Acidic Microenvironment Enhances Cisplatin Resistance in Bladder Cancer via Bcl-2 and XIAP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Cell Culture, and pH Adjustment
2.2. Cell Viability Assay
2.3. Cell Migration Assay
2.4. Western Blotting
2.5. Flow Cytometry Analysis
2.6. Confirmation of Apoptotic Cells
2.7. Statistical Analysis
3. Results
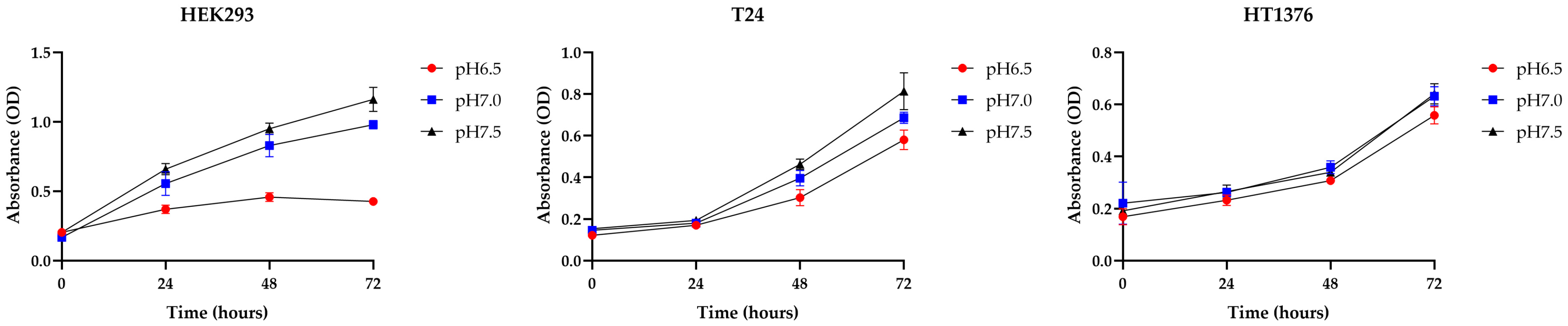
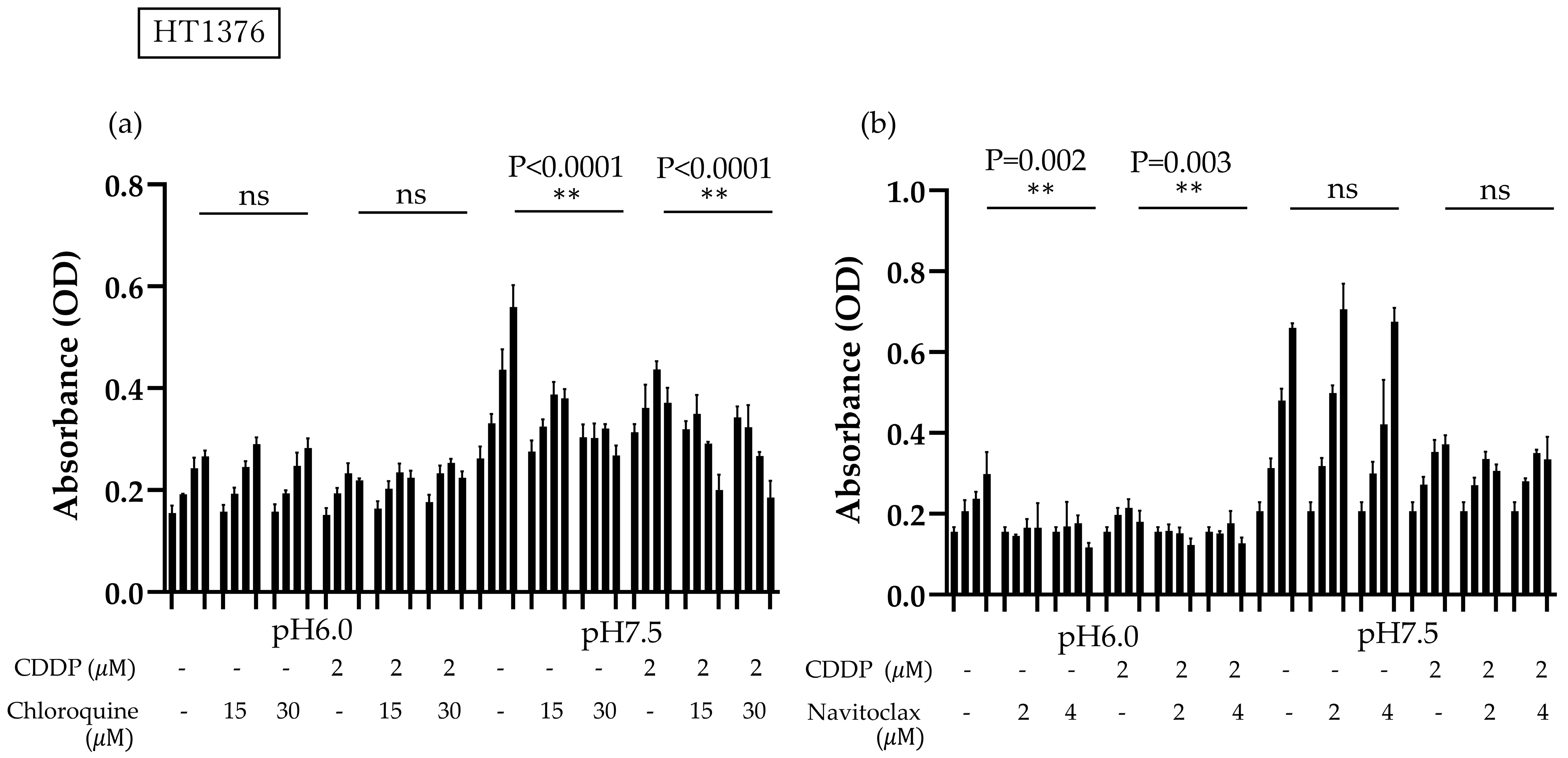
3.1. BC Cells Become More Adaptive in Acidic Environments Compared to Normal Cells, Showing Strong Cisplatin Resistance Under Acidic Conditions
3.2. Acidic Environment Inhibits CDDP Treatment-Induced Mobility of BC Cells
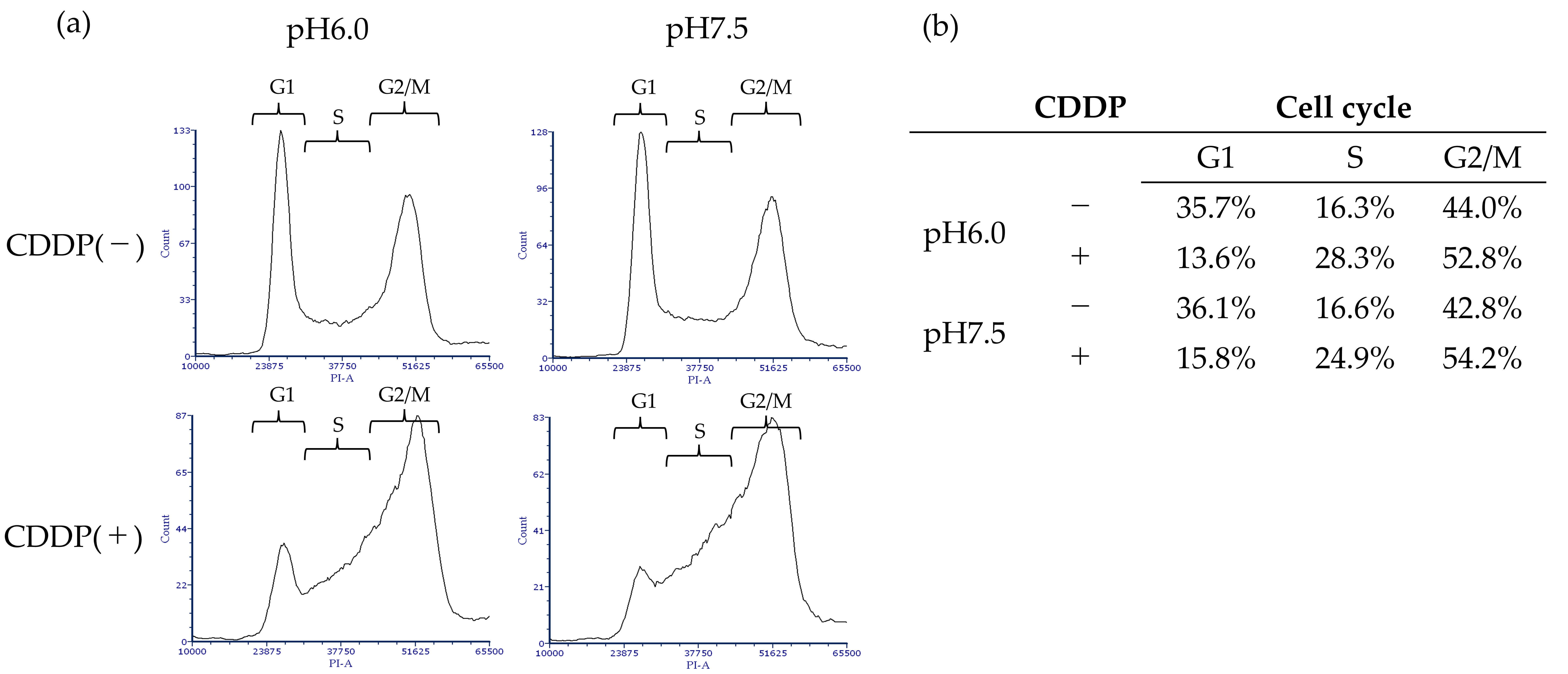
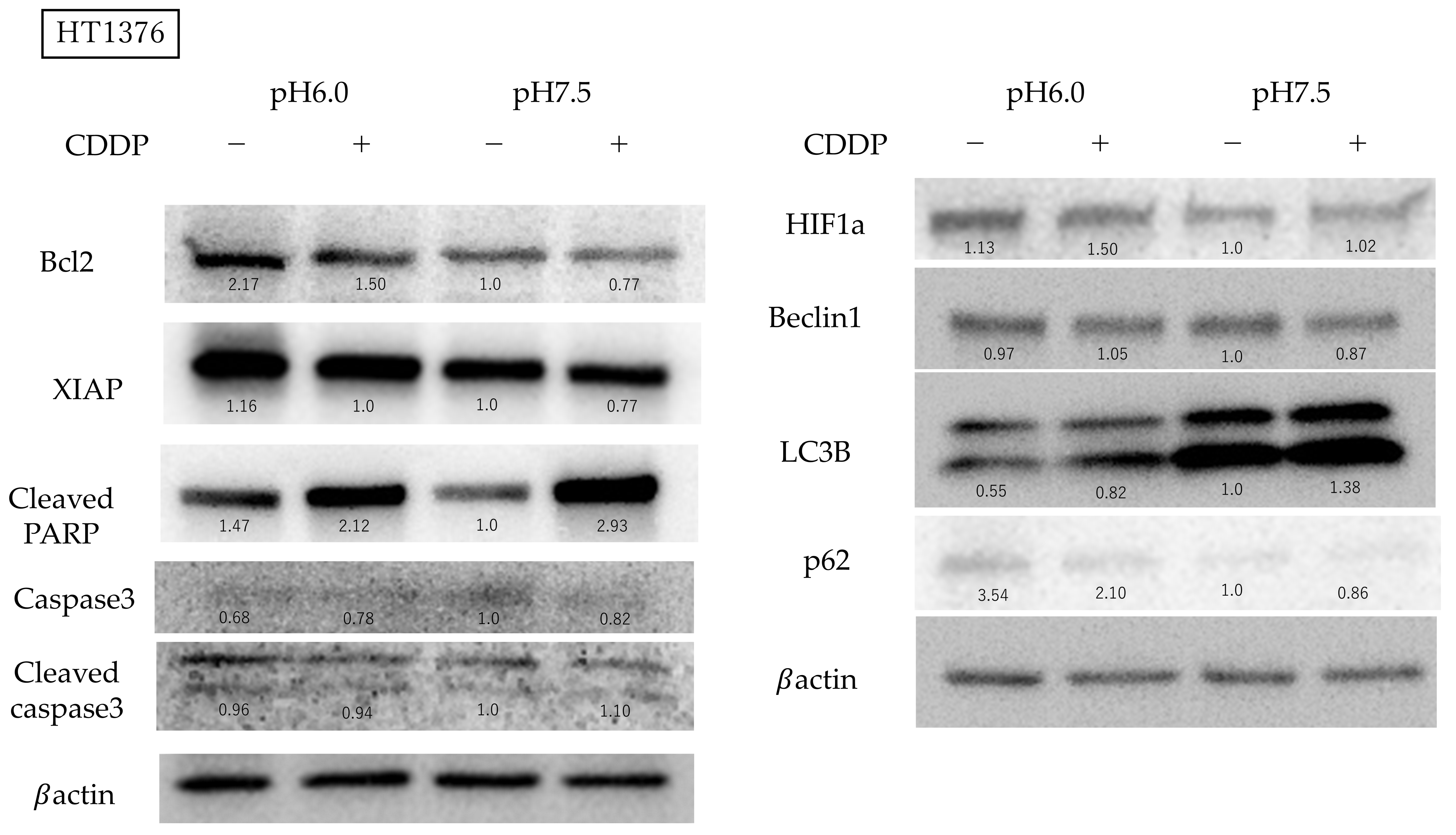
3.3. Bcl-2 Expression Is Activated and Autophagy Is Suppressed in BC Cells Under Acidic Conditions
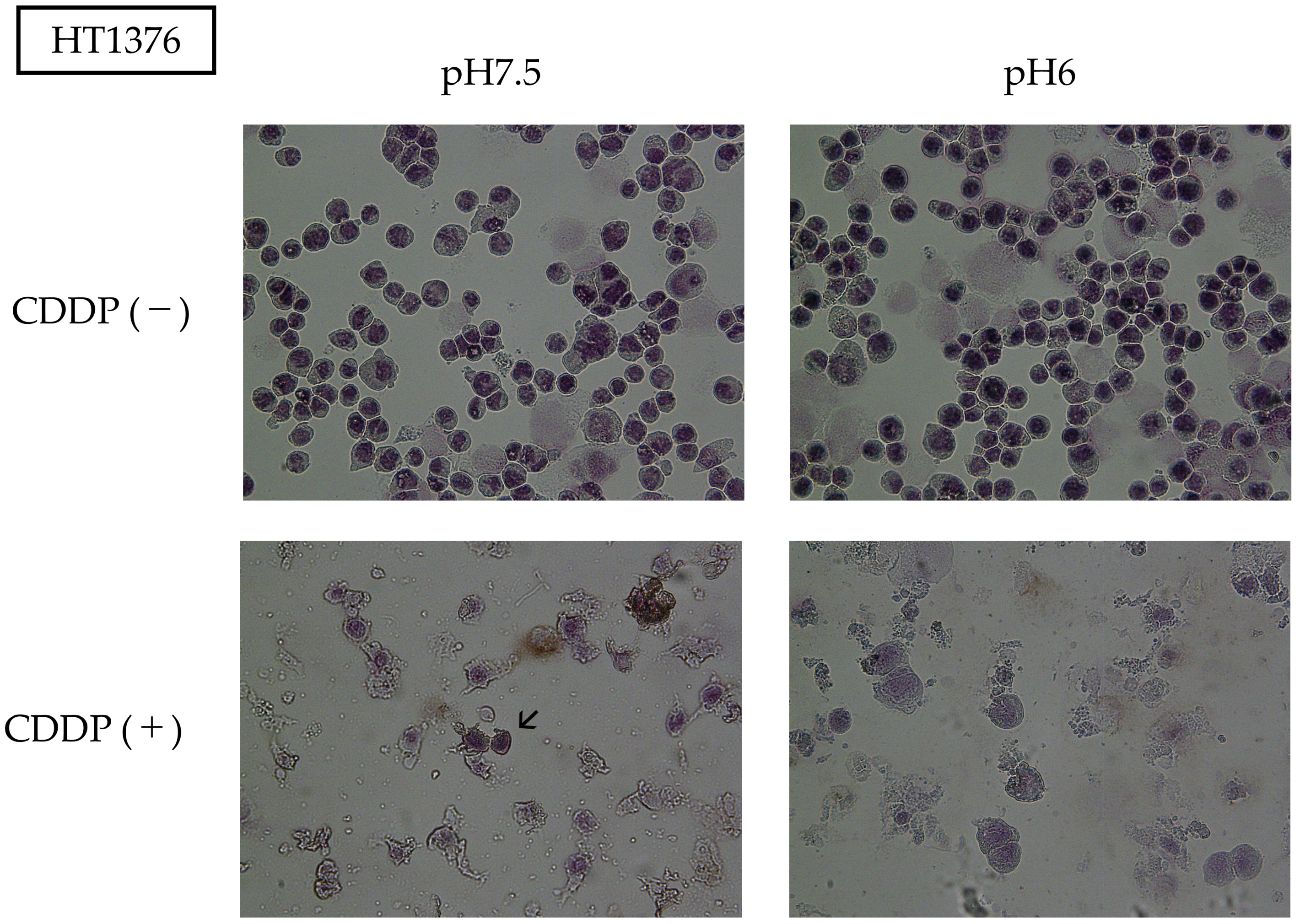
3.4. High Bcl-2 and XIAP Expression Levels in Acidic Environment Suppress Apoptosis and Autophagy-Induced Death in BC Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Es-timates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- A Bueno, A.P.; Clark, O.; Turnure, M.; Moreira, E.S.; Yuasa, A.; Sugiyama, S.; Kirker, M.; Li, S.; Hou, N.; Chang, J.; et al. Treatment patterns in metastatic bladder cancer in Japan: Results of the CancerMPact® survey 2020. Future Oncol. 2024, 20, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Lachand, A.T.; Texier, J.; Texier, P. Surveillance and prognosis of stage T1 superficial bladder tumours. A homogeneous series of 89 cases followed for 1 to 22 years. Prog. Urol. 2001, 11, 472–477. [Google Scholar]
- Lachand, A.T.; Texier, J.; Texier, P. Surveillance and prognosis of stage Ta superficial bladder cancers. A homogeneous series of 138 cases followed for 1 to 18 years. Prog. Urol. 2001, 11, 466–471. [Google Scholar]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Voon der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef]
- von der Maase, H.; Hansen, S.; Roberts, J.; Dogliotti, L.; Oliver, T.; Moore, M.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.; et al. Gemcitabine and Cisplatin Versus Metho-trexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Mul-tinational, Multicenter, Phase III Study. J. Clin. Oncol. 2023, 41, 3881–3890. [Google Scholar] [CrossRef]
- Dogliotti, L.; Cartenì, G.; Siena, S.; Bertetto, O.; Martoni, A.; Bono, A.; Amadori, D.; Onat, H.; Marini, L. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: Results of a randomized phase 2 trial. Eur. Urol. 2007, 52, 134–141. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Raggi, D.; Miceli, R.; Sonpavde, G.; Giannatempo, P.; Mariani, L.; Galsky, M.D.; Bellmunt, J.; Necchi, A.; Galsky, M.D. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 49–61. [Google Scholar] [CrossRef]
- Albers, P.; Park, S.I.; Niegisch, G.; Fechner, G.; Steiner, U.; Lehmann, J.; Heimbach, D.; Heidenreich, A.; Fimmers, R.; Siener, R.; et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: Short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]. Ann. Oncol. 2011, 22, 288–294. [Google Scholar] [CrossRef] [PubMed]
- BellBellmunt, J.; Théodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.; et al. Phase III Trial of Vinflunine Plus Best Supportive Care Compared With Best Supportive Care Alone After a Platinum-Containing Regimen in Patients With Advanced Transitional Cell Carcinoma of the Urothelial Tract. J. Clin. Oncol. 2009, 27, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Ullén, A.; Loriot, Y.; Sridhar, S.S.; Sternberg, C.N.; Bellmunt, J.; et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results from the JAVELIN Bladder 100 Trial after ≥2 Years of Follow-Up. J. Clin. Oncol. 2023, 41, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Powles, T.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Mamtani, R.; et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann. Oncol. 2023, 34, 1047–1054. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
- Prunes, B.d.B.; Nunes, J.S.; da Silva, V.P.; Laureano, N.K.; Gonçalves, D.R.; Machado, I.S.; Barbosa, S.; Lamers, M.L.; Rados, P.V.; Kurth, I.; et al. The role of tumor acidification in aggressiveness, cell dissemination and treatment resistance of oral squamous cell carcinoma. Life Sci. 2022, 288, 120163. [Google Scholar] [CrossRef]
- Ralph, A.C.L.; Valadão, I.C.; Cardoso, E.C.; Martins, V.R.; Oliveira, L.M.S.; Bevilacqua, E.M.A.F.; Geraldo, M.V.; Jaeger, R.G.; Goldberg, G.S.; Freitas, V.M. Environmental control of mammary carcinoma cell expansion by acidification and spheroid formation in vitro. Sci. Rep. 2020, 10, 21959. [Google Scholar] [CrossRef]
- Yang, X.; Yin, H.; Zhang, Y.; Li, X.; Tong, H.; Zeng, Y.; Wang, Q.; He, W. Hypoxia-induced autophagy promotes gemcitabine resistance in human bladder cancer cells through hypoxia-inducible factor 1alpha activation. Int. J. Oncol. 2018, 53, 215–224. [Google Scholar]
- Maeda, T.; Kikuchi, E.; Matsumoto, K.; Miyajima, A.; Oya, M. Urinary pH Is Highly Associated with Tumor Recurrence During Intravesical Mitomycin C Therapy for Nonmuscle Invasive Bladder Tumor. J. Urol. 2011, 185, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.T.; Usman, A.H.; Ali, S.; Raza, H.; Shah, A.N.; Mahmmoud Fadelallah Eljack, M. Intravesical mitomycin C efficacy in acidic and alkaline urinary pH: Impact on recurrence-free survival rate after TURBT. Ann. Med. Surg. 2023, 85, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar]
- Griffiths, J.R. Are cancer cells acidic? Br. J. Cancer 1991, 64, 425–427. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Khandelwal, P.; Abraham, S.N.; Apodaca, G. Cell biology and physiology of the uroepithelium. Am. J. Physiol. Renal Physiol. 2009, 297, F1477–F1501. [Google Scholar] [CrossRef]
- Han, J.H.; Jeong, S.H.; Yuk, H.D.; Jeong, C.W.; Kwak, C.; Ku, J.H. Acidic urine is associated with poor prognosis in patients with bladder cancer undergoing radical cystectomy. Front. Oncol. 2022, 12, 964571. [Google Scholar] [CrossRef]
- Han, J.H.; Jeong, S.H.; Yuk, H.D.; Jeong, C.W.; Kwak, C.; Ku, J.H. Acidic Urine Is Associated With Poor Prognosis of Upper Tract Urothelial Carcinoma. Front. Oncol. 2021, 11, 817781. [Google Scholar] [CrossRef]
- Zhao, H.; Tse, R.T.-H.; Cheng, C.K.-L.; Wong, C.Y.-P.; Kong, A.W.-Y.; Chan, R.C.-K.; Chiu, P.K.-F.; Ng, C.-F.; Teoh, J.Y.-C. In vitro cytotoxicity of human urine and its potential toxic parameters towards bladder cancer cells. PLoS ONE 2022, 17, e0276127. [Google Scholar] [CrossRef]
- Mahoney, B.P.; Raghunand, N.; Baggett, B.; Gillies, R.J. Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem. Pharmacol. 2003, 66, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.N.M.; Hisam, N.S.N.; Liew, S.L.; Ugusman, A. Clinical Review: Navitoclax as a Pro-Apoptotic and Anti-Fibrotic Agent. Front. Pharmacol. 2020, 11, 564108. [Google Scholar]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, B.P.; Salvesen, G.S.; Scott, F.L. Human inhibitor of apoptosis proteins: Why XIAP is the black sheep of the family. EMBO Rep. 2006, 7, 988–994. [Google Scholar] [CrossRef]
- Evans, M.K.; Brown, M.C.; Geradts, J.; Bao, X.; Robinson, T.J.; Jolly, M.K.; Vermeulen, P.B.; Palmer, G.M.; Gromeier, M.; Levine, H.; et al. XIAP Regulation by MNK Links MAPK and NFkappaB Signaling to Determine an Aggressive Breast Cancer Phenotype. Cancer Res. 2018, 78, 1726–1738. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T.; Yang, D.; Wang, J.; Li, X.; He, Z.; Chen, F.; Che, X.; Song, X. Expression of the IAP protein family acts cooperatively to predict prognosis in human bladder cancer patients. Oncol. Lett. 2013, 5, 1278–1284. [Google Scholar] [CrossRef]
- Sahoo, S.; Singh, P.; Kalha, B.; Singh, O.; Pal, R. Gonadotropin-mediated chemoresistance: Delineation of molecular pathways and targets. BMC Cancer 2015, 15, 931. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Jo, E.K.; Silwal, P.; Yuk, J.M. AMPK-Targeted Effector Networks in Mycobacterial Infection. Front. Microbiol. 2019, 10, 520. [Google Scholar] [CrossRef]
- Mann, S.S.; Hammarback, J.A. Molecular Characterization of Light Chain-3—A Microtubule-Binding Subunit of Map1a and Map1b. J. Biol. Chem. 1994, 269, 11492–11497. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Yoshimori, T. Autophagy: A regulated bulk degradation process inside cells. Biochem. Biophys. Res. Commun. 2004, 313, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Bjørkøy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Øvervatn, A.; Stenmark, H.; Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005, 171, 603–614. [Google Scholar] [CrossRef]
- Ando, H.; Eshima, K.; Ishida, T. Neutralization of Acidic Tumor Microenvironment (TME) with Daily Oral Dosing of Sodium Potassium Citrate (K/Na Citrate) Increases Therapeutic Effect of Anti-cancer Agent in Pancreatic Cancer Xenograft Mice Model. Biol. Pharm. Bull. 2021, 44, 266–270. [Google Scholar] [CrossRef]
- Robey, I.F.; Baggett, B.K.; Kirkpatrick, N.D.; Roe, D.J.; Dosescu, J.; Sloane, B.F.; Hashim, A.I.; Morse, D.L.; Raghunand, N.; Gatenby, R.A.; et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009, 69, 2260–2268. [Google Scholar] [CrossRef]
- Ando, H.; Ikeda, A.; Tagami, M.; Matsuo, N.C.A.; Shimizu, T.; Ishima, Y.; Eshima, K.; Ishida, T. Oral administration of sodium bicarbonate can en-hance the therapeutic outcome of Doxil® via neutralizing the acidic tumor microenvironment. J. Control Release 2022, 350, 414–420. [Google Scholar] [CrossRef]
- Au, J.L.-S.; Badalament, R.A.; Wientjes, M.G.; Young, D.C.; Warner, J.A.; Venema, P.L.; Pollifrone, D.L.; Harbrecht, J.D.; Chin, J.L.; Lerner, S.P.; et al. Methods to improve efficacy of intravesical mitomycin C: Results of a randomized phase III trial. J. Natl. Cancer Inst. 2001, 93, 597–604. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiruma, K.; Bilim, V.; Kazama, A.; Shirono, Y.; Murata, M.; Tomita, Y. Acidic Microenvironment Enhances Cisplatin Resistance in Bladder Cancer via Bcl-2 and XIAP. Curr. Issues Mol. Biol. 2025, 47, 43. https://doi.org/10.3390/cimb47010043
Hiruma K, Bilim V, Kazama A, Shirono Y, Murata M, Tomita Y. Acidic Microenvironment Enhances Cisplatin Resistance in Bladder Cancer via Bcl-2 and XIAP. Current Issues in Molecular Biology. 2025; 47(1):43. https://doi.org/10.3390/cimb47010043
Chicago/Turabian StyleHiruma, Kaede, Vladimir Bilim, Akira Kazama, Yuko Shirono, Masaki Murata, and Yoshihiko Tomita. 2025. "Acidic Microenvironment Enhances Cisplatin Resistance in Bladder Cancer via Bcl-2 and XIAP" Current Issues in Molecular Biology 47, no. 1: 43. https://doi.org/10.3390/cimb47010043
APA StyleHiruma, K., Bilim, V., Kazama, A., Shirono, Y., Murata, M., & Tomita, Y. (2025). Acidic Microenvironment Enhances Cisplatin Resistance in Bladder Cancer via Bcl-2 and XIAP. Current Issues in Molecular Biology, 47(1), 43. https://doi.org/10.3390/cimb47010043




