Negative mpMRI Rules Out Extra-Prostatic Extension in Prostate Cancer before Robot-Assisted Radical Prostatectomy
Abstract
:1. Introduction
2. Materials and Methods
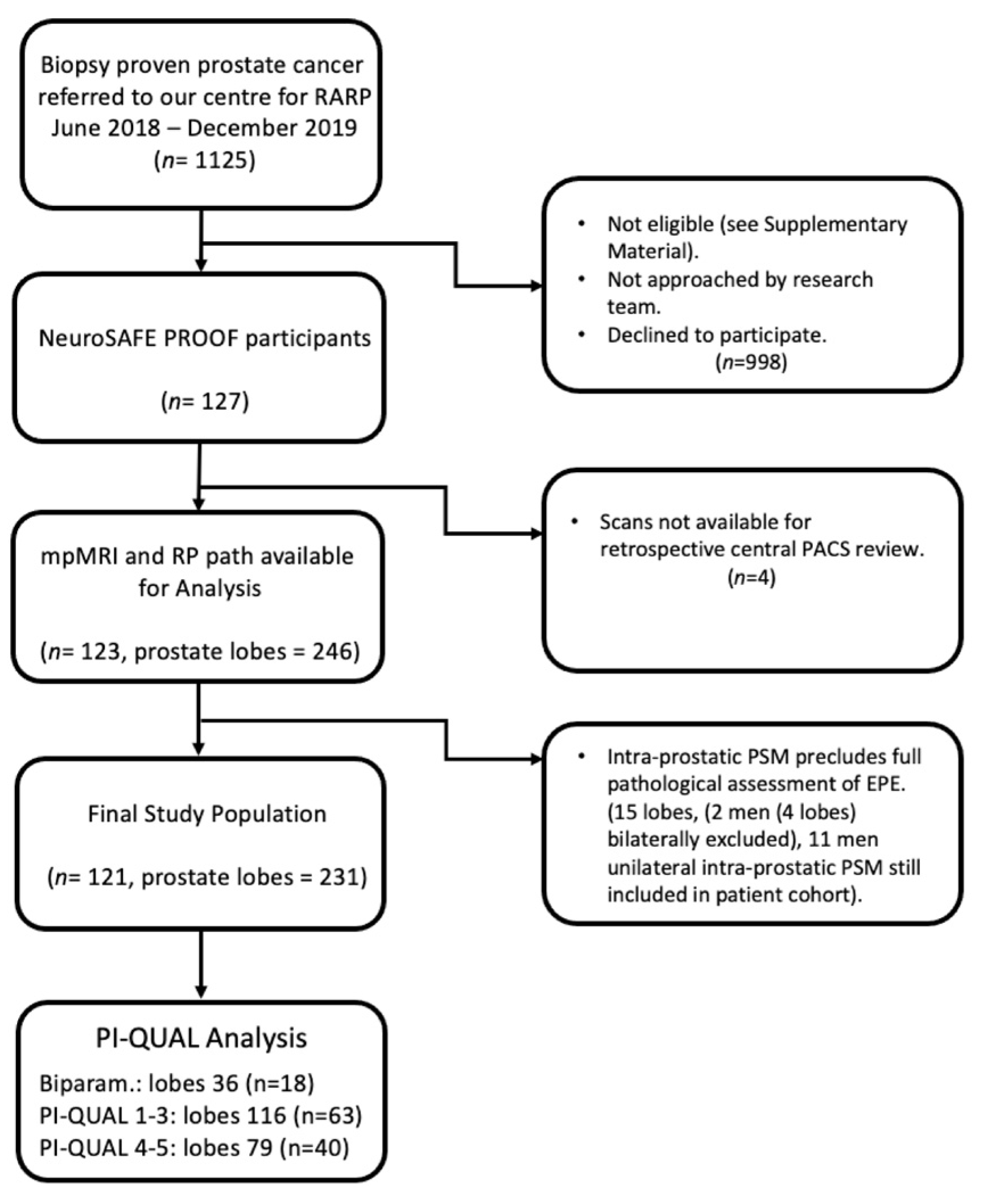
2.1. Study Design and Patient Population
2.2. Standard of Reference
2.3. Imaging Protocols and Evaluation
2.4. Sample Size and Power Calculation
2.5. Statistical Analysis
3. Results
3.1. mpMRI Prediction of EPE
3.2. Scan Quality and Diagnostic Performance
3.3. Accuracy of EPE with Additional Sequences and Clinical Information
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Walz, J.; Burnett, A.L.; Costello, A.J.; Eastham, J.A.; Graefen, M.; Guillonneau, B.; Menon, M.; Montorsi, F.; Myers, R.P.; Rocco, B.; et al. A Critical Analysis of the Current Knowledge of Surgical Anatomy Related to Optimization of Cancer Control and Preservation of Continence and Erection in Candidates for Radical Prostatectomy. Eur. Urol. 2010, 57, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Schiavina, R.; Bianchi, L.; Borghesi, M.; Dababneh, H.; Chessa, F.; Pultrone, C.V.; Angiolini, A.; Gaudiano, C.; Porreca, A.; Fiorentino, M.; et al. MRI Displays the Prostatic Cancer Anatomy and Improves the Bundles Management Before Robot-Assisted Radical Prostatectomy. J. Endourol. 2018, 32, 315–321. [Google Scholar] [CrossRef]
- Marenco, J.; Orczyk, C.; Collins, T.; Moore, C.; Emberton, M. Role of MRI in planning radical prostatectomy: What is the added value? World J. Urol. 2019, 37, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, P.; Sridhara, A.; Ramachandran, N.; Briggs, T.; Nathan, S.; Kelly, J. Achieving Quality Assurance of Prostate Cancer Surgery During Reorganisation of Cancer Services. Eur. Urol. 2015, 68, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Cornford, P. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2020, 79, 243–262. [Google Scholar] [CrossRef]
- Ball, M.W.; Partin, A.W.; Epstein, J.I. Extent of Extraprostatic Extension Independently Influences Biochemical Recurrence-free Survival: Evidence for Further pT3 Subclassification. Urology 2015, 85, 161–164. [Google Scholar] [CrossRef]
- Danneman, D.; Wiklund, F.; Wiklund, N.P.; Egevad, L. Prognostic significance of histopathological features of extraprostatic extension of prostate cancer. Histopathology 2013, 63, 580–589. [Google Scholar] [CrossRef]
- Rapisarda, S.; Bada, M.; Crocetto, F.; Barone, B.; Arcaniolo, D.; Polara, A.; Imbimbo, C.; Grosso, G. The role of multiparametric resonance and biopsy in prostate cancer detection: Comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom. Radiol. 2020, 45, 4178–4184. [Google Scholar] [CrossRef]
- De Rooij, M.; Hamoen, E.H.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Faculty Opinions recommendation of Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur. Urol. 2015, 70, 2189. [Google Scholar] [CrossRef]
- Abrams-Pompe, R.S.; Fanti, S.; Schoots, I.G.; Moore, C.M.; Turkbey, B.; Vickers, A.J.; Walz, J.; Steuber, T.; Eastham, J.A. The Role of Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Primary Staging of Newly Diagnosed Prostate Cancer: A Systematic Review of the Literature. Eur. Urol. Oncol. 2020, 4, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Giganti, F.; Allen, C.; Emberton, M.; Moore, C.M.; Kasivisvanathan, V. Prostate Imaging Quality (PI-QUAL): A New Quality Control Scoring System for Multiparametric Magnetic Resonance Imaging of the Prostate from the PRECISION trial. Eur. Urol. Oncol. 2020, 3, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Giganti, F.; Dinneen, E.; Kasivisvanathan, V.; Haider, A.; Freeman, A.; Kirkham, A.; Punwani, S.; Emberton, M.; Shaw, G.; Moore, C.M.; et al. Inter-reader agreement of the PI-QUAL score for prostate MRI quality in the NeuroSAFE PROOF trial. Eur. Radiol. 2021, 32, 879–889. [Google Scholar] [CrossRef]
- De Rooij, M.; Barentsz, J.O. PI-QUAL v.1: The first step towards good-quality prostate MRI. Eur Radiol. 2022, 32, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Samaratunga, H.; The ISUP Prostate Cancer Group; Montironi, R.; True, L.; Epstein, I.J.; Griffiths, D.F.; Humphrey, A.P.; van der Kwast, T.; Wheeler, T.M.; Srigley, J.R.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 1: Specimen handling. Mod. Pathol. 2010, 24, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magi-Galluzzi, C.; The ISUP Prostate Cancer Group; Evans, A.J.; Delahunt, B.; Epstein, I.J.; Griffiths, D.F.; van der Kwast, T.H.; Montironi, R.; Wheeler, T.M.; Srigley, J.R.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 3: Extraprostatic extension, lymphovascular invasion and locally advanced disease. Mod. Pathol. 2010, 24, 26–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Shtern, F.; Padhani, A.; Tempany, C.M.; Thoeny, H.C.; et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 70, e137–e138. [Google Scholar] [CrossRef]
- Davis, R.; Salmasi, A.; Koprowski, C.; Kim, S.; Kwon, Y.S.; Faiena, I.; Patel, N.; Elsamra, S.E.; Kim, I.Y. Accuracy of Multiparametric Magnetic Resonance Imaging for Extracapsular Extension of Prostate Cancer in Community Practice. Clin. Genitourin. Cancer 2016, 14, e617–e622. [Google Scholar] [CrossRef]
- Alessi, S.; Pricolo, P.; Summers, P.; Femia, M.; Tagliabue, E.; Renne, G.; Petralia, G. Low PI-RADS assessment category excludes extraprostatic extension (>/=pT3a) of prostate cancer: A histology-validated study including 301 operated patients. Eur. Radiol. 2019, 29, 5478–5487. [Google Scholar] [CrossRef] [Green Version]
- Audit NPC. Annual Report 2019-Results from the NPCA Prospective Audit in Enhland and Wales; Royal College of Surgeons: London, UK, 2019. [Google Scholar]
- Allen, D.J.; Hindley, R.; Clovis, S.; O’Donnell, P.; Cahill, D.; Rottenberg, G.; Popert, R. Does body-coil magnetic-resonance imaging have a role in the preoperative staging of patients with clinically localized prostate cancer? BJU Int. 2004, 94, 534–538. [Google Scholar] [CrossRef]
- Falagario, U.; Jambor, I.; Ratnani, P.; Martini, A.; Treacy, P.; Lantz, A.; Wajswol, E.; Papastefanou, G.; Giuseppe, C.; Wiklund, P.; et al. Performance of prostate multiparametric MRI for prediction of prostate cancer extra-prostatic extension according to NCCN risk categories: Implication for surgical planning. Eur. Urol. Open Sci. 2020, 19, e1743–e1744. [Google Scholar] [CrossRef]
- Caglic, I.; Sushentsev, N.; Shah, N.; Warren, A.Y.; Lamb, B.W.; Barrett, T. Comparison of biparametric versus multiparametric prostate MRI for the detection of extracapsular extension and seminal vesicle invasion in biopsy naive patients. Eur. J. Radiol. 2021, 141, 109804. [Google Scholar] [CrossRef] [PubMed]
- Boschheidgen, M.; Schimmöller, L.; Arsov, C.; Ziayee, F.; Morawitz, J.; Valentin, B.; Radke, K.L.; Giessing, M.; Esposito, I.; Albers, P.; et al. MRI grading for the prediction of prostate cancer aggressiveness. Eur. Radiol. 2021, 32, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Mehralivand, S.; Shih, J.H.; Harmon, S.; Smith, C.; Bloom, J.; Czarniecki, M.; Gold, S.; Hale, G.; Rayn, K.; Merino, M.J.; et al. A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI. Radiology 2019, 290, 709–719. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Kongnyuy, M.; Sidana, A.; George, A.K.; Muthigi, A.; Iyer, A.; Ho, R.; Chelluri, R.; Mertan, F.; Frye, T.P.; Su, D.; et al. Tumor contact with prostate capsule on magnetic resonance imaging: A potential biomarker for staging and prognosis. Urol. Oncol. Semin. Orig. Investig. 2016, 35, 30.e1–30.e8. [Google Scholar] [CrossRef]
- Bakir, B.; Onay, A.; Vural, M.; Armutlu, A.; Yıldız, S.; Esen, T. Can Extraprostatic Extension Be Predicted by Tumor-Capsule Contact Length in Prostate Cancer? Relationship With International Society of Urological Pathology Grade Groups. Am. J. Roentgenol. 2020, 214, 588–596. [Google Scholar] [CrossRef]
- Reistaeter, L.A.R.H.O.; Beisland, C.; Honore, A.; Gravdal, K.; Losnegard, A.; Monssen, J.; Alslen, L.A.; Biermann, M. Assessing Extraprostatic Extension with Multiparametric MRI of the Prostate: Mehralivand Extraprostatic Extension Grade or Extraprostatic Extension Likert Scale? Radiol. Imaging Cancer 2020, 2, 1. [Google Scholar]
- Park, K.J.; Kim, M.-H.; Kim, J.K. Extraprostatic Tumor Extension: Comparison of Preoperative Multiparametric MRI Criteria and Histopathologic Correlation after Radical Prostatectomy. Radiology 2020, 296, 87–95. [Google Scholar] [CrossRef]
- Diamand, R.; Ploussard, G.; Roumiguié, M.; Oderda, M.; Benamran, D.; Fiard, G.; Quackels, T.; Assenmacher, G.; Simone, G.; Van Damme, J.; et al. External Validation of a Multiparametric Magnetic Resonance Imaging–based Nomogram for the Prediction of Extracapsular Extension and Seminal Vesicle Invasion in Prostate Cancer Patients Undergoing Radical Prostatectomy. Eur. Urol. 2020, 79, 180–185. [Google Scholar] [CrossRef]
- Gandaglia, G.; Ploussard, G.; Valerio, M.; Mattei, A.; Fiori, C.; Roumiguie, M.; Briganti, A. The Key Combined Value of Multiparametric Magnetic Resonance Imaging, and Magnetic Resonance Imaging-targeted and Concomitant Systematic Biopsies for the Prediction of Adverse Pathological Features in Prostate Cancer Patients Undergoing Radical Prostatectomy. Eur. Urol. 2020, 77, 733–741. [Google Scholar] [PubMed]
- Soeterik, T.F.; van Melick, H.H.; Dijksman, L.M.; Küsters-Vandevelde, H.; Stomps, S.; Schoots, I.G.; Biesma, D.H.; Witjes, J.; van Basten, J.-P.A. Development and External Validation of a Novel Nomogram to Predict Side-specific Extraprostatic Extension in Patients with Prostate Cancer Undergoing Radical Prostatectomy. Eur. Urol. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nyarangi-Dix, J.; Wiesenfarth, M.; Bonekamp, D.; Hitthaler, B.; Schütz, V.; Dieffenbacher, S.; Mueller-Wolf, M.; Roth, W.; Stenzinger, A.; Duensing, S.; et al. Combined Clinical Parameters and Multiparametric Magnetic Resonance Imaging for the Prediction of Extraprostatic Disease—A Risk Model for Patient-tailored Risk Stratification When Planning Radical Prostatectomy. Eur. Urol. Focus 2018, 6, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Losnegård, A.; Reisæter, L.A.R.; Halvorsen, O.J.; Jurek, J.; Assmus, J.; Arnes, J.B.; Honoré, A.; Monssen, J.A.; Andersen, E.; Haldorsen, I.S.; et al. Magnetic resonance radiomics for prediction of extraprostatic extension in non-favorable intermediate- and high-risk prostate cancer patients. Acta Radiol. 2020, 61, 1570–1579. [Google Scholar] [CrossRef]
- Dinneen, E.; Haider, A.; Grierson, J.; Freeman, A.; Oxley, J.; Briggs, T.; Shaw, G. NeuroSAFE frozen section during robot-assisted radical prostatectomy (RARP): Peri-operative and Histopathological Outcomes from the NeuroSAFE PROOF Feasibility Randomised Controlled Trial. BJU Int. 2020, 127, 676–686. [Google Scholar] [CrossRef]
- Petralia, G.; Musi, G.; Padhani, A.R.; Summers, P.; Renne, G.; Alessi, S.; Raimondi, S.; Matei, D.V.; Renne, S.; Jereczek-Fossa, B.A.; et al. Robot-assisted Radical Prostatectomy: Multiparametric MR Imaging–directed Intraoperative Frozen-Section Analysis to Reduce the Rate of Positive Surgical Margins. Radiology 2015, 274, 434–444. [Google Scholar] [CrossRef] [Green Version]


| All (n = 121) | No EPE (n = 82) | EPE Present (n = 39) | p Value † | ||
|---|---|---|---|---|---|
| Patient | Mean age, year ± SD (range) | 56.9 ± 7 | 56.6 ± 7.5 | 57.82 ± 5.6 | 0.34 |
| (40–71) | (40–71) | (48–71) | |||
| Mean PSA, ng/mL ± SD (range) | 8.9 ± 6 | 7.1 ± 3.8 | 12.7 ± 7.9 | <0.001 | |
| (1.2–35) | (1.2–25) | (4.6–35) | |||
| Clinical ISUP, n (%) | <0.001 | ||||
| 1 | 6 (5) | 5 (83.3) | 1 (16.7) | ||
| 2 | 94 (77) | 72 (76.6) | 22 (23.4) | ||
| 3 | 14 (11.6) | 4 (28.6) | 10 (71.4) | ||
| 4 | 6 (5) | 1 (16.7) | 5 (83.3) | ||
| 5 | 1 (0.8) | 0 | 1 (100) | ||
| EAU risk, n (%) | 0.013 | ||||
| Low | 0 | 0 | 0 | ||
| Int | 34 (28.1) | 28 (34.1) | 6 (15.4) | ||
| High | 87 (71.9) | 54 (65.9) | 33 (84.6) | ||
| DRE *, n (%) | <0.001 | ||||
| T1 | 53 (22.9) | 44 (83) | 9 (17) | ||
| T2 | 125 (54.1) | 111 (89) | 14 (11) | ||
| T3 | 53 (22.9) | 33 (62.3) | 20 (37.7) | ||
| mpMRI | Mean time from MRI to operation, days ± SD (range) | 108 ± 68.8 | 114 ± 73.1 | 98 ± 58.3 | 0.23 |
| (2–355) | (2–355) | (6–292) | |||
| Mean prostate volume, cc ± (range) | 36.6 ± 15.0 | 36.3 ± 15.1 | 37.2 ± 14.8 | 0.75 | |
| (12–86) | (13–86) | (14–80) | |||
| Tumour side, n (%) | 0.58 | ||||
| Right | 35 (28.9) | 23 (65.7) | 12 (34.3) | ||
| Left | 22 (18.2) | 16 (72.7) | 6 (27.3) | ||
| Both | 61 (50.4) | 42 (68.9) | 19 (31.1) | ||
| No visible lesion | 3 (2.5) | 1 (33.3) | 2 (66.6) | ||
| Tumour position, n (%) | 0.39 | ||||
| Posterior | |||||
| Anterior | 97 (80.2) | 68 (70.1) | 29 (29.9) | ||
| Both | 8 (6.6) | 4 (50) | 4 (50) | ||
| No visible lesion | 13 (10.7) | 9 (69.2) | 4 (31.8) | ||
| 3 (2.5) | 1 (33.3) | 2 (66.6) | |||
| RP specimen | Mean prostate weight, g ± SD (range) | 43.8 ± 13.9 | 44.6 ± 14.4 | 42.1 ± 12.7 | 0.36 |
| (15–89) | (22–89) | (15–74) | |||
| Mean tumour volume, mls ± SD (range) | 4.3 ± 3.6 | 3.4 ± 2.8 | 6.3 ± 4.5 | <0.001 | |
| (0.25–22.3) | (0.25–12.9) | (1.2–22.30 | |||
| Pathological ISUP, n (%) | 0.003 | ||||
| 1 | |||||
| 2 | 2 (1.7) | 2 (100) | 0 | ||
| 3 | 92 (75.4) | 69 (75) | 23 (25) | ||
| 4 | 23 (18.9) | 10 (43.5) | 13 (56.5) | ||
| 5 | 1 (0.8) | 1 (100) | 0 | ||
| 3 (2.5) | 0 | 3 (100) | |||
| Pathological stage, n (%) | n/a | ||||
| 2a-b | |||||
| 2c | 7 (5.8) | 7 | 0 | ||
| 3a | 75 (62) | 75 | 0 | ||
| 3b | 32 (26.4) | 0 | 32 | ||
| 7 (5.8) | 0 | 7 |
| Reader 1 | Reader 2 | Reader 3 | Readers Combined | |
|---|---|---|---|---|
| SE * | 88.4 | 92.5 | 90.5 | 90.4 |
| (74.9–96) | (79.6–98.4) | (77.4–97.3) | (83.8–94.9) | |
| SP * | 61.7 | 39.5 | 55.5 | 52.3 |
| (54.4–68.7) | (32.4–46.9) | (48–62.9) | (48–56.5) | |
| PPV * | 34.6 | 24.8 | 31.9 | 29.9 |
| (25.7–44.2) | (18.1–32.6) | (23.7–41.1) | (25.3–34.8) | |
| NPV * | 95.9 | 96.1 | 96.2 | 96 |
| (90.6–98.6) | (88.9–99.2) | (90.5–99) | (93.2–97.9) | |
| AUC | 0.84 | 0.77 | 0.83 | 0.82 |
| (0.77–0.92) | (0.68–0.86) | (0.76, 0.9) | (0.77–0.86) |
| Biparametric Scan (n = 18) | PI-QUAL 1–3 (n = 63) | PI-QUAL 4–5 (n = 40) | |
|---|---|---|---|
| SE | 80 (59.3–93.2) | 89.1 (78.8–95.5) | 100 (90.3–100) |
| SP | 48.4 (37.9–59) | 49.8 (43.7–56) | 57 (49.7–64.1) |
| NPV | 90 (78.2–96.7) | 95 (90–98) | 100 (96.7–100) |
| PPV | 29.4 (19–41.7) | 29.8 (23.5–36.9) | 30.3 (22.2–39.4) |
| AUC | 0.76 (0.64–0.88) | 0.78 (0.72–0.84) | 0.92 (0.88–0.96) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinneen, E.; Allen, C.; Strange, T.; Heffernan-Ho, D.; Banjeglav, J.; Lindsay, J.; Mulligan, J.-P.; Briggs, T.; Nathan, S.; Sridhar, A.; et al. Negative mpMRI Rules Out Extra-Prostatic Extension in Prostate Cancer before Robot-Assisted Radical Prostatectomy. Diagnostics 2022, 12, 1057. https://doi.org/10.3390/diagnostics12051057
Dinneen E, Allen C, Strange T, Heffernan-Ho D, Banjeglav J, Lindsay J, Mulligan J-P, Briggs T, Nathan S, Sridhar A, et al. Negative mpMRI Rules Out Extra-Prostatic Extension in Prostate Cancer before Robot-Assisted Radical Prostatectomy. Diagnostics. 2022; 12(5):1057. https://doi.org/10.3390/diagnostics12051057
Chicago/Turabian StyleDinneen, Eoin, Clare Allen, Tom Strange, Daniel Heffernan-Ho, Jelena Banjeglav, Jamie Lindsay, John-Patrick Mulligan, Tim Briggs, Senthil Nathan, Ashwin Sridhar, and et al. 2022. "Negative mpMRI Rules Out Extra-Prostatic Extension in Prostate Cancer before Robot-Assisted Radical Prostatectomy" Diagnostics 12, no. 5: 1057. https://doi.org/10.3390/diagnostics12051057






