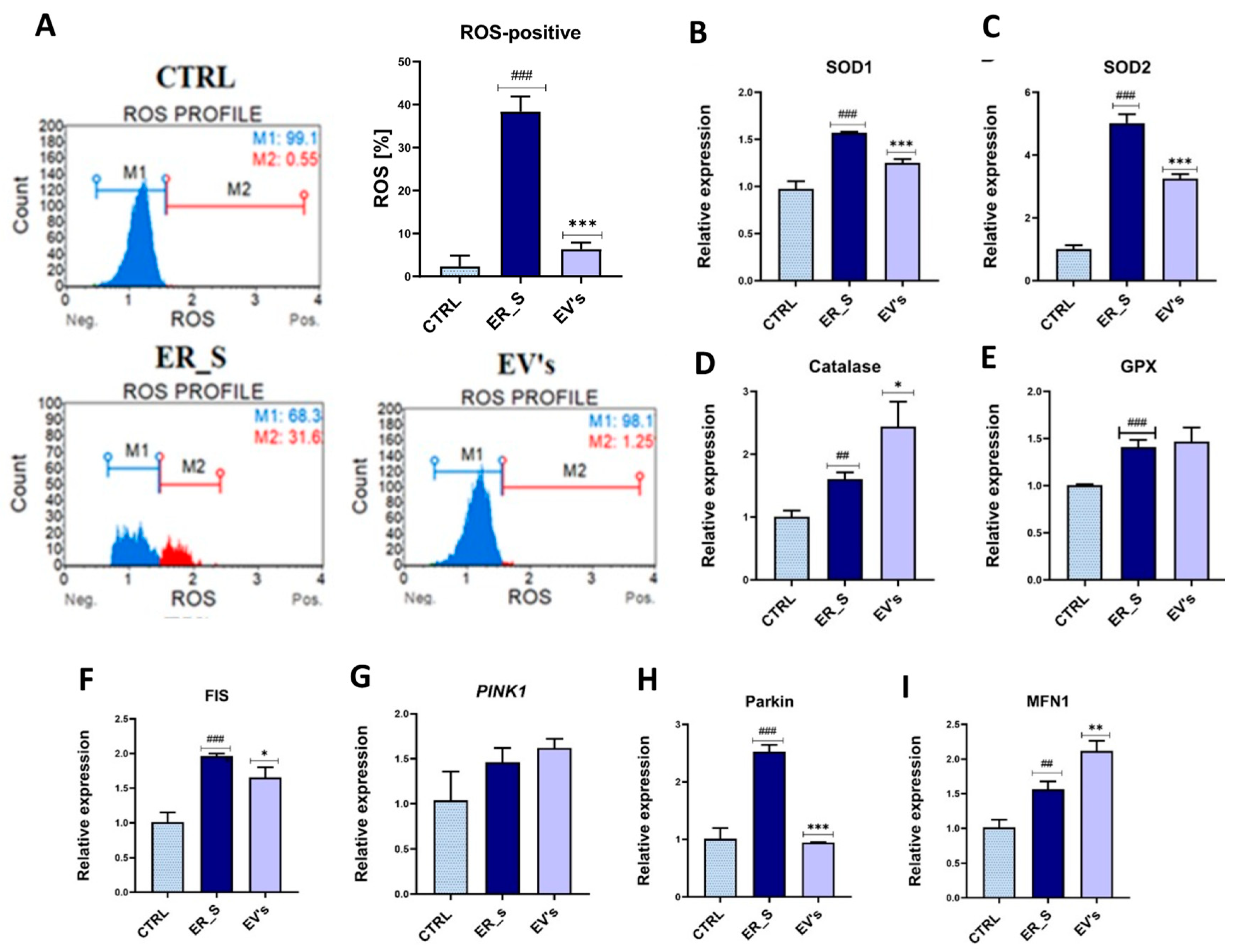
2.10. Oxidative Stress Analysis
2.11. Cell Cycle Analysis
2.12. Apoptosis Assay
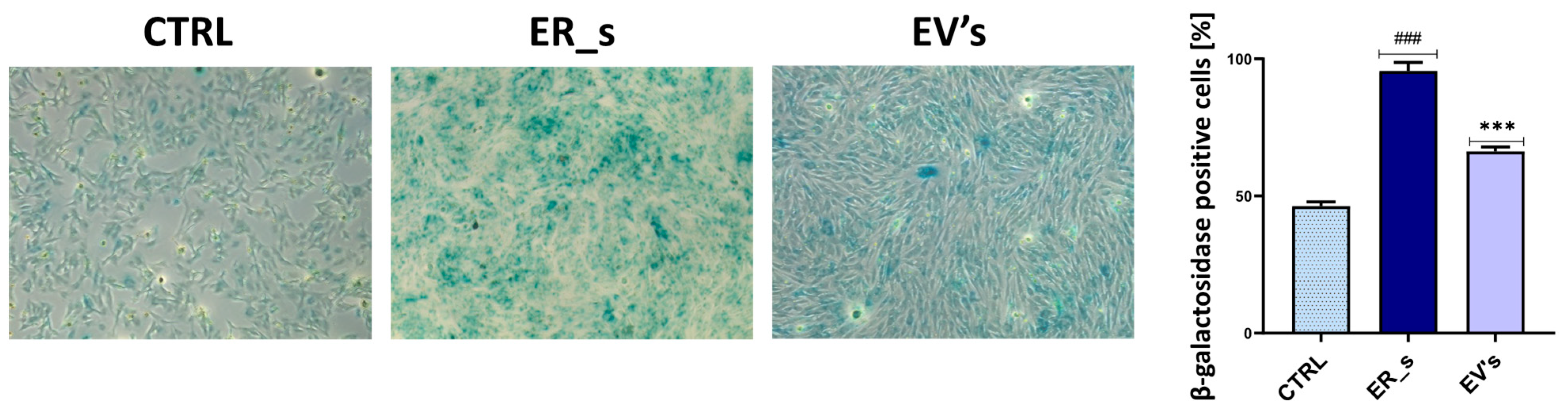
2.13. Senescence β-Galactosidase Staining
2.14. Statistical Analysis
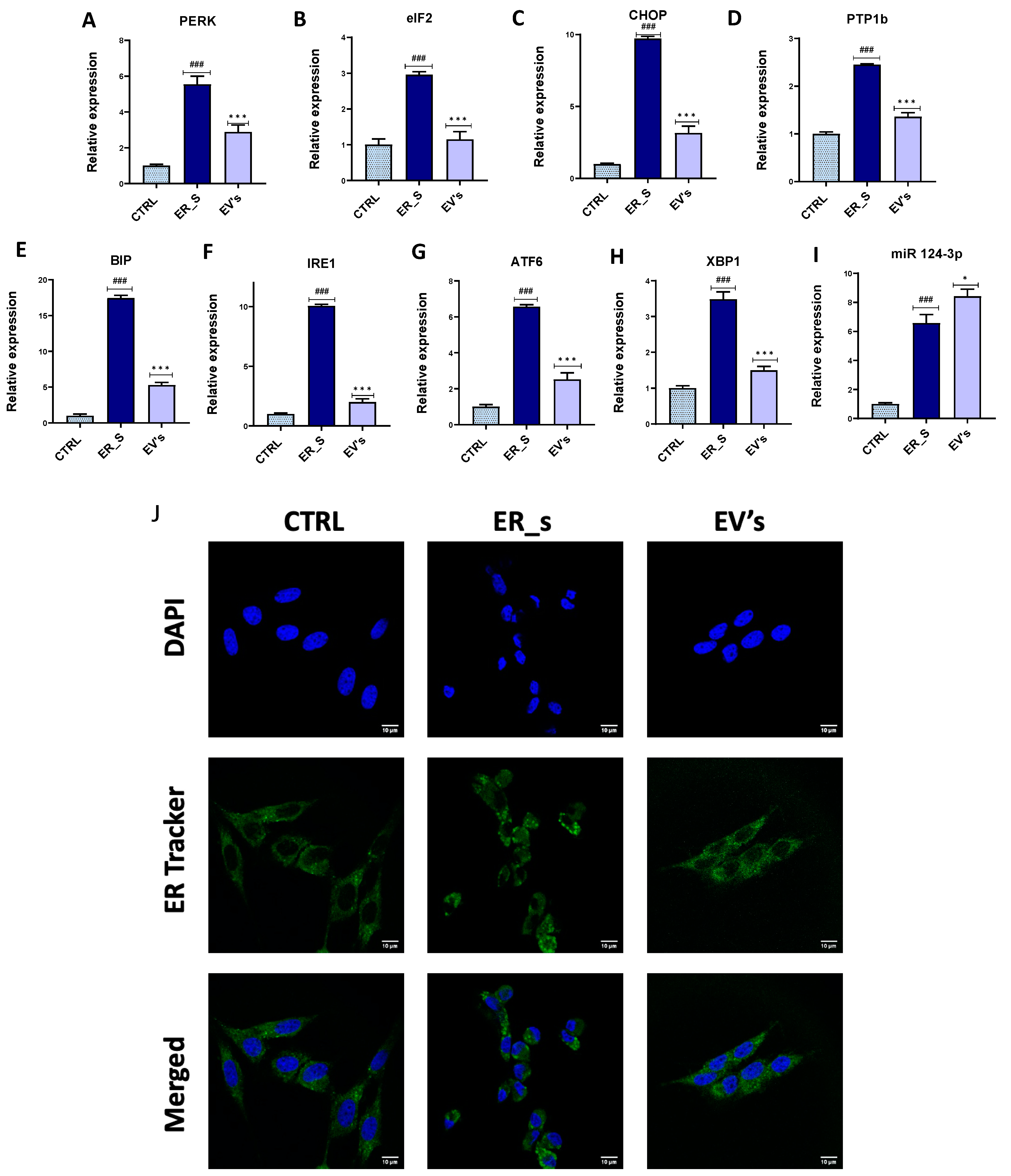
3. Results
3.1. EVs Characterization
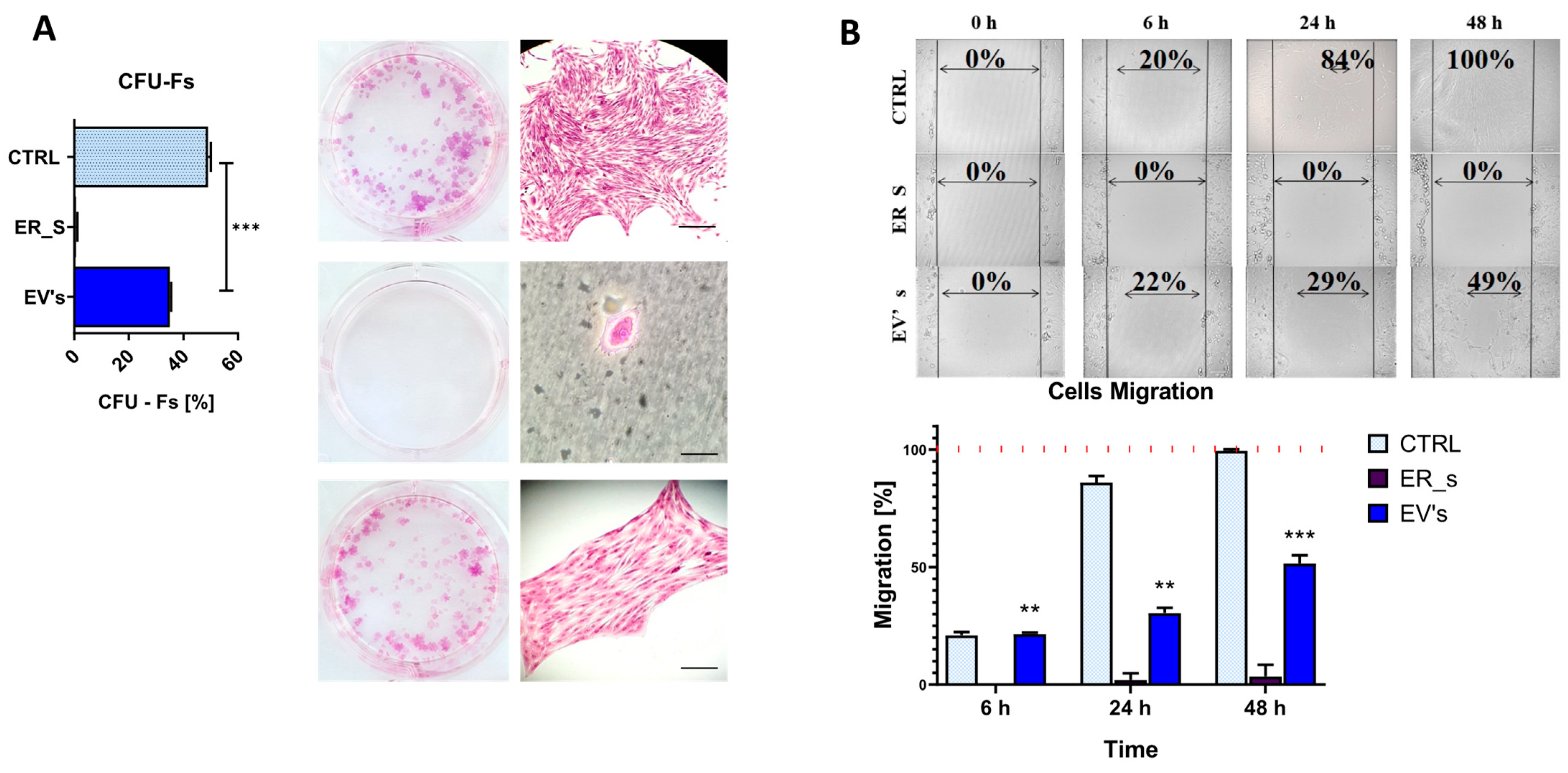
3.2. Effect of EVs on CSSCs Cells’ Stemness
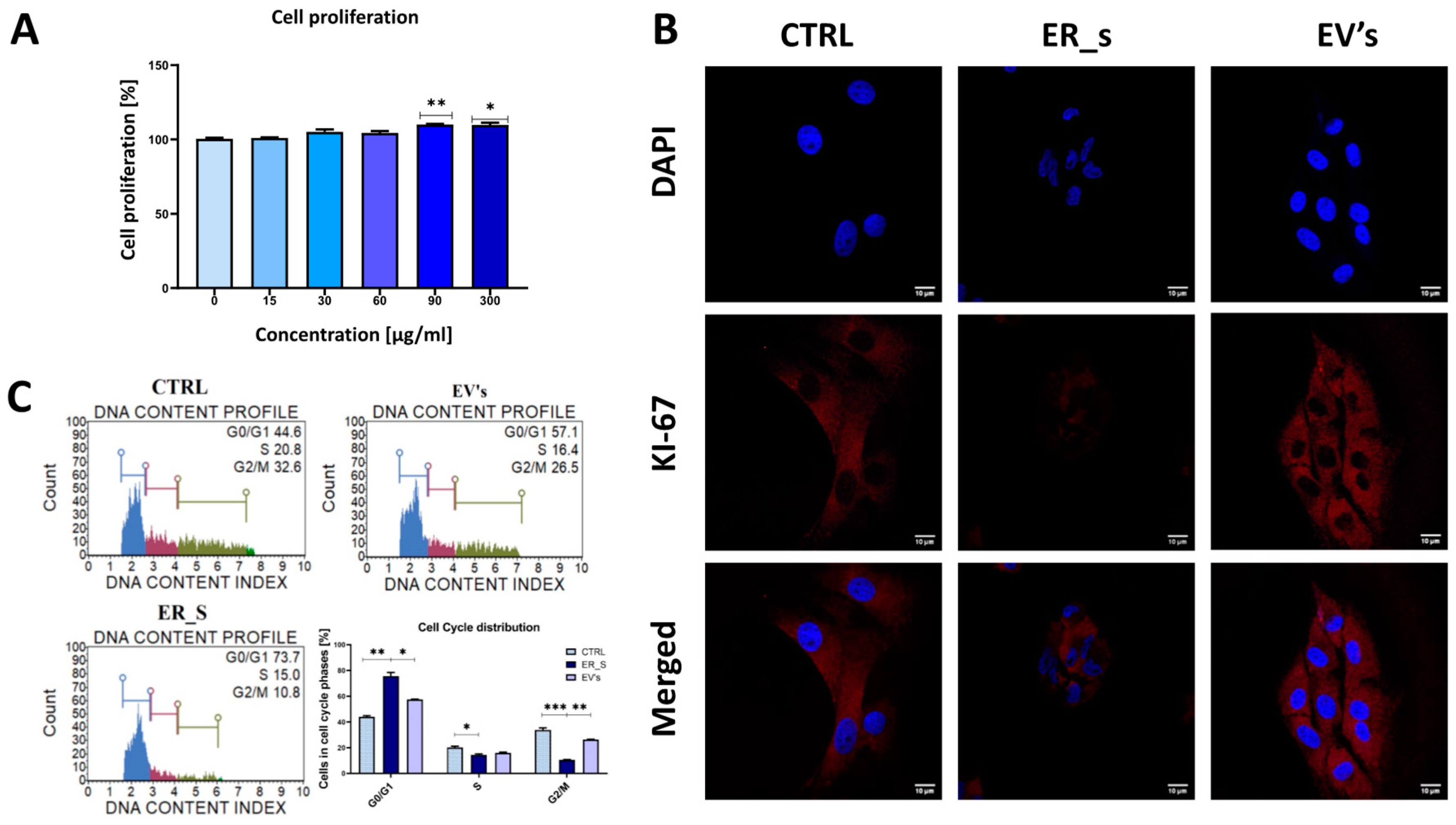
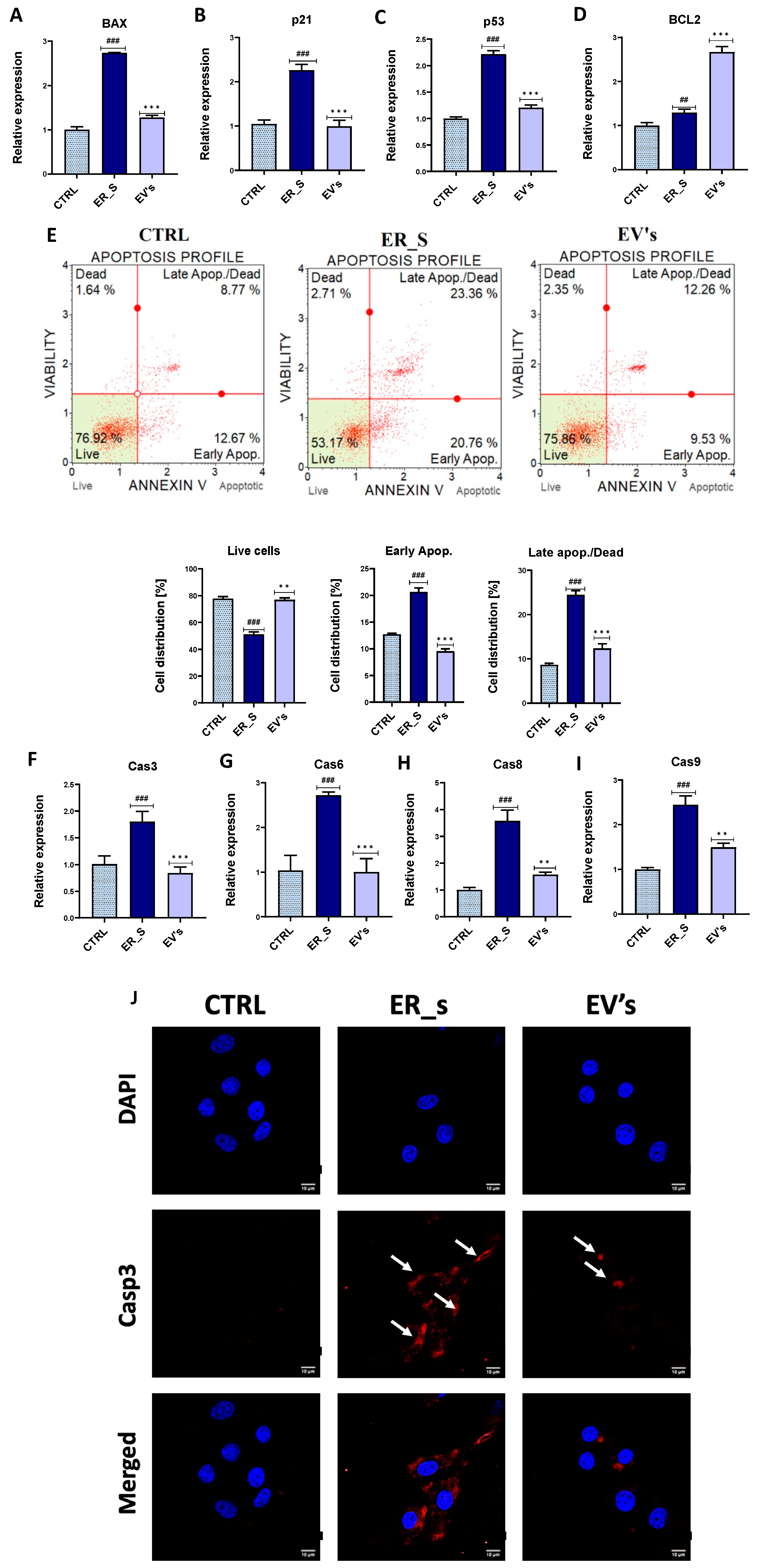
3.3. EVs Promote the Proliferation of Corneal Stromal Stem Cells and Protect from Apoptosis
3.4. EVs Mitigate Inflammation in CSSCs
3.5. EVs Reduce ER Stress in CSSCs
3.6. EVs Promote Corneal Cells Metabolic Homeostasis under ER Stress Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, L.B.; Pinard, C.L. Corneal Ulcers in Horses. Compend. Contin. Educ. Vet. 2013, 35, E4. [Google Scholar] [PubMed]
- Ollivier, F.J.; Brooks, D.E.; Kallberg, M.E.; Komaromy, A.M.; Lassaline, M.E.; Andrew, S.E.; Gelatt, K.N.; Stevens, G.R.; Blalock, T.D.; Setten, G.-B.; et al. Evaluation of Various Compounds to Inhibit Activity of Matrix Metalloproteinases in the Tear Film of Horses with Ulcerative Keratitis. Am. J. Vet. Res. 2003, 64, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Saghizadeh, M.; Soleymani, S.; Harounian, A.; Bhakta, B.; Troyanovsky, S.M.; Brunken, W.J.; Pellegrini, G.; Ljubimov, A.V. Alterations of Epithelial Stem Cell Marker Patterns in Human Diabetic Corneas and Effects of C-Met Gene Therapy. Mol. Vis. 2011, 17, 2177–2190. [Google Scholar] [PubMed]
- Keadle, T.L.; Usui, N.; Laycock, K.A.; Miller, J.K.; Pepose, J.S.; Stuart, P.M. IL-1 and TNF-Alpha Are Important Factors in the Pathogenesis of Murine Recurrent Herpetic Stromal Keratitis. Investig. Ophthalmol. Vis. Sci. 2000, 41, 96–102. [Google Scholar]
- Brooks, D.E.; Andrew, S.E.; Biros, D.J.; Denis, H.M.; Cutler, T.J.; Strubbe, D.T.; Gelatt, K.N. Ulcerative Keratitis Caused by Beta-Hemolytic Streptococcus Equi in 11 Horses. Vet. Ophthalmol. 2000, 3, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Krupa, W.; Topczewska, J.; Garbiec, A.; Karpinski, M. Is the Welfare of Sport Horses Assured by Modern Management Practices? Anim. Sci. Genet. 2022, 18, 57–77. [Google Scholar]
- Practical Horseman. Understanding Ulcers. Available online: https://practicalhorsemanmag.com/health-archive/understanding-ulcers-54340/ (accessed on 8 January 2023).
- de Araujo, A.L.; Gomes, J.Á. Corneal Stem Cells and Tissue Engineering: Current Advances and Future Perspectives. World J. Stem Cells 2015, 7, 806–814. [Google Scholar] [CrossRef]
- Saghizadeh, M.; Kramerov, A.A.; Svendsen, C.N.; Ljubimov, A.V. Concise Review: Stem Cells for Corneal Wound Healing. Stem Cells 2017, 35, 2105–2114. [Google Scholar] [CrossRef]
- Rafat, M.; Jabbarvand, M.; Sharma, N.; Xeroudaki, M.; Tabe, S.; Omrani, R.; Thangavelu, M.; Mukwaya, A.; Fagerholm, P.; Lennikov, A.; et al. Bioengineered Corneal Tissue for Minimally Invasive Vision Restoration in Advanced Keratoconus in Two Clinical Cohorts. Nat. Biotechnol. 2022, 41, 70–81. [Google Scholar] [CrossRef]
- Saccu, G.; Menchise, V.; Giordano, C.; Delli Castelli, D.; Dastrù, W.; Pellicano, R.; Tolosano, E.; Van Pham, P.; Altruda, F.; Fagoonee, S. Regenerative Approaches and Future Trends for the Treatment of Corneal Burn Injuries. J. Clin. Med. 2021, 10, 317. [Google Scholar] [CrossRef]
- Kureshi, A.K.; Dziasko, M.; Funderburgh, J.L.; Daniels, J.T. Human Corneal Stromal Stem Cells Support Limbal Epithelial Cells Cultured on RAFT Tissue Equivalents. Sci. Rep. 2015, 5, 16186. [Google Scholar] [CrossRef] [PubMed]
- Veréb, Z.; Póliska, S.; Albert, R.; Olstad, O.K.; Boratkó, A.; Csortos, C.; Moe, M.C.; Facskó, A.; Petrovski, G. Role of Human Corneal Stroma-Derived Mesenchymal-Like Stem Cells in Corneal Immunity and Wound Healing. Sci. Rep. 2016, 6, 26227. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, K.R. Inflammatory Mechanisms in Corneal Ulceration. Trans. Am. Ophthalmol. Soc. 1985, 83, 610–663. [Google Scholar] [PubMed]
- Pinnamaneni, N.; Funderburgh, J.L. Concise Review: Stem Cells in the Corneal Stroma. Stem Cells 2012, 30, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- West-Mays, J.A.; Dwivedi, D.J. The Keratocyte: Corneal Stromal Cell with Variable Repair Phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Xu, Y.; Yang, E.; Du, Y. Stemness and Regenerative Potential of Corneal Stromal Stem Cells and Their Secretome After Long-Term Storage: Implications for Ocular Regeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3728–3738. [Google Scholar] [CrossRef] [PubMed]
- Oie, Y.; Nishida, K. Regenerative Medicine for the Cornea. BioMed Res. Int. 2013, 2013, e428247. [Google Scholar] [CrossRef] [PubMed]
- Nurković, J.S.; Vojinović, R.; Dolićanin, Z. Corneal Stem Cells as a Source of Regenerative Cell-Based Therapy. Stem Cells Int. 2020, 2020, e8813447. [Google Scholar] [CrossRef] [PubMed]
- Magrelli, F.M.; Merra, A.; Pellegrini, G. Surgery Versus ATMPs: An Example from Ophthalmology. Front. Bioeng. Biotechnol. 2020, 8, 440. [Google Scholar] [CrossRef]
- Maurizi, E.; Adamo, D.; Magrelli, F.M.; Galaverni, G.; Attico, E.; Merra, A.; Maffezzoni, M.B.R.; Losi, L.; Genna, V.G.; Sceberras, V.; et al. Regenerative Medicine of Epithelia: Lessons From the Past and Future Goals. Front. Bioeng. Biotechnol. 2021, 9, 652214. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Peng, C.-H.; Hung, K.-H.; Lee, Y.-Y.; Lin, T.-C.; Jang, S.-F.; Liu, J.-H.; Chen, Y.-T.; Woung, L.-C.; Wang, C.-Y.; et al. Stem Cell Therapy for Corneal Regeneration Medicine and Contemporary Nanomedicine for Corneal Disorders. Cell Transplant. 2015, 24, 1915–1930. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Sotozono, C.; Kinoshita, S. Current Advancements in Corneal Cell–Based Therapy. Asia-Pac. J. Ophthalmol. 2022, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, K.; Shetty, R.; Ghosh, A. Corneal Cell Therapy: With iPSCs, It Is No More a Far-Sight. Stem Cell Res. Ther. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, Y.; Oie, Y.; Nishida, K. Corneal Stem Cell-Based Therapies. In Corneal Regeneration: Therapy and Surgery; Alió, J.L., Alió del Barrio, J.L., Arnalich-Montiel, F., Eds.; Essentials in Ophthalmology; Springer International Publishing: Cham, Switzerland, 2019; pp. 155–172. [Google Scholar] [CrossRef]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and Characterization of Extracellular Vesicles and Future Directions in Diagnosis and Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhang, K.; Zhang, S.; Wang, R.; Nie, Y.; Tao, H.; Han, Z.; Liang, L.; Wang, D.; Liu, J.; et al. Enhanced Proangiogenic Potential of Mesenchymal Stem Cell-Derived Exosomes Stimulated by a Nitric Oxide Releasing Polymer. Biomaterials 2017, 133, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-S.X.; Yin, J.; Xu, K.; Huang, J. Growth Factors and Corneal Epithelial Wound Healing. Brain Res. Bull. 2010, 81, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Hochreiter, B.; Schmid, J.A. Extracellular Vesicles Linking Inflammation, Cancer and Thrombotic Risks. Front. Cell Dev. Biol. 2022, 10, 859863. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Q.; Wu, K.; Liu, L.; Zhao, M.; Yang, H.; Wang, X.; Wang, W. Extracellular Vesicles as Potential Biomarkers and Therapeutic Approaches in Autoimmune Diseases. J. Transl. Med. 2020, 18, 432. [Google Scholar] [CrossRef]
- Wu, W.-C.; Song, S.-J.; Zhang, Y.; Li, X. Role of Extracellular Vesicles in Autoimmune Pathogenesis. Front. Immunol. 2020, 11, 579043. [Google Scholar] [CrossRef] [PubMed]
- Akhmerov, A.; Parimon, T. Extracellular Vesicles, Inflammation, and Cardiovascular Disease. Cells 2022, 11, 2229. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F. Extracellular Vesicles and Neurodegenerative Diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef] [PubMed]
- Regev-Rudzki, N.; Michaeli, S.; Torrecilhas, A.C. Editorial: Extracellular Vesicles in Infectious Diseases. Front. Cell. Infect. Microbiol. 2021, 11, 697919. [Google Scholar] [CrossRef] [PubMed]
- Yeung, V.; Boychev, N.; Farhat, W.; Ntentakis, D.P.; Hutcheon, A.E.K.; Ross, A.E.; Ciolino, J.B. Extracellular Vesicles in Corneal Fibrosis/Scarring. Int. J. Mol. Sci. 2022, 23, 5921. [Google Scholar] [CrossRef] [PubMed]
- Yeung, V.; Zhang, T.C.; Yuan, L.; Parekh, M.; Cortinas, J.A.; Delavogia, E.; Hutcheon, A.E.K.; Guo, X.; Ciolino, J.B. Extracellular Vesicles Secreted by Corneal Myofibroblasts Promote Corneal Epithelial Cell Migration. Int. J. Mol. Sci. 2022, 23, 3136. [Google Scholar] [CrossRef]
- Han, K.-Y.; Tran, J.A.; Chang, J.-H.; Azar, D.T.; Zieske, J.D. Potential Role of Corneal Epithelial Cell-Derived Exosomes in Corneal Wound Healing and Neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef] [PubMed]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-Derived Exosomes on Corneal Epithelial Wound Healing. Investig. Opthalmology Vis. Sci. 2018, 59, 5194. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Yeung, V.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D.; Ciolino, J.B. Extracellular Vesicles in the Cornea: Insights from Other Tissues. Anal. Cell. Pathol. Amst. 2021, 2021, 9983900. [Google Scholar] [CrossRef]
- Zieske, J.D.; Higashijima, S.C.; Spurr-Michaud, S.J.; Gipson, I.K. Biosynthetic Responses of the Rabbit Cornea to a Keratectomy Wound. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1668–1677. [Google Scholar]
- McKay, T.B.; Hutcheon, A.E.K.; Zieske, J.D.; Ciolino, J.B. Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation. Cells 2020, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from Normal and Diabetic Human Corneolimbal Keratocytes Differentially Regulate Migration, Proliferation and Marker Expression of Limbal Epithelial Cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef] [PubMed]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef]
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Toledano Furman, N.E.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S.; Olson, S.D. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells 2018, 36, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, R.; Takam Kamga, P.; Tanasi, I.; Krampera, M. Extracellular Vesicle-Dependent Communication Between Mesenchymal Stromal Cells and Immune Effector Cells. Front. Cell Dev. Biol. 2020, 8, 596079. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, H.; Chiang, W.-C.; Felden, J.; Nguyen, A.; Lin, J.H. ER Stress and Unfolded Protein Response in Ocular Health and Disease. FEBS J. 2019, 286, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, P.; Shinohara, T. Age-Related Cataracts: Role of Unfolded Protein Response, Ca2+ Mobilization, Epigenetic DNA Modifications, and Loss of Nrf2/Keap1 Dependent Cytoprotection. Prog. Retin. Eye Res. 2017, 60, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, D.; Aguila, M.; Bellingham, J.; Kanuga, N.; Adamson, P.; Cheetham, M.E. The Role of the ER Stress-Response Protein PERK in Rhodopsin Retinitis Pigmentosa. Hum. Mol. Genet. 2017, 26, 4896–4905. [Google Scholar] [CrossRef]
- Peters, J.C.; Bhattacharya, S.; Clark, A.F.; Zode, G.S. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3860–3868. [Google Scholar] [CrossRef]
- Sayin, N.; Kara, N.; Pekel, G. Ocular Complications of Diabetes Mellitus. World J. Diabetes 2015, 6, 92–108. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Zhou, Q.; Li, J.; Wang, X.; Zhang, J.; Li, D.; Qi, X.; Liu, T.; Zhao, X.; et al. MANF Promotes Diabetic Corneal Epithelial Wound Healing and Nerve Regeneration by Attenuating Hyperglycemia-Induced Endoplasmic Reticulum Stress. Diabetes 2020, 69, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.M.; Di Zazzo, A.; Bonini, S.; Argüeso, P. Endoplasmic Reticulum Stress Promotes Inflammation-Mediated Proteolytic Activity at the Ocular Surface. Sci. Rep. 2020, 10, 2216. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Hyttinen, J.M.T.; Toropainen, E.; Kaarniranta, K. Endoplasmic Reticulum Stress in Age-Related Macular Degeneration: Trigger for Neovascularization. Mol. Med. 2010, 16, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Berber, P.; Grassmann, F.; Kiel, C.; Weber, B.H.F. An Eye on Age-Related Macular Degeneration: The Role of MicroRNAs in Disease Pathology. Mol. Diagn. Ther. 2017, 21, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Martinon, F.; Rodriguez, D.; Glimcher, L.H. The Unfolded Protein Response: Integrating Stress Signals through the Stress Sensor IRE1α. Physiol. Rev. 2011, 91, 1219–1243. [Google Scholar] [CrossRef] [PubMed]
- Lenna, S.; Trojanowska, M. The Role of Endoplasmic Reticulum Stress and the Unfolded Protein Response in Fibrosis. Curr. Opin. Rheumatol. 2012, 24, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic Interaction of BiP and ER Stress Transducers in the Unfolded-Protein Response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Yao, P.-P.; Sheng, M.-J.; Weng, W.-H.; Long, Y.; Liu, H.; Chen, L.; Lu, J.-J.; Rong, A.; Li, B. High Glucose Causes Apoptosis of Rabbit Corneal Epithelial Cells Involving Activation of PERK-eIF2α-CHOP-Caspase-12 Signaling Pathway. Int. J. Ophthalmol. 2019, 12, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Boyko, T.V.; Bam, R.; Jiang, D.; Wang, Z.; Bhatia, N.; Tran, M.C.; Longaker, M.T.; Koong, A.C.; Yang, G.P. Inhibition of IRE1 Results in Decreased Scar Formation. Wound Repair Regen. 2017, 25, 964–971. [Google Scholar] [CrossRef]
- Park, H.-R.; Sun, R.; Panganiban, R.A.; Christiani, D.C.; Lu, Q. MicroRNA-124 Reduces Arsenic-Induced Endoplasmic Reticulum Stress and Neurotoxicity and Is Linked with Neurodevelopment in Children. Sci. Rep. 2020, 10, 5934. [Google Scholar] [CrossRef]
- Hao, X.; Liu, Y.; Hu, S.; Pan, X.; Chen, P. Association of Macular Corneal Dystrophy with Excessive Cell Senescence and Apoptosis Induced by the Novel Mutant CHST6. Exp. Eye Res. 2022, 214, 108862. [Google Scholar] [CrossRef]
- Buono, L.; Scalabrin, S.; De Iuliis, M.; Tanzi, A.; Grange, C.; Tapparo, M.; Nuzzi, R.; Bussolati, B. Mesenchymal Stem Cell-Derived Extracellular Vesicles Protect Human Corneal Endothelial Cells from Endoplasmic Reticulum Stress-Mediated Apoptosis. Int. J. Mol. Sci. 2021, 22, 4930. [Google Scholar] [CrossRef]
- Nagymihály, R.; Veréb, Z.; Facskó, A.; Moe, M.C.; Petrovski, G. Effect of Isolation Technique and Location on the Phenotype of Human Corneal Stroma-Derived Cells. Stem Cells Int. 2017, 2017, 9275248. [Google Scholar] [CrossRef]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of Extracellular Vesicles: Determining the Correct Approach (Review). Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef]
- Nigro, A.; Finardi, A.; Ferraro, M.M.; Manno, D.E.; Quattrini, A.; Furlan, R.; Romano, A. Selective Loss of Microvesicles Is a Major Issue of the Differential Centrifugation Isolation Protocols. Sci. Rep. 2021, 11, 3589. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Szłapka-Kosarzewska, J.; Śmieszek, A.; Marycz, K. 5-Azacytydine and Resveratrol Reverse Senescence and Ageing of Adipose Stem Cells via Modulation of Mitochondrial Dynamics and Autophagy. J. Cell. Mol. Med. 2019, 23, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.T.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. In Vitro Anti-Pancreatic Cancer Activity of HPLC-Derived Fractions from Helicteres Hirsuta Lour. Stem. Mol. Biol. Rep. 2020, 47, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Choi, J.-W.; Lee, M.-K.; Choi, Y.-H.; Nam, T.-J. Effect of Cyclophilin from Pyropia Yezoensis on the Proliferation of Intestinal Epithelial Cells by Epidermal Growth Factor Receptor/Ras Signaling Pathway. Mar. Drugs 2019, 17, 297. [Google Scholar] [CrossRef]
- Oláh, M.; Farkas, E.; Székács, I.; Horvath, R.; Székács, A. Cytotoxic Effects of Roundup Classic and Its Components on NE-4C and MC3T3-E1 Cell Lines Determined by Biochemical and Flow Cytometric Assays. Toxicol. Rep. 2022, 9, 914–926. [Google Scholar] [CrossRef]
- Ekström, K.; Crescitelli, R.; Pétursson, H.I.; Johansson, J.; Lässer, C.; Olofsson Bagge, R. Characterization of Surface Markers on Extracellular Vesicles Isolated from Lymphatic Exudate from Patients with Breast Cancer. BMC Cancer 2022, 22, 50. [Google Scholar] [CrossRef]
- Davidson, S.M.; Boulanger, C.M.; Aikawa, E.; Badimon, L.; Barile, L.; Binder, C.J.; Brisson, A.; Buzas, E.; Emanueli, C.; Jansen, F.; et al. Methods for the Identification and Characterization of Extracellular Vesicles in Cardiovascular Studies: From Exosomes to Microvesicles. Cardiovasc. Res. 2023, 119, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Alorda-Clara, M.; Reyes, J.; Trelles-Guzman, M.G.; Florido, M.; Roca, P.; Pons, D.G.; Oliver, J. Isolation and Characterization of Extracellular Vesicles in Human Bowel Lavage Fluid. Int. J. Mol. Sci. 2023, 24, 7391. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Vila, M.; Yoshioka, Y.; Ochiya, T. Biological Functions Driven by mRNAs Carried by Extracellular Vesicles in Cancer. Front. Cell Dev. Biol. 2021, 9, 620498. [Google Scholar] [CrossRef]
- Sun, Y.; Dos Santos, A.; Balayan, A.; Deng, S.X. Evaluation of Cryopreservation Media for the Preservation of Human Corneal Stromal Stem Cells. Tissue Eng. Part C Methods 2020, 26, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Santra, M.; Geary, M.L.; Rubin, E.; Hsu, M.Y.S.; Funderburgh, M.L.; Chandran, C.; Du, Y.; Dhaliwal, D.K.; Jhanji, V.; Yam, G.H.-F. Good Manufacturing Practice Production of Human Corneal Limbus-Derived Stromal Stem Cells and in Vitro Quality Screening for Therapeutic Inhibition of Corneal Scarring. Stem Cell Res. Ther. 2024, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, A.; Marycz, K.; Kornicka, J.; Groborz, S.; Meissner, J.; Mularczyk, M. Tetrahydrocannabivarin (THCV) Protects Adipose-Derived Mesenchymal Stem Cells (ASC) against Endoplasmic Reticulum Stress Development and Reduces Inflammation during Adipogenesis. Int. J. Mol. Sci. 2023, 24, 7120. [Google Scholar] [CrossRef]
- Abdelwahid, E.; Li, H.; Wu, J.; Irioda, A.C.; de Carvalho, K.A.T.; Luo, X. Endoplasmic Reticulum (ER) Stress Triggers Hax1-Dependent Mitochondrial Apoptotic Events in Cardiac Cells. Apoptosis Int. J. Program. Cell Death 2016, 21, 1227–1239. [Google Scholar] [CrossRef]
- Jackisch, L.; Murphy, A.M.; Kumar, S.; Randeva, H.; Tripathi, G.; McTernan, P.G. Tunicamycin-Induced Endoplasmic Reticulum Stress Mediates Mitochondrial Dysfunction in Human Adipocytes. J. Clin. Endocrinol. Metab. 2020, 105, dgaa258. [Google Scholar] [CrossRef]
- Tiganis, T.; Leaver, D.D.; Ham, K.; Friedhuber, A.; Stewart, P.; Dziadek, M. Functional and Morphological Changes Induced by Tunicamycin in Dividing and Confluent Endothelial Cells. Exp. Cell Res. 1992, 198, 191–200. [Google Scholar] [CrossRef]
- Zheng, H. Partnership in Action: The Endoplasmic Reticulum Regulates the Cytoskeleton. J. Plant Physiol. 2021, 266, 153540. [Google Scholar] [CrossRef]
- Plummer, C.E. Corneal Response to Injury and Infection in the Horse. Vet. Clin. N. Am. Equine Pract. 2017, 33, 439–463. [Google Scholar] [CrossRef] [PubMed]
- Willmann, D.; Fu, L.; Melanson, S.W. Corneal Injury. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Barrientez, B.; Nicholas, S.E.; Whelchel, A.; Sharif, R.; Hjortdal, J.; Karamichos, D. Corneal Injury: Clinical and Molecular Aspects. Exp. Eye Res. 2019, 186, 107709. [Google Scholar] [CrossRef] [PubMed]
- Kratochvílová, K.; Moráň, L.; Paďourová, S.; Stejskal, S.; Tesařová, L.; Šimara, P.; Hampl, A.; Koutná, I.; Vaňhara, P. The Role of the Endoplasmic Reticulum Stress in Stemness, Pluripotency and Development. Eur. J. Cell Biol. 2016, 95, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Dana, R. Comparison of Topical Interleukin-1 vs. Tumor Necrosis Factor-Alpha Blockade with Corticosteroid Therapy on Murine Corneal Inflammation, Neovascularization, and Transplant Survival (an American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2007, 105, 330–343. [Google Scholar] [PubMed]
- Wilson, S.E.; Esposito, A. Focus on Molecules: Interleukin-1: A Master Regulator of the Corneal Response to Injury. Exp. Eye Res. 2009, 89, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Mohan, R.R.; Kim, W.-J.; Wilson, S.E. Modulation of TNF-α–Induced Apoptosis in Corneal Fibroblasts by Transcription Factor NF-κB. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1327–1336. [Google Scholar]
- Fujita, S.; Saika, S.; Kao, W.W.-Y.; Fujita, K.; Miyamoto, T.; Ikeda, K.; Nakajima, Y.; Ohnishi, Y. Endogenous TNFα Suppression of Neovascularization in Corneal Stroma in Mice. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3051–3055. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Mehta, A.; Zhao, J.L.; Lee, K.; Marinov, G.K.; Garcia-Flores, Y.; Lu, L.-F.; Rudensky, A.Y.; Baltimore, D. An NF-κB-microRNA Regulatory Network Tunes Macrophage Inflammatory Responses. Nat. Commun. 2017, 8, 851. [Google Scholar] [CrossRef]
- Mohan, R.R.; Liang, Q.; Kim, W.J.; Helena, M.C.; Baerveldt, F.; Wilson, S.E. Apoptosis in the Cornea: Further Characterization of Fas/Fas Ligand System. Exp. Eye Res. 1997, 65, 575–589. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-kappaB-Dependent Induction of microRNA miR-146, an Inhibitor Targeted to Signaling Proteins of Innate Immune Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Zhuang, Z.; Qin, X.; Hu, H.; Tian, S.; Lu, Z.; Zhang, T.; Bai, Y. Down-Regulation of microRNA-155 Attenuates Retinal Neovascularization via the PI3K/Akt Pathway. Mol. Vis. 2015, 21, 1173–1184. [Google Scholar]
- Pulkkinen, K.H.; Ylä-Herttuala, S.; Levonen, A.-L. Heme Oxygenase 1 Is Induced by miR-155 via Reduced BACH1 Translation in Endothelial Cells. Free Radic. Biol. Med. 2011, 51, 2124–2131. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G.; Labbaye, C. miR-146 and miR-155: Two Key Modulators of Immune Response and Tumor Development. Non-Coding RNA 2017, 3, 22. [Google Scholar] [CrossRef]
- Atarod, S.; Ahmed, M.M.; Lendrem, C.; Pearce, K.F.; Cope, W.; Norden, J.; Wang, X.-N.; Collin, M.; Dickinson, A.M. miR-146a and miR-155 Expression Levels in Acute Graft-Versus-Host Disease Incidence. Front. Immunol. 2016, 7, 156430. [Google Scholar] [CrossRef]
- Chipurupalli, S.; Samavedam, U.; Robinson, N. Crosstalk Between ER Stress, Autophagy and Inflammation. Front. Med. 2021, 8, 758311. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Joo, H.S.; Hong, E.B.; Lee, H.J.; Lee, J.M. Therapeutic Application of Exosomes in Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 1144. [Google Scholar] [CrossRef] [PubMed]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, B.; Jiang, H.; Wang, Y.; Qu, M.; Duan, H.; Zhou, Q.; Shi, W. Macrophage Depletion Impairs Corneal Wound Healing after Autologous Transplantation in Mice. PLoS ONE 2013, 8, e61799. [Google Scholar] [CrossRef]
- Fini, M. Keratocyte and Fibroblast Phenotypes in the Repairing Cornea. Prog. Retin. Eye Res. 1999, 18, 529–551. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Chan, G.C.F.; Zhou, Y.; Lai, X.; Lian, M. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stromal Cells Protect Cardiac Cells Against Hypoxia/Reoxygenation Injury by Inhibiting Endoplasmic Reticulum Stress via Activation of the PI3K/Akt Pathway. Cell Transplant. 2020, 29, 096368972094567. [Google Scholar] [CrossRef] [PubMed]
- Bitirim, C.V.; Ozer, Z.B.; Aydos, D.; Genc, K.; Demirsoy, S.; Akcali, K.C.; Turan, B. Cardioprotective Effect of Extracellular Vesicles Derived from Ticagrelor-Pretreated Cardiomyocyte on Hyperglycemic Cardiomyocytes through Alleviation of Oxidative and Endoplasmic Reticulum Stress. Sci. Rep. 2022, 12, 5651. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.; Shastri, M.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef] [PubMed]
- Victor, P.; Sarada, D.; Ramkumar, K.M. Crosstalk between Endoplasmic Reticulum Stress and Oxidative Stress: Focus on Protein Disulfide Isomerase and Endoplasmic Reticulum Oxidase 1. Eur. J. Pharmacol. 2021, 892, 173749. [Google Scholar] [CrossRef] [PubMed]
- Dutra Silva, J.; Su, Y.; Calfee, C.S.; Delucchi, K.L.; Weiss, D.; McAuley, D.F.; O’Kane, C.; Krasnodembskaya, A.D. Mesenchymal Stromal Cell Extracellular Vesicles Rescue Mitochondrial Dysfunction and Improve Barrier Integrity in Clinically Relevant Models of ARDS. Eur. Respir. J. 2021, 58, 2002978. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.; Hwang, J.S.; Bo Noh, K.; Park, S.H.; Seo, J.H.; Shin, Y.J. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote the Regeneration of Corneal Endothelium Through Ameliorating Senescence. Investig. Ophthalmol. Vis. Sci. 2023, 64, 29. [Google Scholar] [CrossRef]
- Saccu, G.; Menchise, V.; Gai, C.; Bertolin, M.; Ferrari, S.; Giordano, C.; Manco, M.; Dastrù, W.; Tolosano, E.; Bussolati, B.; et al. Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Promote Corneal Wound Repair by Regulating Inflammation and Angiogenesis. Cells 2022, 11, 3892. [Google Scholar] [CrossRef]



| Antibodies | Concentrations | CAT Numbers | Company |
|---|---|---|---|
| KI-67 | 1:1000 | orb10033 | Biorbyt (Janki, Poland) |
| IL-1β | 1:500 | ab9722 | Abcam (Cambridge, UK) |
| Cas3 | 1:1000 | c8487 | Sigma (Poznan, Poland) |
| CytC | 1:500 | Nb100-56503 | Novous (Poznan, Poland) |
| Gene | Primer Sequence (5′->3′) |
|---|---|
| BAX | F: GGCACCTCTTCCCTCCTTTCT |
| R: CGATGCGCTTGAGACACTCG | |
| BCL2 | F: TTCTTTGAGTTCGGTGGGGT |
| R: GGGCCGTACAGTTCCACAA | |
| p21 | F: GAAGAGAAACCCCCAGCTCC |
| R: TGACTGCATCAAACCCCACA | |
| p53 | F: TACTCCCCTGCCCTCAACAA |
| R: AGGAATCAGGGCCTTGAGGA | |
| Cas3 | F: GGCAGACTTCCTGTATGCGT |
| R: CCATGGCTACCTTGCGGTTA | |
| Cas6 | F: GCCTACAGAAGAAGTTTGGGG |
| R: AGGGTTAGGTGCCAGAAGAA | |
| Cas8 | F: ACTGTGATGTTGCTGGGACT |
| R: CTTTCTCCTGGTGCATCTATCG | |
| Cas9 | F: CACCTTCCCAGGCTTTGTCT |
| R: GGCTCTGGCCTCAGTAAGTT | |
| IL-1α | F: CCAAGCTGAACTTCAAGGAGAGCGT |
| R: CTCAGCACATGCTCAGCAAGTGAC | |
| IL-1β | F: TATGTGTGTGATGCAGCTGTG |
| R: ACTCAAATTCCACGTTGCCC | |
| IL-6 | F: CGTCACTCCAGTTGCCTTCT |
| R: GCCAGTACCTCCTTGCTGTT | |
| TNF α | F: TCCTACCCGTCCAAGGTCAA |
| R: CTCATACCAGGGCTTGGCTT | |
| IL-4 | F: CGTCACTCCAGTTGCCTTCT |
| R: GCCAGTACCTCCTTGCTGTT | |
| IL-10 | F: CTAGGGAACGAAGCATCCAGG |
| R: TCAGGAGAGAGGTACCACAGG | |
| TGF β | F: ATTCCTGGCGCTACCTCAGT |
| R: GCTGGAACTGAACCCGTTGAT | |
| PERK | F: GTGACTGCAATGGACCAGGA |
| R: TCACGTGCTCACGAGGATATT | |
| eIF2 | F: AGTCTTCAGGCATTGGCTCC |
| R: CCGAGTGGGACATGTATCGG | |
| CHOP | F: AGCCAAAATCAGAGCCGGAA |
| R: GGGGTCAAGAGTGGTGAAGG | |
| ATF6 | F: CAGGGTGCACTAGAACAGGG |
| R: AATGTGTCTCCCCTTCTGCG | |
| PTP1B | F: TGGAAGGAGCTCTCCCATGA |
| R: GGAAGGGCTTCCAGTGAGTC | |
| BIP | F: CTGTAGCGTATGGTGCTGCT |
| R: CATGACACCTCCCACGGTTT | |
| IRE1 | F: GAATCAGACGAGCACCCGAA |
| R: TTTCTTGCAGAGGCCGAAGT | |
| XBP1 | F: TTACGCGAGAAAACTCATGGCC |
| R: GGGTCCAAGTTGAACAGAATGC | |
| SOD1 | F: CATTCCATCATTGGCCGCAC |
| R: GAGCGATCCCAATCACACCA | |
| SOD2 | F: GGACAAACCTGAGCCCCAAT |
| R: TTGGACACCAGCCGATACAG | |
| Cat | F: ACCAAGGTTTGGCCTCACAA |
| R: TTGGGTCAAAGGCCAACTGT | |
| GPX | F: TCGAGCCCAACTTCACACTC |
| R: AAGTTCCAGGCGACATCGTT | |
| FIS | F: GGTGCGAAGCAAGTACAACG |
| R: GTTGCCCACAGCCAGATAGA | |
| MNF1 | F: AAGTGGCATTTTTCGGCAGG |
| R: TCCATATGAAGGGCATGGGC | |
| Parkin | F: TCCCAGTGGAGGTCGATTCT |
| R: CCCTCCAGGTGTGTTCGTTT | |
| PINK1 | F: GCACAATGAGCCAGGAGCTA |
| R: GGGGTATTCACGCGAAGGTA | |
| CD63 | F: GTTCTTCTGCTGGCCTTCTG |
| R: CTGCATCCTGTCCACAATCG | |
| CD9 | F: CCGATTCGACTCTCAGACCA |
| R: CTGTTCTACTCCACCAGCCA | |
| CD81 | F: CACAGACCACCAACCTTCTC |
| R: CAGGCAAAGAGGATGACGAG | |
| GAPDH | F: GATGCCCCAATGTTTGTGA |
| R: AAGCAGGGATGATGTTCTGG |
| Primer miRNAs | Primer Sequence (5′->3′) |
|---|---|
| miR155 | TTAATGCTAATCGTGATAGGGGTT |
| miR146a-5p | TGAGAACTGAATTCCATGGGTT |
| miR124-3p | TAAGGCACGCGGTGAATGCCAA |
| miR17-5p | CAAAGTGCTTACAGTGCAGGTAG |
| miR101-1/2 | TACAGTACTGTGATAACTGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meissner, J.M.; Chmielińska, A.; Ofri, R.; Cisło-Sankowska, A.; Marycz, K. Extracellular Vesicles Isolated from Equine Adipose-Derived Stromal Stem Cells (ASCs) Mitigate Tunicamycin-Induced ER Stress in Equine Corneal Stromal Stem Cells (CSSCs). Curr. Issues Mol. Biol. 2024, 46, 3251-3277. https://doi.org/10.3390/cimb46040204
Meissner JM, Chmielińska A, Ofri R, Cisło-Sankowska A, Marycz K. Extracellular Vesicles Isolated from Equine Adipose-Derived Stromal Stem Cells (ASCs) Mitigate Tunicamycin-Induced ER Stress in Equine Corneal Stromal Stem Cells (CSSCs). Current Issues in Molecular Biology. 2024; 46(4):3251-3277. https://doi.org/10.3390/cimb46040204
Chicago/Turabian StyleMeissner, Justyna M., Aleksandra Chmielińska, Ron Ofri, Anna Cisło-Sankowska, and Krzysztof Marycz. 2024. "Extracellular Vesicles Isolated from Equine Adipose-Derived Stromal Stem Cells (ASCs) Mitigate Tunicamycin-Induced ER Stress in Equine Corneal Stromal Stem Cells (CSSCs)" Current Issues in Molecular Biology 46, no. 4: 3251-3277. https://doi.org/10.3390/cimb46040204
APA StyleMeissner, J. M., Chmielińska, A., Ofri, R., Cisło-Sankowska, A., & Marycz, K. (2024). Extracellular Vesicles Isolated from Equine Adipose-Derived Stromal Stem Cells (ASCs) Mitigate Tunicamycin-Induced ER Stress in Equine Corneal Stromal Stem Cells (CSSCs). Current Issues in Molecular Biology, 46(4), 3251-3277. https://doi.org/10.3390/cimb46040204






