Genetic Variants Associated with Body Mass Index Changes in Korean Adults: The Anseong and Ansan Cohorts of the Korean Genome and Epidemiology Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Statistical Analysis
2.3. Ethics Statement
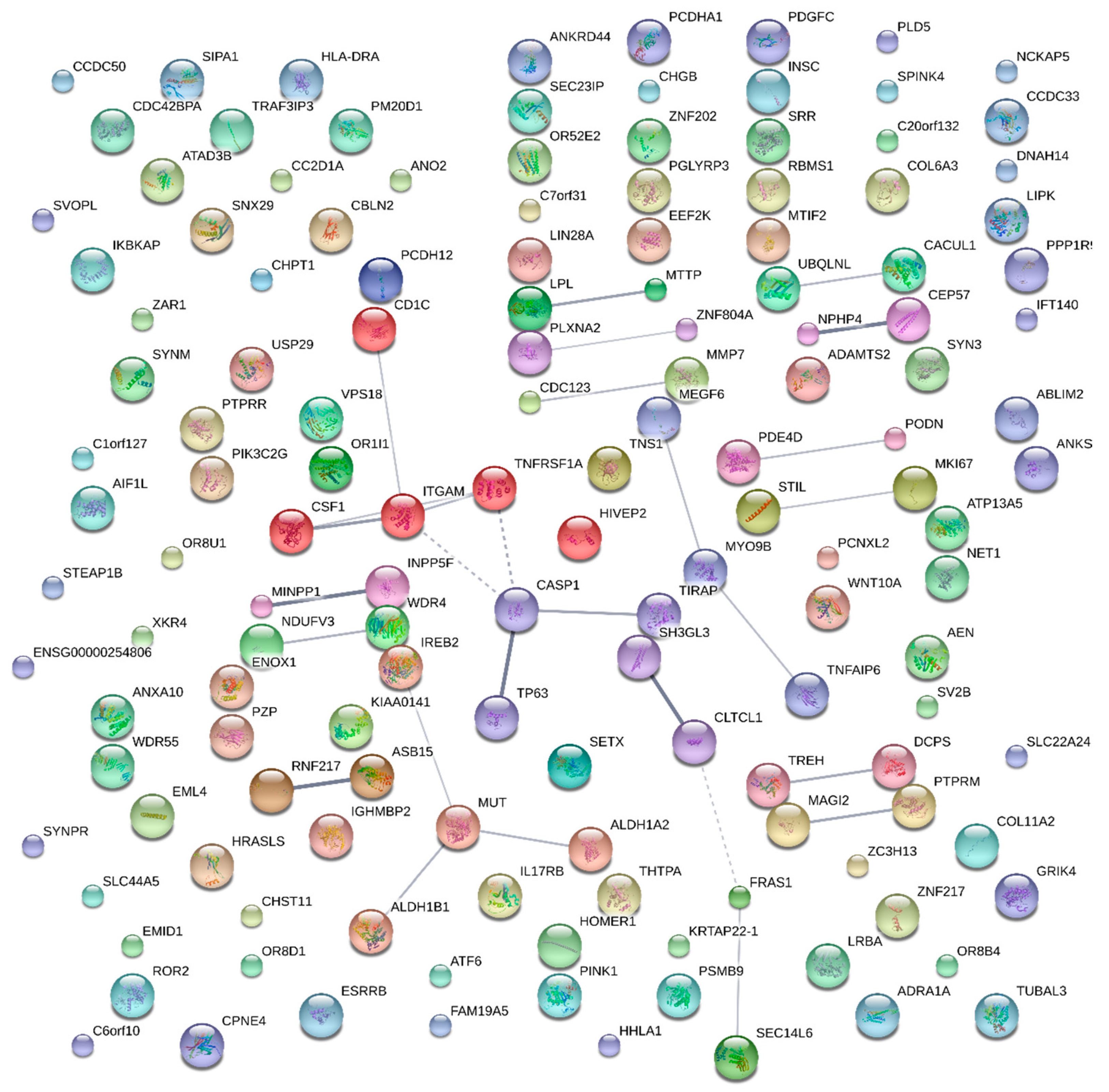
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keys, A.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; Taylor, H.L. Indices of relative weight and obesity. J. Chronic Dis. 1972, 25, 329–343. [Google Scholar] [CrossRef]
- Eknoyan, G. Adolphe Quetelet (1796–1874)–the average man and indices of obesity. Nephrol. Dial. Transpl. 2008, 23, 47–51. [Google Scholar] [CrossRef]
- Bray, G.A. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef]
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef]
- Lau, D.C.W.; Douketis, J.D.; Morrison, K.M.; Hramiak, I.M.; Sharma, A.M.; Ur, E.; Obesity Canada Clinical Practice Guidelines Expert Panel. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. Can. Med. Assoc. J. 2007, 176, S1–S13. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Malaspina, D.; Boden-Albala, B.; Heymsfield, S.B. Inadequate sleep as a risk factor for obesity: Analyses of the nhanes i. Sleep 2005, 28, 1289–1296. [Google Scholar] [CrossRef]
- Pelletier, C.; Imbeault, P.; Tremblay, A. Energy balance and pollution by organochlorines and polychlorinated biphenyls. Obes. Rev. 2003, 4, 17–24. [Google Scholar] [CrossRef]
- Traversy, G.; Chaput, J.P. Alcohol consumption and obesity: An update. Curr. Obes. Rep. 2015, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Bottai, M.; Frongillo, E.A.; Sui, X.; O’Neill, J.R.; McKeown, R.E.; Burns, T.L.; Liese, A.D.; Blair, S.N.; Pate, R.R. Use of quantile regression to investigate the longitudinal association between physical activity and body mass index. Obesity 2014, 22, E149–E156. [Google Scholar] [CrossRef]
- Segal, N.L.; Allison, D.B. Twins and virtual twins: Bases of relative body weight revisited. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 437–441. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef]
- Akbarian, S.A.; Salehi-Abargouei, A.; Pourmasoumi, M.; Kelishadi, R.; Nikpour, P.; Heidari-Beni, M. Association of brain-derived neurotrophic factor gene polymorphisms with body mass index: A systematic review and meta-analysis. Adv. Med. Sci. 2018, 63, 43–56. [Google Scholar] [CrossRef]
- Cho, N.H.; Jang, H.C.; Choi, S.H.; Kim, H.R.; Lee, H.K.; Chan, J.C.; Lim, S. Abnormal liver function test predicts type 2 diabetes: A community-based prospective study. Diabetes Care 2007, 30, 2566–2568. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The string database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef]
- Kim, D.M.; Ahn, C.W.; Nam, S.Y. Prevalence of obesity in Korea. Obes. Rev. 2005, 6, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Walley, A.J.; Asher, J.E.; Froguel, P. The genetic contribution to non-syndromic human obesity. Nat. Rev. Genet. 2009, 10, 431–442. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Battegay, E.J.; Rupp, J.; Iruela-Arispe, L.; Sage, E.H.; Pech, M. Pdgf-bb modulates endothelial proliferation and angiogenesis in vitro via pdgf beta-receptors. J. Cell Biol. 1994, 125, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Virakul, S.; Dalm, V.A.; Paridaens, D.; van den Bosch, W.A.; Mulder, M.T.; Hirankarn, N.; van Hagen, P.M.; Dik, W.A. Platelet-derived growth factor-bb enhances adipogenesis in orbital fibroblasts. Invest. Ophthalmol. Vis. Sci. 2015, 56, 5457–5464. [Google Scholar] [CrossRef]
- Muller, Y.L.; Hanson, R.L.; Knowler, W.C.; Fleming, J.; Goswami, J.; Huang, K.; Traurig, M.; Sutherland, J.; Wiedrich, C.; Wiedrich, K.; et al. Identification of genetic variation that determines human trehalase activity and its association with type 2 diabetes. Hum. Genet. 2013, 132, 697–707. [Google Scholar] [CrossRef]
- Croteau-Chonka, D.C.; Marvelle, A.F.; Lange, E.M.; Lee, N.R.; Adair, L.S.; Lange, L.A.; Mohlke, K.L. Genome-wide association study of anthropometric traits and evidence of interactions with age and study year in Filipino women. Obesity 2011, 19, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Savas, S.; Schmidt, S.; Jarjanazi, H.; Ozcelik, H. Functional nssnps from carcinogenesis-related genes expressed in breast tissue: Potential breast cancer risk alleles and their distribution across human populations. Hum. Genom. 2006, 2, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Proud, C.G. Regulation and roles of elongation factor 2 kinase. Biochem. Soc. Trans. 2015, 43, 328–332. [Google Scholar] [CrossRef]
- Komine, Y.; Takao, K.; Miyakawa, T.; Yamamori, T. Behavioral abnormalities observed in zfhx2-deficient mice. PLoS ONE 2012, 7, e53114. [Google Scholar] [CrossRef]
- Kawaguchi, D.M.; Glatt, S.J. Grik4 polymorphism and its association with antidepressant response in depressed patients: A meta-analysis. Pharmacogenomics 2014, 15, 1451–1459. [Google Scholar] [CrossRef]
- Whalley, H.C.; Pickard, B.S.; McIntosh, A.M.; Zuliani, R.; Johnstone, E.C.; Blackwood, D.H.; Lawrie, S.M.; Muir, W.J.; Hall, J. A grik4 variant conferring protection against bipolar disorder modulates hippocampal function. Mol. Psychiatry 2009, 14, 467–468. [Google Scholar] [CrossRef]
- Kawase, T.; Ichikawa, H.; Ohta, T.; Nozaki, N.; Tashiro, F.; Ohki, R.; Taya, Y. P53 target gene aen is a nuclear exonuclease required for p53-dependent apoptosis. Oncogene 2008, 27, 3797–3810. [Google Scholar] [CrossRef]
- Hoefele, J.; Wilhelm, C.; Schiesser, M.; Mack, R.; Heinrich, U.; Weber, L.T.; Biskup, S.; Daumer-Haas, C.; Klein, H.G.; Rost, I. Expanding the mutation spectrum for Fraser syndrome: Identification of a novel heterozygous deletion in fras1. Gene 2013, 520, 194–197. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean | Standard Deviation | Max | Min | N |
|---|---|---|---|---|---|
| BMI | 24.607 | 3.032 | 40.259 | 12.535 | 21,518 |
| Age | 53.765 | 8.401 | 75 | 40 | 21,518 |
| Sex | 1.523 | 0.499 | 2 | 1 | 21,518 |
| 24.607 | 0.041 | 24.673 | 24.505 | 21,518 | |
| 0.000 | 3.032 | 15.750 | −11.994 | 21,518 |
| Variable | Male | Female | Difference (t-Value) |
|---|---|---|---|
| Age | 53.329 | 54.164 | −0.834 (−8.18) *** |
| BMI | 24.430 | 24.768 | −0.338 (−7.28) *** |
| 24.609 | 24.605 | 0.004 (7.28) *** | |
| −0.179 | 0.163 | −0.342 (−8.27) *** | |
| N | 10,269 | 11,249 | Sum = 21,518 |
| SNP Id | Gene | Mutations | Class | p-Value | |||
|---|---|---|---|---|---|---|---|
| All | Male | Female | |||||
| rs7105363 | GRIK4, LOC105369532 | intron variant | Silent | protective | * 0.000 | 0.033 | * 0.005 |
| rs8027765 | AEN | Missense | N140D | protective | * 0.000 | 0.010 | * 0.006 |
| rs2373011 | ANKS1B | intron variant | Silent | protective | * 0.000 | 0.011 | * 0.005 |
| rs2229165 | CSF1 | intron variant, missense | Silent, G438R | protective | * 0.001 | 0.038 | * 0.008 |
| rs9935059 | EEF2K | missense, transcript variant | H23R | risk | * 0.000 | 0.014 | * 0.006 |
| rs34670941 | FRAS1 | Missense | V3626A | risk | * 0.000 | 0.021 | * 0.007 |
| rs4691380 | PDGFC | intron variant | Silent | risk | * 0.000 | * 0.001 | * 0.003 |
| rs4982766 | THTPA, ZFHX2 | intron variant, missense | Silent, V1545A | risk | * 0.001 | 0.036 | * 0.008 |
| rs2276064 | TREH | Missense | R486W | risk | * 0.001 | * 0.004 | 0.044 |
| Term | Count | % | p-Value | Genes |
|---|---|---|---|---|
| Cluster 1 | ||||
| Null | 4 | 57.1 | 0.006 | TNFRSF1A, CSF1, TIRAP, ITGAM |
| Tuberculosis | 3 | 42.9 | 0.009 | TNFRSF1A, TIRAP, ITGAM |
| Type 2 Diabetes, edema, rosiglitazone | 5 | 71.4 | 0.009 | TNFRSF1A, CSF1, TIRAP, CASP1, ITGAM |
| Pharmacogenomic | 5 | 71.4 | 0.020 | TNFRSF1A, CSF1, TIRAP, CASP2, ITGAM |
| Unknown | 4 | 57.1 | 0.029 | TNFRSF1A, CSF1, TIRAP, ITGAM |
| Immune | 4 | 57.1 | 0.139 | TNFRSF1A, CSF1, TIRAP, ITGAM |
| Cluster 2 | ||||
| Acquired immunodeficiency syndrome | 3 | 75.0 | 0.014 | MUT, ALDH1B, IREB2 |
| Cardiovascular | 4 | 100.0 | 0.036 | ALDH1A2, MUT, ALDH1B, IREB2 |
| Infection | 3 | 75.0 | 0.074 | MUT, ALDH1B, IREB2 |
| Cluster 3 | ||||
| Splice variant | 3 | 75.0 | 0.150 | SH3GL3, FRAS1, CLTCL1 |
| Membrane | 3 | 75.0 | 0.301 | SH3GL3, FRAS1, CLTCL2 |
| Phosphoprotein | 3 | 75.0 | 0.353 | SH3GL3, FRAS1, CLTCL3 |
| Alternative splicing | 3 | 75.0 | 0.522 | SH3GL3, FRAS1, CLTCL4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-I.; Kim, S.-K.; Kang, S.-W. Genetic Variants Associated with Body Mass Index Changes in Korean Adults: The Anseong and Ansan Cohorts of the Korean Genome and Epidemiology Study. Curr. Issues Mol. Biol. 2024, 46, 9074-9081. https://doi.org/10.3390/cimb46080536
Lee S-I, Kim S-K, Kang S-W. Genetic Variants Associated with Body Mass Index Changes in Korean Adults: The Anseong and Ansan Cohorts of the Korean Genome and Epidemiology Study. Current Issues in Molecular Biology. 2024; 46(8):9074-9081. https://doi.org/10.3390/cimb46080536
Chicago/Turabian StyleLee, Sang-Im, Su-Kang Kim, and Sang-Wook Kang. 2024. "Genetic Variants Associated with Body Mass Index Changes in Korean Adults: The Anseong and Ansan Cohorts of the Korean Genome and Epidemiology Study" Current Issues in Molecular Biology 46, no. 8: 9074-9081. https://doi.org/10.3390/cimb46080536






